Abstract
Many skin disorders are associated with increased numbers of activated mast cells and are worsened by stress; however, the mechanism underlying these processes is not understood. Corticotropin-releasing hormone (CRH) is secreted under stress from the hypothalamus, but also in the skin, where it induces mast cell activation and vascular permeability. We investigated the effect of CRH in a number of animal models by using i.v. Evans blue extravasation as a marker of vascular permeability. Intradermal CRH is among the most potent peptides at 100 nM, its effect being nearly comparable to that of neurotensin (NT). Pretreatment of skin injection sites with the NT receptor antagonist SR48692 blocks CRH-induced vascular permeability, which is diminished in NT−/− mice, implying that NT is necessary for the effect of CRH. CRH and NT precursor mRNA are shown to be expressed in both dorsal root ganglia and skin, whereas the latter also expresses mRNA for prohormone convertase 5, an enzyme that cleaves pro-NT into its active form. We also show that the effect of both CRH and NT is absent in W/Wv mast cell-deficient mice; however, only a fraction of skin mast cells express CRH receptors, as shown by FACS analysis of CRH receptor (CRHR) and c-kit double-positive disaggregated mouse skin mast cells. These findings suggest that CRH induces skin vascular permeability through NT acting on mast cells and that both peptides should be considered in the pathogenesis of skin disorders exacerbated by stress.
Keywords: inflammation, mast cells, stress, urticaria
Acute emotional stress in humans precipitates or worsens skin conditions that involve mast cells (1), including atopic dermatitis (2), psoriasis (3), and urticaria (4). Acute restraint stress in rats has been shown to induce degranulation of skin mast cells (5), an effect mimicked by intradermal injection of corticotropin-releasing hormone (CRH) (6). CRH also increases vascular permeability when injected intradermally, an effect absent in W/Wv mast cell-deficient mice and blocked by the mast cell stabilizer disodium cromoglycate (cromolyn) (7). CRH also induces mast cell-dependent vasodilation (8) in the microvasculature of human skin (9). CRH mRNA and peptide are expressed in human skin; in contrast, mouse skin apparently does not express mRNA for CRH and contains only CRH peptide (10). CRH receptors (CRHR) are expressed in both human and rodent skin (10, 11).
Skin mast cells may have important functions as “sensors” of environmental and emotional stress (12), possibly through direct activation by CRH and related peptides (13). As a result, mast cells could play an important role in the pathophysiology of inflammatory diseases worsened by stress (14). However, it is not yet known whether CRH acts alone to activate skin mast cells.
Neuropeptides, especially neurotensin (NT), could be involved in the pathogenesis of inflammatory skin disorders, especially those exacerbated by stress (15). NT is one of the most potent inducers of vascular permeability when injected into rodent skin (7), and NT receptors (NTR) have been identified on mast cells (16). NT also significantly increases histamine release from isolated rat skin (17) and in skin blisters (18) in a mast cell-dependent manner. However, the role of NT in stress-induced skin conditions has not been investigated.
Mast cells are found in large numbers (10,000–20,000 mast cells per mm3) beneath the epithelial surface of the skin (19). They are located in the subpapillary region, around blood vessels, lymphatic structures, and epithelial appendages of the skin, where they participate in acute and late-phase hypersensitivity reactions (20). Degranulation of mast cells leads to the release of multiple mediators with potent vasodilatory, inflammatory, and nociceptive properties (21). For example, histamine increases vascular permeability (6) and stimulates cutaneous sensory nerves (22), leading to pruritus. Biopsies of acute urticarial lesions show interstitial edema and endothelial swelling, secondary to mast cell activation (4). The lichenified plaques in the skin of patients with atopic dermatitis contain a higher number of mast cells, as compared with clinically uninvolved skin (23), and so do skin lesions in patients with psoriasis (3).
We hypothesized that stress may induce the release of CRH and NT from dorsal root ganglia (DRG), leading to skin mast cell activation and increased vascular permeability. Our results show that CRH and NT induce skin vascular permeability, but the effect of both is blocked by a NTR antagonist and is absent in NT−/− mice, whereas both CRH and NT precursor are expressed by DRG. CRH released under stress may be acting together or sequentially with NT to induce skin vascular permeability.
Results
Effect of Intradermal Administration of Peptides.
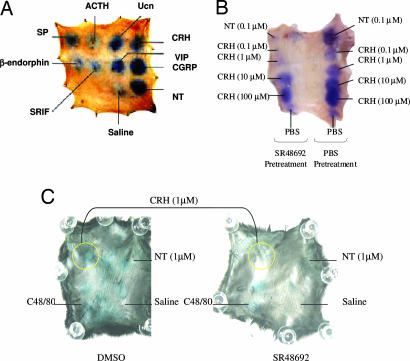
The effect of intradermal administration of various peptides (100 nM) on vascular permeability was studied in the rat skin. At this dose NT and calcitonin gene-related peptide gave the largest responses, followed by CRH, urocortin, vasoactive intestinal peptide, substance P (SP), somatostatin, β-endorphin, and adrenocorticotropin hormone (Fig. 1A).
Fig. 1.
Effect of intradermal CRH and NT, as well as pretreatment with NTR antagonist SR48692 in rat and mouse skin. (A) Comparison of various neuropeptides to induce vascular permeability in rat skin. (B) Rats were anesthetized and injected with 0.6 ml of 1% Evans blue via the tail vein. Ten minutes after the injection, SR48692 (10 μM) or PBS was injected intradermally in the skin. Pretreated sites were then injected 10 min later with NT (0.1 μM) or different concentrations of CRH (0.1–100 μM). The rats were killed 10 min later, and the skin was removed, turned over, mounted, and photographed. (C) Mice were pretreated with an i.p. injection of 1% DMSO (Left) or 0.05 mg/kg SR48692 (Right) for 60 min before intradermal injections of NT and CRH. After 60 min, the mice were anesthetized and injected via the tail vein with 0.2 ml of 1% Evans blue. CRH and NT (0.05 ml of 1 μM) were injected intradermally in the dorsal skin. The animals were killed after 10 min, and the skin was removed and photographed.
