Abstract
Enhancement of oligodendrocyte survival through activation of leukemia inhibitory factor receptor (LIFR) signaling is a candidate therapeutic strategy for demyelinating disease. However, in other cell types, LIFR signaling is under tight negative regulation by the intracellular protein suppressor of cytokine signaling 3 (SOCS3). We, therefore, postulated that deletion of the SOCS3 gene in oligodendrocytes would promote the beneficial effects of LIFR signaling in limiting demyelination. By studying wild-type and LIF-knockout mice, we established that SOCS3 expression by oligodendrocytes was induced by the demyelinative insult, that this induction depended on LIF, and that endogenously produced LIF was likely to be a key determinant of the CNS response to oligodendrocyte loss. Compared with wild-type controls, oligodendrocyte-specific SOCS3 conditional-knockout mice displayed enhanced c-fos activation and exogenous LIF-induced phosphorylation of signal transducer and activator of transcription 3. Moreover, these SOCS3-deficient mice were protected against cuprizone-induced oligodendrocyte loss relative to wild-type animals. These results indicate that modulation of SOCS3 expression could facilitate the endogenous response to CNS injury.
Keywords: oligodendrocyte, SOCS3, cuprizone, signal transduction, conditional knockout
Demyelinating diseases of the CNS often involve oligodendrocyte death (1). The in vitro viability of oligodendrocytes is potentiated by several cytokines, including leukemia inhibitory factor (LIF), ciliary neurotrophic factor (CNTF), insulin-like growth factor I (IGF-I), and neurotrophin 3 (NT-3) (2, 3). These findings suggest therapeutic approaches, and recent work indicates that signaling through the LIF receptor (LIFR) complex by LIF or CNTF ameliorates demyelination in experimental autoimmune encephalomyelitis (4, 5). This effect can be induced by exogenous LIF but also probably represents an innate response because expression of LIF and CNTF is induced during inflammatory demyelination and CNS injury (4, 6). Both LIF and CNTF signal through the LIFR, which comprises LIFRβ and glycoprotein 130 (gp130); CNTF also requires the glycosylphosphatidylinositol-anchored nonsignaling CNTF receptor to effect its binding (7).
Signaling through LIFR involves activation of the signal transducers and activators of transcription (STATs) and is usually terminated rapidly by a negative feedback loop involving STAT-induced expression of the intracellular protein suppressor of cytokine signaling 3 (SOCS3), a member of a molecular family comprising SOCS1–7 and cytokine-inducible SH2-containing protein (CIS) (8). In several systems, SOCS proteins function as negative regulators, induced in response to cytokine stimulation as well as inhibiting further signaling by the same cytokine receptors. The SOCS1 and SOCS3 proteins are highly related, both being implicated in negative regulation of Janus kinase (JAK)–STAT signaling (9), the predominant pathway activated by LIFR. Expression of the SOCS3 gene is up-regulated by the STATs (10), and the SOCS3 protein binds to phosphotyrosine-759 on gp130 (11).
The above results suggest that SOCS proteins could limit the beneficial effects that LIFR signaling exerts to limit central demyelination. However, SOCS3-knockouts are embryonically lethal because of placental deficits (12, 13). Here, we utilized a conditional-knockout approach in oligodendrocytes, and we established that SOCS3 modulates cuprizone-induced demyelination.
Results
Oligodendrocytes Express SOCS3 in Response to LIFR Signaling in Vitro and During Demyelination.
To address whether SOCS3 has a role in modulating LIFR/gp130 signaling within oligodendrocytes, we utilized Northern blot analysis to assess expression of SOCS3 within primary oligodendrocytes isolated from rat optic nerves. The SOCS3 mRNA was almost undetectable in unstimulated cells, but it was induced after stimulation by LIF. This induction was rapid, occurring within 30 min of stimulation (Fig. 1a). In addition, the SOCS3 protein was also present at very low levels in the CG-4 cell line derived from oligodendrocytes (14), but it was induced by LIF within 1 h (Fig. 1b). The mRNA for SOCS1 was not induced by LIF stimulation (data not shown), suggesting that SOCS3 is likely to be the more relevant protein in modulating LIFR signaling within oligodendrocytes.
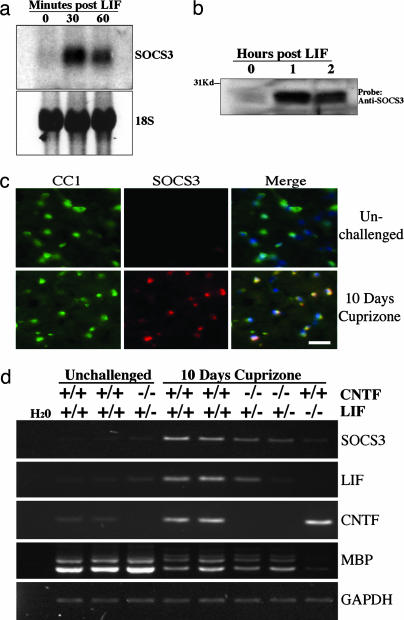
Fig. 1.
SOCS3 is induced by LIFR signaling by oligodendrocytes in vitro and in vivo in the context of cuprizone-mediated demyelination. (a) Northern blot analysis of SOCS3 expression in primary rat oligodendrocytes treated with LIF. (b) Western blot analysis of SOCS3 expression in CG-4-derived oligodendrocytes treated with LIF. (c) Immunostaining for CC1 and SOCS3 in the white matter of an unchallenged mouse and a mouse given 0.2% cuprizone for 10 days. Merged images include DAPI nuclear stain (blue). (Scale bar, 25 μm.) (d) RT-PCR analysis of SOCS3, myelin basic protein (MBP), LIF, and CNTF in samples isolated from the corpus callosum by laser capture microdissection (LCM). Mice were either unchallenged or given cuprizone for 10 days, and they were wild type or mutant for the LIF and CNTF genes as indicated. Template loading uniformity between samples was assessed by amplification of GAPDH.
