Abstract
N-glycosylation of a mAb may have a major impact on its therapeutic merits. Here, we demonstrate that expression of a hybrid enzyme (called xylGalT), consisting of the N-terminal domain of Arabidopsis thaliana xylosyltransferase and the catalytic domain of human β-1,4-galactosyltransferase I (GalT), in tobacco causes a sharp reduction of N-glycans with potentially immunogenic core-bound xylose (Xyl) and fucose (Fuc) residues as shown by Western blot and MALDI-TOF MS analysis. A radioallergosorbent test inhibition assay with proteins purified from leaves of WT and these transgenic tobacco plants using sera from allergic patients suggests a significant reduction of potential immunogenicity of xylGalT proteins. A mAb purified from leaves of plants expressing xylGalT displayed an N-glycan profile that featured high levels of galactose, undetectable xylose, and a trace of fucose. Hence, a transgenic plant expressing the hybrid GalT might yield more effective and safer monoclonals for therapeutic purposes than WT plants and even transgenic plants expressing the unchanged GalT.
Keywords: biopharmaceutical, glycosylation, immunogenicity
Plants have the potential to become cost-effective and safe factories for the production of recombinant therapeutic proteins, particularly when relatively large volumes are required. Biopharmaceuticals, and especially mAbs, are a quickly expanding group of therapeutics, with hundreds of products under development (1, 2). Yet, the range of applications that can be accommodated from this promising source is limited by the atypical N-glycan composition of plant-derived mAbs due to differences in the biosynthesis of N-linked glycans between plants and mammals (3). Because N-linked glycans have been suggested to play an essential role in determining the efficiency of IgG interactions with Fcγ receptors, plant-produced mAbs may be unsuitable for some of the intended therapeutic aims (4).
The criteria specifying “plantibodies” will be determined by therapeutic and commercial considerations. From a therapeutic point of view, they will have to be as close to naturally occurring IgG as possible and as effective and safe as mAbs that are currently being produced with mammalian cell cultures. Typical human and mouse IgG contains three major glycoforms bound to its single N-glycosylation site, the majority being biantennary, monogalactosylated N-glycans with a core-bound fucose (Fuc) (5).
Biosynthesis of N-linked glycans is initiated with the transfer of a lipid-linked oligosaccharide moiety (Glc3Man9GlcNAc2, in which Man is mannose and GlcNAc is N-acetylglucosamine) to a nascent polypeptide chain in the endoplasmic reticulum (ER) (6). In fact, it appears that the end product of ER-associated N-glycan synthesis may be identical in all eukaryotes examined up until now with the exception of trypanosomatids (7). It is beyond the ER and mainly in the Golgi that the wide diversity in eukaryotic N-glycan structures is created. Through a series of trimming reactions by glycosidases in the ER and cis-Golgi compartments, the so-called high-Man N-glycans (Man5–9GlcNAc2) are produced. The actual formation of complex-type N-glycans is initiated with the addition of the first GlcNAc onto Man5GlcNAc2.
The final N-glycan structures in a given cell are shaped by the combined action of glycosyltransferases and glycosidases lining the membranes of the Golgi stack. Plant and mammalian mannosidase II (Man-II), for example, will act only when a terminal GlcNAc residue is present on the α-1,3 arm of the N-glycan (8–10). In addition, the sequential distribution of the enzymes also may play an important role in determining N-glycan structure, as was shown by expression of chimeric glycosyltransferases in BHK-21 cell cultures (11).
In a previous study, we showed that expression of human β-1,4-galactosyltransferase I (GalT) in tobacco plants results in the introduction of terminal β-1,4-galactose residues on endogenous plant N-glycans as well as on N-glycans of recombinant antibodies coexpressed in these plants (12). However, the galactose carrying N-glycans still contained the plant-specific β-1,2-xylose and α-1,3-Fuc residues linked to the core. These xylose (Xyl) and Fuc epitopes have been implicated in the allergenicity of certain plant and parasite proteins, which may pose a problem when plants are going to be used for the production of therapeutic mAbs (13–15).
A number of studies using hybrid glycosyltransferases have established that the cytoplasmic tail, transmembrane domains, and stem regions (i.e., the CTS domains) of several of these enzymes, including those of human GalT and plant core β-1,2-xylosyltransferase (XylT), play a decisive role in their sub-Golgi distribution (11, 16, 17). In the current study, we examined whether altering the localization of human GalT in plant cells by swapping its natural CTS domain with one from Arabidopsis thaliana XylT, could be used to modify N-glycosylation in plants and to reduce fucosylation and xylosylation of the chitobiose core. The results indicate that expression of the hybrid enzyme in tobacco causes high-level galactosylation of N-glycans and a steep decrease in the level of N-glycans with core-bound Xyl and Fuc. Concomitantly, radioallergosorbent test (RAST) assays indicate that the allergenic potential of proteins from a typical transgenic line is greatly reduced. The N-glycans of a mAb produced in a transgenic plant expressing the xylGalT gene are almost completely devoid of Xyl and Fuc residues.
Results
Construction of Chimeric GalT Gene and Tobacco Transformation.
An A. thaliana cDNA encoding XylT was isolated from a cDNA library by a previously described PCR-based sibling selection procedure (18). XylT activity was confirmed by immunostaining of transfected CHO cells with a Xyl-specific antibody purified from rabbit anti-horseradish peroxidase (HRP) antiserum (19). The DNA fragment covering the N-terminal part of XylT comprising the localization signals was amplified by PCR and fused with a PCR fragment containing the catalytic domain of human GalT. The resulting ORF encodes a fusion protein containing the first 53 amino acids of A. thaliana XylT fused with amino acids 69–398 of human GalT. The transformations with a plant transformation vector featuring the hybrid gene under the control of the CaMV 35S promoter displayed lower transformation efficiencies than earlier experiments with the full-length GalT (data not shown). In addition, pollen production and seed set were greatly reduced.
Immunological Analysis of Tobacco Leaf Proteins.
