Abstract
Pseudomonas aeruginosa is both a ubiquitous environmental bacterium and an opportunistic human pathogen. A remarkable metabolic versatility allows it to occupy a multitude of ecological niches, including wastewater treatment plants and such hostile environments as the human respiratory tract. P. aeruginosa is able to degrade and metabolize biocidic SDS, the detergent of most commercial personal hygiene products. We identify SdsA1 of P. aeruginosa as a secreted SDS hydrolase that allows the bacterium to use primary sulfates such as SDS as a sole carbon or sulfur source. Homologues of SdsA1 are found in many pathogenic and some nonpathogenic bacteria. The crystal structure of SdsA1 reveals three distinct domains. The N-terminal catalytic domain with a binuclear Zn2+ cluster is a distinct member of the metallo-β-lactamase fold family, the central dimerization domain ensures resistance to high concentrations of SDS, whereas the C-terminal domain provides a hydrophobic groove, presumably to recruit long aliphatic substrates. Crystal structures of apo-SdsA1 and complexes with substrate analog and products indicate an enzymatic mechanism involving a water molecule indirectly activated by the Zn2+ cluster. The enzyme SdsA1 thus represents a previously undescribed class of sulfatases that allows P. aeruginosa to survive and thrive under otherwise bacteriocidal conditions.
Keywords: SDS hydrolase, sulfur metabolism, human pathogen, sulfatase classification
Sulfur is the fifth most common element in organic compounds after carbon, hydrogen, oxygen, and nitrogen. Although sulfur is generally available in the biosphere in the form of organic compounds, free sulfate ions are frequently growth limiting. In particular sulfate ester compounds, ubiquitously produced by living organisms and, hence, found throughout the biosphere, account for a large fraction of sulfur content in aerobic soils (1).
Microorganisms use a range of sulfatases of widely varying substrate specificities to use sulfate esters as carbon and/or sulfur source (2–4). At least three mechanistically distinct groups of sulfatases exist: arylsulfatases, the best studied group (hereby assigned to group I), are predominantly eukaryotic. They are characterized by an active-site serine or cysteine posttranslationally modified to formylglycine that mediates the cleavage of the CO-S bond of sulfate esters, producing inorganic sulfate and the corresponding alcohol (1, 5). The Fe(II) α-ketoglutarate-dependent dioxygenase superfamily of enzymes constitutes a second group (group II) of sulfatases. These enzymes oxidatively cleave sulfate esters into inorganic sulfate and the corresponding aldehyde and require α-ketoglutarate as a cosubstrate (6). Evidence for a third group of sulfatases (group III) has accumulated over many years (7–11). However, the fact that Pseudomonas aeruginosa strains express up to six alkyl- or arylsulfatases (12), compounded by a lack of sequence data for the zymographically identified enzymes, confused the issue and prevented their identification as a distinct group. The gene sdsA from Pseudomonas sp. ATCC19151 was implicated in the degradation of SDS and named accordingly (7). Although alkylsulfatase activity for the gene product was not demonstrated at the time, SdsA, unrelated by sequence to sulfatases of group I and II, is the first member of group III sulfatases. Homologues of SdsA (sequence identities up to 37%) occur in numerous eubacteria. Eukaryotic homologues, presumably acquired from α-proteobacteria, are less frequent but include Bds1 (Yol164w) of Saccharomyces cerevisiae (13) and EAL47917 of the human pathogen Entamoeba histolytica (14). The N-terminal domain of SdsA and its homologues harbors a Zn2+-binding motif (THxHxDHxGG-102-E-18-AE-44-H) identifying them as metallo-β-lactamase (MBL)-related enzymes (15).
SdsA is 30% identical by amino acid sequence to a previously unnamed homologue from P. aeruginosa strain PAO1, gene number PA0740, the subject of this study. Because both enzymes have an identical domain structure and share the ability to degrade linear alkylsulfates, we henceforth will refer to the gene product of PA0740 as SdsA1.
We present the crystal structure of SdsA1 from P. aeruginosa strain PAO1 at 1.9 Å resolution, the class-III sulfatase. Two of its domains, the N-terminal metallo-β-lactamase-like and the C-terminal, SCP-2-like domains are structurally related to known folds. The third domain is a previously undescribed SDS-resistant dimerization domain. Complexes of SdsA1 binding a substrate analogue or sulfate ester cleavage products within the active site provide structural clues with respect to the catalytic mechanism.
Results
SdsA1 Is an Essential, Secreted SDS-Hydrolyzing Enzyme.
P. aeruginosa is able to use 0.1% SDS as a sole carbon and/or sulfur source. A mutant with an isogenic sdsA1 transposon insertion is no longer able to grow under these conditions. Colonies of P. aeruginosa, when grown on minimal media agarose plates with SDS as a sole sulfur source, develop a distinct, white halo (see Fig. 5, which is published as supporting information on the PNAS web site) that we ascribe to water-insoluble 1-dodecanol (1DO) produced during SdsA1-mediated cleavage of SDS. This identification was confirmed by mass spectrometry after SDS cleavage by purified SdsA1. The second cleavage product is soluble SO42−. The inferred secretion of SdsA1 is supported by the identification of an N-terminal 19-residue signal peptide (www.predisi.de). Sequencing SdsA1, heterologously expressed in Escherichia coli, confirms the posttranslational loss of residues 1–19.
Hydrolysis of SDS by SdsA1 results in the release of a proton. The reaction thus could be followed by monitoring the net change of pH. The KM value of SdsA1 for SDS hence was estimated to be 9 ± 3 μM. Correspondingly, we find that 0.26 μg SdsA1 (1U) is required to degrade 1 μmol of SDS at room temperature within 1 min. Apart from SDS, SdsA1 also cleaves shorter primary alkylsulfates such as decyl sulfate, octyl sulfate, and hexyl sulfate. Sulfated sugars (glucosamin-2-sulfate and glucose-3-sulfate) and arylsulfates (para-nitrocatecholsulfate and para-nitrophenylsulfate) are not cleaved (data not shown).