Increased SOCS3 expression has been reported within the context of demyelination induced by the copper-binding chemical cuprizone (15), but the cells responsible for this expression have not been identified. We, therefore, assessed the expression profile of SOCS3 by using immunofluorescence in mice treated with cuprizone for 10 days. This early time point was chosen because it has been reported that there is a strong down-regulation of myelin genes but relatively little oligodendrocyte loss at this time point (15). In control animals, expression of SOCS3 was restricted to neurons, as described in refs. 16 and 17; this neuronal expression was not obviously altered in response to cuprizone. However, the white matter of animals exposed to cuprizone exhibited a greater number of SOCS3-positive cells than unchallenged controls (Fig. 1c). The majority of these cells were oligodendrocytes, as shown by their expression of the oligodendrocyte-specific marker CC1. The increased oligodendroglial SOCS3 expression extended to white-matter tracts outside the corpus callosum such as the optic nerves and stria medullaris of the thalamus, indicating that cuprizone administration causes early reactive changes, even in regions that do not frankly demyelinate.
We next explored whether either LIF or CNTF was responsible for the cuprizone-mediated induction of SOCS3. RT-PCR was performed on samples obtained from the medial corpus callosum by laser capture microdissection (LCM). After 10 days of cuprizone, the expression of the SOCS3, LIF, and CNTF genes was induced within the corpus callosum of wild-type mice. Two CNTF−/−/LIF+/− mice subjected to the same experimental paradigm displayed absent CNTF and reduced LIF gene expression, as expected. Expression of the SOCS3 gene was also reduced in the CNTF−/−/LIF+/− mutant mice relative to wild-type mice, confirming that its induction is downstream from CNTF and/or LIF. Furthermore, there was almost no induction of the SOCS3 gene in two CNTF+/+/LIF−/− mice challenged with cuprizone (as shown for one of the mice in Fig. 1d), strongly suggesting that LIF is the predominant inducer of SOCS3 in this system.
LIF Is an Endogenous Survival Factor for Oligodendrocytes.
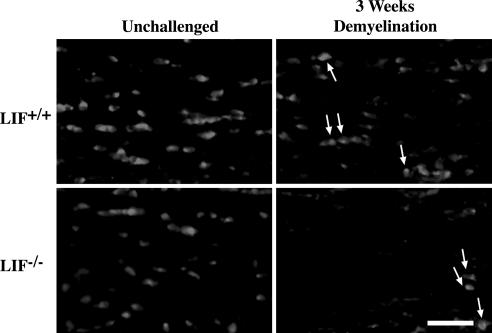
We next determined whether LIF was also the principal cytokine responsible for limiting oligodendroglial loss in the context of cuprizone-induced injury by analyzing wild-type and LIF-knockout mice. Although it has been reported that female LIF−/− mice display mild reductions of MBP expression within restricted regions of the brain (18), assessment of the density of CC1-positive oligodendrocytes within the corpus callosi of three unchallenged LIF−/− mice and three wild-type controls revealed no statistically significant difference between the two genotypes (P > 0.05; Fig. 2 and Table 1), nor did the density of oligodendrocytes within the corpus callosum appear to be obviously affected by gender among the knockouts (data not shown). In contrast, after 3 weeks of cuprizone, a time point at which wild-type mice usually display only moderate demyelination, a cohort of four LIF−/− mice displayed an 84.8% reduction in oligodendrocytes (compared with 36.2% loss in four wild-types mice, P < 0.01), a finding corroborated in a second, independent, cohort of mice (data not shown).
Fig. 2.
Deletion of LIF increases the severity of cuprizone-mediated oligodendrocyte loss. Representative frozen sections within the medial corpus callosum of LIF−/− mice and wild-type controls were stained for the oligodendrocyte marker CC1. Mice were either unchallenged or given cuprizone for 3 weeks. Arrows indicate examples of CC1-positive cells. (Scale bar, 25 μm.)
Table 1.
LIF deficiency accentuates cuprizone-mediated loss of CC1-positive oligodendrocytes
| Genotype | Unchallenged | 3 wk cuprizone |
|---|---|---|
| LIF+/+ | 2,102 ± 210 | 1,342 ± 225 |
| LIF−/− | 2,057 ± 285 | 312 ± 167** |
Numbers represent cells per mm2, ± SD.
**, P < 0.01 vs. WT mice.
Generation of Oligodendrocyte SOCS3 Conditional Knockouts.