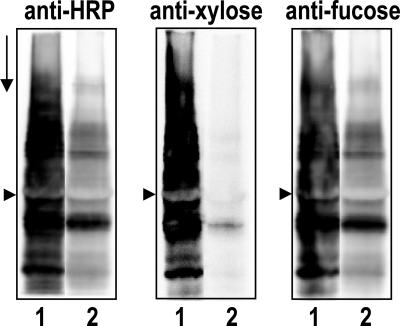
Based on Western blot analysis of transgenic plants with the lectin RCA (Ricinus communis agglutinin) to screen for galactosylated N-glycans (data not shown), a typical transgenic line, xylGalT12, was selected from a number of lines expressing hybrid GalT for further Western blot analysis with anti-HRP antibodies and fractions thereof (19). In Fig. 1, a Western blot showed clearly that binding of the anti-HRP and its β-1,2-Xyl- or α-1,3-Fuc-specific fractions with xylGalT leaf proteins (lane 2) was strongly reduced compared with binding with WT leaf proteins (lane 1).
Fig. 1.
Western blots of total leaf protein from WT (lane 1) and line xylGalT12 (lane 2) plants. The blots were probed with anti-HRP, anti-Xyl, and anti-Fuc antibodies as indicated. The arrowheads mark the position of the large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase, and the arrow points in the direction of electrophoresis.
N-Glycan Analysis of the Transgenic Plants.
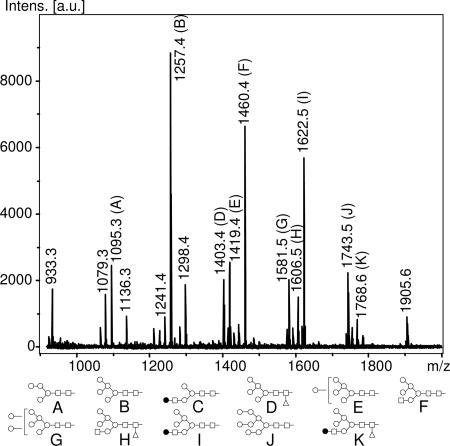
MALDI-TOF analysis of leaf proteins from xylGalT12 plants revealed a highly complex pattern of almost 40 N-glycans, of which only 16 are represented by a relative peak area of >1.5% (Fig. 2 and Table 1). One major class of N-glycans consisted of high-Man oligosaccharides (Man5–9GlcNAc2) totaling up to 34% of the total relative peak area. Another very abundant class of N-glycans was made up of hybrid oligosaccharides, of which the products at m/z = 1,460.4 and 1,622.5 were by far the most abundant components, together accounting for 27% of total N-glycans, as estimated by relative peak area. These oligosaccharides, containing one GlcNAc and at least one additional hexose residue linked to the trimannosyl core structure, are almost completely absent in WT plants (Table 2). In line with the Western blot results described above, it was not surprising to find that the abundance of N-glycans containing Xyl and Fuc was strongly reduced from ≈86% in WT plants to 26% (Table 2). Small amounts of the characteristic WT N-glycan GlcNAc2Man3(Xyl)(Fuc)GlcNAc2 and its degradation products, GlcNAcMan3(Xyl)(Fuc)GlcNAc2 and Man3(Xyl)(Fuc)GlcNAc2, were also detectable.
Fig. 2.
MALDI-TOF spectra of [M+Na+] ions from m/z of 900–2,000 from xylGalT12 N-glycans. Diagrams indicating the proposed structures of peaks representing >4% of total peak area or mentioned in the text have been added below the spectrum, and ions representing >1.5% of total peak area have been denoted with their corresponding m/z value.
Table 1.
MALDI-TOF analysis of N-glycans [M+Na+] from xylGalT12 leaf proteins treated with β-1,4-galactosidase (+β-galase) or left untreated (−β-galase)
| Observed m/z | Composition | Proposed structure | −β-galase | +β-galase |
|---|---|---|---|---|
| 933.3 | H3N2 | Man3GlcNAc2 | 2.6 | 2.2 |
| 1,079.3 | H3N2F1 | Man3(Fuc)GlcNAc2 | 2.5 | 2.9 |
| 1,095.3 | H4N2 | Man4GlcNAc2 | 4.2 | 4.1 |
| 1,136.3 | H3N3 | GlcNAcMan3GlcNAc2 | 1.6 | 2.2 |
| 1,241.4 | H4N2F1 | Man4(Fuc)GlcNAc2 | 1.7 | 1.7 |
| 1,257.4 | H5N2 | Man5GlcNAc2 | 16.6 | 17.0 |
| 1,298.4 | H4N3 | GlcNAcMan4GlcNAc2 | 3.2 | 4.2 |
| 1,403.4 | H5N2F1 | Man5(Fuc)GlcNAc2 | 4.1 | 4.2 |
| 1,419.4 | H6N2 | Man6GlcNAc2 | 5.0 | 5.0 |
| 1,460.4 | H5N3 | GlcNAcMan5GlcNAc2 | 14.7 | 28.1 |
| 1,581.5 | H7N2 | Man7GlcNAc2 | 4.9 | 4.5 |
| 1,606.5 | H5N3F1 | GlcNAcMan5(Fuc)GlcNAc2 | 3.3 | 4.6 |
| 1,622.5 | H6N3 | GalGlcNAcMan5GlcNAc2 | 12.5 | 0.3 |
| 1,743.5 | H8N2 | Man8GlcNAc2 | 5.4 | 5.4 |
| 1,768.6 | H6N3F1 | GalGlcNAcMan5(Fuc)GlcNAc2 | 1.9 | 0.2 |
| 1,905.6 | H9N2 | Man9GlcNAc2 | 2.5 | 2.3 |
| Total | 86.7 | 88.9 |
Values indicate percentage of total peak area. N-glycans representing <1.5% of total peak area in untreated samples have been omitted. F, fucose; H, hexose; N, N-acetylglucosamine.
Table 2.
MALDI-TOF MS analysis of N-glycans from WT and a random selection of RCA-positive tobacco xylGalT lines
| Line | High-Man | Hybrids | Xyl/Fuc |
|---|---|---|---|
| WT | 13.3 | 0.5 | 86.4 |
| 1 | 29.6 | 21.8 | 56.8 |
| 3 | 49.8 | 41.2 | 17.9 |
| 5 | 30.2 | 50.4 | 31.6 |
| 7 | 47.7 | 39.7 | 19.9 |
| 8 | 38.2 | 39.0 | 29.5 |
| 10 | 35.4 | 54.4 | 26.4 |
| 12 | 34.4 | 41.5 | 26.2 |
| 14 | 31.8 | 45.2 | 33.7 |
| 18 | 27.2 | 36.9 | 51.4 |
| 19 | 38.1 | 50.2 | 22.7 |
Numbers indicate the percentage of total peak area represented by Man5–9 peaks (“high-Man”), N-glycans with only one GlcNAc and at least one hexose residue in addition to the trimannosyl core structure (“Hybrids”), and N-glycans containing at least one Xyl or Fuc residue (“Xyl/Fuc”).