Structure Determination and Refinement.
SdsA1 crystallized in the space group P6522. The structure was solved by multiple anomalous dispersion techniques by using 10 Se sites of SeMet-derivatized SdsA1. Native SdsA1 was refined to a resolution of 1.9 Å (16) with an R factor (Rfree) of 16% (22%). The final electron density is of excellent quality for residues 21–654 except for disordered residues 206–208 and 626–631. Four residues indirectly participating in Zn2+ binding (Asp-138, Ala-238, Thr-279, and Ala-298) are well defined in the electron density (Fig. 6, which is published as supporting information on the PNAS web site), yet adopt sterically strained main chain conformations (see Table 1). The identification of the two metal-binding sites of SdsA1 as Zn2+ was confirmed by anomalous scattering techniques (Fig. 7, which is published as supporting information on the PNAS web site). The ability of other metal ions to substitute for either Zn2+ was not systematically tested. Substrate (SDS), product (SO42−) and substrate analogue (1-decane sulfonic acid, 1DA), soaked into separate crystals, have been interpreted to represent various states of the enzymatic reaction. Crystallographic data are summarized in Table 1.
Structure of SdsA1.
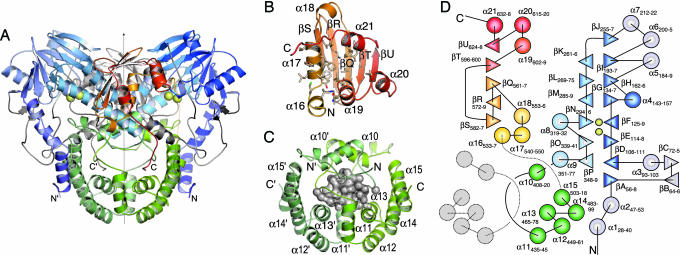
SdsA1 is a symmetric dimer (Fig. 1A). Each monomer consists of three domains: the N-terminal, catalytic, αββα-sandwich domain (residues 1–379, blue); an α-helical, dimerization domain (380–520, green), intricately interlocking the monomers; and a mixed, C-terminal domain (521–655, orange).
Fig. 1.
Structure of SdsA1. (A) A cartoon representation of the SdsA1 dimer. Structural domains are color-coded: N-terminal domain, blue; dimerization domain, green; C-terminal domain, orange-red. A disordered loop connecting α-helices α15 and α16 is indicated by a dotted line, and Zn ions are represented by yellow spheres. (B) The C-terminal domain of SdsA1. Amino acid side chains lining the hydrophobic groove are shown in stick representation. (C) A large water-filled cavity within the dimerization domain of SdsA1. Van der Waals surfaces of enclosed water molecules (red spheres) are shown in translucent gray. (D) Schematic representation of the SdsA1 secondary structure: α-helices are represented by circles, β-strands by triangles, loops by lines, and Zn ions by yellow spheres. The second gray-colored dimerization domain is included to emphasize the extensive intertwining of the SdsA1 dimer.
The N-terminal, catalytic domain consists of a 14-stranded β-sandwich (βA, βD-βJ, and βK-βP) decorated by α-helices on either side (Fig. 2A). Two Zn ions, Zn1 and Zn2, located at the protein core-facing end of the β-sandwich, mark the active site. Zn1 is coordinated by Asp-173, His-174, Glu-299, and His-344; Zn2 by His-169 His-171, and Glu-280. All of these residues are located in extended loops βH/α5, βL/βM, βN/α8, and βO/βP. Much shorter loops are found at the opposite end of the β-sandwich. The core-facing end is covered by a dome-like structure shielding the active site. It consists of α-helices α1 to α3, α5 to α7, the loops βB/βC and βN/α8 from the N-terminal domain, and helix α15 and loop α9/α10 from the dimerization domain. Laterally a funnel-shaped opening provides access to the active site (Fig. 2A).
Fig. 2.
The catalytic domain of SdsA1. (A) Cartoon with coloring as in Fig. 1. Side chains coordinating Zn ions (yellow spheres) are shown as sticks. The active-site dome (gray) is not conserved in other MBL fold enzymes. The sulfate recognition loop βN/α8 is shown in purple. (B) Structure-based sequence alignment of SdsA1 and B. fragilis β-lactamase (PDB: 1A7T). Secondary structure elements of SdsA1 are depicted by rods and arrows. Conserved residues are marked in red; those conserved in all enzymes (see Table 2, which is published as supporting information on the PNAS web site) are marked by asterisks, and metal ion ligands are marked by yellow circles.
SdsA1 is observed to be a dimer both in solution (analytical gel filtration) and in the crystal structure. The dimerization domain, encompassing the central section of the protein, is exclusively α-helical (α10–α15). Each domain provides five C-terminal α-helices (α11–α15), creating a vessel-like structure (Fig. 1C). Extended loops preceding the α-helices symmetrically twist around a central void, again preceded by “lid” α-helix α10. The dimerization domains thus encircle each other by a complete rotation, creating a central water-filled cavity (gray surface in Fig. 1C). Monomers hence interlock to a degree that would require complete unfolding or cleavage of one polypeptide chain to physically separate them. This observation, in part, may resolve the question of how SDS-degrading enzymes resist denaturation by SDS (10).
The C-terminal domain consists of a five-stranded mixed β-sheet (βQ–βU) covered by six short α-helices, α16–α21 on one side (Fig. 1 B and D). Together, the β-sheet and α-helices create a deep groove perpendicular to the β-strands. The groove or slide is lined almost exclusively by hydrophobic residues (Fig. 1B).