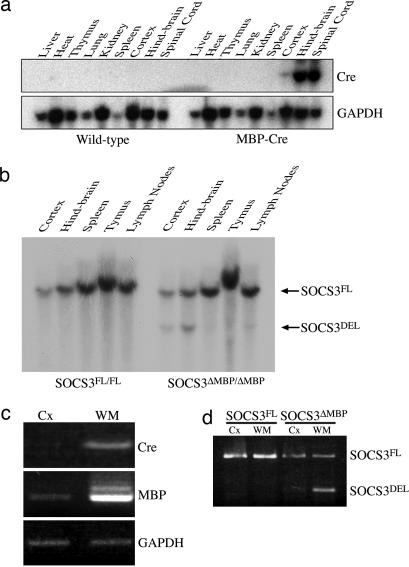
Our next goal was to assess whether the SOCS3 expression induced in oligodendrocytes limited the benefits of endogenous cytokine signaling in the context of central demyelination. To address this question, SOCS3 was deleted from myelinating oligodendrocytes by crossing mice in which the coding region of SOCS3 is loxP-flanked [SOCS3FL/FL (19)] with transgenic mice expressing Cre recombinase from a 1.96-kb fragment of the MBP promoter (20). Cre mRNA was highly expressed within the spinal cord and hindbrain but not detected in other organs, indicating that the expression of the transgene mimicked that of the endogenous promoter (Fig. 3a). Southern blot analysis of genomic DNA isolated from the CNS and other organs indicated that Cre-mediated recombination of the loxP-flanked SOCS3 allele was restricted to the CNS (cortex and hindbrain, Fig. 3b). To establish the spatial profile of recombination of SOCS3 within the brain, LCM was utilized to isolate and analyze samples from the corpus callosum and cerebral cortex of a SOCS3FL/FL and a SOCS3ΔMBP/ΔMBP mouse. RT-PCR analysis of samples from the SOCS3ΔMBP/ΔMBP mouse revealed stronger expression of MBP and Cre mRNA within the corpus callosum relative to cortex (Fig. 3c). The recombined (deleted) allele of SOCS3 was also readily detectable by PCR from corpus callosal samples, whereas only the unrecombined (floxed) allele was detectable from cortical samples and samples obtained from a SOCS3FL/FL animal (Fig. 3d), indicating that the MBP–Cre transgene effects the deletion of SOCS3 within the brain in a predominantly white matter-specific distribution. In contrast to wild-type mice (Fig. 1c), no colocalization was seen between SOCS3 and CC1 in a SOCS3ΔMBP/ΔMBP mouse treated with cuprizone (data not shown).
Fig. 3.
Generation and confirmation of conditional knockout of SOCS3 within oligodendrocytes. (a) Northern blot analysis of Cre expression in MBP–Cre transgenic mice. (b) BamHI digestion and Southern blot analysis of DNA from the CNS and immune organs of a SOCS3FL/FL and a SOCS3ΔMBP/ΔMBP mouse distinguish between the loxP-flanked (SOCS3FL, 9 kb) and excised allele (SOCS3DEL, 4.9 kb). The image shown is representative of three mice examined for each genotype. (c) RT-PCR analysis of genomic DNA isolated from the LCM samples [cortex (Cx) or white matter (WM)] of a SOCS3ΔMBP/ΔMBP mouse, showing white-matter predominance of Cre and MBP gene expression. (d) PCR analysis of genomic DNA isolated from the LCM samples (cortex or white matter) of a SOCS3FL/FL and a SOCS3ΔMBP/ΔMBP mouse, distinguishing between the SOCS3 loxP-flanked (FL) and excised (DEL) alleles.
Molecular Consequences of Oligodendrocyte-Specific Deletion of SOCS3.
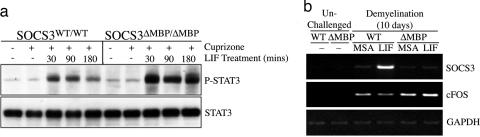
To determine how the deletion of SOCS3 influenced key signaling events, cuprizone was administered to SOCS3WT/WT and SOCS3ΔMBP/ΔMBP mice for 10 days. The responsiveness of SOCS3WT/WT and SOCS3ΔMBP/ΔMBP mice to LIF was first assessed at the level of STAT3 activation after a single injection of LIF. Wild-type mice displayed a modest phosphorylation of STAT3 within the hindbrain 30 min after receiving LIF, which was significantly reduced by 3 h after LIF stimulation. In contrast, the SOCS3ΔMBP/ΔMBP mice displayed a LIF-induced phosphorylation of STAT3 that was heightened relative to wild-type mice (Fig. 4a, one mouse per sample, blot representative of two independent experiments). Next, we compared the induction of a subset of LIF-responsive target genes in the corpus callosum of SOCS3WT/WT and SOCS3ΔMBP/ΔMBP mice that were given cuprizone for 10 days and treated with LIF or carrier protein [murine serum albumin (MSA)] only. Analysis with RT-PCR revealed that wild-type mice displayed a strong induction of SOCS3 mRNA 1 h after exogenous LIF administration, whereas, as expected, no such induction was seen in the SOCS3ΔMBP/ΔMBP mice (Fig. 4b, one mouse per sample, gel representative of two independent experiments). By utilizing RT-PCR (Fig. 4b) and real-time PCR, we also established that the immediate-early gene c-fos was up-regulated in all mice treated with cuprizone for 10 days, presumably in response to locally produced cytokines. This induction was observed to be 3-fold greater in a cohort of four SOCS3ΔMBP/ΔMBP mice relative to four wild-type mice (14.2 ± 3.1-fold vs. 4.6 ± 1.6-fold; P < 0.05), but, unlike the above-described pattern of potentiation of phosphorylation of STAT3, the expression of c-fos was not significantly altered by the administration of exogenous LIF (14.2 ± 3.1-fold without LIF vs. 8.1 ± 1.6-fold with LIF; P = 0.15) in SOCS3ΔMBP/ΔMBP mice challenged with cuprizone. Immunofluorescence analysis of c-Fos expression within the corpus callosum confirmed the real-time PCR expression pattern, and it also confirmed that the collosal c-Fos expression in SOCS3ΔMBP/ΔMBP mice was found predominantly in cells arranged in linear arrays, typical of oligodendrocytes (data not shown).
