Abstract
Arginine-vasopressin (AVP) is a hormone that is essential for both osmotic and cardiovascular homeostasis, and exerts important physiological regulation through three distinct receptors, V1a, V1b, and V2. Although AVP is used clinically as a potent vasoconstrictor (V1a receptor-mediated) in patients with circulatory shock, the physiological role of vasopressin V1a receptors in blood pressure (BP) homeostasis is ill-defined. In this study, we investigated the functional roles of the V1a receptor in cardiovascular homeostasis using gene targeting. The basal BP of conscious mutant mice lacking the V1a receptor gene (V1a−/−) was significantly (P < 0.001) lower compared to the wild-type mice (V1a+/+) without a notable change in heart rate. There was no significant alteration in cardiac functions as assessed by echocardiogram in the mutant mice. AVP-induced vasopressor responses were abolished in the mutant mice; rather, AVP caused a decrease in BP, which occurred in part through V2 receptor-mediated release of nitric oxide from the vascular endothelium. Arterial baroreceptor reflexes were markedly impaired in mutant mice, consistent with a loss of V1a receptors in the central area of baroreflex control. Notably, mutant mice showed a significant 9% reduction in circulating blood volume. Furthermore, mutant mice had normal plasma AVP levels and a normal AVP secretory response, but had significantly lower adrenocortical responsiveness to adrenocorticotropic hormone. Taken together, these results indicate that the V1a receptor plays an important role in normal resting arterial BP regulation mainly by its regulation of circulating blood volume and baroreflex sensitivity.
Keywords: knockout mouse, adrenal cortex
The neurohypophyseal hormone arginine vasopressin (AVP) is involved in a plethora of physiological regulatory processes that occur via stimulation of specific V1a, V1b, and V2 receptors (1). These receptors have distinct pharmacological profiles and couple with specific intracellular second messengers (1). Vasopressin plays a prominent role in the cardiovascular system and influences arterial blood pressure (BP) at multiple sites in a complex fashion. The role of AVP has been well characterized in the regulation of BP in pathophysiological conditions such as severe hypovolemia/hypotension episodes (2). However, its contribution to BP homeostasis in normal physiological situations is ill-defined (3). Vasopressin is a potent stimulator of vascular smooth muscle contraction in vitro, and V1a receptors mediate its vasoconstrictor effect (3). However, a relatively large amount of vasopressin is required to raise BP in vivo under normal physiological conditions (4); this is thought to be because vasopressin also acts on the brain, decreasing cardiac output by inhibiting sympathetic efferent activity and potentiating baroreflexes (5). AVP has been shown to enhance baroreflex function via activation of V1 receptors in the area postrema (6–8). In addition, vasopressin causes vasodilatation in some blood vessels, perhaps via release of nitric oxide (NO) from the vascular endothelium (9).
Highly selective peptide and nonpeptide V1a receptor antagonists have been developed (10–12), and have been shown to successfully inhibit the AVP-induced pressor response; however, they have little effect on basal levels of BP (13). Hence, the V1a receptor appears to play only a minor role in maintaining BP homeostasis under normal physiological conditions. To better understand the physiological roles of the V1a receptor, we have generated mice lacking this receptor and monitored their cardiovascular function. Unlike previous observations with selective V1a receptor antagonists, the V1a receptor-deficient mice exhibit notably lower resting BP. In investigating the mechanism of this lowered BP, we found reduced circulating blood volume and impaired baroreflex sensitivity in V1a receptor-deficient mice. Our results revealed that the V1a receptor plays an important role in maintaining resting BP, not via direct vasoconstriction, but by regulating the neural and hormonal actions of AVP.
Results
Establishment and Characterization of V1a Receptor-Null Mice.
To inactivate the V1a receptor gene, parts of the first exon and the first intron were removed by gene targeting (Fig. 1A). Mutation of the wild-type V1a receptor gene in the mouse genome was documented by Southern blot analysis of diagnostic restriction digests in the offspring of heterozygous intercrosses (Fig. 1B). Analysis of the genotype frequencies after intercrosses of heterozygous mutant mice revealed no significant deviation from Mendelian expectations. V1a receptor-deficient mice (V1a−/−) grew and developed normally. The V1a−/− mice expressed no V1a receptor mRNA transcripts in any tissue examined (Fig. 1C). Maximum [3H]AVP binding in the liver was 299 ± 8 fmol per mg of protein (n = 4) in control wild-type (V1a+/+) mice, whereas it was not detectable in the V1a−/− mice. Furthermore, the pressor response of isolated perfused mesenteric arterial beds to AVP (range, 5–500 nM) was completely lost in V1a−/−, whereas the arterial responsiveness to KCl (150 mM) was comparable between V1a+/+ and V1a−/− mice (data not shown).
Fig. 1.
Generation of V1a receptor-deficient mice. (A) Strategy for V1a receptor gene targeting. The 1.2-kb SacII–HindIII region was replaced with the Neo cassette (30). The 1.8-kb diphtheria toxin cassette (DT) was used as a negative selection marker (34) to obtain the targeting vector NeoDT. Neo, PGK-neo cassette; DT, diphtheria toxin-A fragment gene; B, BamHI; E, EcoRI; H, HindIII; S, SacII; X, XhaI. (B) Southern blot analysis of tail DNA. DNA was digested with BamHI/XbaI, and the blot was hybridized with the 3′ probe. The 7.6-kb band was derived from the wild-type allele (WT), and the 5.8-kb band was derived from the targeted allele (MT). (C) RT-PCR analysis. The V1a receptor and GAPDH mRNA transcripts were detected as 532- and 662-bp fragments, respectively. The V1a−/− mice expressed no V1a receptor mRNA transcripts in any tissue examined. M, 100-bp DNA marker; B, brain; Hi, hippocampus; P, pituitary; Ma, mesenteric artery; H, heart; L, lung; Li, liver; Pa, pancreas; S, spleen; K, kidney; Ad, adrenal gland; U, uterus.