Although separated by the dimerization domain in terms of sequence, the N-terminal and C-terminal domains of SdsA1 are spatially directly adjacent. Together they form a hydrophobic chute leading toward the active site pocket (see below). Hydrophobic substrates thus may be recruited by the C-terminal domain or hydrophobic products presented.
Structurally Related Domains.
The αββα-sandwich fold of the SdsA1 N-terminal domain, which was first described for metallo-β-lactamases (17), has been observed in other metalloenzymes (18–20) and predicted for quite a few more (15). These enzymes are mostly hydrolases, inter alia cleaving β-lactams, phosphoesters, and N-acyl homoserine lactones but also include a rubredoxin oxidase (17–22). We confirm the similarity of the β-sandwich of SdsA1 and its short-loop outer end to that of related αββα-enzymes (Table 2; refs. 15 and 23). The metal-binding end (except for the Zn2+-binding loops) is responsible for substrate recognition and, hence, unique to each enzyme. Correspondingly, the extended loops and secondary structure elements covering the active-site of SdsA1 are much shorter or absent in related structures (Fig. 2B). Despite sequence identities of only ≈10%, the C-terminal domain of SdsA1 is structurally similar to several eukaryotic sterol-binding domains such as the SCP-X domain of human peroxisomal multifunctional enzyme type 2 (24), and sterol-carrier protein 2 from rabbit or yellow fever mosquito (25, 26). The role of binding hydrophobic molecules in these proteins is presumably true of the C-terminal domain SdsA1 as well.
Active Site.
The active-site Zn ions denoted Zn1 and Zn2 are 3.3 Å apart. They are bridged by a water or, presumably, by a shared hydroxyl ion W1, a typical feature of binuclear Zn2+ centers (27). Zn1 has a distorted trigonal-bipyramidal coordination sphere where His-174, His-344, and W1 provide the equatorial and Asp-173 and Glu-299 the apical ligands (Fig. 3A). Zn2 is tetrahedrally coordinated by W1, His-169, His-171, and Glu-280. W1, additionally, hydrogen bonds Asp-173 (2.7 Å) and water W2 (2.7 Å), whereas W2 also hydrogen bonds Glu-299 (2.7 Å). A glutamate in the direct vicinity of the Zn ions is unique to SdsA1. It replaces an aspartate, histidine, or cysteine of related enzymes (Fig. 2B and 3A Inset). W2 thus has two negatively charged neighbors, W1 and Glu-299, causing it to be highly polarized.
Fig. 3.
Active site of SdsA1. (A) The Zn2+-binding site. Zn ions are depicted as yellow spheres, water molecules or hydroxyl ions as red spheres, and metal-binding residues as stick models. Selected hydrogen bonds and interactions are indicated by gray dotted lines, and distances are provided in angstroms. Backbone colors indicate temperature factors: red, high structural flexibility; blue, low structural flexibility. Loop βL/βM adopts two distinct conformations depending on Zn2 occupancy. Closed conformation, red (high B factor); open conformation, green. Corresponding conformations of Tyr-325 are also shown. The van der Waals surface of Ile-239 is shown in translucent orange. Zn2+ ligands of SdsA1 and MBL proteins listed in Table 2 are compared (Inset). (B) Active site of SdsA1 with SDS analogue 1DA (vivid colors) superimposed on reaction product structures, SO42− (light blue) and 1DO (light green). Zn2+ ligands are shown as thin gray sticks, and substrate/product-binding residues are shown as thicker, colored sticks. A dotted yellow circle indicates the equatorial plane of the distorted trigonal-bipyramidal configuration of S1DA. (B Inset) 2Fo − Fc electron density of 1DA (1σ, blue) depicts the relative location of the proposed nucleophile W2 relative to S1DA. (C) A schematic representation of the 1DA bound to the active site. H bonds and salt bridges are marked by dotted lines, hydrophobic interactions are marked by gray arcs, and distances are in angstroms.
Zn1 is fully occupied in all structures of SdsA1. Zn2 is lost more easily. In SeMet-substituted SdsA1, Zn2 is only half-occupied, possibly due to chelation by DTT added to prevent oxidation of the selenium. In crystals of native SdsA1 (no DTT), both Zn sites are fully occupied without Zn2+ having been added. Loop βL/βM (residues 276–284, including the Zn2 ligand Glu-280) adopts two distinct conformations in response to Zn2. When present, Zn2 binds Glu-280 locking βL/βM into a closed conformation. Loss of Zn2 releases βL/βM, which folds away, with Thr-276 and Pro-282 serving as hinges (Fig. 3A), whereas Tyr-325 rotates by ≈65°, and hydrogen bonds to Gln-274-O and Thr-276-N. This rearrangement, together with the formation of a hydrogen bond between Pro-282-O and Gly-192-N, leads to the stabilization of βL/βM in the open conformation. The electron densities of both βL/βM conformations hence are clearly defined in the particular structures. A concentration of 3 mM ZnCl2 ensures full Zn2 occupancy and a closed βL/βM conformation. Variable Zn2+ occupancies have been observed for other binuclear Zn2+ clusters (28, 29). The distinct conformations of βL/βM are, however, specific to SdsA1.
Binding of Substrates, Products, and Substrate Analogues to the Active Site.
To obtain structural information pertaining to the reaction mechanism of SdsA1, crystals were soaked in 2% SDS (final concentration) 10 min before cryocooling. The resulting elongated difference electron density within the hydrophobic active-site channel was interpreted as a linear alcohol in two conformations bound near the active site. The missing sulfate group indicates the presence of 1DO, the cleavage product of SDS (Fig. 3B; see also Fig. 8, which is published as supporting information on the PNAS web site).