Fig. 4.
Oligodendrocyte SOCS3 conditional knockouts display heightened sensitivity to LIFR signaling in the context of cuprizone-mediated demyelination. (a) LIF-induced tyrosine phosphorylation of STAT3 in hindbrains from SOCS3WT/WT and SOCS3ΔMBP/ΔMBP mice after 10 days of receiving cuprizone. (b) RT-PCR analysis of SOCS3 and c-Fos in LCM-isolated sections from the corpus callosum of SOCS3WT/WT and SOCS3ΔMBP/ΔMBP mice. Mice were either unchallenged or given cuprizone for 10 days, followed by an injection of either LIF or mouse serum albumin (MSA) 1 h before being killed. Template loading was assessed by amplification of GAPDH.
Deletion of SOCS3 Inhibits Oligodendrocyte Loss in the Context of Demyelination.
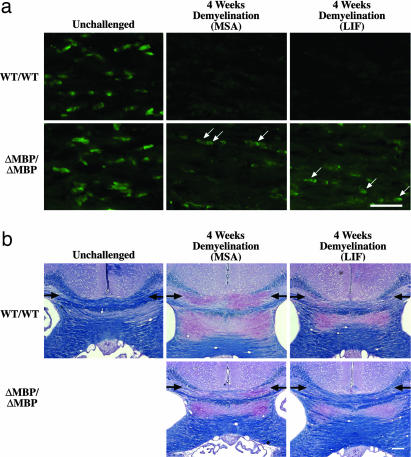
We next assessed the effects of SOCS3 on oligodendrocyte numbers in cuprizone-mediated demyelination. We exposed four SOCS3WT/WT and four SOCS3ΔMBP/ΔMBP mice to cuprizone for 4 weeks, a time at which wild-type mice display widespread loss of oligodendrocytes within the medial corpus callosum (21, 22). After 4 weeks of receiving cuprizone, the wild-type mice displayed almost complete loss of CC1-positive oligodendrocytes (Fig. 5a and Table 2; P < 0.001). Although the SOCS3ΔMBP/ΔMBP mice displayed some oligodendrocyte loss within the corpus callosum in response to cuprizone (P < 0.05), this loss was attenuated by 49% compared with wild-type mice (P < 0.05). Diminished oligodendrocyte loss in SOCS3ΔMBP/ΔMBP mice was also confirmed in a second, independent, cohort of mice.
Fig. 5.
Oligodendrocyte SOCS3 conditional knockouts are protected against cuprizone-mediated demyelination. (a) Representative frozen sections within the medial corpus callosum of SOCS3WT/WT and SOCS3ΔMBP/ΔMBP mice stained for the oligodendrocyte marker CC1. Mice were either unchallenged or given cuprizone for 4 weeks together with daily treatment with either LIF or MSA from days 7 to 28. Arrows indicate examples of CC1-positive cells, which are more numerous in SOCS3ΔMBP/ΔMBP mice compared with wild-type mice after 4 weeks of cuprizone, whether they were treated with LIF or vehicle (MSA). (Scale bar, 25 μm.) (b) Representative LFB–PAS-stained paraffin sections from an unchallenged mouse and cuprizone-challenged SOCS3WT/WT and SOCS3ΔMBP/ΔMBP mice treated daily with either LIF or MSA from days 7 to 28. The medial corpus callosi are indicated by arrows, demonstrating the most overt demyelination, as determined by loss of LFB staining (blue) and uncovering of PAS staining (red) in wild-type mice challenged with cuprizone, with partial protection in SOCS3ΔMBP/ΔMBP mice receiving vehicle and almost complete protection in SOCS3ΔMBP/ΔMBP mice receiving LIF. (Scale bar, 200 μm.)
Table 2.
SOCS3 deficiency protects CC1-positive oligodendrocytes independent of exogenous LIF
| Genotype | Unchallenged | 4 wk cuprizone |
|
|---|---|---|---|
| MSA | LIF | ||
| WT/WT | 2,305 ± 140 | 66 ± 32 | 178 ± 218 |
| ΔMBP/ΔMBP | 1,976 ± 133 | 1,000 ± 534* | 882 ± 572* |
Numbers represent cells per mm2, ± SD.
*, P < 0.05 vs. WT mice.
To assess whether this reduction in oligodendrocyte loss led to a preservation of myelin, an independent cohort of mice was assessed for luxol fast blue (LFB) staining of myelin after 4 weeks of cuprizone administration (Fig. 5b). Although eight wild-type mice challenged in this way displayed severe reductions in myelin levels within the corpus callosum relative to unchallenged controls, this reduction was attenuated by 18% in eight SOCS3ΔMBP/ΔMBP mice with the image analysis program (National Institutes of Health), although not reaching statistically significant levels (relative myelin levels compared with unchallenged controls: wild-type 40.7% ± 7.1%, SOCS3ΔMBP/ΔMBP 47.5% ± 9.8%; P > 0.05).
Deletion of SOCS3 Promotes the Capacity of Exogenous LIF to Ameliorate Myelin Loss.
