Determining how cells distinguish between adaptive and maladaptive signals when they appear to share the same molecular pathways has been a vexing biological problem. The ability to identify distinctive features of a pathophysiological response compared with a physiological response would allow for the rational design of approaches to eliminate or diminish undesirable consequences of pathophysiological responses while preserving the beneficial effects of physiological signals. These issues are especially pertinent in the heart and in understanding the development of heart failure, for which >500,000 new cases are diagnosed in the United States each year. Major factors contributing to worsening heart failure include a number of compensatory neurohormonal signals intended to counteract decreased cardiac output, such as hyperadrenergic stimulation (1). In a recent issue of PNAS, Balijepalli et al. (2) provide new insight into how adrenergic signaling pathways are organized in the heart.
Adrenergic signaling in the myocardium contributes to the control of heart rate (chronotropy), strength of contraction (inotropy), and rate of relaxation (lusitropy) by changing the levels of intracellular Ca2+ or by altering the sensitivity of critical regulatory proteins to Ca2+. Signaling is mediated predominantly by two distinct β-adrenergic receptors, β1 and β2, which differ in their abundance, distribution, and downstream signal transducers (3). Approximately 75% of the cardiac β-adrenergic receptors are β1, which appear to be distributed globally throughout the sarcolemma. β1 receptors couple to the Gs heterotrimeric G protein. The less-abundant β2 receptors reside predominantly in caveolae (4), specialized compartments of the plasma membrane organized by caveolins. Caveolae are flask-shaped membrane invaginations rich in cholesterol and glycosphingolipids that house and coordinate multiple signaling components, many of which appear to be dedicated to Ca2+ signaling (5). Besides their distinct homes, β2 receptors also differ from β1 in that they couple to both Gs and Gi. Nevertheless, stimulation of either β1 or β2 activates adenylyl cyclase to increase intracellular cAMP. In turn, cAMP activates protein kinase A, resulting in the phosphorylation of key elements of the contractile apparatus and of proteins that control internal Ca2+ levels. Prominent among the PKA targets are the L-type voltage-gated Ca2+ channels (CaV1.2), which open upon membrane depolarization, allowing Ca2+ to enter the cell. The “receptors” for this Ca2+ signal are the ryanodine receptors (RyR2), Ca2+ release channels on the sarcoplasmic reticulum (SR) that flood the cytoplasm with additional Ca2+ that then initiates contraction (Fig. 1). PKA phosphorylation of L-type Ca2+ channels potentiates inward Ca2+ current and thereby augments contraction.
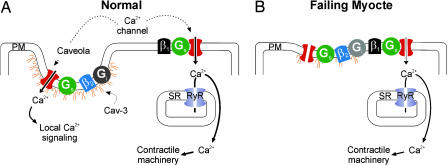
Fig. 1.
Caveolar localization of the β2-adrenergic receptor/L-type Ca2+ channel signaling complex. (A) The β2 receptor and its accompanying Gi and Gs proteins are depicted within a caveola, organized by Cav-3. Localized L-type Ca2+ channel potentiation in response to β2 agonists may contribute to Ca2+ signaling cascades that are distinct from the larger Ca2+ pool involved in excitation–contraction coupling. The β1 receptors, coupled solely to Gs, are depicted on the plasma membrane (PM) and outside of caveolae. Most L-type Ca2+ channels are closely opposed to RyR in the SR. Influx of Ca2+ through L-type Ca2+ channels triggers opening of RyRs and release of Ca2+ from the SR to activate the contractile machinery. (B) In heart failure, signaling through β2 receptors blunts β1 potentiation of L-type Ca2+ channels through a Gi-dependent mechanism (19). This blunted response may result from dysregulation of caveolar organization, thus disturbing the compartmentation of the β1 and β2 receptors.
Electrophysiological studies of L-type Ca2+ current after adrenergic stimulation revealed important consequences of the localization and G protein-coupling differences between β1 and β2 receptor subtypes. By isolating L-type Ca2+ channels within a patch pipette, Chen-Izu et al. (6) determined that remote stimulation (outside of the pipette) of β1 increased Ca2+ channel current within the pipette, suggesting that β1 signaling included a diffusive second messenger. In contrast, β2-specific agonists were effective only when included within the pipette. This membrane-delimited β2 signaling depended on Gi, because inactivation of Gi with pertussis toxin made β2 signaling diffusive. Several other studies have provided additional evidence for important functional consequences of differential signaling through the β-adrenergic receptors. For example, sustained signaling through β1 receptors led to myocyte apoptosis; this β1-mediated proapoptotic signal depended on Ca2+ influx through L-type Ca2+ channels and activation of Ca2+ calmodulin-dependent kinase II (CaMKII) (7). On the other hand, β2 activation was protective against apoptotic signals (8–10). Like the membrane-delimited activation of L-type Ca2+ currents, coupling of β2 to Gi was also necessary for prosurvival signaling; Gi inactivation with pertussis toxin blocked protection (8).