Basal Cardiovascular Hemodynamics.
To evaluate the role of V1a receptors in the regulation of cardiovascular function, basal mean arterial blood pressures (MAPs) and heart rates (HRs) of both genotypes were first measured. The systolic and diastolic BP and MAP were all significantly lower in V1a−/− mice than in V1a+/+ mice (Table 1). However, there was no significant difference in HR between the two groups, as monitored by either tail-cuff or intraarterial recording (Table 1). When cardiac function was assessed by echocardiogram, there was no significant difference between the two groups in left ventricular dimensions, ejection fraction, or cardiac output/body weight ratios (Table 2). There was no difference in heart weight (Table 2), and histological examination of cardiac myocytes showed no detectable abnormality (data not shown).
Table 1.
Basal BP and HR in V1a receptor-null and wild-type mice
| Parameter | V1a+/+, mean ± SD | V1a−/−, mean ± SD |
|---|---|---|
| Tail-cuff | ||
| SBP | 121 ± 2 | 108 ± 3* |
| DBP | 90 ± 2 | 82 ± 2* |
| HR | 515 ± 18 | 485 ± 14 |
| n | 18 | 18 |
| Carotid artery (conscious) | ||
| SBP | 131 ± 1 | 123 ± 1** |
| MAP | 107 ± 1 | 99 ± 1** |
| DBP | 89 ± 1 | 80 ± 1** |
| HR | 667 ± 7 | 684 ± 7 |
| n | 69 | 58 |
Arterial pressures were measured as described in Materials and Methods. Values are the means ± SEM.
*, P < 0.05;
**, P < 0.01 compared to V1a+/+ mice. SBP, systolic BP, DBP, diastolic BP.
Table 2.
Echocardiography parameters in V1a receptor-null and wild-type mice
| Parameter | V1a+/+ | V1a−/− |
|---|---|---|
| Echocardiogram | ||
| LVDd, mm | 1.93 ± 0.15 | 1.70 ± 0.06 |
| LVDs, mm | 0.92 ± 0.1 | 0.80 ± 0.04 |
| %FS | 53 ± 1 | 53 ± 2 |
| EF, % | 89 ± 1 | 89 ± 1 |
| VTI, cm | 3.4 ± 0.1 | 3.2 ± 0.1 |
| SV, μl | 29.9 ± 0.4 | 28.2 ± 0.8 |
| CO, ml/min | 19.0 ± 0.3 | 17.2 ± 0.5* |
| CO/BW, ml/min per g | 0.74 ± 0.04 | 0.71 ± 0.03 |
| HR, bpm | 638 ± 13 | 613 ± 18 |
| Body weight, g | 26.2 ± 1.5 | 24.5 ± 0.7 |
| n | 8 | 7 |
| Heart/body weight, mg/g | 4.0 ± 0.1 | 4.1 ± 0.1 |
| n | 12 | 11 |
LVDd and LVDs, maximum left ventricular dimensions in diastolic and systolic phases, respectively. %FS, % fractional shortening. EF, ejection fraction. VTI, Doppler velocity time integral in the left ventricular outflow. SV, stroke volume. CO, cardiac output. CO/BW, cardiac output divided by body weight.
*, P < 0.05.
Laboratory Analysis.
Basal levels of urea nitrogen, creatinine, electrolytes, inorganic phosphate, calcium, blood cell counts, serum albumin, total and direct bilirubin, liver enzymes, total cholesterol, triglyceride, free fatty acids, and serum osmolality did not significantly differ in V1a+/+ and V1a−/− mice (data not shown). Serum AVP levels were also comparable in both groups of mice either under basal conditions (2.8 ± 1.0 vs. 4.3 ± 0.9 pg/ml, both n = 6) or following stimulation of hemorrhage (9.7 ± 2.4 vs. 6.9 ± 1.5 pg/ml, both n = 6). To assess the V2 receptor-mediated antidiuretic effect, we monitored urine output for 24 h and during water loading with or without 1-deamino, 8 d-arginine vasopressin (DDAVP). Neither the basal urine output for 24 h nor the urine osmolality was different between V1a+/+ and V1a−/− mice (24-h urine volume: 2.1 ± 0.1 ml vs. 1.8 ± 0.2 ml, both n = 10, respectively; urine osmolality: 2,200 ± 160 mOsm vs. 2,300 ± 160, both n = 10, respectively). Urine volumes during water loading (5% of body weight) were 1.0 ± 0.1 and 1.2 ± 0.1 ml/4 h in V1a+/+ (n = 10) and V1a−/− mice (n = 10), respectively. In addition, urine output in response to DDAVP (100 ng/kg) during water loading was also not different between V1a+/+ and V1a−/− mice: 0.11 ± 0.04 and 0.17 ± 0.04 ml/4 h in V1a+/+ (n = 10) and V1a−/− mice (n = 10), respectively. Also, the urine osmolality monitored in these experiments was similar in the two groups of mice (data not shown). These studies show that AVP secretion and V2 receptor-mediated responses are intact in V1a-deficient mice.