Groups of five wild-type and five SOCS3ΔMBP/ΔMBP mice were treated daily with LIF or MSA between days 7 and 28 in the context of a 28-day course of cuprizone to ascertain whether exogenous LIF could further reduce oligodendrocyte loss. Neither group treated with LIF displayed any significant reduction in the loss of oligodendrocytes relative to controls of the corresponding genotype that were treated with carrier protein (MSA) only (P = 0.32 and 0.76, respectively); however, LIF-treated SOCS3ΔMBP/ΔMBP mice still displayed significantly higher densities of oligodendrocytes than their LIF-treated wild-type counterparts (P < 0.05) (Fig. 5a and Table 2). On the other hand, when myelination was assessed in an independent group of 10 LIF and 8 vehicle-treated SOCS3ΔMBP/ΔMBP mice by analysis of LFB staining density with the image program, there was a significant rescue of myelin loss in the LIF-treated animals (relative myelin levels compared with unchallenged SOCS3ΔMBP/ΔMBP mice: vehicle alone 47.5% ± 9.8%, LIF treatment 90.1% ± 5.9%; P < 0.05). No such statistically significant benefit of LIF was seen in wild-type animals (Fig. 5b).
Discussion
Our data establish that activation of the LIFR complex after cuprizone-induced injury limits oligodendrocyte loss and induces the inhibitor SOCS3 in these cells. Deletion of SOCS3 further reduces oligodendrocyte loss and provides a substrate that enables exogenous LIF to protect against demyelination.
Although a number of cytokine systems influence the remyelination phase after cuprizone-mediated demyelination, including TNF-α, IGF-I, FGF-2, and IL-1β (22–25), less is known about the cytokines that modulate the initial loss of oligodendrocytes. Although IGF-I reduces the loss of oligodendrocytes when transgenically overexpressed, mice lacking the IGF-I receptor within oligodendrocytes fail to show any acceleration in cuprizone-mediated loss of oligodendrocytes, displaying deficiencies confined to the subsequent remyelination phase (21, 25). These findings, in combination with our observations that the loss of oligodendrocytes is exacerbated in LIF−/− mice, suggest that LIFR signaling is an important endogenous mechanism for limiting oligodendroglial injury and loss in vivo. Moreover, given that both LIF and CNTF were induced within the corpus callosum in response to cuprizone, it is likely that cytokines signaling through the LIFR complex will show a high degree of redundancy.
A number of observations suggest that SOCS3 is a negative regulator of LIF signaling in cuprizone-induced demyelination. Not only is the expression of both LIF and SOCS3 induced by cuprizone, but also the induction of SOCS3 is almost completely abrogated in LIF−/− mice. Deletion of the LIF and SOCS3 genes also results in opposing phenotypes; LIF−/− mice display accelerated loss, whereas SOCS3ΔMBP/ΔMBP mice display enhanced oligodendrocyte survival with cuprizone. Furthermore, the phenotype displayed by the SOCS3ΔMBP/ΔMBP mice coincided with increased expression of the LIF-responsive immediate-early gene c-fos and potentiated activation of STAT3. Interestingly, c-fos was selectively enhanced in those cuprizone-challenged SOCS3ΔMBP/ΔMBP mice that were not exposed to exogenous LIF, whereas STAT3 phosphorylation was enhanced by the administration of LIF; whether this apparent dissociation accounts for composite concentration-dependent effects that LIF might exert on oligodendrocyte biology awaits further investigation. It is also possible that the beneficial effects of SOCS3 deficiency could have been mediated by enhanced signaling of other cytokines. However, the known cytokine responsiveness of oligodendrocytes is fairly limited, being restricted to the LIF family, IGF-I, NT-3, and neuregulin. Neither NT-3 nor neuregulin is reported to interact with SOCS3, and, as indicated above, the beneficial effects of IGF-I in this context appear restricted to the remyelination phase.
Perhaps unexpectedly, administration of exogenous LIF to wild-type mice failed to reduce cuprizone-mediated demyelination significantly, as measured by either oligodendrocyte density or LFB staining. Given that this exogenous administration resulted in a further increase in SOCS3 but not c-fos mRNA levels within the corpus callosum (Fig. 4), it seems likely that the LIF-induced SOCS3 was rapidly and effectively inhibiting any further signaling through the LIFR complex. Other investigators have also reported that attempts to enhance LIF signaling within the heart by overexpressing the gp130 receptor subunit resulted in heightened SOCS3 induction in response to LIF but without other LIF-responsive genes being similarly increased (26). These findings establish the difficulty of potentiating LIFR signaling past endogenous levels in the presence of SOCS3.
Provision of exogenous LIF to SOCS3ΔMBP/ΔMBP mice did not affect oligodendrocyte numbers, which implies that some oligodendrocyte loss is driven by non-LIF-dependent mechanisms but that this residual population can still be induced to limit demyelination by exogenous LIF in the absence of the negative regulatory activity of SOCS3. In combination, these results suggest that the modulation of SOCS3 and enhancement of LIFR signaling provide exciting potential therapeutic strategies for the treatment of central demyelinating disease.
Materials and Methods
Animal Resources.
The SOCS3 loxP-flanked strain (line 350, C57BL/6 background) was generated as described in ref. 19. The MBP–Cre lines were generated in conjunction with F. Koentgen of Ozgene (Bently, Western Australia, Australia) from a plasmid kindly provided by Alex Gow (Wayne State University School of Medicine, Detroit) by microinjection into the male pronucleus of fertilized C57BL/6 oocytes. Homozygous loxP-flanked SOCS3 mice positive for the MBP–Cre transgene (SOCS3ΔMBP/ΔMBP) were obtained by crossing MBP–Cre mice onto the 350 line for two generations. CNTF−/−/LIF−/− and CNTF−/−/LIF+/− mice on the C57BL/6 background were obtained by crossing CNTF+/−/LIF+/− mice that were a kind gift from M. Sendtner (Institute for Clinical Neurobiology, University of Wurzburg). LIF-knockout mice on a mixed DBA2 and C57BL/6 background were a kind gift from Phillipe Brulet (Institut Pasteur, Paris); oligodendrocyte counts from these animals were compared with those from wild-type littermates. Oligodendrocyte purifications were performed on Sprague–Dawley rat pups (postnatal day 7) from Monash University (Melbourne, Australia). Experiments were conducted in accordance with guidelines of the Howard Florey Animal Ethics Committee.