A comparison of MALDI-TOF spectra of xylGalT12 N-glycans treated with a β-1,4-galactose-specific galactosidase from Streptococcus pneumoniae with untreated N-glycans from this line revealed a number of significant changes (Table 1). The most striking difference concerned a peak at m/z = 1,622.5 that almost completely disappeared upon galactosidase digestion, demonstrating that this was indeed very likely to be GalGlcNAcMan5GlcNAc2. Conversely, the peak area of a digestion product at m/z = 1,460.4 was greatly increased. The fact that the area of this peak at m/z = 1,460.4 increased with an amount equivalent to the original 1,622.5 peak suggested that it was made up of GlcNAcMan5GlcNAc2 and not GalMan4GlcNAc2, another hybrid N-glycan with an equal m/z. Otherwise, the increase of this 1,460.4 peak would have been less steep, or the peak area might have even diminished.
The galactosidase-induced decrease of the peak area of the oligosaccharide at m/z = 1,768.6 suggested that this N-glycan was galactosylated as well, thereby confirming its identity as GalGlcNAcMan5(Fuc)GlcNAc2 (Table 1). This product appeared to be quantitatively converted to GlcNAcMan5(Fuc)GlcNAc2 at m/z = 1,606.5 upon galactosidase digestion, as was reflected in an equivalent increase in peak area.
The N-glycan profile of xylGalT12 was quite typical of tobacco plants containing high levels of galactosylated N-glycans resulting from expression of the hybrid GalT (Table 2). These transgenics contained equally high levels of high-Man and hybrid N-glycans as line 12, which also coincided with a sharp decrease of Xyl or Fuc residues.
The steep reduction of N-glycans with Xyl or Fuc residues in xylGalT12 did not appear to be due to a decrease of XylT or core α-1,3-fucosyltransferase activity, because activities of both of these enzymes were comparable in microsomal preparations from WT and xylGalT12 leaves (Table 3). As expected, the WT leaves did not display GalT activity, but this activity could be detected clearly in xylGalT12 plants.
Table 3.
Enzyme activities of glycosyltransferases in microsomal preparations of WT and xylGalT12 plants
| Enzyme | Acceptor | Enzyme activity, pmol/min per mg of protein |
|
|---|---|---|---|
| WT | xylGalT12 | ||
| GalT | GlcNAcβ-S-pNP | — | 57.0 |
| XylT | GlcNAc-β1-2-Man-α1-3(Man-α1-6)Man-β-R | 0.2 | 0.2 |
| FucT | GlcNAcβ1-2-Man-α1-3(GlcNAc-β1-2-Man-α1-6)Man-β1-4GlcNAc-β1-4(Fuc-α1-6)GlcNAc-GP | 6.8 | 3.8 |
R, O-(CH2)8-COOCH3; GP, IgG glycopeptide; FucT, core α-1,3-fucosyltransferase; —, not detected.
Effect of xylGalT Expression on IgE Binding.
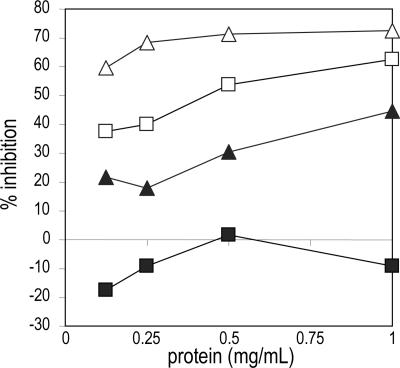
In view of the alleged immunogenic role of the core-bound Xyl and Fuc residues, the allergenic potential of proteins from line xylGalT12 compared with proteins from WT plants was assayed with two sera (pf 192 and pf 41) containing high levels of IgE directed against glycoepitopes, with pf 192 having a preference for Xyl and pf 41 having a preference for Fuc (15). In a RAST inhibition assay, it was shown that IgE binding of these two sera to grass pollen proteins was at least 10 times less efficiently inhibited by xylGalT12 leaf proteins than by those from the WT plant (Fig. 3).
Fig. 3.
RAST inhibition with total protein from leaves of WT (open symbols) and xylGalT12 (filled symbols) plants and anti-CCD-positive sera from patients pf 41 (triangles) and pf 192 (squares).
N-Glycosylation of a mAb in xylGalT12.
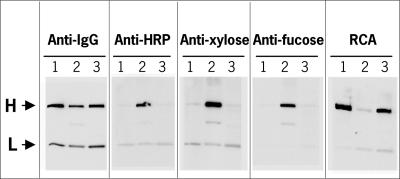
The xylGalT12 line was emasculated and fertilized by pollen from line MGR48-31-4, a single-copy transgenic tobacco line homozygous for the mAb MGR48. From a number of F1 plants, line XGM8 expressing both MGR48 and xylGalT genes as detected by anti-IgG and RCA blot analysis, respectively, was selected for Western blot and N-glycan analysis of the mAb. To this end, protein G chromatography was used to isolate and purify MGR48 IgG from XGM8 leaves from hybridoma and from transgenic tobacco line MGR48-31-4 (20). Western blot analysis with anti-IgG showed that approximately equal amounts of MGR48 had been loaded in all lanes (Fig. 4). The heavy chain of MGR48 from WT tobacco stained strongly upon probing with anti-HRP, as expected (Fig. 4, anti-HRP, lane 2). In contrast, there was no clear difference between anti-HRP binding of the heavy chain from the hybridoma control and the antibody prepared from the xylGalT-expressing line, which suggested a sharp reduction of core fucosylation and xylosylation in xylGalT12 as observed for the endogenous glycoproteins (compare Fig. 1, lanes 1 and 2). Predictably, the anti-Xyl and anti-Fuc antibodies revealed results that are very similar to those obtained with the total anti-HRP probe (Fig. 4). The RCA lectin blot showed that MGR48 purified from hybridoma and xylGalT transformed plants were galactosylated to a similar extent (Fig. 4).