Levels of serum catecholamines and urine catecholamine metabolites were comparable in V1a+/+ and V1a−/− mice; total amounts of serum catecholamines were 5.5 ± 0.8 ng/ml in V1a+/+ and 5.2 ± 0.7 ng/ml in V1a−/−, respectively (both n = 8). Urinary excretion of vanillylmandelic acid was 338 ± 48 and 311 ± 23 ng/day and excretion of homovanillic acid was 756 ± 119 and 700 ± 71 ng/day for V1a+/+ and V1a−/− mice, respectively (both n = 10). Plasma angiotensin II concentrations were not significantly different between V1a+/+ and V1a−/− mice (38 ± 5 and 31 ± 3 pg/ml for V1a+/+ and V1a−/−, respectively, both n = 7). Urinary excretion of cAMP, cGMP, and aldosterone was similar in both groups of mice (cAMP, 36 ± 3 nmol per 24 h in V1a+/+ and 43 ± 3 nmol per 24 h in V1a−/−, respectively, both n = 10; cGMP, 10 ± 1 nmol per 24 h in V1a+/+ and 8 ± 0.8 nmol per 24 h in V1a−/−, respectively, both n = 10; aldosterone, 4.3 ± 0.5 ng per 24 h in V1a+/+ and 4.6 ± 0.3 ng per 24 h in V1a−/−, respectively, both n = 10). Intake of water and food during measurements were similar in both V1a+/+ and V1a−/− mice (data not shown).
Pressor Response to AVP.
Pressor responses to vasoactive agents were examined in nonanesthetized mice. Administration of AVP caused a prompt and potent pressor response in a dose-dependent fashion in the V1a+/+ mice. However, the AVP-induced pressor response was abolished in the V1a−/− mice and AVP now caused a decrease in BP (Fig. 2 A and B). The AVP-induced decrease in BP was almost completely blocked by pretreatment with the V2 receptor-selective antagonist, [adamantaneacetyl1, O-ethyl-d-Tyr-2, Val-4, aminobutyryl6, Arg-8, 9]-vasopressin (Fig. 2C). Furthermore, pretreatment with the inhibitor of nitric oxide synthesis, Nω-nitro-l-arginine (l-NNA) markedly shortened the duration of the AVP-induced BP decrease (Fig. 2D).
Fig. 2.
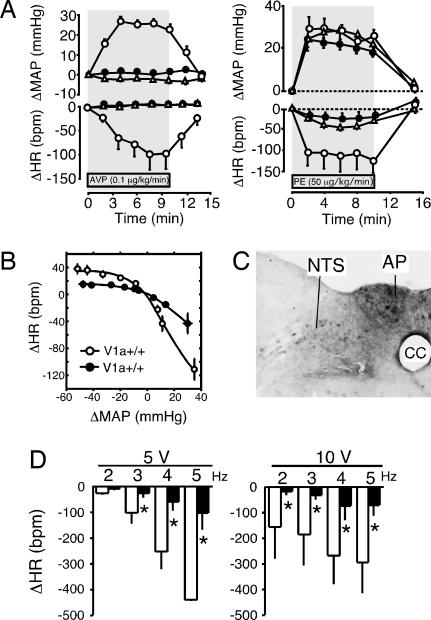
Cardiovascular responses to AVP. (A) Representative hemodynamic measurements of BP and HR in V1a+/+ (Upper) and V1a−/− (Lower) mice after stimulation with AVP (≈0.1–100 μg/kg). (B) The concentration-response curve for the AVP-induced pressor (Left) and HR (Right) response. The changes in MAP (in mmHg) and HR (bpm) are shown. The concentration–response curves of MAP and HR for AVP in V1a−/− mice (filled circles) were significantly (P < 0.05) different from those in V1a+/+ mice (open circles). Data points are the means ± SEM from analyses of 8–10 mice. (C) Representative hemodynamic measurements of BP for V1a−/− mice in the absence (Upper) or presence (Lower) of the V2 receptor antagonist, [adamantaneacetyl1, O-ethyl-d-Tyr-2, Val-4, aminobutyryl6, Arg-8,9]-vasopressin (100 μg/kg per min i.v.) 10 min before AVP injection (≈10–100 μg/kg). (D) Representative hemodynamic measurements of BP and HR in V1a−/− mice in the presence (Right) or absence (Left) of the NO inhibitor l-NNA (200 μg/kg per min i.v.) 60 min before AVP injection (100 μg/kg).
Assessment of Peripheral Vascular Resistance.
To investigate the involvement of peripheral vascular resistance in the lowered MAP in V1a−/− mice, MAP and HR were measured after treatment with i.v. bunazosin (100 μg/kg), a peripheral α1-adrenergic receptor blocker, and hexamethonium (20 mg/kg), an autonomic ganglion blocker, in conscious unrestrained animals. Although the bunazosin-induced decrease in MAP was similar in both groups of mice (17 ± 4 vs. 19 ± 5 mmHg for V1a+/+ and V1a−/− mice, respectively), the reflex increase in HR was significantly attenuated in V1a−/− mice (54 ± 8 vs. 20 ± 15 bpm for V1a+/+ and V1a−/− mice, respectively, n = 5). Hexamethonium treatment lowered MAP and HR to a similar extent in both groups of mice (decreases in MAP were 33 ± 5 vs. 39 ± 2 mmHg and decreases in HR were 84 ± 19 vs. 93 ± 11 bpm for V1a+/+ and V1a−/−, respectively, n = 5). Also, endogenous nitric oxide production in V1a−/− mice was indirectly examined by systemic administration of l-NNA. In V1a−/− mice, the increase in MAP during continuous infusion of l-NNA (200 mg/kg per min) for 90 min was significantly (P < 0.05) less than that of V1a+/+ mice (27 ± 4 and 10 ± 5 mmHg for V1a+/+ and V1a−/− mice, respectively), indicating that nitric oxide synthesis at baseline was not enhanced in V1a−/− mice.