NTR Antagonist SR48692 Blocks the Intradermal Effects of CRH on Vascular Permeability.
In view of the fact that we had shown that the NTR antagonist SR48692 could block stress-induced skin mast cell activation (5), we investigated the role of NT in CRH-induced vascular permeability using the NTR antagonist SR48692. We pretreated the skin injection sites in rats with either saline or SR48692 (10 μM) 10 min before injection with CRH (0.1–1 μM). This experiment had to be performed in rats because the mouse skin is too thin to permit a double injection without causing damage or nonspecific leakage of fluid. Pretreatment with SR48692 blocked the effect of 0.1 μM NT, as well as that of 0.1 and 1 μM CRH (Fig. 1B), whereas its solvent, DMSO (0.1%), had no inhibitory effect (results not shown).
C57BL/6 mice were also pretreated with an i.p. injection of 0.25 ml of 10 μM SR48692 to yield 0.05 mg/kg (for 30-g mice). This pretreatment eliminated the effect of intradermal administration of 1 μM NT and significantly reduced the effect of 1 μM CRH (Fig. 1C).
Effect of Intradermal CRH in NT−/− Mice.
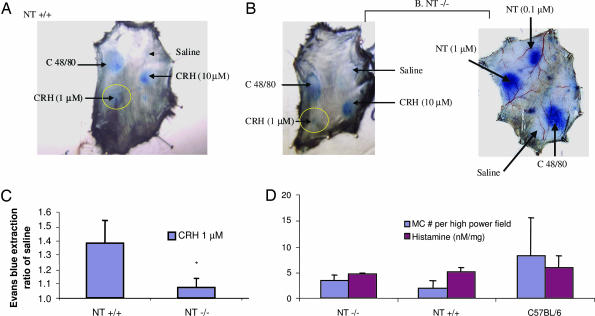
To confirm the requirement for NT, apparent from the use of the NTR antagonist, we investigated whether CRH could induce vascular permeability in NT−/− mice. CRH (1 μM) did not increase vascular permeability in NT−/− mice (Fig. 2A and B; compare the yellow circles). Compound 48/80 increased extravasation of Evans blue in the skin of both the NT+/+ (Fig. 2A) and NT−/− (Fig. 2B) mice, indicating that both NT+/+ and NT−/− mice could respond to the stimulus; NT (0.1 μM or higher) also induced vascular permeability in the NT−/− mice (Fig. 2B). When Evans blue was extracted from the skin by incubation in formamide, there was a significant (P = 0.036, n = 3) decrease in Evans blue extravasation (reported in arbitrary units) induced by 1 μM CRH in the NT−/− mice (1.075 ± 0.064) as compared with the NT+/+ mice (1.380 ± 0.157), whereas there was no significant (P = 0.253, n = 3) difference in response to C48/80 (Fig. 2C). The absence of CRH-induced vascular permeability in the NT−/− mice could not be accounted for by any differences in skin mast cell number or histamine content among NT−/−, NT+/+, and C57BL/6 mice because there were no statistical differences among them (Fig. 2D).
Fig. 2.
The effect of intradermal CRH on extravasation in NT−/− mice. NT+/+ (A) and NT−/− mice (B) were anesthetized with an i.p. injection of ketamine/xylazine, and the fur on the back was shaved. The mice were injected via the tail vein with 0.2 ml of 1% Evans blue. Ten minutes later, 0.05 ml of saline, compound 48/80 (10 μg/ml), CRH (1 μM or 10 μM), or NT (0.1 or 1 μM) was injected. The animals were killed 10 min later by decapitation. The skin was removed, turned over, and photographed. (C) Evans blue was extracted by incubation in N,N-dimethylformamide, and the concentration was calculated from a standard curve. CRH-induced permeability (yellow circles) is reported as a ratio of normal saline (saline) to control for interanimal variability (∗, P = 0.036, n = 3). (D) Mast cell number and skin histamine content were determined in mouse skin isolated from C57BL/6, NT+/+, and NT−/− mice (n = 2; three sections for each). Skin samples were isolated and either sectioned, adhered to slides, and stained with toluidine blue for mast cell counts or homogenized in PBS for histamine analysis.
Expression of CRH and NT in DRG and Skin.
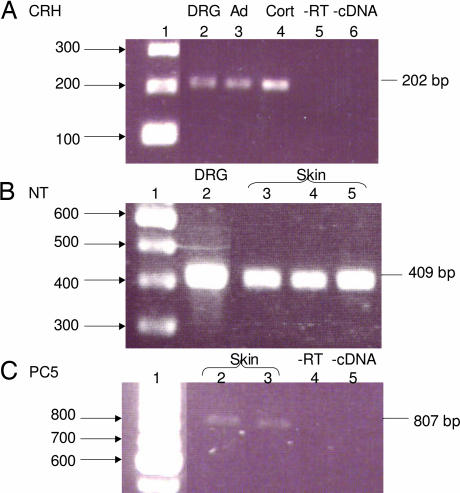
We then investigated a possible source of CRH and NT that might be released in the skin under stress. CRH (Fig. 3A) and NT precursor (Fig. 3B) peptide mRNA was expressed in mouse DRG. NT precursor mRNA was also expressed in mouse skin (Fig. 3B); moreover, skin expressed prohormone convertase 5 (PC5), an enzyme required to convert pro-NT to NT (Fig. 3C).
Fig. 3.