Induction of Demyelination.
Cuprizone-mediated demyelination was induced by feeding mice at 6–10 weeks of age a diet of powdered feed (Barastoc; Ridley AgriProducts, Pakenham, Victoria) containing 0.2% wt/wt cuprizone (Sigma) for up to 4 weeks.
LIF Treatment.
Mice were injected s.c. with LIF daily (Amrad, Melbourne, Australia, 25 μg/kg of body weight) made up in 100 μl of 0.1% MSA (Sigma) or an equivalent dose of 0.1% MSA alone. For assessment of LIF responsiveness at day 10 of cuprizone treatment, mice received i.v. injections of 2 μg of LIF in 100 μl of 0.1% MSA at specified times before being killed.
Cell Culture.
Oligodendrocyte precursors were purified from optic nerves of 7-day-old rats and were expanded in SATO medium with 10 ng/ml platelet-derived growth factor and NT-3 (PeproTech, Rocky Hill, NJ) as described in ref. 27. Cells were induced to differentiate by the addition of thyroid hormone for 48 h and treated with 100 ng/ml LIF for 30 and 60 min. The bipotential glial cell line CG-4 (kind gift from O. Bögler, Center for Molecular Genetics, University of California, San Diego) was cultured in SATO medium with 30% B104 conditioned medium as reported in ref. 14 and induced to differentiate for 48 h by withdrawal of conditioned medium before cytokine treatment.
Histology.
For fluorescence microscopy, 10-μm cryostat sections were probed with primary antibodies at 1:200 followed by detection with appropriate fluorochrome-conjugated secondary antibodies (Molecular Probes, 1:500). Primary antibodies used were APC-7/CC1 (Oncogene Science) and rabbit polyclonal anti-SOCS3 (produced by J.-G.Z.). Paraffin sections (10 μm) were used for assessment of demyelination with LFB–periodic acid/Schiff (PAS) reagent.
Oligodendrocyte Density Analysis.
Three 10-μm coronal cryostat sections of the brain between bregma −0.58 mm and −0.9 mm at least 50 μm apart were taken from each animal. Sections were stained with DAPI and the CC1 antibody and photographed at a magnification of ×200 at the midline of the corpus callosum (corresponding to the medial 425 μm of each section). The number of CC1-immunopositive cells with DAPI-positive nuclei within the corpus callosum was counted for each image, and the area of the corpus callosum was determined in square millimeters with the image 1.63 program (National Institutes of Health). All counts and analyses were performed blind to the genotype and treatment of the animal.
Analysis of LFB Staining.
LFB–PAS-stained paraffin sections were selected for each animal at as close to bregma −0.75 mm as the series of sections would allow. Sections were photographed with a ×10 objective, and the images were imported into photoshop (Adobe Systems, San Jose, CA). Within photoshop, the blue channel of each image was selected and exported as a gray scale image into image 1.63, which was used to take a density reading of the LFB staining within the central 870 μm of the corpus callosum. Density measurements for each mouse were normalized against unchallenged corpus callosum optical density values by using the following formula: transformed value = (density reading/unchallenged density average) × 100. All analysis was performed blind to the genotype and treatment of the animal.
Western Blots.
Hindbrains were lysed in radioimmunoprecipitation assay buffer with protease inhibitors (28) and 100 μg of protein run on 10% Tris–glycine gels (Invitrogen), and they were transferred to poly(vinylidene difluoride) membrane (Pall). Membranes were assayed for STAT3 or phospho-STAT3 as described in ref. 28.
Northern Blot Analysis.
RNA samples were generated from cultured oligodendrocytes or mouse organs with Miniprep and Midiprep kits, respectively (Qiagen, Valencia, CA) as per the manufacturer's instructions. Northern blotting was performed according to standard methodology with probes generated from the 3′ UTR region of SOCS3 (plasmid kindly supplied by Lynn Hartley, The Walter and Eliza Hall Institute).
LCM.
Frozen sections (16 μm) of unfixed brains were collected on membrane-coated slides (PALM, Zeiss) and immersed in acetone at −20°C for 5 min. The slides were air-dried at room temperature for 10 min, stained in 1% cresyl violet/95% vol/vol ethanol for 1 min, and rinsed through three changes of 100% ethanol for 2 min each. The slides were air-dried, and laser-dissected tissue (typically 5 × 105 μm2 taken from 5–10 sections) was isolated from either the medial corpus callosum or the lateral cerebral cortex with the PALM MicroBeam MicroLaser System and PALM robosoftware (Millennium Science, Surrey Hills, Victoria, Australia). Tissue was collected into the cap of a 200-μl tube containing 5 μl of either DNA isolation buffer [1 mM EDTA/20 mM Tris, pH 8.0/0.5% Igepal CA-630 (Stepan, Northfield, IL)/200 μg/ml proteinase K] or PicoPure RNA Isolation kit extraction buffer (Arcturus, Mountain View, CA) for RNA isolation.
PCR.
Laser-capture DNA samples were amplified by using primers that distinguish among the wild-type, loxP-flanked, and deleted SOCS3 alleles. Primer sequences were as follows: forward, 5′-TCTTGTGTCTCTCCCCATCC-3′; coding reverse, 5′-TGACGCTCAACGTGAAGAAG-3′; and 3′ UTR reverse, 5′-ACGTCTGTGATGCTTTGCTG-3′.