Arterial Baroreceptor Reflex.
The effects of the absence of V1a receptors on BP and HR were assessed by comparing responses to pharmacological manipulations. As shown in Fig. 3A, continuous infusion of AVP (0.1 μg/kg per min, 10 min) induced a prompt increase in BP, which lasted for 10 min in V1a+/+ mice. The maximum increase in MAP was 26 ± 4 mmHg (n = 8) in V1a+/+ mice, whereas a V1a receptor antagonist, [β-mercapto-β, β-cyclopentamethyl-enepropionyl1, O-methyl-Tyr-2, Arg-8]-vasopressin (10 μg/kg per min) completely suppressed the AVP-induced pressor response (Fig. 3A). The AVP-induced pressor response was completely lost in V1a−/− mice. Interestingly, however, continuous infusion of phenylephrine (PE, 50 μg/kg per min, 10 min) induced a similar increase in BP in all groups, but baroreceptor reflex control of HR was markedly attenuated in both the V1a−/− mice and V1a+/+ mice treated with V1a antagonist (Fig. 3B). In all experiments, administration of the V1a receptor-antagonist had no significant effect on basal resting BP levels in V1a+/+ mice (data not shown).
Fig. 3.
BP and HR responses to phenylephrine (PE) and sodium nitroprusside (SNP). (A) MAP and HR changes during stimulation with AVP (Left) and PE (Right). AVP (0.1 μg/kg per min) or PE (50 μg/kg per min) was administered into the right femoral vein by using a microsyringe pump. V1a receptor antagonist [β-mercapto-β, β-cyclopentamethylenepropionyl1, O-methyl-Tyr-2, Arg-8]-vasopressin (10 μg/kg per min) was coadministered with the continuous infusion of AVP or PE. Points represent the mean ± SEM of measurements from eight mice. Open and filled circles, V1a+/+ and V1a−/− mice, respectively, without antagonist treatment. Open triangles, V1a+/+ mice receiving the V1a receptor antagonist infusion. (B) Relationship between MAP and HR changes in wild-type and V1a-null mice (arterial baroreceptor reflex). After bolus i.v. injection with PE (≈0.1–10.0 μg/kg) or SNP (≈1–300 μg/kg) in V1a+/+ (open circles) or V1a−/− mice (filled circles), changes in HR were plotted against those of MAP. Data points are the means ± SEM of analyses from 8–10 mice. (C) In situ hybridization using antisense probe for V1a mRNA in brainstem of wild-type mice. AP, area postrema; NTS, nucleus of the solitary tract; cc, central canal of medulla. (D) HR changes after electrical stimulation of the vagus. The right vagus nerve was electrically stimulated at 5 V (Right) and 10 V (Left) by using stimulation frequencies of 2–5 Hz in V1a+/+ (open columns) or V1a−/− mice (filled columns) anesthetized with urethane (1.5 g/kg, i.p.). Data points are the means ± SEM of analyses from 8–10 mice. ∗, P < 0.05 as compared with V1a+/+ mice.
To further examine cardiac autonomic nervous responsiveness, different doses of the vasopressor PE, or the vasodepressor sodium nitroprusside (SNP), were administered, and changes of peak HR were plotted against those of MAP to assess the arterial baroreceptor reflex. Baroreceptor reflex control of HR in V1a−/− mice was markedly attenuated compared to that in V1a+/+ mice (Fig. 3B). The results showed impaired baroreflex sensitivity in V1a−/− mice as well as in the mice treated with V1a receptor antagonist.
We further explored the locus responsible for the impaired baroreflex in V1a−/− mice. We first examined the sympathetic– and parasympathetic–cardiac junctions. Direct pharmacological stimulation of cardiac cholinergic receptors with oxotremorine (3.0 μg/kg) caused a similar extent of HR decrease in V1a+/+ and V1a−/− mice (HR decrease was 98 ± 5 and 95 ± 4 bpm, respectively, both n = 4). Similarly, direct pharmacological stimulation of cardiac adrenergic receptors with dobutamine (10 μg/kg) or isoproterenol (10 μg/kg) caused a comparable HR increase in both groups (HR increases were 8 ± 3 and 9 ± 4 bpm with dobutamine, and 15 ± 5 and 16 ± 5 bpm with isoproterenol for V1a+/+ and V1a−/− mice, respectively, both n = 4). Furthermore, the bradycardia in response to electrical stimulation of the vagus nerve was markedly attenuated in V1a−/− mice (Fig. 3D), as was observed when BP was raised by AVP or PE. Taken together, these results indicated that the impaired HR response in the V1a−/− mice was probably caused by an impaired vagal afferent-HR reflex. Therefore, we examined V1a receptor expression in the nucleus of the solitary tract (NTS) where the vagal afferents terminate. As shown in Fig. 3C, in situ hybridization (ISH) analysis showed the V1a receptor to be highly expressed in the area of the NTS and area postrema in V1a+/+ mice, whereas no positive signal was detected in the same area of V1a−/− mice.