Crystals were furthermore soaked in mother liquor supplemented with 100 mM Li2SO4 for 24 h. A SO42− is clearly visible in the electron density bound by Arg-312, Arg-317, Thr-310, Asn-307, His-405, and W2 (Fig. 3B; see also Fig. 9, which is published as supporting information on the PNAS web site). Its position does not overlap with that of the 1DO (see above), indicating that both products could simultaneously occupy the active site.
To prevent substrate hydrolysis, SDS was substituted by 10 mM 1DA, an uncleavable SDS analogue, in soaking experiments. At 1.9 Å resolution, the difference density indicates one 1DA molecule bound within the active site (vivid colors; Fig. 3B; see also Fig. 10, which is published as supporting information on the PNAS web site). An occupancy of 0.6 for 1DA resulted in matching temperature factors to those of surrounding protein atoms, whereas residual electron density near 1DAO1 was modeled as a solvent molecule with an occupancy of 0.4 (Fig. 3B Inset). Overall, the position of 1DA is similar to that of the reaction products (pale colors). The sulfur atom, however, is located slightly nearer the binuclear Zn2+ site and the bridging hydroxyl than the free SO42− ion. 1DA is not disordered and the sulfonic acid group is bound by the same residues that coordinate SO42−. The movement relative to SO42− is accommodated by side-chain rotations rather than any changes in backbone conformations (Fig. 3 B and C). The sulfonic methylene group of 1DA (substituting for the substrate sulfate ester oxygen) is found to be located opposite Nη1 and Nη2 of Arg-312, indicating that Arg-312 may activate the ester oxygen in the substrate complex by its positive charge. His-306, on the other hand, appears to function as a “van der Waals bumper,” preventing the sulfate from approaching Zn1 and Zn2. The electron density of the R-SO3− group indicates a distorted tetrahedral sulfur geometry, which we accounted for by lowering the angular restraints of the sulfur substituents facing W2 during refinement to allow a better modeling of the observed density (Fig. 3B Inset). Interestingly, all residues involved in sulfate coordination, except His-405, are part of the loop βN/α8. This sulfate-recognition loop itself forms part of the active-site dome (Fig. 2 A and B), is correspondingly conserved among SdsA1 homologues and instrumental in creating the specificity for the R-O-SO3− group (Fig. 3C).
Discussion
SdsA1: A Representative of the Metallo-β-Lactamase Fold.
SdsA1 is a member of the MBL fold family (15). Until recently, only three of the 17 protein groups proposed to contain this fold had been characterized structurally, MBLs (group 1 or G1), glyoxylases II (G2), and rubredoxin oxidoreductases (G3). Recent crystal structures of other family members include acid phosphorylcholine esterase Pce (G9) (21, 22), the tRNA3′-processing endoribonuclease tRNaseZ (G6) (30), the N-acyl homoserine lactone hydrolase (G12) (20), and the methyl parathion hydrolase (G15) (31). The structure of SdsA1 adds group 13 to this divergent family.
The MBL fold clearly represents a stable scaffold that repeatedly has been used during evolution to catalyze a diverse range of chemical reactions. Structurally, this diversity is achieved by varying the sequence and length of loops near the active site to accommodate various substrates. By combining the MBL domain with auxiliary substrate-, product- or cofactor-binding domains, the range of functions has been extended yet further, as exemplified by G9 (Pce), G3 (rubredoxin oxidoreductase), and G13 (SdsA1).
Catalytic Mechanism of SdsA1.
In hydrolytic enzymes bearing a binuclear metal cluster (32), a hydroxyl ion bridging the metal ions or a Zn2+-bound water is thought to generally function as the nucleophile. Such a mechanism is widely accepted for phosphate ester hydrolysis (35) and several supporting crystal structures are available (22, 34, 35). Typically, the product PO43− is found bridging the metal ions in these structures.
In SdsA1, by contrast, neither product SO42− nor SO3− group of 1DA directly coordinate Zn2+. Instead, the sulfur is removed by 5.0 Å (SO3−) or 5.7 Å (SO42−) from the Zn2+-bridging hydroxyl W1. A direct nucleophilic attack on the sulfur atom by W1, therefore, appears unlikely. The specificity of SO42− and R-SO3− recognition (Figs. 3 B and C, 10, and 11) indicate the observed positions to be catalytically relevant. Similarly, the lack of an obvious hinge region within the active site cavity argues against a significant translocation of the sulfate ion toward the hydroxyl as part of an active-site closure. Direct access to the Zn2+ cluster and W1 is severely limited furthermore because of the accumulation of large residues such as the two Zn2+ ligands Glu-280 and Glu-299, unique to SdsA1, as well as Ile-239 and His-306 (Fig. 3).
If, as proposed, W1 is not the nucleophile, another water molecule could assume this function. We identify W2 as a possible candidate. In the complex of SdsA1 and 1DA, W2 fills the gap between W1 and S1DA and, at 3.0 Å, interacts strongly with S1DA. Polarizing interactions with hydroxyl W1 and Glu-299 (Fig. 3A) may induce W2 to mount a nucleophilic attack on a substrate sulfate ester in a first step of an addition-elimination reaction. The directionality of the attack would be enhanced by the adjacent hydrophobic Ile-239-Cδ (Fig. 4A), whereas Asn-310, Arg-317, and His-405 would serve to polarize the SO42− group of the substrate (SO3− in 1DA). The relative positions of W2, S1DA, and C11DA in the 1DA complex (angle W2-S1DA -C11DA: ≈120°; O21DA, W2, and C11DA lying in one plane; Fig. 4B) thus appear to mimic a trigonal bipyramidal transition state that would be relieved by loss of the leaving group alcohol in the elimination step of the reaction, resulting in the overall cleavage of the CO-S bond. However, W2 and the leaving group occupy adjacent positions in the sulfur ligand sphere, arguing against a concerted mechanism in which both would occupy axial positions in a trigonal bipyramidal transition state. Either the observed state does not represent a productive cleavage intermediate or a more complex addition-elimination reaction possibly involving pseudorotation of ligands would be required (36).