Balijepalli et al. (2) provide a new wrinkle to this compartmentation story. They demonstrate for the first time that L-type Ca2+ channels can be found within caveolae in cardiac myocytes and that β2 activation of L-type Ca2+ channels requires intact caveolae. Electron microscopy showed α1C, the L-type Ca2+ channel pore-forming subunit, within caveolae in neonatal cardiac myocytes. Sucrose density gradients revealed the cosedimentation of α1C with caveolin-3 (Cav-3), the predominant caveolin isoform in the heart. As previously found, the β2 receptor was enriched in this fraction, and all of the important components of the β2-adrenergic signaling complex (Gs, Gi, adenylyl cyclase, and PKA) were also present. Interestingly, disruption of caveolae with 10 mM methyl β-cyclodextrin (MβCD) or inhibition of Cav-3 by small interfering RNA prevented β2 stimulation of L-type Ca2+ channel current, suggesting that caveolar localization was necessary. Previously, it was known that Kv1.5 potassium channels are preferentially localized in caveolae (11), and a fraction of cardiac Na+ channels cosediment with Cav-3 (12), although the functional consequences of the location of either ion channel within caveolae have not yet been determined.
The demonstration that β2-mediated potentiation of L-type Ca2+ currents was caveolae-dependent may have important consequences for the understanding and treatment of cardiac hypertrophy and heart failure. Approximately 90% of L-type Ca2+ channels in adult cardiac myocytes are found within T tubules (13), a specialized architecture of tubular invaginations of the sarcolemma, where they face RyRs in the juxtaposed SR. This organization ensures instantaneous release of SR Ca2+ stores throughout the cytoplasm after membrane depolarization. Although a small population of RyRs have been found in regions of the SR that are not associated with T tubules or the plasmalemma (14), definitive demonstration and localization of “orphan” L-type Ca2+ channels have been more elusive. The presence of L-type Ca2+ channels not necessarily associated with the SR, as implied in this new study by Balijepalli et al. (2), raises the possibility that Ca2+ signaling through this subpopulation of channels may provide a specialized function different from excitation–contraction coupling, such as contributing to the signaling cascades that initiate cardiac hypertrophy. Although several lines of evidence have implicated Ca2+ signaling in the development of hypertrophy, it has been difficult to understand how a myocyte can distinguish between hypertrophy-inducing signals and the much larger pool of internal Ca2+ that rapidly rises and falls over a 10-fold concentration range with each heart beat (15). A recent report suggests that the hypertrophic signal endothelin-1 increases nuclear Ca2+ by an inositol 1,4,5-trisphosphate (IP3)-triggered release from IP3 receptors in the nuclear membrane (16), the first convincing demonstration of a separable Ca2+ signal in myocytes. Balijepalli et al. (2) present another example supporting the idea of cardiac Ca2+-signaling microdomains, this one being in the cytoplasm rather than the nucleus. The emerging picture here is that focal Ca2+ changes, whether modest or large, at the site of Ca2+ entry can signal downstream pathways, and that this signaling is restricted because of fast termination and rapid diffusion or extrusion, with little change in overall Ca2+ concentration. Such a mechanism would allow for specific signaling despite the normal “background” Ca2+ fluctuations that are a part of excitation–contraction coupling. It is interesting to speculate that protective adrenergic signaling through β2 receptors may be related to caveolar localization because CaMKII, necessary for the contrasting β1-mediated apoptotic signal, has not been reported in caveolae.
The dependence on caveolar localization for β2-mediated activation of L-type Ca2+ channels and the role of both β2 and Ca2+ signaling in cardiovascular physiology also place a new focus on caveolae and Cav-3 in particular. Could dysregulation of caveolar organization contribute to heart failure or cardiac hypertrophy by affecting this signaling complex? The development of heart failure in Cav-3−/− mice (17) makes this an intriguing possibility and suggests that pharmacological modulation of caveolae may be a fruitful avenue for drug development. Further, in at least one report (18), heart failure was accompanied by Cav-3 down-regulation. If destabilization or loss of caveolae contributed to heart failure such that β2 signaling was no longer segregated (Fig. 1), it might provide a cogent explanation for the observation that adrenergic activation of L-type Ca2+ currents in the failing heart is blunted because of β2 stimulation of a Gi-dependent pathway (19).
These observations also raise many new questions. For example, how is a subpopulation of L-type Ca2+ channels targeted to caveolae? Because a postsynaptic density protein 95/discs large/ZO-1 (PDZ)-binding motif in the C terminus of β2 is important for receptor trafficking and coupling to Gi (20), it is interesting to consider that a PDZ motif in the C terminus of α1C, previously shown to be important for excitation–transcription coupling in neurons (21), might provide the caveolar localization signal to L-type Ca2+ channels. Also, certain key experiments in the Balijepalli et al. (2) study were performed in neonatal myocytes, which lack the elegant T tubule architecture important for excitation–contraction coupling in adult myocytes. It will be essential to determine whether caveolar localization of L-type Ca2+ channels is critical for β2-regulated L-type Ca2+ currents in the adult and whether this arrangement is perturbed in heart failure. Regardless, this study by Balijepalli et al. (2) opens new possibilities for the modulation of pathophysiological signaling of the β-adrenergic system that may lead to novel therapies for heart failure.