Circulating Blood Volume.
In an effort to determine the mechanism(s) of the lowered resting BP observed in V1a−/− mice, we next examined circulating blood volumes by using radiolabeled albumin. V1a−/− mice showed a significant 9% reduction of blood volume (3.5 ± 0.1 vs. 3.1 ± 0.1 ml for V1a+/+ and V1a−/− mice, respectively, both n = 8), although total body weight was similar in the two groups (31.2 ± 0.4 and 31.3 ± 0.9 g for V1a+/+ and V1a−/−mice, respectively). Also, both groups of mice had similar hematocrit values (41 vs. 40% in V1a+/+ and V1a−/−, both n = 7) and red blood cell counts (8.7 vs. 8.4 × 106 cells per μl in V1a+/+ and V1a−/−, both n = 7).
Function and Morphology of Adrenal Cortex.
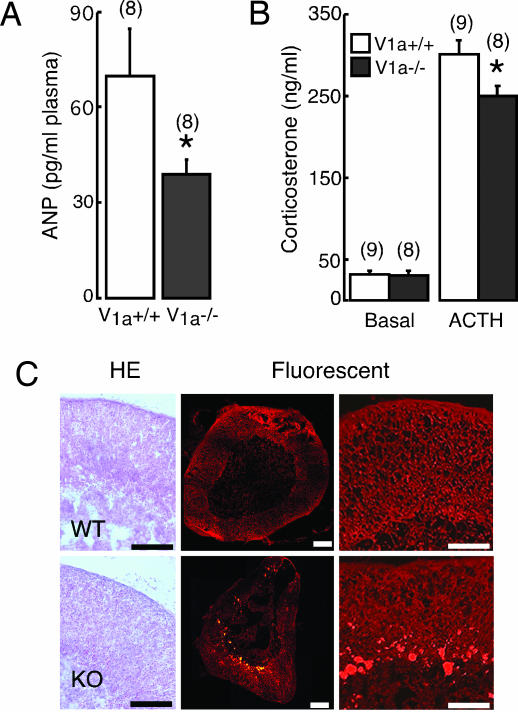
Although baseline levels of hormones examined were mostly similar between the two groups, basal levels of atrial natriuretic peptide (ANP) were significantly (P < 0.05) lower in V1a−/− mice compared with V1a+/+ mice (Fig. 4A). In addition, baseline corticosterone levels were similar, but the ACTH-stimulated corticosterone response was significantly (P < 0.05) lower in V1a−/− mice (Fig. 4B). In accordance with impaired corticosterone responsiveness to ACTH, histological analysis of adrenal gland in V1a−/− mice showed unclear adrenocortical zonation with markedly enhanced depositions of autofluorescent granules, probably lipofuscin, in the reticular layer (Fig. 4C).
Fig. 4.
Analysis of ANP concentrations and adrenal histology. Blood concentrations of ANP (A) and corticosterone concentrations before and after ACTH stimulation (5 μg/kg) (B) are shown. ∗, P < 0.05 compared with V1a+/+ mice. (C) Sections of adrenal glands from 8-week-old mice with (Left) or without (Center and Right) hematoxylin and eosin staining were examined under bright-field microscopy (Left) and under green fluorescent light (Center and Right). Both pictures are representatives of at least eight different animals. (Scale bars, 100 μm.)
Discussion
Using gene targeting to generate a mouse model lacking the V1a receptor, we investigated the functional role of the V1a receptor subtype in the cardiovascular system. Consistent with the loss of V1a receptor expression, V1a−/− mice showed altered pressor responses to AVP stimulation, and vascular contractile responses to AVP were lost. V1a−/− mice showed lower resting BP under nonanesthetized conditions without a notable increase in HR. Although there was no significant alteration in cardiac function between the V1a−/− and V1a+/+ mice, mutant mice showed a significant 9% reduction of circulating blood volume. Also, V1a−/− mice had markedly impaired baroreflex sensitivity. Responses to vasoactive hormones including AVP were mostly intact, but ACTH-stimulated corticosterone responsiveness was attenuated. Taken together, we conclude that reduced circulating blood volume and impaired baroreflex sensitivity, rather than a loss of vascular contractile response to AVP, may mainly contribute to the lower resting BP in V1a receptor-null mice. The present study provides evidence that the V1a receptor is critically involved in the maintenance of physiological BP levels.
Our present study confirmed and extended previous pharmacological studies showing that V1a receptors mediate AVP-stimulated pressor responses. Interestingly, administration of higher concentrations of AVP caused a BP decrease in V1a−/− mice, which was inhibited by pretreatment either with the V2 receptor-selective antagonist or with the NO inhibitor. This finding indicated that AVP can release NO from the endothelium via the V2 receptor, thereby leading to vasorelaxation. V1a−/− mice clearly demonstrated the presence of the V2 receptor/NO system in their vascular endothelium, as has been suggested by pharmacological studies (14, 15). Therefore, the magnitude of the BP increase seen after in vivo administration of AVP actually represents a summation of V1a receptor-mediated vasoconstriction and V2 receptor/NO-mediated vasodilatation.