RT-PCR.
RNA from LCM samples was isolated by using the PicoPure RNA Isolation kit as per the manufacturer's instructions. RNA (≈10 ng) was reverse-transcribed, and genes of interest were amplified for 30 cycles by using standard procedures. Primers used were as follows: GAPDH forward, 5′-ACCACAGTCCATCCCATCAC-3, and reverse, 5′-TCCACCACCCTGTTGCTGTA-3′ (29); MBP forward, 5′-ATGGCATCACAGAAGAGA-3′, and reverse, 5′-CATGGGAGATCCAGAGCG-3′ (GenBank accession no. L00398); SOCS3 forward, 5′-ACCAGCGCCACTTCTTCACG-3′, and reverse, 5′-GTGGAGCATCATACTGATCC-3′ (30); c-fos forward, 5′-GGCTCTCCTGTCAACACACA-3′, and reverse, 5′-CCGCTTGGAGTGTATCTGTC-3′ (31); LIF forward, 5′-CGCCTAACATGACAGACTTCCCAT-3′, and reverse, 5′-AGGCCCCTCATGACGTCTATAGTA-3′ (32); CNTF forward, 5′-GGGGATGGCTTTCGCAGA-3′, and reverse, 5′-GGTACGGTAAGCCTGGAGGT-3′ (National Center for Biotechnology Information accession no. NM_053007).
Quantitative PCR for c-fos.
Brains from day-10 cuprizone-treated mice were collected in RNAlater (Ambion, Austin, TX) and stored for 2 weeks at 4°C. The corpus callosum was microdissected from the brain of each animal, and RNA was isolated with the PicoPure RNA Isolation kit according to the manufacturer's instructions. RNA (8.5 ng) was reverse-transcribed, and quantitative PCR for 18 S and c-fos RNAs was performed on a Sequence Detector 7700 (Applied Biosystems) using SYBR green chemistry (Applied Biosystems) according to the manufacturer's instructions. PCRs were analyzed with the sequence detection system software (Applied Biosystems), with results shown as the relative expression from cohorts of wild-type and SOCS3ΔMBP/ΔMBP mice by using the equivalent untreated control as a baseline and accounting for 18S RNA levels. Primers were as follows: 18 S forward, 5′-CGGCTACCACATCCAAGGAA-3′, and reverse, 5′-GCTGGAATTACCGCGGCT-3′; c-fos forward, 5′-AGGAGGCCTTCACCCTGC-3′, and reverse, 5′-TGACTGGCTCCAAGGATGG-3′.
Statistics.
Data are expressed as the means ± SD. Comparisons were evaluated with an unpaired Student's t test assuming unequal variances. *, P < 0.05; **, P < 0.01.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia and by the National Multiple Sclerosis Society of the United States of America. Recombinant LIF was a generous gift from the Australian Medical Research and Development Corp.
Abbreviations
- CNTF
ciliary neurotrophic factor
- IGF-I
insulin-like growth factor I
- LCM
laser capture microdissection
- LFB
luxol fast blue
- LIF
leukemia inhibitory factor
- LIFR
leukemia inhibitory factor receptor
- MBP
myelin basic protein
- MSA
murine serum albumin
- NT-3
neurotrophin 3
- PAS
periodic acid/Schiff
- SOCS
suppressor of cytokine signaling
- STAT
signal transducer and activator of transcription.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Lucchinetti C., Bruck W., Parisi J., Scheithauer B., Rodriguez M., Lassmann H. Ann. Neurol. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 2.Kahn M. A., De Vellis J. Glia. 1994;12:87–98. doi: 10.1002/glia.440120202. [DOI] [PubMed] [Google Scholar]
- 3.Barres B. A., Schmid R., Sendnter M., Raff M. C. Development (Cambridge, U.K.) 1993;118:283–295. doi: 10.1242/dev.118.1.283. [DOI] [PubMed] [Google Scholar]
- 4.Butzkueven H., Zhang J. G., Soilu-Hanninen M., Hochrein H., Chionh F., Shipham K. A., Emery B., Turnley A. M., Petratos S., Ernst M., et al. Nat. Med. 2002;8:613–619. doi: 10.1038/nm0602-613. [DOI] [PubMed] [Google Scholar]
- 5.Linker R. A., Maurer M., Gaupp S., Martini R., Holtmann B., Giess R., Rieckmann P., Lassmann H., Toyka K. V., Sendtner M., Gold R. Nat. Med. 2002;8:620–624. doi: 10.1038/nm0602-620. [DOI] [PubMed] [Google Scholar]
- 6.Sriram K., Benkovic S. A., Hebert M. A., Miller D. B., O'Callaghan J. P. J. Biol. Chem. 2004;279:19936–19947. doi: 10.1074/jbc.M309304200. [DOI] [PubMed] [Google Scholar]
- 7.Stahl N., Davis S., Wong V., Taga T., Kishimoto T., Ip N. Y., Yancopoulos G. D. J. Biol. Chem. 1993;268:7628–7631. [PubMed] [Google Scholar]
- 8.Krebs D. L., Hilton D. J. Stem Cells. 2001;19:378–387. doi: 10.1634/stemcells.19-5-378. [DOI] [PubMed] [Google Scholar]