Fig. 4.
Western blots of purified antibody MGR48 from hybridoma cells (lane 1), a WT tobacco line expressing MGR48 (lane 2), and a tobacco line expressing both MGR48 and xylGal (lane 3). The blots were probed with anti-IgG, anti-HRP, anti-Xyl, and anti-Fuc antibodies and RCA as indicated. The position of the heavy (H) and light (L) chains is indicated with arrows.
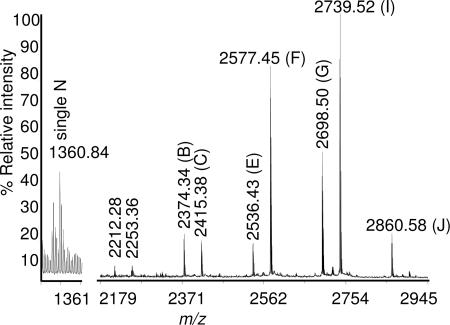
N-glycosylation of the purified MGR48 antibody from xylGalT12 plants was analyzed in more detail by MALDI-TOF MS of an HPLC-fractionated tryptic digest (Fig. 5 and Table 4). This analysis showed that a small but significant proportion of the glycopeptide from the hinge region contained just a single GlcNAc residue at m/z = 1,360.8, possibly resulting from an endogenous endoglycosidase. The N-glycans attached to the two most abundant glycopeptides of the IgG (i.e., GalGlcNAcMan5GlcNAc2 and GlcNAcMan5GlcNAc2) were also among the most abundant oligosaccharides found in leaf glycoproteins as a whole. Hence, it was somewhat surprising to find that Man7GlcNAc2 instead of Man5GlcNAc2 appeared to prevail as the main high-Man structure attached to MGR48. However, the most remarkable finding of this analysis related to the almost complete absence of N-glycans containing Xyl or Fuc; only 0.4% of glycopeptides contained a fucosylated N-glycan, and glycopeptides with Xyl were completely lacking.
Fig. 5.
MALDI-TOF spectrum of the glycopeptides comprising the tryptic EEQFNSTFR fragments from MGR48 produced in tobacco line XGM8. Diagrams depicting the proposed structures of the N-glycans carried by glycopeptides with >3% MALDI intensity can be found below the spectrum in Fig. 2.
Table 4.
MALDI-TOF analysis of the glycosylated Fc peptide EEQFNSTFR from mAb MGR48 purified from XGM8
| Proposed N-glycan structure | m/z, theoretical | m/z, observed | MALDI intensity, % |
|---|---|---|---|
| N | 1,360.60 | 1,360.84 | 11.7 |
| Man4GlcNAc2 | 2,211.94 | 2,212.28 | 1.2 |
| GlcNAcMan3GlcNAc2 | 2,252.96 | 2,253.36 | 1.2 |
| Man5GlcNAc2 | 2,374.00 | 2,374.34 | 4.9 |
| GalGlcNAcMan3GlcNAc2 | 2,415.02 | 2,415.38 | 4.0 |
| Man6GlcNAc2 | 2,536.06 | 2,536.43 | 3.7 |
| GlcNAcMan5GlcNAc2 | 2,577.08 | 2,577.45 | 23.4 |
| Man7GlcNAc2 | 2,698.12 | 2,698.50 | 13.9 |
| GalGlcNAcMan5GlcNAc2 | 2,739.14 | 2,739.52 | 29.3 |
| Man8GlcNAc2 | 2,860.18 | 2,860.58 | 4.9 |
Glycopeptides with MALDI intensity <1% have been omitted. N, single GlcNAc.
Discussion
The N-glycan composition of glycoproteins in leaves from transgenic tobacco plants expressing the hybrid xylGalT gene changed much more dramatically compared with WT plants than previously, when the cDNA encoding the full-length human GalT gene was introduced (12). Because MALDI-TOF MS of N-linked glycans using 2,5-dihydroxybenzoic acid can be assumed to provide an adequate reflection of the actual N-glycan makeup, it is very clear that the N-glycan pattern was more akin now to the N-glycans in a tobacco cell culture transformed with a slightly truncated cDNA lacking the first 13 amino acid residues of the cytoplasmic tail of the full-length human GalT (21, 22). Both types of transgenic tobacco systems revealed a big increase in the level of high-Man and hybrid oligosaccharides and a concomitant decrease in biantennary N-glycans compared with WT plants.
Presumably, the main galactosylated hybrid, GalGlcNAcMan5GlcNAc2, is formed when the initial step in the synthesis of complex N-glycans (the addition of the first GlcNAc to the Man at the Man-α-1,3 arm) is followed straight away by galactosylation. This type of hybrid oligosaccharide was first found to be produced during in vitro assays with rat liver Golgi preparations using swainsonine, an inhibitor of Man-II (9, 23). The resulting galactosylated, hybrid N-glycan had to be treated with jack bean β-galactosidase to allow further trimming by Man-II, suggesting that galactosylation at the Man-α-1,3 arm inhibited any further processing of the ensuing molecule by other Golgi enzymes, such as the competing Man-II, N-acetylglucosaminyltransferase (GnT) II, and GnT-III (23–25). Apparently, the tobacco Man-II analogue mimics the substrate specificity of its animal counterparts and is likewise prevented from employing the monogalactosylated N-glycan as an acceptor.
Under normal circumstances, the most abundant hybrid GlcNAcMan5GlcNAc2 is an intermediate in the formation of complex N-glycans and does not accumulate. Although its presence could signal a block in the conversion of GlcNAcMan5GlcNAc2, we think that this oligosaccharide is much more likely to be derived from the highly abundant GalGlcNAcMan5GlcNAc2 by the action of an endogenous β-galactosidase; the commercially available jack bean β-1,3/4-galactosidase being a prime example of this type of activity in plants. Similarly, the high abundance of Man5GlcNAc2 compared with the other high-Man N-glycans in xylGalT12 plants is likely to be due largely to hexosaminidase activity on GlcNAcMan5GlcNAc2 (i.e., the glycosidase that is also involved in the formation of paucimannosidic N-glycans in WT plants).