CRH, NT, and PC5 mRNA expression in mouse skin and DRG. (A) Total RNA isolated from mouse DRG was subjected to RT-PCR analysis by using oligonucleotide primer pairs that amplify a region corresponding to CRH. The DNA products amplified were resolved on a 1.2% agarose gel and visualized with ethidium bromide. The size of the predicted DNA product is indicated on the right of the gel (202 bp). Lane 1, 100-bp ladder; lane 2, DRG; lane 3, mouse adrenal; lane 4, mouse cortex; lane 5, without reverse transcriptase (-RT); lane 6, without cDNA (-cDNA). (B) Total RNA isolated from mouse DRG and skin was subjected to RT-PCR analysis by using oligonucleotide primer pairs that amplify a region corresponding to pro-NT. The DNA was amplified by PCR and resolved on a 1.2% agarose gel. The predicted DNA product is 409 bp. Lane 1, 100-bp DNA ladder; lane 2, DRG; lanes 3–5, mouse skin. (C) Total RNA isolated from mouse skin was subjected to RT-PCR analysis by using oligonucleotide primer pairs that amplify a region corresponding to PC5. The DNA was amplified by PCR and resolved on a 1.2% agarose gel. The predicted DNA product is 807 bp. Lane 1, 100-bp DNA ladder; lanes 2 and 3, mouse skin; lane 4, without reverse transcriptase (-RT); lane 5, without cDNA (-cDNA).
Effect of CRH and NT in W/Wv Mice.
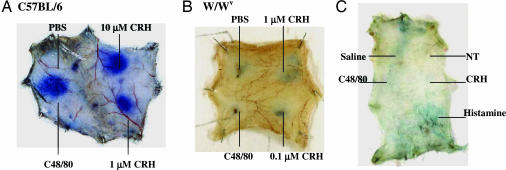
The increase in vascular permeability (Fig. 4A) was absent in W/Wv mice, as shown for CRH and NT (Fig. 4B), indicating that these effects are mast cell-dependent.
Fig. 4.
Effect of intradermal CRH and NT on vascular permeability in C57BL/6 (A), and W/Wv (B and C) mast cell-deficient mice. Anesthetized mice were injected with 0.2 ml of 1% Evans blue via the tail vein; 10 min later, PBS, CRH, NT (100 nM), or C48/80 (1 μg/ml) was injected intradermally. Mice were killed 10 min after the intradermal injections, and the skin was removed and photographed.
CRHR Expression on Skin Mast Cells.
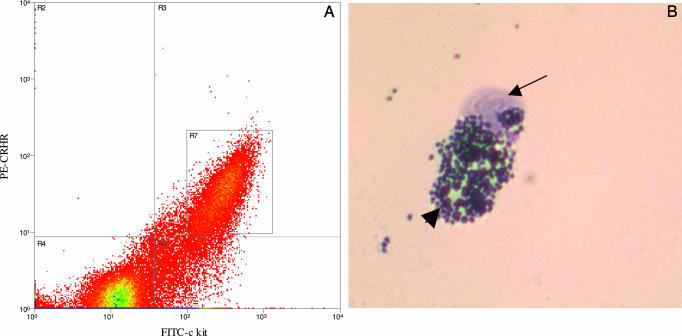
Given that CRH-induced skin vascular permeability was mast cell-dependent, we investigated whether skin mast cells expressed CRHR. Disaggregated skin mast cells were separated by using double labeling for c-kit, the surface receptor for their growth factor c-kit ligand (stem cell factor), and CRHR. A fraction comprising ≈25% of the starting mast cell number was isolated by FACS analysis (Fig. 5) and was confirmed by toluidine blue staining. Lack of a suitable NTR antibody precluded similar analysis for NTR-positive mast cells.
Fig. 5.
FACS analysis of disaggregated mouse skin mast cells. (A) The x axis corresponds to FITC-conjugated c-kit, and the y axis corresponds to phycoerythrin-conjugated CRHR (nonspecific, recognizes both R1 and R2). The cells labeled in quadrant R3 are positive for both c-kit and CRHR. Quadrant R4 is the negative control and is set for nonspecific fluorescence. R7 (box) contains those cells that did not stain with 7-amino-actinomycin D, the viable cells, and the cell population subsequently sorted for further analysis. (B) Intact mast cell with several granules. Those cells that were double-labeled with both c-kit and CRHR and did not take up 7-amino-actinomycin D were sorted by using a MoFlo instrument and collected in PBS/0.5% BSA. The cells were prepared on glass slides and stained with toluidine blue. The arrow indicates the nucleus, and the arrowhead points to the mast cell granules.
Discussion
Our present findings show that CRH and NT are potent inducers of skin vascular permeability and that the effect of CRH depends largely on NT, because it is inhibited by the NTR antagonist SR48692 and is diminished in NT−/− mice (24). Our results also show that mRNA for CRH and NT is present in DRG, from where their respective proteins may be synthesized and released into the skin under stress. A fraction of disaggregated mouse skin mast cells was shown to express CRHR, suggesting that the potent increase in skin vascular permeability may be largely due to its indirect effect through NT. In situ hybridization and immunohistochemistry also showed that a number of perifollicular mast cells express CRHR (25). Human mast cells were recently shown to express mRNA and protein for a number of CRHR isoforms (26).
The NTR antagonist SR48692 used here was previously shown to inhibit the interaction of NT with its binding sites on brain membranes (27), as well as to block NT stimulation of mast cell secretion in vivo and in vitro (28, 29). Moreover, the same compound was reported to inhibit the effect of stress on skin (5), heart (30), and bladder (31) mast cell activation, as well as gastrointestinal function (32). NT involvement in skin mast cell activation is supported by the fact that NT stimulates rat peritoneal (33, 34), skin (17), and human jejunum (35) mast cells. Rat serosal mast cells were reported to express NTR (16); moreover, NT is rapidly degraded by stimulated rat mast cells (36), suggesting a possible mechanism for blocking further activation by NT. NT-positive cells have been reported in the small intestine of humans (37, 38) and in the heart (39, 40), but there has not been any report of skin cells positive for NT. The apparent lack of appropriate antibodies has hampered such studies in rodent and human tissues.