- 9.Starr R., Willson T. A., Viney E. M., Murray L. J., Rayner J. R., Jenkins B. J., Gonda T. J., Alexander W. S., Metcalf D., Nicola N. A., Hilton D. J. Nature. 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- 10.Auernhammer C. J., Bousquet C., Melmed S. Proc. Natl. Acad. Sci. USA. 1999;96:6964–6969. doi: 10.1073/pnas.96.12.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicholson S. E., De Souza D., Fabri L. J., Corbin J., Willson T. A., Zhang J. G., Silva A., Asimakis M., Farley A., Nash A. D., et al. Proc. Natl. Acad. Sci. USA. 2000;97:6493–6498. doi: 10.1073/pnas.100135197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts A. W., Robb L., Rakar S., Hartley L., Cluse L., Nicola N. A., Metcalf D., Hilton D. J., Alexander W. S. Proc. Natl. Acad. Sci. USA. 2001;98:9324–9329. doi: 10.1073/pnas.161271798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahashi Y., Carpino N., Cross J. C., Torres M., Parganas E., Ihle J. N. EMBO J. 2003;22:372–384. doi: 10.1093/emboj/cdg057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Louis J. C., Magal E., Muir D., Manthorpe M., Varon S. J. Neurosci. Res. 1992;31:193–204. doi: 10.1002/jnr.490310125. [DOI] [PubMed] [Google Scholar]
- 15.Jurevics H., Largent C., Hostettler J., Sammond D. W., Matsushima G. K., Kleindienst A., Toews A. D., Morell P. J. Neurochem. 2002;82:126–136. doi: 10.1046/j.1471-4159.2002.00954.x. [DOI] [PubMed] [Google Scholar]
- 16.Mori H., Hanada R., Hanada T., Aki D., Mashima R., Nishinakamura H., Torisu T., Chien K. R., Yasukawa H., Yoshimura A. Nat. Med. 2004;10:739–743. doi: 10.1038/nm1071. [DOI] [PubMed] [Google Scholar]
- 17.Polizzotto M. N., Bartlett P. F., Turnley A. M. J. Comp. Neurol. 2000;423:348–358. [PubMed] [Google Scholar]
- 18.Bugga L., Gadient R. A., Kwan K., Stewart C. L., Patterson P. H. J. Neurobiol. 1998;36:509–524. doi: 10.1002/(sici)1097-4695(19980915)36:4<509::aid-neu5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 19.Croker B. A., Krebs D. L., Zhang J. G., Wormald S., Willson T. A., Stanley E. G., Robb L., Greenhalgh C. J., Forster I., Clausen B. E., et al. Nat. Immunol. 2003;4:540–545. doi: 10.1038/ni931. [DOI] [PubMed] [Google Scholar]
- 20.Gow A., Friedrich V. L., Jr., Lazzarini R. A. J. Cell Biol. 1992;119:605–616. doi: 10.1083/jcb.119.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mason J. L., Ye P., Suzuki K., D'Ercole A. J., Matsushima G. K. J. Neurosci. 2000;20:5703–5708. doi: 10.1523/JNEUROSCI.20-15-05703.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arnett H. A., Mason J., Marino M., Suzuki K., Matsushima G. K., Ting J. P. Nat. Neurosci. 2001;4:1116–1122. doi: 10.1038/nn738. [DOI] [PubMed] [Google Scholar]
- 23.Gao X., Gillig T. A., Ye P., D'Ercole A. J., Matsushima G. K., Popko B. Mol. Cell. Neurosci. 2000;16:338–349. doi: 10.1006/mcne.2000.0883. [DOI] [PubMed] [Google Scholar]
- 24.Mason J. L., Suzuki K., Chaplin D. D., Matsushima G. K. J. Neurosci. 2001;21:7046–7052. doi: 10.1523/JNEUROSCI.21-18-07046.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mason J. L., Xuan S., Dragatsis I., Efstratiadis A., Goldman J. E. J. Neurosci. 2003;23:7710–7718. doi: 10.1523/JNEUROSCI.23-20-07710.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tone E., Kunisada K., Kumanogoh A., Negoro S., Funamoto M., Osugi T., Kishimoto T., Yamauchi-Takihara K. Cytokine. 2000;12:1512–1518. doi: 10.1006/cyto.2000.0751. [DOI] [PubMed] [Google Scholar]
- 27.Barres B. A., Hart I. K., Coles H. S., Burne J. F., Voyvodic J. T., Richardson W. D., Raff M. C. Cell. 1992;70:31–46. doi: 10.1016/0092-8674(92)90531-g. [DOI] [PubMed] [Google Scholar]
- 28.Brysha M., Zhang J. G., Bertolino P., Corbin J. E., Alexander W. S., Nicola N. A., Hilton D. J., Starr R. J. Biol. Chem. 2001;276:22086–22089. doi: 10.1074/jbc.M102737200. [DOI] [PubMed] [Google Scholar]
- 29.Tokumoto Y. M., Durand B., Raff M. C. Dev. Biol. 1999;213:327–339. doi: 10.1006/dbio.1999.9397. [DOI] [PubMed] [Google Scholar]
- 30.Bjorbaek C., Elmquist J. K., El-Haschimi K., Kelly J., Ahima R. S., Hileman S., Flier J. S. Endocrinology. 1999;140:2035–2043. doi: 10.1210/endo.140.5.6736. [DOI] [PubMed] [Google Scholar]
- 31.Duval D., Reinhardt B., Kedinger C., Boeuf H. FASEB J. 2000;14:1577–1584. doi: 10.1096/fj.14.11.1577. [DOI] [PubMed] [Google Scholar]
- 32.Chesnokova V., Auernhammer C. J., Melmed S. Endocrinology. 1998;139:2209–2216. doi: 10.1210/endo.139.5.6016. [DOI] [PubMed] [Google Scholar]