Our study also showed that the V1a receptor has an important role in maintaining resting arterial BP. BP is a function of vascular resistance and cardiac output, and cardiac output is in turn determined by cardiac function and circulating blood volume. The V1a receptor gene was expressed in several tissues including blood vessels and heart, albeit at very low levels in the heart; however, cardiac function, in particular contractility, was normal in V1a−/− mice. The AVP-stimulated contractile response was lost in isolated perfused mesenteric arteries of V1a−/− mice, showing that the V1a receptor can contribute to the regulation of function of the mesenteric artery, the muscular-type resistance vessel responsible for BP control. However, despite the potency of vasopressin as a direct vasoconstrictor, it is of note that in intact animals vasopressin-induced pressor responses are minimal and occur only with concentrations of vasopressin significantly higher than those required for maximal antidiuresis (3). In fact, AVP elicited a pressor response only when much higher amounts of AVP (≈1 ng/ml) than the normal circulating levels (1–3 pg/ml in mouse and human) were administered (Fig. 2A). Therefore, the role of AVP in the maintenance of resting arteriolar tone and systemic blood pressure via V1a receptor activation appears to be minimal, and the hypotension developed in V1a−/− mice cannot be explained simply by loss of V1a receptor-mediated vasoconstriction and a consequent reduction of vasomotor tone. This interpretation that V1a receptor-mediated vasoconstriction plays a less significant role in the maintenance of normal BP needs caution, because it is difficult to extrapolate from observations in knockout mice, which have had a lifetime to develop counterregulatory responses to the normal adult animal.
The occurrence of lower baseline BP in V1a receptor-deficient mice was an unexpected observation, because AVP-deficient mutant Brattleboro rats remain normotensive (16). Furthermore, previous pharmacological studies with selective V1a receptor antagonists showed that blocking the V1a receptor did not lead to any significant change in basal BP levels (17–21). The present study also confirmed a minor effect of V1a receptor antagonists on basal BP levels, even though they markedly inhibited baroreflex function. The discrepancy in BP between the V1a receptor-deficient mice and mice treated with V1a receptor antagonists cannot be fully explained. In investigating the mechanism(s) responsible for the observed hypotension in V1a receptor-deficient mice, we found that the circulating blood volume was significantly reduced by 9%. Hematological and blood electrolyte analysis showed no significant difference between mutant and control mice, suggesting that the V1a−/− mouse adapted to lower blood volume. The reduced circulating blood volume was attributable mainly, if not totally, to the lowered resting BP in the V1a−/− mouse. However, the primary mechanism responsible for the reduced circulating blood volume is uncertain. We observed morphofunctional alterations in the adrenal cortex of V1a−/− mice, which might be related to the reduced circulating blood volume. The V1a receptor is known to stimulate steroid secretion in human adrenal glands (22). Further studies are clearly needed to investigate the role of the V1a receptor in the regulation of circulating blood volume.
V1a−/− mice exhibited markedly altered baroreflex sensitivity, showing that the V1a receptor is critically involved in the regulation of baroreflex control. Our finding extended the previous observation that AVP-deficient mutant Brattleboro rats had attenuated baroreflex sensitivity (23). Also, this finding has clinical implication for the use of V1a antagonists. Although V1a receptors have been suggested to be a therapeutic target in the treatment of a variety of cardiovascular diseases (2, 19, 20), this study indicates that impairing the baroreflex function by inhibiting the V1a receptor might lead to untoward side effects, because maintaining the BP and HR within an optimal range could be difficult (24).
Basic pathways mediating reflex control of HR involve baroreceptors, afferents to the central nervous system (CNS), the cardiovascular center in the CNS, and sympathetic and parasympathetic efferents to the heart (25). Impairment at any point along these pathways would lead to an altered baroreflex. The present results point to a defect in the CNS and/or baroreceptors, because cardiac function, the sympathetic–cardiac junction, and the parasympathetic–cardiac junction seemed intact in V1a−/− mice. In addition, the bradycardiac response to electrical stimulation of the vagus nerve was markedly impaired in the V1a−/−mice, suggesting a defect in the CNS. The electrical stimulation of the vagus nerve in this experiment included both afferent and efferent vagal excitation. An altered HR response in V1a−/− mice, however, was probably caused by an impaired vagal afferent-HR reflex, because there seemed to be no difference in the efferent rim (as shown in the experiments with oxotremorine). Stimulation of the vagal afferent has been shown to inhibit neurons of the cardiovascular center (26) and the vagal afferents terminate in the nucleus of the solitary tract (NTS), where the V1a receptor is richly expressed (27). Our in situ hybridization (ISH) analysis confirmed that V1a receptors in such areas of baroreflex control are lost in V1a−/− mice.
In conclusion, our knockout mouse study has demonstrated the complex physiological role of the V1a receptor in the cardiovascular system. V1a receptor knockout mice will continue to be of value for investigating regulatory mechanisms in a variety of physiological responses to AVP.
Materials and Methods
Generation of V1a Receptor-Null Mice.
Mouse V1a gene 5.8-kb (EcoRI/SacII) and 5.0-kb (HindIII) fragments (28) were used for construction of the targeting vector (Fig. 1). Knockout mice were generated according to the procedure described in ref. 29. Briefly, F1 heterozygotes were generated by mating chimeric mice to C57BL/6J mice and homozygotes (F2) were obtained by mating between the F1 heterozygotes. The knockouts used in this analysis were F3, F4, and F5, which carried the genetic background of 129Sv and C57BL/6J strains. V1a+/+ littermates were used for the analysis as control mice. These mice were analyzed at 8 to 12 weeks of age. All experiments were performed in accordance with the Declaration of Helsinki-approved Institutional Guidelines.
RT-PCR Analysis.