In addition to NT (34), SP (41), vasoactive intestinal peptide, and calcitonin gene-related peptide can also activate skin mast cells (14). Antidromic stimulation of unmyelinated sensory nerves leads to the release of neuropeptides such as SP (42–44). SP and NT activate mast cells (17), inducing leukocyte infiltration, thus amplifying the initial inflammatory response (41). SR48692 could interfere with a common step for CRH and NT at or downstream from surface receptors. There is some evidence that cationic peptides bypass mast cell surface receptors and act on a G protein directly (45, 46). For instance, SP and compound 48/80 (47), as well as the bee venom peptide mastoparan (48), were shown to act directly on G proteins, “mimicking” the action of ligand binding to specific receptors. In fact, compound 48/80 may interact with the same receptor as NT and SP (49); moreover, SP-induced skin vascular leakage was blocked by N-acetyl-NT (50), and NT-induced colonic mast cell degranulation was blocked by a SP receptor antagonist (51). Pretreatment of mast cells with neuraminidase decreased the ability of SP and compound 48/80 to trigger rat mast cells (52, 53), suggesting that binding of these cationic molecules to negatively charged surface components may be a prerequisite for their mast cell stimulatory action.
Mast cells are found close to neurons in the skin (54) and dura (55). Parasympathetic nerve stimulation can augment or trigger mast cell secretion (56), and mast cell-derived histamine can then stimulate peripheral neurons (22). Mast cells can secrete various preformed (histamine, kinins, and proteases) and newly synthesized (leukotrienes, prostaglandins, and cytokines) proinflammatory mediators. Vasodilatory molecules other than histamine include nitric oxide, tryptase, TNF-α, vasoactive intestinal peptide, and VEGF (57). An isoform of VEGF is particularly vasodilatory (58), and VEGF was shown to induce dilation of microvessels (59). Moreover, overexpression of VEGF in mouse skin can lead to the development of skin inflammation resembling psoriasis (60, 61). It is of interest that CRH can induce selective release of VEGF from human mast cells (26), whereas intradermal injection of CRH also leads to histamine secretion, implying that the in vivo action may require other triggers such as NT.
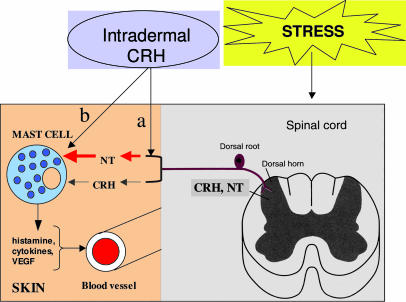
The most plausible scheme (Fig. 6) to explain our results would be that intradermal CRH stimulates release of NT from post-DRG nerve endings, leading to skin mast cell activation together with or sequentially with CRH; vasodilatory and proinflammatory molecules released from mast cells, such as histamine, cytokines, and VEGF, could then increase vascular permeability. In conclusion, this is the first instance, to our knowledge, that NT is shown to be involved in the action of CRH. Mast cell–neuron interactions (62) and mast cell activation may be involved in the pathophysiology of skin conditions such as atopic dermatitis (2, 63), urticaria (4, 64), and psoriasis (3, 63, 65, 66), all of which involve mast cells and are exacerbated by stress (67–69). Local administration of CRHR or NTR antagonists that could penetrate the skin may be useful for the treatment of stress-induced dermatoses.
Fig. 6.
Proposed model for stress-induced skin mast cell activation and increased vascular permeability. CRH and NT pro-peptide are present in the DRG and are released into the skin after stress. In the skin, NT precursor is cleaved to its active peptide and stimulates mast cells, together or sequentially with CRH, to release vasodilatory and proinflammatory molecules such as histamine, cytokines, and VEGF. Intradermal injection of CRH may activate the post-DRG nerve endings to release NT into the skin, which in turn stimulates mast cells (a) and/or directly activate mast cells in addition to its indirect effect through NT (b).
Materials and Methods
Experimental Animals.
Sprague–Dawley rats (Charles River Breeding Laboratories), C57BL/6 mice, and W/Wv mast cell-deficient mice and their +/+ normal littermates (The Jackson Laboratory) were used at ≈7 weeks of age. NT knockout (−/−) breeding pairs were provided by Paul Dobner (University of Massachusetts Medical School, Worcester). The mice were bred in-house and genotyped by PCR analysis of tail DNA (see Supporting Text, which is published as supporting information on the PNAS web site). All mice were kept on a 14:10 h dark:light cycle and were provided food and water ad libitum. These studies were approved by the Tufts University Institutional Animal Care and Use Committee (Protocol 45-03).
Skin Vascular Permeability After Intradermal Injections.
Male rats (350 g) or 5- to 7-week-old mice [C57BL/6 mice and NT−/− mice and their wild-type littermates were used; NT−/− mice that had been backcrossed five times with C57BL/6J (5N) show altered responses to antipsychotic drugs (24)]. Mice were anesthetized with a single i.p. injection of ketamine/xylazine (80 mg/kg ketamine and 10 mg/kg xylazine), and the fur on the subscapular area of their backs was removed with an electric shaver. A total of 0.6 ml (for rats) or 0.2 ml (for mice) of sterile-filtered 1% Evans blue dissolved in sterile normal saline (0.9% NaCl) was injected via the tail vein; 10 min later 0.05 ml of triggers or normal saline was injected intradermally in the dorsal region by using a tuberculin syringe. The triggers included compound 48/80, a synthetic mast cell secretagogue used as a “positive” control, CRH, NT, calcitonin gene-related peptide, vasoactive intestinal peptide, urocortin, SP, adrenocorticotropin hormone, β-endorphin, and somatostatin from Santa Cruz Biotechnology. The animals were killed 10 min after the intradermal injections by decapitation. The skin was removed, mounted, and photographed. Skin samples were removed by using a circular template to retain reproducibility, and the Evans blue dye was extracted by incubating them in N,N-dimethyl formamide overnight at 55°C. The following day, the Evans blue fluorescence from individual skin samples was measured at an excitation of 620 nm and an emission of 680 nm. The concentration of Evans blue was determined from a standard curve and was normalized by dividing with the value corresponding to skin samples injected with normal saline.