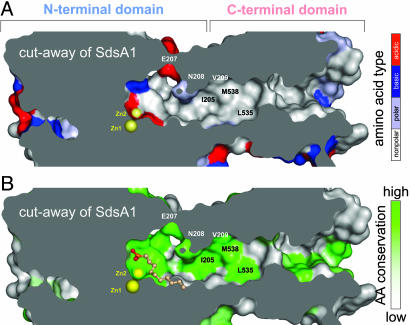
Fig. 4.
The hydrophobic chute connecting the C-terminal domain with the active site of SdsA1. The van der Waals surface of SdsA1 (colored) has been cut away to reveal the hydrophobic chute; the molecular interior is colored gray. Zn ions (yellow spheres) mark the active site. Selected amino acids are marked. (A) Predominantly hydrophobic residues (gray) line the chute, whereas polar (light blue), basic (blue), and acidic (red) amino acids mark the active site or the molecular surface. (B) Amino acids lining the chute are strongly conserved (green) in contrast to the molecular surface (gray). Bound 1DA is shown in ball-and-stick representation.
Substrate Specificity of SdsA1 and Other Sulfatases in P. aeruginosa.
Pseudomonas species have been described to express up to six distinct sulfatases (8, 12, 37, 38), presumably to accommodate the range of sulfate ester compounds encountered in their natural habitats. P. aeruginosa harbors at least three sulfatases, one each of the three mechanistic groups. Arylsulfatase AtsA (5) and the medium-chain alkylsulfatase AtsK (6, 39) are cytosolic enzymes induced by sulfate limitation. They are coexpressed with an ABC-type transporter that allows for the uptake of sulfated compounds. Disruption of this transporter system, correspondingly, abrogates the utilization of arylsulfates or medium-chain alkylsulfates. The ability to grow on SDS is independent of this mechanism (40). We have identified SdsA1 as a third sulfatase of P. aeruginosa that cleaves both short and medium-length alkylsulfates, including SDS. As a result, it allows P. aeruginosa to use SDS as a sole carbon or sulfur source. In contrast to AtsA and AtsK, SdsA1 is an extracellular enzyme. AtsA and AtsK are regulated, respectively, by CysB (40, 41) and SftR (PA0191) (42). SdsA1 appears to be regulated by PA0739, 54% identical to the LysR-type regulator SdsB shown to have this function in Pseudomonas sp. ATCC19151 (7). Cleavage of SDS by SdsA1 nevertheless, is presumably coincidental because SDS has been introduced to the environment only through its use in detergents during the last century (13). Chlorinated and nonchlorinated alkyl diol disulfates have been proposed to represent physiological substrates of prokaryotic alkylsulfatases (8) and a related synthetic compound, octadecan 1,12-disulfate, has been demonstrated to be a substrate for both primary and secondary alkylsulfatases of Pseudomonas C12B (43).
Structurally, the active site of SdsA1 appears larger than required to bind primary alkylsulfates (Fig. 4A). Conserved hydrophilic residues near the active site not involved in SDS, 1DO, or 1DA binding (Fig. 4B) imply that SdsA1 may recognize and cleave a much wider range of substrates. Although glucosamin-2-sulfate and glucose-3-sulfate are not cleaved by SdsA1, mono- or disaccharide sulfates are easily accommodated within the active-site pocket in silico. Linked to hydrophobic tails to accommodate the hydrophobicity of the active-site channel and the C-terminal domain, sulfated sugars such as sulfoglycolipids may represent physiological substrates for SdsA1. Using these widely occurring compounds (44) would allow P. aeruginosa to survive in niches not available to other bacteria.
Surprisingly, the role of SdsA1 may not be confined to the acquisition of sulfur. We noticed that the inactivation of SdsA1 by a transposon resulted in higher concentrations of N-acyl homoserine lactones and elevated protease secretion during stationary growth (data not shown). Although it remains to be seen whether sulfur deficiency (45) caused by the lack of SdsA1 (or another, as-yet-unknown function of SdsA1) accounts for this phenomenon, our data indicate that the role of SdsA1 is not limited to sulfur metabolism but indirectly affects quorum sensing in P. aeruginosa as well.
Methods
Cloning, Protein Expression, and Purification.
The gene PA0740 (SdsA1) of P. aeruginosa PAO1 was amplified from genomic DNA by using oligonucleotides 5′-GACGGCCATATGAGCCGTCTGCTTGCACTCCTG-3′ and 5′-GCGCAGATCTGCCTTCGGACTTCGCCGCCGGCGT-3′ as forward and reverse PCR primers and cloned into expression vector pBBR22bII, a derivative of pBBR22b (46), by using restriction enzymes NdeI and BglII. SdsA1-His6 fusion protein was expressed in E. coli Tuner cells (Novagen) grown in LB medium supplemented with 37 μg/ml chloramphenicol. Cells were grown to an OD600 of 1.0 at 37°C, and protein expression was induced by 0.5 mM isopropyl-β-d-thiogalactoside overnight at 20°C. SeMet-substituted SdsA1-His6 was produced as described in ref. 47. Cells were centrifuged, resuspended, and lysed by French press. Cell debris were removed by centrifugation. Purification was achieved by Ni-NTA affinity chromatography (Qiagen), anion exchange chromatography (MonoQ; GE Healthcare) and gel filtration (Superdex 200 HR 16/60; GE Healthcare). The molecular mass in solution was estimated by using gel filtration calibration kits HMW and LMW (GE Healthcare). Protein of >95% purity was concentrated to 3 mg/ml and stored in 50 mM Tris·HCl, pH 8, at 4°C. To prevent oxidation of selenium atoms, 3 mM DTT was added to the SeMet-substituted protein.