Total RNA (5 μg) was treated with RNase-free DNase (Takara, Tokyo) and reverse-transcribed by using random hexamers, as described (29). The upstream and downstream primers of the V1a receptor gene (5′ to 3′) were ATTGCTGGGCTACCTTCATCC and CCTTGGCGAATTTCTGCGCT. The GAPDH primers (5′ to 3′) were GGTCATCATCTCCGCCCCTTC upstream and CCACCACCCTGTTGCTGTAG downstream.
Serum and Urine Laboratory Analysis.
Plasma hormone levels and urine cGMP and cAMP were measured by using commercially available kits (Assay Designs). Total plasma catecholamine levels (epinephrine, norepinephrine, and dopamine) and urinary levels of catecholamine metabolites were determined by HPLC using commercially available reagents (Tosho, Tokyo) as described (30). For the corticosteroid stimulation test, blood samples were collected 30 min after i.p. injection of 5 μg/kg ACTH. Corticosterone concentration was measured by liquid chromatography tandem mass spectrometry (API 4000; Applied Biosystems).
Histological Analysis.
For ISH analysis using free-floating sections, radiolabeled cRNA probes were made by using [33P]UTP (New England Nuclear) (31). The use of sense cRNA probe revealed no specific hybridization signals in brain sections.
Measurement of BP and HR.
Tail cuff monitoring.
Systolic and diastolic BP and HR were measured in conscious male mice as described (30).
Direct intraarterial recording.
MAP and HR were measured in nonanesthetized male mice as described (30).
Echocardiographic Analysis.
Quantitative echocardiographic measurements were performed on lightly anesthetized, spontaneously breathing mice by using the SONOS-5500 system (Philipus Medical Systems, Andover, MA) as described (30).
Arterial Baroreceptor Reflex.
MAP and HR were continuously monitored, and MAP was altered by between 50 and 150 mmHg with a bolus injection of either SNP or PE to obtain MAP-HR relations (32). For direct electrical stimulation of the vagus nerve, the right cervical vagus nerve was isolated and both afferent and efferent fibers were electrically stimulated while monitoring BP and HR.
Measurement of Plasma Volume.
Plasma volume was determined by measuring the dilution of intravenously injected 131I-labeled human serum albumin through the tail vein (33). Plasma volume was calculated based on the relative dilution of the original injected label.
Statistical Analysis.
All values are expressed as means ± SEM. Statistical analysis was performed by using two-way analysis of variance with Tukey's posttest or Student's t test. P values <0.05 were considered statistically significant.
Acknowledgments
This work was supported in part by research grants from the Scientific Fund of the Ministry of Education, Science, and Culture of Japan (to G.T., A.T., and T.-a.K.); the Japan Health Science Foundation and the Ministry of Human Health and Welfare (to A.T. and T.-a.K.); the Promotion and Mutual Aid Corporation for Private Schools of Japan (to S.T.); and the 21st Century Center of Excellence Program “Knowledge Information Infrastructure for Genome Science” (to G.T.).
Abbreviations
- AVP
arginine vasopressin
- BP
blood pressure
- HR
heart rate
- MAP
mean arterial blood pressure
- l-NNA
Nω-nitro-l-arginine
- PE
phenylephrine.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Thibonnier M., Coles P., Thibonnier A., Shoham M. Prog. Brain Res. 2002;139:179–196. doi: 10.1016/s0079-6123(02)39016-2. [DOI] [PubMed] [Google Scholar]
- 2.Laszlo F. A., Laszlo F., Jr., De Wied D. Pharmacol. Rev. 1991;43:73–108. [PubMed] [Google Scholar]
- 3.Jackson E. K. In: Goodman & Gilman's The Pharmacological Basis of Therapeutics. Hardman J. G., Limbird L. E., Gilman A. G., editors. New York: MacGraw-Hill; 1996. pp. 715–731. [Google Scholar]
- 4.Johnston C. I. J. Hypertens. 1985;3:557–569. doi: 10.1097/00004872-198512000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Abboud F. M., Floras J. S., Aylward P. E., Guo G. B., Gupta B. N., Schmid P. G. Blood Vessels. 1990;27:106–115. doi: 10.1159/000158801. [DOI] [PubMed] [Google Scholar]
- 6.Bishop V. S., Hay M. Front. Neuroendocrinol. 1993;14:57–75. doi: 10.1006/frne.1993.1003. [DOI] [PubMed] [Google Scholar]
- 7.Hasser E. M., Bishop V. S. Circ. Res. 1990;67:265–271. doi: 10.1161/01.res.67.2.265. [DOI] [PubMed] [Google Scholar]
- 8.Scheuer D. A., Bishop V. S. Am. J. Physiol. 1996;270:H1963–71. doi: 10.1152/ajpheart.1996.270.6.H1963. [DOI] [PubMed] [Google Scholar]