NTR Antagonist Pretreatment.
SR48692 (Sanofi Recherche, Paris) was prepared (1 mM stock solution) in DMSO and was stored at −20°C in the dark. Male Sprague–Dawley rats were anesthetized and injected with 0.6 ml of 1% Evans blue via the tail vein; 10 min later peptides were injected intradermally at the concentration indicated. For some experiments, the injection sites were pretreated for 10 min with either saline or SR48692 (10 μM) before the intradermal injection of the triggers. The rats were killed 10 min later, and the skin was removed and photographed. For mouse experiments, the SR48692 stock solution was diluted 1:100 in saline, and 0.25 ml was injected i.p. to yield 0.05 mg/kg SR48692 (dose based on a 30-g mouse) in a final DMSO concentration of 1%. DMSO was injected alone to serve as a vehicle control. One hour after the i.p. injection of SR48692 or DMSO mice were injected with 0.2 ml of 1% Evans blue via the tail vein, and vascular permeability induced by intradermal NT, CRH, and 48/80 was measured as described above.
Skin Histamine Content.
The amount of histamine present in the skin of knockout mice and their wild-type littermates was determined by homogenizing skin samples in PBS and measuring the histamine in the supernatant by ELISA (Immunotech–Beckman Coulter) performed in duplicate. The histamine content is reported as nanomolar histamine per milligram of tissue.
Skin Mast Cell Numbers.
Skin was dissected from wild-type mice, as well as from knockout mice and their wild-type littermates, and frozen in tissue-embedding medium. The skin was sectioned and adhered to slides. The slides were stained with acidified toluidine blue (1%, pH < 2) for 60 min at 24°C, and the number of mast cells was counted. Three sections per skin sample were counted, and the mean number of mast cells is reported.
Isolation of Mouse Skin and DRG.
Male C57BL/6 mice (The Jackson Laboratory) were kept in plastic cages (five mice per cage) in a light/dark cycle and were provided with food and water ad libitum. The mice were killed (ketamine/xylazine followed by decapitation), and the skin or spinal cords were dissected. The skin was immediately frozen at −80°C. The DRG were isolated from the spinal cords and pooled (n = 4 mice) before freezing at −80°C. RNA was isolated from the skin and DRG by using TRI Reagent and used for RT-PCR.
RT-PCR for CRH, NT, and PC5.
RNA was isolated by using TRIzol reagent (Invitrogen). The absorbance at 260 and 280 nm was measured by using a Uvikon spectrophotometer to determine the concentration and quality of RNA. cDNA was synthesized by using Moloney murine leukemia virus reverse transcriptase, and PCR was performed to amplify specific gene sequences by using Platinum Taq and oligonucleotide primers synthesized in the Tufts University Core Facility (Table 1). PCR products were displayed on a 1.2% agarose gel, visualized with ethidium bromide under UV light, and captured on film. The band size was compared to a 100-bp DNA ladder.
Table 1.
Oligonucleotide primer pairs used for RT-PCR
| Gene | Forward and reverse primers | PCR product, bp |
|---|---|---|
| CRH | 5′-AGCCCTTGAATTTCTTGCA-3′ | 202 |
| 5′-AACACGCGGAAAAAGTTA-3′ | ||
| NT/NN | 5′-AGCTCCTGGAGTCTGTGCTC-3′ | 409 |
| 5′-CATACAGCTGCCGTTTCAGA-3′ | ||
| PC5 | 5′-CCCAAGTGGCCAAGTATGTG-3′ | 807 |
| 5′-GGCAATGATTCCAGCAGCCATGGG-3′ |
Isolation of Mouse Skin Mast Cells for FACS Analysis.
Mice (n = 2–4) were anesthetized with an i.p. injection of ketamine/xylazine and killed by decapitation. The fur on the back was shaved, and the skin was dissected and rinsed one time in 70% ethanol on ice and twice in cold Hanks’ balanced salt solution (without calcium or magnesium). EDTA (1 mM) was added to the Hanks’ balanced salt solution during the washing and cutting of the tissue to prevent mast cell activation from calcium release. The tissue was cut into small pieces (2 mm2) and incubated (for 2 h at 37°C) in a 25-ml solution of collagenase (2.0 mg/ml Type 1A; Sigma) and hyaluronidase (0.5 mg/ml Type 1S, Sigma) in Hanks’ balanced salt solution. The incubation mixture was gently gassed throughout with a mixture of 95% O2 and 5% CO2 while shaking (≈150 rpm). The undigested material was filtered through moistened gauze, and the cells were collected by centrifugation (5 min at 150 × g) and washed once in Hanks’ balanced salt solution.
FACS Analysis of Skin Mast Cells.