Besides the large increase in the level of high-Man N-glycans and the parallel decrease in hybrid and complex N-glycans, the striking decline in core-bound Xyl and Fuc is another feature that is typical of N-glycans in xylGalT transgenic plants and the BY2 cells transformed with the truncated GalT. Of course, the high level of high-Man N-glycans by itself is already responsible for a major part of this decline, but the remaining N-glycans, mostly hybrids, carry very few Xyl and Fuc substitutions. This observation is in accordance with the known inability of plant XylT and core α-1,3-fucosyltransferase to donate sugar residues to β-1,4-galactose-capped N-glycans in vitro (26–28).
Because the hybrid GalT appeared to compete very successfully with Man-II, it is very likely that it was present in the same compartments as N-acetylglucosaminyltransferase I or as Man-II, which might actually turn out to be identical if plants resemble animals in this respect as well (29). Using immunoelectronmicroscopic detection of a GFP that was N-terminally tagged with the 36 amino acid residues of the Arabidopsis XylT, the hybrid fluorescent protein has been shown to be concentrated mainly in the medial Golgi (17). Because our xylGalT comprises as much as 53 amino acids of the N terminus of the same xylosyltransferase (i.e., the region containing the cytoplasmic tail and the transmembrane domain), we suggest that this hybrid enzyme is also likely to be located in the medial Golgi. For now, it is unclear what causes the obvious similarities between N-glycan profiles from tobacco BY2 cells expressing a truncated form of human GalT and leaves from tobacco plants expressing the xylGalT gene. The truncation removes a cytoplasmic dibasic motif that has been found to be important for COPII (coat protein complex II)-mediated ER-to-Golgi export of membrane proteins, but this fact does not by itself explain the apparently medial-Golgi localization of the truncated enzyme (30, 31). Although subcellular localization differences exist between the naturally occurring truncated and full-length GalT in mammalian cells, available reports on sub-Golgi compartmentalization do not support the notion of the truncated enzyme residing in the medial Golgi (32).
Our finding that sera from allergic patients with IgE against cross-reacting carbohydrate determinants (CCDs) displayed a significantly reduced response with protein from xylGalT plants compared with protein from WT plants may be of considerable biotechnological significance. The clinical relevance of CCD-specific IgE is still a matter of debate but is deemed limited for the majority of patients (33). On the other hand, it was demonstrated that approximately one-third of the CCD-positive sera from patients with tomato allergy have biologically relevant CCD-specific IgE antibodies (34).
Apart from safety issues regarding the N-glycan composition of a mAb produced in plants, the therapeutic efficacy of plant-derived antibodies will be another major determinant of their acceptance. Although it proved to be virtually impossible to create mouse mAbs directed against plant-specific features of N-linked glycans, rabbits elicited IgGs with high affinity toward core-bound Xyl and Fuc when they were immunized with (neo)glycoproteins carrying plant N-glycans (35–37). Hence, the fact that low levels of IgGs against N-glycans with core-bound α-1,3-Fuc and Xyl were encountered in 25–50% of a population of healthy blood donors emphasizes the relevance of plant-derived antibodies lacking core substitutions that could reduce the half-life of the therapeutic protein, especially in therapies in which the biopharmaceutical would be administered on a regular basis (38).
The abundant hybrid N-glycans in xylGalT plants are not expected to induce immune responses because they not only represent biosynthetic intermediates from the ubiquitous N-glycosylation pathway operating in each cell but can also be found as part of human leukocyte cell surface glycoproteins, for example (39). On the other hand, mAbs carrying high-Man N-glycans are likely to be rapidly cleared from serum as a result of the action of the Man receptor (40). A further improvement in the processing of the high-Man and hybrid N-glycans using additional genetic modifications might lead to plant mAb production systems suitable for even the most demanding applications.
Materials and Methods
Construction of xylGalT-Containing Vector.
A DNA fragment covering the N-terminal part of the A. thaliana xylosyltransferase (GenBank accession no. AJ277603) was amplified by using the following primers: XylTpvuF, ATACTCGAGTTAACAATGAGTAA-ACGGAATC; XylTpvuR, TTCTCGATCGCCGATTGGTTA-TTC. The C-terminal part of the hybrid gene was made up of a PCR fragment of the human GalT (GenBank accession no. X55415) amplified by using the following primers: GalTpvuF, GCCGCCGCGATCGGGCAGTCCTCC; GalTrev, AACGGATCCACGCTAGC-TCGGTGTCCCGAT. The PCR fragments comprising the N- and C-terminal parts of the hybrid gene were digested with XhoI/PvuI and PvuI/BamHI, respectively, and ligated simultaneously into XhoI/BamHI-digested pBluescriptSK+. Subsequently, the fragment containing the gene was cloned as an HpaI/BamHI fragment into SmaI/BamHI-digested plant expression vector pBINPLUS between the cauliflower mosaic virus (CaMV) 35S promoter and nos terminator and was designated xylGalT.
Plant Transformation, Growth, and Crossing.
The plant transformation vector xylGalT was used to transform Nicotiana tabacum (cv. Samsun NN) as described in ref. 12. MGR48 is a mouse monoclonal IgG1 that has been expressed in tobacco plants (41). A homozygous plant expressing this antibody was crossed bidirectionally with selected line xylGalT12. Offspring was analyzed for the presence of mAb and galactosylated N-glycans. Transgenic seedlings and plants were grown in a greenhouse at 21°C.
Western Blot Analysis.
Total protein extracts, gels, and blots were prepared essentially as described in ref. 12. Equal loading was verified by using Ponceau S staining of the blots. Immunological detection of β-1,4-galactosylated proteins was performed with biotinylated RCA (Vector Laboratories) and HRP-conjugated streptavidin (Rockland, Gilbertsville, PA), whereas core-bound Xyl and Fuc were probed with rabbit anti-HRP-IgG (Rockland) and Xyl- and Fuc-specific fractions thereof, followed by HRP-conjugated goat anti-rabbit-IgG (12). Purified antibodies (300 ng of each) that had been separated on precast 12% SDS/PAGE gels (Bio-Rad) were blotted onto nitrocellulose by using CAPS (3-cyclohexylamino-1-propane sulfonic acid) buffer as described in refs. 20 and 42. Blots were probed with anti-mouse IgG, rabbit anti-HRP, rabbit anti-Xyl, rabbit anti-Fuc antibodies, and biotinylated RCA. Detection of bound primary antibodies was performed with Lumi-Light and Lumi-Imager (Roche Diagnostics) after incubation with HRP-conjugated sheep anti-mouse and goat anti-rabbit antibodies, whereas bound RCA was visualized with HRP-labeled streptavidin.