Bacterial Growth.
P. aeruginosa strain TBCF10839 wild type and the isogenic SdsA1-transposon mutant were grown on E3 (48) agarose containing 3.5 g of NaNH4HPO4·4 H2O, 7.5 g of K2HPO4·3H2O, 3.7 g/liter KH2PO4, and 0.1% (vol/vol) trace salts solution. One liter of trace salts solution contained 2.86 g of FeCl3·6H2O, 1.98 g of MnCl2·4H2O, 3.31 g of CoCl2·6H2O, 1.47 g of CaCl2·2H2O, 0.17 g of CuCl2·2H2O, and 0.61 g of ZnCl2. Combinations of either 0.1% (wt/vol) SDS and 10 mM citrate, SDS, and 1 mM MgSO4 or SDS alone were provided as the sole carbon and sulfur sources. Growth of bacterial colonies was monitored at 37°C for 3–5 days.
Biochemical Characterization of Sulfatase Activity.
Hydrolysis of SDS releases a proton. The change in pH during SdsA1-mediated SDS hydrolysis therefore was recorded photometrically by using a phenol red/Hepes indicator/buffer pair at pH 7.5 (49). A typical reaction contained 0.2 mM Hepes, 100 μM phenol red, 1–700 μM SDS, and 80 pmol SdsA1. Initial rates of SDS hydrolysis were determined by monitoring the decrease in absorbance at 557 nm at 25°C. Standard curves were prepared by substituting SDS with equal amounts of HCl. The cleavage product 1DO was extracted by using chloroform and its trimethylsilyl derivative confirmed by GC/MS.
Crystallization.
SdsA1 crystals were grown at 20°C by hanging-drop vapor-diffusion with equal volumes (2 μl) of protein (3 mg/ml) and reservoir solutions (12% PEG 4000/10% isopropanol/200 mM LiCl/100 mM sodium citrate, pH 6). For cryoprotection, 30% PEG 400 was added to the reservoir solution. Crystals (space group P6522, a = b = 86 Å, c = 364 Å) grew after 12 h. Isomorphous SeMet-substituted crystals, obtained by microseeding, were used for x-ray data collection.
Structure Determination.
X-ray diffraction data sets were collected at three wavelengths (inflection, 0.9796 Å; peak, 0.9790 Å; high-energy remote, 0.9256 Å) at beamline BW7A (European Molecular Biology Laboratory Hamburg outstation, Germany) on a MarCCD detector (Marresearch, Norderstedt, Germany). Data were processed by using the hkl (50) and ccp4 (51) suites. Statistics are listed in Table 1. Phasing was achieved by multiple anomalous dispersion techniques. shelxd was used to locate Se-sites (52, 53). After phase calculation, phase extension, and phase improvement by density modification (shelxe; refs. 53 and 54), ≈50% of the polypeptide (≈300 amino acids) could be traced by using arp/warp (55) and resolve (56). The second half was built manually (o; ref. 57). The structure of SdsA1 was refined to a resolution of 1.9 Å (refmac5; ref. 16).
SDS, 1DA, and Li2SO4 were dissolved in reservoir solution and added 1:1 to crystal mother liquor. SDS was soaked at 2% for 10 min, 1DA at 10 mM for 2 days, and Li2SO4 at 100 mM for 2 days. Diffraction data were collected at EMBL beamlines X11 and X13 (Deutsches Elektronen-Synchrotron, Hamburg, Germany) and beamline PX I (Swiss Light Source, Paul Scherrer Institute, Villigen, Switzerland). o (57) and coot (58) were used for model building, structural analysis, and substrate modeling. Structures were validated by using procheck (59) and whatif (60). Figures were prepared by using pymol (www.pymol.org).
Supplementary Material
Acknowledgments
We thank Dr. Andrea Schmidt (European Molecular Biology Laboratory Hamburg outstation) for help during data collection, Dr. Martin Fieber-Erdmann (Berliner Elektronenspeicherring-Gesellschaft für Synchrotronstrahlung) for x-ray fluorescence scans, Dr. Hartmut Niemann for data collection of the SdsA1 complexes and helpful comments on the manuscript, Björn Niebel for kinetic analyses, Dr. Manfred Nimtz for mass spectrometric identification of SdsA1 cleavage products, and Rita Getzlaff for N-terminal sequencing of proteins. Synchrotron beam time at beamlines BW7A, X11, and X13 (European Molecular Biology Laboratory, Deutsches Elektronen-Synchrotron) and PX I (Swiss Light Source) is gratefully acknowledged. Financial support was provided by the Fords der Chemischen Industrie (to D.W.H.) and the Bundesministerium für Bildung und Forschung within the framework of the Competence Network PathoGenoMik Grants FKZ: PTJ/BIO-01K003092001-031U213B and PTJ-BIO/0313134 (to H.K. and B.T.).
Abbreviations
- Gn
group n
- MBL
metallo-β-lactamase
- 1-DA
1-decane-sulfonic-acid
- 1-DO
1-dodecanol.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The atomic coordinates have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 2CFU, 2CFZ, 2CG2, and 2CG3).