The cells were resuspended in PBS and incubated in PBS (negative control), 10 μg/ml FITC-conjugated rat anti-mouse c-kit monoclonal antibody (BD Biosciences Pharmingen), 20 μl/ml phycoerythrin-conjugated rabbit anti-mouse CRHR polyclonal antibody (Santa Cruz Biotechnology), or both antibodies together (positive double-stained cells). The cells were incubated on ice for 15 min and then collected by centrifugation and washed one time in PBS. 7-Amino-actinomycin D intercalates into double-stranded nucleic acids and is excluded from viable cells but can penetrate the cell membranes of dying or dead cells. 7-Amino-actinomycin D was added for ≈20 min at 4°C in the dark. The cells were sorted by using a MoFlo instrument (Dako). The data were analyzed by using summit software (Cytomation). After sorting, the cells were adhered to slides, stained with toluidine blue, and photographed.
Statistics.
Photographs of rodent skins shown are representative of at least 10 different experiments where similar results were obtained. For the quantification of CRH-induced vascular permeability in the skin of wild-type and NT−/− mice, the Evans blue concentration (ng/ml) in the skin samples was first calculated from a standard curve, and all values for the same animal were divided by the corresponding concentration for saline injection to control for interanimal variability. The mean number of mast cells and histamine content in the skin of NT−/−, NT+/+, and C57BL/6 mice was compared by using ANOVA. A Bonferroni post hoc test was performed to control for multiple comparisons. spss software (SPSS, Chicago) was used for all statistical analysis. Statistical significance was set as P ≤ 0.05.
Supplementary Material
Acknowledgments
We thank Dr. Danielle Gully (Sanofi Recherche) for providing SR48692, Dr. James Marchand (Department of Anatomy and Cell Biology, Tufts University) for help with dissecting the DRG, and Dr. David Cochrane (Department of Biology, Tufts University) for advice. We also thank Ms. Jessica Christian for her patience and word processing skills. This work was supported in part by National Institutes of Health Grant AR47652 (to T.C.T.).
Abbreviations
- CRH
corticotropin-releasing hormone
- DRG
dorsal root ganglia
- NT
neurotensin
- SP
substance P
- CRHR
CRH receptor
- NTR
NT receptor
- PC5
prohormone convertase 5.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Katsarou-Katsari A., Filippou A., Theoharides T. C. Int. J. Immunopathol. Pharmacol. 1999;12:7–11. [PubMed] [Google Scholar]
- 2.Sugiura H., Uehara M. Acta Derm. Venereol. 1993;73:296–299. doi: 10.2340/000155557293295. [DOI] [PubMed] [Google Scholar]
- 3.Ozdamar S. O., Seckin D., Kandemir B., Turanli A. Y. Dermatology. 1996;192:189–190. doi: 10.1159/000246359. [DOI] [PubMed] [Google Scholar]
- 4.Huston D. P., Bressler R. B. Med. Clin. North Am. 1992;76:805–840. doi: 10.1016/s0025-7125(16)30327-3. [DOI] [PubMed] [Google Scholar]
- 5.Singh L. K., Pang X., Alexacos N., Letourneau R., Theoharides T. C. Brain Behav. Immun. 1999;13:225–239. doi: 10.1006/brbi.1998.0541. [DOI] [PubMed] [Google Scholar]
- 6.Theoharides T. C., Singh L. K., Boucher W., Pang X., Letourneau R., Webster E., Chrousos G. Endocrinology. 1998;139:403–413. doi: 10.1210/endo.139.1.5660. [DOI] [PubMed] [Google Scholar]
- 7.Singh L. K., Boucher W., Pang X., Letourneau R., Seretakis D., Green M., Theoharides T. C. J. Pharmacol. Exp. Ther. 1999;288:1349–1356. [PubMed] [Google Scholar]
- 8.Crompton R., Clifton V. L., Bisits A. T., Read M. A., Smith R., Wright I. M. J. Clin. Endocrinol. Metab. 2003;88:5427–5432. doi: 10.1210/jc.2003-030377. [DOI] [PubMed] [Google Scholar]
- 9.Clifton V. L., Crompton R., Smith R., Wright I. M. J. Clin. Endocrinol. Metab. 2002;87:267–270. doi: 10.1210/jcem.87.1.8149. [DOI] [PubMed] [Google Scholar]
- 10.Slominski A., Wortsman J., Pisarchik A., Zbytek B., Linton E. A., Mazurkiewicz J. E., Wei E. T. FASEB J. 2001;15:1678–1693. doi: 10.1096/fj.00-0850rev. [DOI] [PubMed] [Google Scholar]
- 11.Slominski A., Pisarchik A., Tobin D. J., Mazurkiewicz J., Wortsman J. Endocrinology. 2004;145:941–950. doi: 10.1210/en.2003-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paus R., Theoharides T. C., Arck P. Trends Immunol. 2006;27:32–39. doi: 10.1016/j.it.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Theoharides T. C., Donelan J. M., Papadopoulou N., Cao J., Kempuraj D., Conti P. Trends Pharmacol. Sci. 2004;25:563–568. doi: 10.1016/j.tips.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Theoharides T. C., Cochrane D. E. J. Neuroimmunol. 2004;146:1–12. doi: 10.1016/j.jneuroim.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 15.Ostlere L. S., Cowen T., Rustin M. H. Clin. Exp. Dermatol. 1995;20:462–467. doi: 10.1111/j.1365-2230.1995.tb01378.x. [DOI] [PubMed] [Google Scholar]