- 9.Aki Y., Tamaki T., Kiyomoto H., He H., Yoshida H., Iwao H., Abe Y. J. Cardiovasc. Pharmacol. 1994;23:331–336. [PubMed] [Google Scholar]
- 10.Yamamura Y., Ogawa H., Chihara T., Kondo K., Onogawa T., Nakamura S., Mori T., Tominaga M., Yabuuchi Y. Science. 1991;252:572–574. doi: 10.1126/science.1850553. [DOI] [PubMed] [Google Scholar]
- 11.Mayinger B., Hensen J. Exp. Clin. Endocrinol. Diabetes. 1999;107:157–165. doi: 10.1055/s-0029-1212091. [DOI] [PubMed] [Google Scholar]
- 12.Serradeil-Le Gal C., Wagnon J., Garcia C., Lacour C., Guiraudou P., Christophe B., Villanova G., Nisato D., Maffrand J. P., Le Fur G., et al. J. Clin. Invest. 1993;92:224–231. doi: 10.1172/JCI116554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirsch A. T., Majzoub J. A., Ren C. J., Scales K. M., Creager M. A. J. Appl. Physiol. 1993;75:1984–1988. doi: 10.1152/jappl.1993.75.5.1984. [DOI] [PubMed] [Google Scholar]
- 14.Moncada S., Palmer R. M., Higgs E. A. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 15.Furchgott R. F. Annu. Rev. Pharmacol. Toxicol. 1984;24:175–197. doi: 10.1146/annurev.pa.24.040184.001135. [DOI] [PubMed] [Google Scholar]
- 16.Valtin H. Ann. N.Y. Acad. Sci. 1982;394:1–9. doi: 10.1111/j.1749-6632.1982.tb37405.x. [DOI] [PubMed] [Google Scholar]
- 17.Chapman J. T., Hreash F., Laycock J. F., Walter S. J. J. Physiol. 1986;375:421–434. doi: 10.1113/jphysiol.1986.sp016125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sladek C. D., Blair M. L., Sterling C., Mangiapane M. L. Hypertension. 1988;12:506–512. doi: 10.1161/01.hyp.12.5.506. [DOI] [PubMed] [Google Scholar]
- 19.Burrell L. M., Phillips P. A., Stephenson J. M., Risvanis J., Rolls K. A., Johnston C. I. Hypertension. 1994;23:737–743. doi: 10.1161/01.hyp.23.6.737. [DOI] [PubMed] [Google Scholar]
- 20.Burrell L. M., Phillips P. A., Risvanis J., Aldred K. L., Hutchins A. M., Johnston C. I. Hypertension. 1995;26:828–834. doi: 10.1161/01.hyp.26.5.828. [DOI] [PubMed] [Google Scholar]
- 21.Thibonnier M., Kilani A., Rahman M., DiBlasi T. P., Warner K., Smith M. C., Leenhardt A. F., Brouard R. Hypertension. 1999;34:1293–1300. doi: 10.1161/01.hyp.34.6.1293. [DOI] [PubMed] [Google Scholar]
- 22.Guillon G., Trueba M., Joubert D., Grazzini E., Chouinard L., Cote M., Payet M. D., Manzoni O., Barberis C., Robert M., Gallo-Payet N. Endocrinology. 1995;136:1285–1295. doi: 10.1210/endo.136.3.7867583. [DOI] [PubMed] [Google Scholar]
- 23.Imai Y., Nolan P. L., Johnston C. I. Circ. Res. 1983;53:140–149. doi: 10.1161/01.res.53.2.140. [DOI] [PubMed] [Google Scholar]
- 24.Guyton A. C., Hall J. E. In: Textbook of Medical Physiology. Guyton A. C., Hall J. E., editors. Philadelphia: Saunders; 2000. pp. 184–194. [Google Scholar]
- 25.Kumada M., Terui N., Kuwaki T. Prog. Neurobiol. 1990;35:331–361. doi: 10.1016/0301-0082(90)90036-g. [DOI] [PubMed] [Google Scholar]
- 26.Sun M. K., Guyenet P. G. Am. J. Physiol. 1987;252:R699–R709. doi: 10.1152/ajpregu.1987.252.4.R699. [DOI] [PubMed] [Google Scholar]
- 27.Qu L., Hay M., Bishop V. S. Am. J. Physiol. 1997;272:R519–R525. doi: 10.1152/ajpregu.1997.272.2.R519. [DOI] [PubMed] [Google Scholar]
- 28.Kikuchi S., Tanoue A., Goda N., Matsuo N., Tsujimoto G. Jpn. J. Pharmacol. 1999;81:388–392. doi: 10.1254/jjp.81.388. [DOI] [PubMed] [Google Scholar]
- 29.Tanoue A., Ito S., Honda K., Oshikawa S., Kitagawa Y., Koshimizu T. A., Mori T., Tsujimoto G. J. Clin. Invest. 2004;113:302–309. doi: 10.1172/JCI19656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanoue A., Nasa Y., Koshimizu T., Shinoura H., Oshikawa S., Kawai T., Sunada S., Takeo S., Tsujimoto G. J. Clin. Invest. 2002;109:765–775. doi: 10.1172/JCI14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamamoto S., Shigeyoshi Y., Ishida Y., Fukuyama T., Yamaguchi S., Yagita K., Moriya T., Shibata S., Takashima N., Okamura H. J. Comp. Neurol. 2001;430:518–532. [PubMed] [Google Scholar]
- 32.Oh-hashi Y., Shindo T., Kurihara Y., Imai T., Wang Y., Morita H., Imai Y., Kayaba Y., Nishimatsu H., Suematsu Y., et al. Circ. Res. 2001;89:983–990. doi: 10.1161/hh2301.100812. [DOI] [PubMed] [Google Scholar]
- 33.Panel on Diagnostic Applications of Radioisotopes in Haematology. Br. J. Haematol. 1973;25:801–814. [PubMed] [Google Scholar]
- 34.Yagi T., Nada S., Watanabe N., Tamemoto H., Kohmura N., Ikawa Y., Aizawa S. Anal. Biochem. 1993;214:77–86. doi: 10.1006/abio.1993.1459. [DOI] [PubMed] [Google Scholar]