References
- 1.Kertesz M. A. FEMS Microbiol. Rev. 2000;24:135–175. doi: 10.1016/S0168-6445(99)00033-9. [DOI] [PubMed] [Google Scholar]
- 2.Fitzgerald J. W., Payne W. J. Microbios. 1972;5:87–100. [PubMed] [Google Scholar]
- 3.Beil S., Kehrli H., James P., Staudenmann W., Cook A. M., Leisinger T., Kertesz M. A. Eur. J. Biochem. 1995;229:385–394. doi: 10.1111/j.1432-1033.1995.0385k.x. [DOI] [PubMed] [Google Scholar]
- 4.Kahnert A., Kertesz M. A. J. Biol. Chem. 2000;275:31661–31667. doi: 10.1074/jbc.M005820200. [DOI] [PubMed] [Google Scholar]
- 5.Boltes I., Czapinska H., Kahnert A., von Bülow R., Dierks T., Schmidt B., von Figura K., Kertesz M. A., Uson I. Structure (London) 2001;9:483–491. doi: 10.1016/s0969-2126(01)00609-8. [DOI] [PubMed] [Google Scholar]
- 6.Müller I., Kahnert A., Pape T., Sheldrick G. M., Meyer-Klaucke W., Dierks T., Kertesz M., Uson I. Biochemistry. 2004;43:3075–3088. doi: 10.1021/bi035752v. [DOI] [PubMed] [Google Scholar]
- 7.Davison J., Brunel F., Phanopoulos A., Prozzi D., Terpstra P. Gene. 1992;114:19–24. doi: 10.1016/0378-1119(92)90702-q. [DOI] [PubMed] [Google Scholar]
- 8.Dodgson K. S., White G. F. Sulfatases of Microbial Origin. Boca Raton, FL: CRC; 1982. pp. 9–48. [Google Scholar]
- 9.Barbeyron T., Potin P., Richard C., Collin O., Kloareg B. Microbiology. 1995;141:2897–2904. doi: 10.1099/13500872-141-11-2897. [DOI] [PubMed] [Google Scholar]
- 10.Hsu Y. C. Nature. 1963;200:1091–1092. doi: 10.1038/2001091b0. [DOI] [PubMed] [Google Scholar]
- 11.Hsu Y. C. Nature. 1965;207:385–388. doi: 10.1038/207385a0. [DOI] [PubMed] [Google Scholar]
- 12.Lillis V., Dodgson K. S., White G. F., Payne W. J. Appl. Environ. Microbiol. 1983;46:988–994. doi: 10.1128/aem.46.5.988-994.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall C., Brachat S., Dietrich F. S. Eukaryotic Cell. 2005;4:1102–1115. doi: 10.1128/EC.4.6.1102-1115.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loftus B., Anderson I., Davies R., Alsmark U. C., Samuelson J., Amedeo P., Roncaglia P., Berriman M., Hirt R. P., Mann B. J., et al. Nature. 2005;433:865–868. doi: 10.1038/nature03291. [DOI] [PubMed] [Google Scholar]
- 15.Daiyasu H., Osaka K., Ishino Y., Toh H. FEBS Lett. 2001;503:1–6. doi: 10.1016/s0014-5793(01)02686-2. [DOI] [PubMed] [Google Scholar]
- 16.Murshudov G. N., Vagin A. A., Dodson E. J. Acta Crystallogr. D. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 17.Carfi A., Pares S., Duee E., Galleni M., Duez C., Frere J. M., Dideberg O. EMBO J. 1995;14:4914–4921. doi: 10.1002/j.1460-2075.1995.tb00174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cameron A. D., Ridderstrom M., Olin B., Mannervik B. Structure (London) 1999;7:1067–1078. doi: 10.1016/s0969-2126(99)80174-9. [DOI] [PubMed] [Google Scholar]
- 19.Frazao C., Silva G., Gomes C. M., Matias P., Coelho R., Sieker L., Macedo S., Liu M. Y., Oliveira S., Teixeira M., et al. Nat. Struct. Biol. 2000;7:1041–1045. doi: 10.1038/80961. [DOI] [PubMed] [Google Scholar]
- 20.Liu D., Lepore B. W., Petsko G. A., Thomas P. W., Stone E. M., Fast W., Ringe D. Proc. Natl. Acad. Sci. USA. 2005;102:11882–11887. doi: 10.1073/pnas.0505255102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garau G., Lemaire D., Vernet T., Dideberg O., Di Guilmi A. M. J. Biol. Chem. 2005;280:28591–28600. doi: 10.1074/jbc.M502744200. [DOI] [PubMed] [Google Scholar]
- 22.Hermoso J. A., Lagartera L., Gonzalez A., Stelter M., Garcia P., Martinez-Ripoll M., Garcia J. L., Menendez M. Nat. Struct. Mol. Biol. 2005;12:533–538. doi: 10.1038/nsmb940. [DOI] [PubMed] [Google Scholar]
- 23.Holm L., Sander C. J. Mol. Biol. 1993;233:123–138. doi: 10.1006/jmbi.1993.1489. [DOI] [PubMed] [Google Scholar]
- 24.Haapalainen A. M., van Aalten D. M., Merilainen G., Jalonen J. E., Pirila P., Wierenga R. K., Hiltunen J. K., Glumoff T. J. Mol. Biol. 2001;313:1127–1138. doi: 10.1006/jmbi.2001.5084. [DOI] [PubMed] [Google Scholar]
- 25.Choinowski T., Hauser H., Piontek K. Biochemistry. 2000;39:1897–1902. doi: 10.1021/bi992742e. [DOI] [PubMed] [Google Scholar]
- 26.Dyer D. H., Lovell S., Thoden J. B., Holden H. M., Rayment I., Lan Q. J. Biol. Chem. 2003;278:39085–39091. doi: 10.1074/jbc.M306214200. [DOI] [PubMed] [Google Scholar]
- 27.Alberts I. L., Nadassy K., Wodak S. J. Protein Sci. 1998;7:1700–1716. doi: 10.1002/pro.5560070805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fabiane S. M., Sohi M. K., Wan T., Payne D. J., Bateson J. H., Mitchell T., Sutton B. J. Biochemistry. 1998;37:12404–12411. doi: 10.1021/bi980506i. [DOI] [PubMed] [Google Scholar]