- 16.Feldberg R. S., Cochrane D. E., Carraway R. E., Brown E. B., Sawyer R., Hartunian M., Wentworth D. Inflamm. Res. 1998;47:245–250. doi: 10.1007/s000110050325. [DOI] [PubMed] [Google Scholar]
- 17.Cochrane D. E., Emigh C., Levine G., Carraway R. E., Leeman S. E. Ann. N.Y. Acad. Sci. 1982;400:396–397. [Google Scholar]
- 18.Cochrane D. E., Boucher W., Bibb P. Int. Arch. Allergy Immunol. 1986;80:225–230. doi: 10.1159/000234057. [DOI] [PubMed] [Google Scholar]
- 19.Schmolke B., Amon U., Zemcke N., Wolff H. H. Agents Actions. 1994;41:C49–C50. doi: 10.1007/BF02007762. [DOI] [PubMed] [Google Scholar]
- 20.Charlesworth E. N. Chem. Immunol. 1995;62:84–107. [PubMed] [Google Scholar]
- 21.Serafin W. E., Austen K. F. N. Engl. J. Med. 1987;317:30–34. doi: 10.1056/NEJM198707023170106. [DOI] [PubMed] [Google Scholar]
- 22.Christian E. P., Undem B. J., Weinreich D. J. Physiol. 1989;409:297–312. doi: 10.1113/jphysiol.1989.sp017498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sugiura H., Hirota Y., Uehara M. Acta Derm. Venereol. Suppl. (Stockh.) 1989;144:115–118. doi: 10.2340/00015555144115118. [DOI] [PubMed] [Google Scholar]
- 24.Dobner P. R., Fadel J., Deitemeyer N., Carraway R. E., Deutch A. Y. Proc. Natl. Acad. Sci. USA. 2001;98:8048–8053. doi: 10.1073/pnas.141042198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donelan J., Marchand J. E., Kempuraj D., Papadopoulou N., Papaliodis D., Theoharides T. C. 2006;126:932–935. doi: 10.1038/sj.jid.5700153. [DOI] [PubMed] [Google Scholar]
- 26.Cao J., Papadopoulou N., Kempuraj D., Boucher W. S., Sugimoto K., Cetrulo C. L., Theoharides T. C. J. Immunol. 2005;174:7665–7675. doi: 10.4049/jimmunol.174.12.7665. [DOI] [PubMed] [Google Scholar]
- 27.Gully D., Canton M., Borgegrain R., Leanjean F., Molimard J. C., Poncelet M., Gueudet C., Heaulme M., Leyris R., Brouard A., et al. Proc. Natl. Acad. Sci. USA. 1993;90:65–69. doi: 10.1073/pnas.90.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller L. A., Cochrane D. E., Carraway R. E., Feldberg R. S. Br. J. Pharmacol. 1995;114:1466–1470. doi: 10.1111/j.1476-5381.1995.tb13371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nisato D., Guiraudou P., Barthelemy G., Gully D., Le Furm G. Life Sci. 1994;54:PL95–PL100. doi: 10.1016/0024-3205(94)00411-0. [DOI] [PubMed] [Google Scholar]
- 30.Pang X., Alexacos N., Letourneau R., Seretakis D., Gao W., Cochrane D. E., Theoharides T. C. J. Pharmacol. Exp. Ther. 1998;287:307–314. [PubMed] [Google Scholar]
- 31.Alexacos N., Pang X., Boucher W., Cochrane D. E., Sant G. R., Theoharides T. C. Urology. 1999;53:1035–1040. doi: 10.1016/s0090-4295(98)00627-x. [DOI] [PubMed] [Google Scholar]
- 32.Castagliuolo I., Leeman S. E., Bartolac-Suki E., Nikulasson S., Qiu B., Carraway R. E., Pothoulakis C. Proc. Natl. Acad. Sci. USA. 1996;93:12611–12615. doi: 10.1073/pnas.93.22.12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krüger P. G., Aas P., Onarheim J., Helle K. B. Acta Physiol. Scand. 1982;114:467–469. doi: 10.1111/j.1748-1716.1982.tb07011.x. [DOI] [PubMed] [Google Scholar]
- 34.Carraway R., Cochrane D. E., Lansman J. B., Leeman S. E., Paterson B. M., Welch H. J. J. Physiol. 1982;323:403–414. doi: 10.1113/jphysiol.1982.sp014080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selbekk B. H., Flaten O., Hanssen L. E. Scand. J. Gastroenterol. 1980;15:457–460. doi: 10.3109/00365528009181500. [DOI] [PubMed] [Google Scholar]
- 36.Cochrane D. E., Carraway R. E., Boucher W., Feldberg R. S. Peptides. 1991;12:1187–1194. doi: 10.1016/0196-9781(91)90193-s. [DOI] [PubMed] [Google Scholar]
- 37.Helmstaedter V., Taugner C., Feurle G. E., Forssmann W. G. Histochemistry. 1977;53:35–41. doi: 10.1007/BF00511208. [DOI] [PubMed] [Google Scholar]
- 38.Sundler F., Hakanson R., Hammer R. A., Alumets J., Carraway R., Leeman S. E., Zimmerman E. A. Cell Tissue Res. 1977;178:313–321. doi: 10.1007/BF00218696. [DOI] [PubMed] [Google Scholar]
- 39.Ceconi C., Condorelli E., Quinzanini M., Rodella A., Ferrari R., Harris P. Cardiovasc. Res. 1989;23:674–682. doi: 10.1093/cvr/23.8.674. [DOI] [PubMed] [Google Scholar]
- 40.Reinecke M., Weihe E., Carraway R. E., Leeman S. E., Forssmann W. G. Neuroscience. 1982;7:1785–1795. doi: 10.1016/0306-4522(82)90036-7. [DOI] [PubMed] [Google Scholar]