Acknowledgments
This work was supported by National Institutes of Health Grant HL 71165 (to G.S.P.) and American Heart Association Grant 0555875T. G.S.P. is a recipient of the Irma T. Hirschl Career Scientist Award and is the Esther Aboodi Assistant Professor of Medicine. M.S.G. is supported by the Columbia University M.D.–Ph.D. Program.
Conflict of interest statement: No conflicts declared.
See companion article on page 7500 in issue 19 of volume 103.
References
- 1.Packer M. Circulation. 1988;77:721–730. doi: 10.1161/01.cir.77.4.721. [DOI] [PubMed] [Google Scholar]
- 2.Balijepalli R. C., Foell J. D., Hall D. D., Hell J. W., Kamp T. J. Proc. Natl. Acad. Sci. USA. 2006;103:7500–7505. doi: 10.1073/pnas.0503465103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinberg S. F. J. Mol. Cell. Cardiol. 2004;37:407–415. doi: 10.1016/j.yjmcc.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 4.Rybin V. O., Xu X., Lisanti M. P., Steinberg S. F. J. Biol. Chem. 2000;275:41447–41457. doi: 10.1074/jbc.M006951200. [DOI] [PubMed] [Google Scholar]
- 5.Cohen A. W., Hnasko R., Schubert W., Lisanti M. P. Physiol. Rev. 2004;84:1341–1379. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- 6.Chen-Izu Y., Xiao R.-P., Izu L. T., Cheng H., Kuschel M., Spurgeon H., Lakatta E. G. Biophys. J. 2000;79:2547–2556. doi: 10.1016/S0006-3495(00)76495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu W. Z., Wang S. Q., Chakir K., Yang D., Zhang T., Brown J. H., Devic E., Kobilka B. K., Cheng H., Xiao R. P. J. Clin. Invest. 2003;111:617–625. doi: 10.1172/JCI16326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Communal C., Singh K., Sawyer D. B., Colucci W. S. Circulation. 1999;100:2210–2212. doi: 10.1161/01.cir.100.22.2210. [DOI] [PubMed] [Google Scholar]
- 9.Zaugg M., Xu W., Lucchinetti E., Shafiq S. A., Jamali N. Z., Siddiqui M. A. Q. Circulation. 2000;102:344–350. doi: 10.1161/01.cir.102.3.344. [DOI] [PubMed] [Google Scholar]
- 10.Chesley A., Lundberg M. S., Asai T., Xiao R.-P., Ohtani S., Lakatta E. G., Crow M. T. Circ. Res. 2000;87:1172–1179. doi: 10.1161/01.res.87.12.1172. [DOI] [PubMed] [Google Scholar]
- 11.Martens J. R., Sakamoto N., Sullivan S. A., Grobaski T. D., Tamkun M. M. J. Biol. Chem. 2001;276:8409–8414. doi: 10.1074/jbc.M009948200. [DOI] [PubMed] [Google Scholar]
- 12.Yarbrough T. L., Lu T., Lee H.-C., Shibata E. F. Circ. Res. 2002;90:443–449. doi: 10.1161/hh0402.105177. [DOI] [PubMed] [Google Scholar]
- 13.Shannon T. R., Wang F., Puglisi J., Weber C., Bers D. M. Biophys. J. 2004;87:3351–3371. doi: 10.1529/biophysj.104.047449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franzini-Armstrong C., Protasi F., Tijskens P. Ann. N.Y. Acad. Sci. 2005;1047:76–85. doi: 10.1196/annals.1341.007. [DOI] [PubMed] [Google Scholar]
- 15.Molkentin J. D. J. Clin. Invest. 2006;116:623–626. doi: 10.1172/JCI27824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu X., Zhang T., Bossuyt J., Li X., McKinsey T. A., Dedman J. R., Olson E. N., Chen J., Brown J. H., Bers D. M. J. Clin. Invest. 2006;116:675–682. doi: 10.1172/JCI27374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woodman S. E., Park D. S., Cohen A. W., Cheung M. W. C., Chandra M., Shirani J., Tang B., Jelicks L. A., Kitsis R. N., Christ G. J., et al. J. Biol. Chem. 2002;277:38988–38997. doi: 10.1074/jbc.M205511200. [DOI] [PubMed] [Google Scholar]
- 18.Petrashevskaya N. N., Bodi I., Koch S. E., Akhter S. A., Schwartz A. Cardiovasc. Res. 2004;63:561–572. doi: 10.1016/j.cardiores.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 19.He J.-Q., Balijepalli R. C., Haworth R. A., Kamp T. J. Circ. Res. 2005;97:566–573. doi: 10.1161/01.RES.0000181160.31851.05. [DOI] [PubMed] [Google Scholar]
- 20.Xiang Y., Kobilka B. Proc. Natl. Acad. Sci. USA. 2003;100:10776–10781. doi: 10.1073/pnas.1831718100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weick J. P., Groth R. D., Isaksen A. L., Mermelstein P. G. J. Neurosci. 2003;23:3446–3456. doi: 10.1523/JNEUROSCI.23-08-03446.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]