- 29.Rasia R. M., Ceolin M., Vila A. J. Protein Sci. 2003;12:1538–1546. doi: 10.1110/ps.0301603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishii R., Minagawa A., Takaku H., Takagi M., Nashimoto M., Yokoyama S. J. Biol. Chem. 2005;280:14138–14144. doi: 10.1074/jbc.M500355200. [DOI] [PubMed] [Google Scholar]
- 31.Dong Y. J., Bartlam M., Sun L., Zhou Y. F., Zhang Z. P., Zhang C. G., Rao Z., Zhang X. E. J. Mol. Biol. 2005;353:655–663. doi: 10.1016/j.jmb.2005.08.057. [DOI] [PubMed] [Google Scholar]
- 32.Wilcox D. E. Chem. Rev. 1996;96:2435–2458. doi: 10.1021/cr950043b. [DOI] [PubMed] [Google Scholar]
- 33.Knowles J. R. Annu. Rev. Biochem. 1980;49:877–919. doi: 10.1146/annurev.bi.49.070180.004305. [DOI] [PubMed] [Google Scholar]
- 34.Klabunde T., Sträter N., Fröhlich R., Witzel H., Krebs B. J. Mol. Biol. 1996;259:737–748. doi: 10.1006/jmbi.1996.0354. [DOI] [PubMed] [Google Scholar]
- 35.Lindqvist Y., Johansson E., Kaija H., Vihko P., Schneider G. J. Mol. Biol. 1999;291:135–147. doi: 10.1006/jmbi.1999.2962. [DOI] [PubMed] [Google Scholar]
- 36.Westheimer F. H. Acc. Chem. Res. 1968;1:70–78. [Google Scholar]
- 37.Bateman T. J., Dodgson K. S., White G. F. Biochem. J. 1986;236:401–408. doi: 10.1042/bj2360401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dodgson K. S., Fitzgerald J. W., Payne W. J. Biochem. J. 1974;138:53–62. doi: 10.1042/bj1380053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quadroni M., James P., Dainese-Hatt P., Kertesz M. A. Eur. J. Biochem. 1999;266:986–996. doi: 10.1046/j.1432-1327.1999.00941.x. [DOI] [PubMed] [Google Scholar]
- 40.Hummerjohann J., Laudenbach S., Retey J., Leisinger T., Kertesz M. A. J. Bacteriol. 2000;182:2055–2058. doi: 10.1128/jb.182.7.2055-2058.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hummerjohann J., Küttel E., Quadroni M., Ragaller J., Leisinger T., Kertesz M. A. Microbiology. 1998;144:1375–1386. doi: 10.1099/00221287-144-5-1375. [DOI] [PubMed] [Google Scholar]
- 42.Kahnert A., Mirleau P., Wait R., Kertesz M. A. Environ. Microbiol. 2002;4:225–237. doi: 10.1046/j.1462-2920.2002.00289.x. [DOI] [PubMed] [Google Scholar]
- 43.Payne W. J., Painter B. G. Microbios. 1971;3:199–206. [PubMed] [Google Scholar]
- 44.Ishizuka I. Prog Lipid Res. 1997;36:245–319. doi: 10.1016/s0163-7827(97)00011-8. [DOI] [PubMed] [Google Scholar]
- 45.Lazazzera B. A. Curr. Opin. Microbiol. 2000;3:177–182. doi: 10.1016/s1369-5274(00)00072-2. [DOI] [PubMed] [Google Scholar]
- 46.Rosenau F., Jäger K. E. In: Overexpression and Secretion of Biocatalysts in Pseudomonas. Svendsen A., editor. New York: Marcel Dekker; 2004. pp. 617–631. [Google Scholar]
- 47.Guerrero S. A., Hecht H. J., Hofmann B., Biebl H., Singh M. Appl. Microbiol. Biotechnol. 2001;56:718–723. doi: 10.1007/s002530100690. [DOI] [PubMed] [Google Scholar]
- 48.Vogel H. J., Bonner D. M. J. Biol. Chem. 1956;218:97–106. [PubMed] [Google Scholar]
- 49.Thomas P. W., Stone E. M., Costello A. L., Tierney D. L., Fast W. Biochemistry. 2005;44:7559–7569. doi: 10.1021/bi050050m. [DOI] [PubMed] [Google Scholar]
- 50.Otwinowski Z., Minor W. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 51.Collaborative Computational Project, Number 4. Acta Crystallogr. D. 1994;50:760–763. [Google Scholar]
- 52.Schneider T. R., Sheldrick G. M. Acta Crystallogr. D. 2002;58:1772–1779. doi: 10.1107/s0907444902011678. [DOI] [PubMed] [Google Scholar]
- 53.Pape T., Schneider T. R. J. Appl. Crystallogr. 2004;37:843–844. [Google Scholar]
- 54.Sheldrick G. M. Z. Kristallogr. 2002;217:644–650. [Google Scholar]
- 55.Perrakis A., Morris R., Lamzin V. S. Nat. Struct. Biol. 1999;6:458–463. doi: 10.1038/8263. [DOI] [PubMed] [Google Scholar]
- 56.Terwilliger T. J. Synchrotron Radiat. 2004;11:49–52. doi: 10.1107/s0909049503023938. [DOI] [PubMed] [Google Scholar]
- 57.Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard Acta Crystallogr. A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 58.Emsley P., Cowtan K. Acta Crystallogr. D. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 59.Laskowski R. A., MacArthur M. W., Moss D. S., Thornton J. M. J. Appl. Crystallogr. 1993;26:283–291. [Google Scholar]
- 60.Vriend G. J. Mol. Graphics. 1990;8:52–56. doi: 10.1016/0263-7855(90)80070-v. 29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.