N-Glycan Purification.
Protein for N-glycan purification was extracted from 250 mg of leaf material, which had been powderized in liquid nitrogen, by vigorous vortexing in 750 μl of buffer (50 mM Hepes-KOH, pH 7.5/20 mM sodium metabisulfite/5 mM EDTA/0.1% SDS/1.7% insoluble polyvinylpolypyrrolidone) at room temperature in a 2-ml Eppendorf tube. Debris was removed by two successive centrifugations for 10 min at 20,000 × g at 5°C. Protein was precipitated on ice for at least 30 min or overnight at 4°C and then pelleted by centrifugation for 10 min at 20,000 × g at 5°C. Pellets were washed twice with ice-cold 90% acetone, air-dried, and resuspended in 0.5 ml of 10 mM HCl/1.5 mg/ml pepsin. Digestion was continued for 24 h at 37°C and terminated by neutralization to pH 7 with 1 M NH4OH and 10 min of heating at 95°C. After lyophilization, the residue was dissolved in 0.5 ml of 100 mM NaAc (pH 5.0) or in 0.5 ml of 50 mM sodium phosphate buffer, pH 7.5/1% Nonidet P-40, and insoluble material was removed by centrifugation. N-glycans were released by 24 h of treatment at 37°C with 0.15 milliunits of PNGase A solution (Roche Diagnostics) or 4.5 milliunits of PNGase F (New England Biolabs). N-glycans were purified by chromatography on 2 ml of Dowex 50 AG 50W-X2 (Bio-Rad) followed by a 500-mg C18 column (Varian). The eluate from the C18 column was applied to an 8-ml, 150-mg Ultra-Clean Carbograph column (Alltech Associates) from which the bound N-glycans were eluted in 25% acetonitrile (43). The oligosaccharides were lyophilized. For galactosidase treatments, N-glycans were incubated with 1.5 milliunits of S. pneumoniae β-1,4-galactosidase (Calbiochem) in 50 mM sodium phosphate buffer (pH 6.0) and purified away from salts and enzyme by using an Ultra-Clean Carbograph column as described above.
N-Glycan Analysis.
Purified N-glycans were dissolved in 5 mM NaAc and mixed with an equal volume of 1% 2,5-dihydroxybenzoic acid in 50% acetonitrile. One-microliter aliquots were spotted onto a stainless-steel sample plate and dried under a stream of air at room temperature. Positive-ion MALDI-TOF spectra of [M+Na]+ adducts were recorded on an Ultraflex mass spectrometer (Bruker, Billerica, MA) fitted with delayed extraction and a nitrogen laser (337 nm). A maltodextrin series was used as an external molecular-weight standard. Spectra were generated from the sum of 200–300 laser pulses.
Microsome Preparation.
Microsomes were isolated from fresh leaves of mature, full-grown plants at the stage of early flowering. After removal of the midvein, leaves were cut into small pieces and homogenized in a precooled stainless-steel Waring blender; we used 3 ml/g of fresh-weight tobacco leaves of ice-cold microsome isolation buffer, consisting of 250 mM sorbitol/5 mM Tris/2 mM DTT/7.5 mM EDTA set at pH 7.8 by using a 1 M solution of Mes. A protease inhibitor mixture (Complete Mini; Roche Diagnostics) was added. Debris and leaf material were removed by filtering through 88-μm nylon cloth, followed by centrifugation for 10 min at 12,000 × g at 4°C using a Sorvall SS34 rotor. The supernatant containing microsomes was centrifuged in a fixed-angle Centrikon TFT 55.38 rotor for 60 min at 100,000 × g at 4°C in TYPE tubes in a Centricon T-2070 ultracentrifuge. The pellet was resuspended in microsome isolation buffer without EDTA to which glycerol (4% final concentration) was added. The suspension was either used immediately for enzyme measurements or stored at −80°C.
Glycosyltransferase Assays.
Microsomal preparations were washed and resuspended in 500 μl of 10 mM cacodylate buffer (pH 7.2). Fucosyltransferase assays were performed as described by van Tetering et al. (44), and galactosyltransferase assays were performed as described by van Die et al. (45). Xylosyltransferase assays were carried out in 25 μl of reaction mixture: 10 mM cacodylate buffer (pH 7.2)/4 mM ATP/20 mM MnCl2/0.4% Triton X-100/1 mM GlcNAcβ-1–2-Man-α1–3-[Man-α1–6]Man-β-R [in which R is O-(CH2)8-COOCH3, kindly provided by O. Hindsgaul (University of Alberta, Edmonton, Canada)]/0.1 mM UDP-[14C]-Xyl [5 Ci/mmol (1 Ci = 37 GBq), kindly provided by H. Kamerling (Utrecht University, Utrecht, The Netherlands)].
RAST Inhibition.
A RAST inhibition was performed as described in ref. 46. Briefly, peptides from proteinase K-treated grass pollen proteins were coupled to CNBr-activated Sepharose 4B at 40 μg of protein per mg of Sepharose. Human serum samples (50 μl) were preincubated for 2 h at room temperature with serially diluted leaf proteins from WT and transgenic tobacco plants before addition of 0.5 mg of Sepharose-coupled grass pollen peptides in a final volume of 350 μl of PBS/0.3% BSA/0.1% Tween 20. After overnight incubation at room temperature, unbound material was washed away, and 50 μl of 125I-labeled sheep anti-human IgE (Sanquin, Amsterdam) was added. After another overnight incubation at room temperature and washing, radioactivity was measured on a gamma counter. For the uninhibited control, 50 μl of PBS/0.3% BSA/0.1% Tween 20 was added instead of the inhibitor.
Analysis of Purified mAb MGR48.