- 41.Matsuda H., Kawakita K., Kiso Y., Nakano T., Kitamura Y. J. Immunol. 1989;142:927–931. [PubMed] [Google Scholar]
- 42.Goetzl E. J., Chernov T., Renold F., Payan D. G. J. Immunol. 1985;135:802s–805s. [PubMed] [Google Scholar]
- 43.Payan D. G., Levine J. D., Goetzl E. J. J. Immunol. 1984;132:1601–1604. [PubMed] [Google Scholar]
- 44.Foreman J., Jordan C. Agents Actions. 1983;13:105–116. doi: 10.1007/BF01967311. [DOI] [PubMed] [Google Scholar]
- 45.Mousli M., Bueb J.-L., Bronner C., Rouot B., Landry Y. Trends Pharmacol. Sci. 1990;11:358–362. doi: 10.1016/0165-6147(90)90179-c. [DOI] [PubMed] [Google Scholar]
- 46.Szekeres P. G., Traynor J. R. Regul. Pept. 1994;54:293–294. [Google Scholar]
- 47.Mousli M., Bronner C., Landry Y., Bockaert J., Rouot B. FEBS Lett. 1990;259:260–262. doi: 10.1016/0014-5793(90)80023-c. [DOI] [PubMed] [Google Scholar]
- 48.Higashijima T., Uzu S., Nakajima T., Ross E. M. J. Biol. Chem. 1988;263:6491–6494. [PubMed] [Google Scholar]
- 49.Foreman J. C., Jordan C. C., Piotrowski W. Br. J. Pharmacol. 1982;77:531–539. doi: 10.1111/j.1476-5381.1982.tb09328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao G. C., Wei E. T. Regul. Pept. 1995;58:117–121. doi: 10.1016/0167-0115(95)00070-r. [DOI] [PubMed] [Google Scholar]
- 51.Castagliuolo I., Wang C.-C., Valenick L., Pasha A., Nikulasson S., Carraway R. E., Pothoulakis C. J. Clin. Invest. 1999;103:843–849. doi: 10.1172/JCI4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mousli M., Bronner C., Bueb J.-L., Tschirhart E., Gies J.-P., Landry Y. J. Pharmacol. Exp. Ther. 1989;250:329–335. [PubMed] [Google Scholar]
- 53.Cocchiara R., Bongiovanni A., Albeggiani G., Azzolina A., Lampiasi N., DiBlasi F., Geraci D. J. Neuroimmunol. 1997;75:9–18. doi: 10.1016/s0165-5728(96)00229-9. [DOI] [PubMed] [Google Scholar]
- 54.Wiesner-Menzel L., Schulz B., Vakilzadeh F., Czarnetzki B. M. Acta Derm. Venereol. 1981;61:465–469. doi: 10.2340/0001555561465469. [DOI] [PubMed] [Google Scholar]
- 55.Rozniecki J. J., Dimitriadou V., Lambracht-Hall M., Pang X., Theoharides T. C. Brain Res. 1999;849:1–15. doi: 10.1016/s0006-8993(99)01855-7. [DOI] [PubMed] [Google Scholar]
- 56.Fantozzi R., Masini E., Blandina P., Mannaioni P. F., Bani-Sacchi T. Nature. 1978;273:473–474. doi: 10.1038/273473a0. [DOI] [PubMed] [Google Scholar]
- 57.Boesiger J., Tsai M., Maurer M., Yamaguchi M., Brown L. F., Claffey K. P., Dvorak H. F., Galli S. J. J. Exp. Med. 1998;188:1135–1145. doi: 10.1084/jem.188.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grutzkau A., Kruger-Krasagakes S., Baumeister H., Schwarz C., Kogel H., Welker P., Lippert U., Henz B. M., Moller A. Mol. Biol. Cell. 1998;9:875–884. doi: 10.1091/mbc.9.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laham R. J., Li J., Tofukuji M., Post M., Simons M., Sellke F. W. Ann. Vasc. Surg. 2003;17:245–252. doi: 10.1007/s10016-001-0299-x. [DOI] [PubMed] [Google Scholar]
- 60.Xia Y. P., Li B., Hylton D., Detmar M., Yancopoulos G. D., Rudge J. S. Blood. 2003;102:161–168. doi: 10.1182/blood-2002-12-3793. [DOI] [PubMed] [Google Scholar]
- 61.Brown L. F., Olbricht S. M., Berse B., Jackman R. W., Matsueda G., Tognazzi K. A., Manseau E. J., Dvorak H. F., Van de Water L. J. Immunol. 1995;154:2801–2807. [PubMed] [Google Scholar]
- 62.Williams R. M., Bienenstock J., Stead R. H. Chem. Immunol. 1995;61:208–235. [PubMed] [Google Scholar]
- 63.Mihm M. C., Soter N. A., Dvorak H. F., Austen K. F. J. Invest. Dermatol. 1976;67:305–311. doi: 10.1111/1523-1747.ep12514346. [DOI] [PubMed] [Google Scholar]
- 64.Smith C. H., Kepley X., Schwartz L. B., Lee T. H. J. Allergy Clin. Immunol. 1995;96:360–364. doi: 10.1016/s0091-6749(95)70055-2. [DOI] [PubMed] [Google Scholar]
- 65.Yamamoto T., Matsuuchi M., Watanabe K., Katayama I., Nishioka K. Dermatology. 1997;195:73–74. doi: 10.1159/000245694. [DOI] [PubMed] [Google Scholar]
- 66.Petersen L. J., Hansen U., Kristensen J. K., Nielsen H., Skov P. S., Nielsen H. J. Acta Derm. Venereol. 1998;78:190–193. doi: 10.1080/000155598441503. [DOI] [PubMed] [Google Scholar]
- 67.Al’Abadie M. S., Kent G. G., Gawkrodger D. J. Br. J. Dermatol. 1994;130:199–203. doi: 10.1111/j.1365-2133.1994.tb02900.x. [DOI] [PubMed] [Google Scholar]
- 68.Farber E. M., Nickoloff B. J., Recht B., Fraki J. E. J. Am. Acad. Dermatol. 1986;14:305–311. doi: 10.1016/s0190-9622(86)70034-0. [DOI] [PubMed] [Google Scholar]
- 69.Harvima I. T., Viinamäki H., Naukkarinen A., Paukkonen K., Neittaanmäki H., Horsmanheimo M. Psychother. Psychosom. 1993;60:168–176. doi: 10.1159/000288690. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.