MGR48 was purified as described in ref. 12. mAb (100 μg) was reduced, alkylated, and digested with trypsin as described in ref. 47. The HPLC system, column, and mobile phases were the same as in ref. 47. Two hundred microliters of the tryptic digest mixture was fractionated at a flow rate of 1 ml/min. The separation was achieved by using the following gradient: 100% solvent A isocratic for 2 min, 0–18% solvent B for 18 min, and 18–100% solvent B for 22 min. Elution of peptides was monitored by UV absorption at 205 nm. One-milliliter fractions were collected, dried, redissolved in 6 μl of 50% acetonitrile/0.1% trifluoroacetic acid, and examined by MALDI-TOF MS to identify fractions containing glycopeptides. Fractions containing tryptic fragment EEQFNSTFR and its glycoforms were quantitatively combined and reanalyzed by MALDI-TOF MS to obtain a complete glycosylation profile. A Voyager DE-STR (Applied Biosystems) MALDI-TOF mass spectrometer was used, and 1,500 acquisitions were averaged for each spectrum. Operating conditions, instrument calibration, and spectral analysis are described in ref. 47.
Acknowledgments
We thank Dr. W. H. Anderson for his encouragement. This work was supported by Wageningen University and Research Center and The Dow Chemical Company and was carried out according to License GGO 97-230 of the Netherlands Ministry of Housing, Spatial Planning, and the Environment.
Abbreviations
- Fuc
fucose
- ER
endoplasmic reticulum
- GalT
β-1,4-galactosyltransferase I
- HRP
horseradish peroxidase
- Man-II
mannosidase II
- RAST
radioallergosorbent test
- RCA
Ricinus communis agglutinin
- XylT
core β-1,2-xylosyltransferase
- Xyl
xylose
- CCD
cross-reacting carbohydrate determinant
- GlcNAc
N-acetylglucosamine
- Man
mannose.
Footnotes
Conflict of interest statement: A patent application has been filed regarding the expression of hybrid galactosyltransferase in plants.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Peterson R. K. D., Arntzen C. J. Trends Biotechnol. 2004;22:64–66. doi: 10.1016/j.tibtech.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Ma J. K.-C., Drake P. M. W., Christou P. Nat. Rev. Genet. 2003;4:794–805. doi: 10.1038/nrg1177. [DOI] [PubMed] [Google Scholar]
- 3.Gomord V., Sourrouille C., Fitchette A.-C., Bardor M., Pagny S., Lerouge P., Faye L. Plant Biotechnol. J. 2004;2:83–100. doi: 10.1111/j.1467-7652.2004.00062.x. [DOI] [PubMed] [Google Scholar]
- 4.Krapp S., Mimura Y., Jefferis R., Huber R., Sondermann P. J. Mol. Biol. 2003;325:979–989. doi: 10.1016/s0022-2836(02)01250-0. [DOI] [PubMed] [Google Scholar]
- 5.Saba J. A., Kunkel J. P., Jan D. C. H., Ens W. E., Standing K. G., Butler M., Jamieson J. C. Anal. Biochem. 2002;305:16–31. doi: 10.1006/abio.2002.5651. [DOI] [PubMed] [Google Scholar]
- 6.Schachter H. Glycobiology. 1991;1:453–461. doi: 10.1093/glycob/1.5.453. [DOI] [PubMed] [Google Scholar]
- 7.Jones D. C., Mehlert A., Güther M. L. S., Ferguson M. A. J. J. Biol. Chem. 2005;280:35929–35942. doi: 10.1074/jbc.M509130200. [DOI] [PubMed] [Google Scholar]
- 8.Tabas I., Kornfeld S. J. Biol. Chem. 1979;254:11655–11663. [PubMed] [Google Scholar]
- 9.Tulsiani D. R. P., Hubbard S. C., Robbins P. W., Touster O. J. Biol. Chem. 1982;257:3660–3668. [PubMed] [Google Scholar]
- 10.Kaushal G. P., Szumilo T., Pastuszak I., Elbein A. D. Biochemistry. 1990;29:2168–2176. doi: 10.1021/bi00460a030. [DOI] [PubMed] [Google Scholar]
- 11.Grabenhorst E., Conradt H. S. J. Biol. Chem. 1999;274:36107–36116. doi: 10.1074/jbc.274.51.36107. [DOI] [PubMed] [Google Scholar]
- 12.Bakker H., Bardor M., Molthoff J. W., Gomord V., Elbers I., Stevens L. H., Jordi W., Lommen A., Faye L., Lerouge P., Bosch D. Proc. Natl. Acad. Sci. USA. 2001;98:2899–2904. doi: 10.1073/pnas.031419998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Ree R., Hoffman D. R., van Dijk W., Brodard V., Mahieu K., Koeleman C. A., Grande M., van Leeuwen W. A., Aalberse R. C. J. Allergy Clin. Immunol. 1995;95:970–978. doi: 10.1016/s0091-6749(95)70097-8. [DOI] [PubMed] [Google Scholar]
- 14.van Die I., Gomord V., Kooyman F. N., van den Berg T. K., Cummings R. D., Vervelde L. FEBS Lett. 1999;463:189–193. doi: 10.1016/s0014-5793(99)01508-2. [DOI] [PubMed] [Google Scholar]
- 15.van Ree R., Cabanes-Macheteau M., Akkerdaas J., Milazzo J.-P., Loutelier-Bourhis C., Rayon C., Villalba M., Koppelman S., Aalberse R., Rodriguez R., et al. J. Biol. Chem. 2000;275:11451–11458. doi: 10.1074/jbc.275.15.11451. [DOI] [PubMed] [Google Scholar]
- 16.Nilsson T., Lucocq J. M., Mackay D., Warren G. EMBO J. 1991;10:3567–3575. doi: 10.1002/j.1460-2075.1991.tb04923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pagny S., Bouissonnie F., Sarkar M., Follet-Gueye M. L., Driouich A., Schachter H., Faye L., Gomord V. Plant J. 2003;33:189–203. doi: 10.1046/j.0960-7412.2002.01604.x. [DOI] [PubMed] [Google Scholar]
- 18.Bakker H., Lommen A., Jordi W., Stiekema W., Bosch D. Biochem. Biophys. Res. Commun. 1999;261:829–832. doi: 10.1006/bbrc.1999.1117. [DOI] [PubMed] [Google Scholar]
- 19.Faye L., Gomord V., Fitchette-Lainé A.-C., Chrispeels M. J. Anal. Biochem. 1993;209:104–108. doi: 10.1006/abio.1993.1088. [DOI] [PubMed] [Google Scholar]
- 20.Elbers I. J. W., Stoopen G. M., Bakker H., Stevens L. H., Bardor M., Molthoff J. W., Jordi W. J. R. M., Bosch D., Lommen A. Plant Physiol. 2001;126:1314–1322. doi: 10.1104/pp.126.3.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palacpac N. Q., Yoshida S., Sakai H., Kimura Y., Fujiyama K., Yoshida T., Seki T. Proc. Natl. Acad. Sci. USA. 1999;96:4692–4697. doi: 10.1073/pnas.96.8.4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harvey D. J. Int. J. Mass Spectrom. 2003;226:1–35. [Google Scholar]
- 23.Tulsiani D. R. P., Touster O. J. Biol. Chem. 1983;258:7578–7585. [PubMed] [Google Scholar]
- 24.Schachter H., Narasimhan S., Gleeson P., Vella G. Can. J. Biochem. Cell Biol. 1983;61:1049–1066. doi: 10.1139/o83-134. [DOI] [PubMed] [Google Scholar]
- 25.Bendiak B., Schachter H. J. Biol. Chem. 1987;262:5784–5790. [PubMed] [Google Scholar]
- 26.Johnson K. D., Chrispeels M. J. Plant Physiol. 1987;84:1301–1308. doi: 10.1104/pp.84.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Staudacher E., Dalik T., Wawra P., Altmann F., Marz L. Glycoconj. J. 1995;12:780–786. doi: 10.1007/BF00731239. [DOI] [PubMed] [Google Scholar]
- 28.Zeng Y., Bannon G., Thomas V. H., Rice K., Drake R., Elbein A. J. Biol. Chem. 1997;272:31340–31347. doi: 10.1074/jbc.272.50.31340. [DOI] [PubMed] [Google Scholar]
- 29.Nilsson T., Hoe M. H., Slusarewicz P., Rabouille C., Watson R., Hunte R., Watzele G., Berger E. G., Warren G. EMBO J. 1994;13:562–574. doi: 10.1002/j.1460-2075.1994.tb06294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giraudo C. G., Maccioni H. J. F. Mol. Biol. Cell. 2003;14:3753–3766. doi: 10.1091/mbc.E03-02-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuasa K., Toyooka K., Fukuda H., Matsuoka K. Plant J. 2005;41:81–94. doi: 10.1111/j.1365-313X.2004.02279.x. [DOI] [PubMed] [Google Scholar]
- 32.Lopez L. C., Youakim A., Evans S. C., Shur B. D. J. Biol. Chem. 1991;266:15984–15991. [PubMed] [Google Scholar]
- 33.van Ree R. Int. Arch. Allergy Immunol. 2002;129:189–197. doi: 10.1159/000066770. [DOI] [PubMed] [Google Scholar]
- 34.Foetisch K., Westphal S., Lauer I., Retzek M., Altmann F., Kolarich D., Scheurer S., Vieths S. J. Allergy Clin. Immunol. 2003;111:889–896. doi: 10.1067/mai.2003.173. [DOI] [PubMed] [Google Scholar]
- 35.Wilson I. B., Harthill J. E., Mullin N. P., Ashford D. A., Altmann F. Glycobiology. 1998;8:651–661. doi: 10.1093/glycob/8.7.651. [DOI] [PubMed] [Google Scholar]
- 36.Chargelegue D., Vine N. D., van Dolleweerd C. J., Drake P. M. W., Ma J. K.-C. Transgenic Res. 2000;9:187–194. doi: 10.1023/a:1008976219939. [DOI] [PubMed] [Google Scholar]
- 37.Jin C., Bencúrová M., Borth N., Ferko B., Jensen-Jarolim E., Altmann F., Hantusch B. Glycobiology. 2006;16:349–357. doi: 10.1093/glycob/cwj071. [DOI] [PubMed] [Google Scholar]
- 38.Bardor M., Faveeuw C., Fitchette A.-C., Gilbert D., Galas L., Trottein F., Faye L., Lerouge P. Glycobiology. 2003;13:427–434. doi: 10.1093/glycob/cwg024. [DOI] [PubMed] [Google Scholar]
- 39.Asada M., Furukawa K., Kantor C., Gahmberg C. G., Kobata A. Biochemistry. 1991;30:1561–1571. doi: 10.1021/bi00220a017. [DOI] [PubMed] [Google Scholar]
- 40.Lee S. J., Evers S., Roeder D., Parlow A. F., Risteli J., Risteli L., Lee Y. C., Feizi T., Langen H., Nussenzweig M. C. Science. 2002;295:1898–1901. doi: 10.1126/science.1069540. [DOI] [PubMed] [Google Scholar]
- 41.Smant G., Goverse A., Stokkermans J. P. W. G., de Boer J. M., Pomp H., Zilverentant J. F., Overmars H. A., Helder J., Schots A., Bakker J. Phytopathology. 1997;87:839–845. doi: 10.1094/PHYTO.1997.87.8.839. [DOI] [PubMed] [Google Scholar]
- 42.Stevens L. H., Stoopen G. M., Elbers I. J. W., Molthoff J. W., Bakker H. A. C., Lommen A., Bosch D., Jordi W. Plant Physiol. 2000;124:173–182. doi: 10.1104/pp.124.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Packer N. H., Lawson M. A., Jardine D. R., Redmond J. W. Glycoconj. J. 1998;15:737–747. doi: 10.1023/a:1006983125913. [DOI] [PubMed] [Google Scholar]
- 44.van Tetering A., Schiphorst W. E. C. M., van den Eijnden D. H., van Die I. FEBS Lett. 1999;461:311–314. doi: 10.1016/s0014-5793(99)01489-1. [DOI] [PubMed] [Google Scholar]
- 45.van Die I., van Tetering A., Schiphorst W. E. C. M., Sato T., Furukawa K., van den Eijnden D. H. FEBS Lett. 1999;450:52–56. doi: 10.1016/s0014-5793(99)00462-7. [DOI] [PubMed] [Google Scholar]
- 46.Aalberse R. C., Koshte V., Clemens J. G. J. Allergy Clin. Immunol. 1981;68:356–364. doi: 10.1016/0091-6749(81)90133-0. [DOI] [PubMed] [Google Scholar]
- 47.Karnoup A. S., Turkelson V., Anderson W. H. Glycobiology. 2005;15:965–981. doi: 10.1093/glycob/cwi077. [DOI] [PubMed] [Google Scholar]