Abstract
Arginine vasopressin (AVP) and related peptides affect social behaviors in numerous species, but AVP influences on human social functions have not yet been established. Here, we describe how intranasal AVP administration differentially affects social communication in men and women, and we propose a mechanism through which it may exert those influences. In men, AVP stimulates agonistic facial motor patterns in response to the faces of unfamiliar men and decreases perceptions of the friendliness of those faces. In contrast, in women, AVP stimulates affiliative facial motor patterns in response to the faces of unfamiliar women and increases perceptions of the friendliness of those faces. AVP also affected autonomic responsiveness to threatening faces and increased anxiety, which may underlie both communication patterns by promoting different social strategies in stressful contexts in men and women.
Keywords: affiliation, aggression, anxiety, autism, emotion
Central arginine vasopressin (AVP; mammals) and arginine vasotocin (AVT; nonmammalian homologue) systems modulate social behaviors in numerous species from diverse vertebrate groups (1). It has therefore been proposed that social functions of these peptides were highly conserved during vertebrate evolution and, if retained in humans, that variations in the AVP system may be related to individual differences in sociality in our own species, particularly aggressive (2) and/or affiliative (3, 4) tendencies. It has even been hypothesized that extreme variations in the AVP system may be related to the social dysfunctions associated with autism (5–7).
Among the social processes most consistently influenced by AVP and AVT in animal models are those related to social communication, particularly the generation of, and/or responses to, stereotypical signals associated with courtship and aggression. In humans, facial expressions are associated with such forms of emotional communication (8), so we predicted that AVP would influence the motor patterns associated with facial responses to social stimuli, particularly in men, because AVP/AVT systems are most often associated with the regulation of male-typical patterns of social communication (1). Indeed, in a preliminary study, we showed that electromyographic (EMG) responses of the corrugator supercilii, which are associated with threat (9), are increased in men by intranasal AVP administration (10). However, we do not yet know whether AVP also modulates social communication in women, which is an intriguing possibility because sexual dimorphisms typical of AVP/AVT systems in other species in which males have more AVP/AVT-producing cells and/or fiber projections have not been detected in human brains (11). We also do not yet know whether AVP can influence social cognition as well as facial motor patterns related to social communication, nor anything about the mechanisms through which AVP may affect social communication in humans.
To address these fundamental questions and to determine how rapidly social processes are affected by intranasal AVP administration, which leads to peptide elevations in cerebrospinal fluid (CSF) within 10 min of delivery that remain for at least 80 min (12), we gave men and women intranasal AVP or saline and tested their facial EMG, heart rate (HR), and skin conductance (SC) responses to pictures of same-sex models posing various facial expressions of emotion 15 and 50 min after drug administration. Subjects also rated the approachability/friendliness of the faces and completed a state anxiety inventory at the end of the experiment.
Results
AVP administration had sex-specific effects on corrugator EMG responses to same-sex faces 15 min after drug administration, as evidenced by a significant Gender × Drug interaction [F(1,68) = 8.95, P = 0.004]. We therefore analyzed the sexes separately. In men, AVP tended to increase corrugator EMG responses to facial stimuli [Drug main effect: F(1,36) = 3.77, P = 0.06]. Planned, follow-up comparisons showed, as we had previously observed (10), that emotionally neutral faces were the only stimuli to elicit significantly larger EMG responses in men given AVP than in men given saline [F(1,36) = 4.68, P = 0.04; Fig. 1A]. In women, however, intranasal AVP significantly reduced corrugator EMG responses to facial stimuli [Drug main effect: F(1,32) = 5.01, P = 0.03]. Planned follow-up comparisons showed that AVP significantly inhibited responses to happy [F(1,32) = 5.31, P = 0.03] and angry [F(1,32) = 4.33, P = 0.045), but not neutral [F(1,32) = 2.45, P = 0.13] faces (Fig. 1B).
Fig. 1.
Mean and SEM of stimulus-induced changes in corrugator EMG activity in men (A; saline, n = 18; AVP, n = 20) and women (B; saline, n = 18; AVP, n = 20) 15 min after drug administration. AVP significantly increased responses to neutral faces in men, whereas it significantly decreased responses to happy and angry faces in women (∗, P < 0.05).
For the second set of stimulus presentations, 50 min after drug administration, there were no significant Drug main effects or interactive effects between Drug and Gender and/or Face. To examine whether AVP effects may have been masked by a change in reactivity as a result of repeated testing, we compared responses to each class of facial expression between the two sets of stimulus presentations within control subjects. Responses to all stimuli tended to increase across stimulus sets in control men, but only responses to neutral images, which were also the only responses affected by AVP on the first stimulus set, increased significantly [t(17) = 2.23; P = 0.03; Fig. 2]. There were no significant changes across stimulus sets in control women.
Fig. 2.
Mean and SEM of corrugator EMG responses in control men on the two stimulus sets. Responses to neutral faces were significantly larger on set 2 than set 1 (∗, P < 0.05).
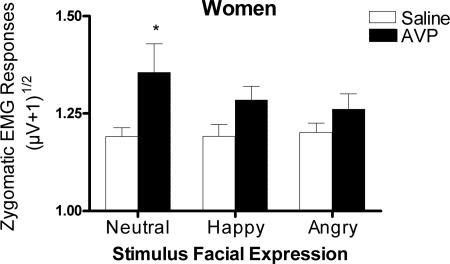
There were no significant Drug main effects or Drug × Gender interactions for zygomaticus responses on the first or second stimulus sets. However, we had specifically hypothesized that AVP would stimulate zygomaticus responses associated with smiling in women, but not in men, based on the argument by Taylor et al. (13) that women exhibit affiliative behaviors in contexts of social anxiety, which we predicted AVP administration would create (see below). We therefore analyzed women’s zygomaticus responses separately from those of men, despite the lack of a significant interaction in the original mixed model, because interaction effects are statistically more difficult to detect than main effects (14). There were no effects of AVP 15 min after drug administration; however, AVP did significantly increase zygomaticus responses in women 50 min after drug administration [Drug main effect, F(1,34) = 6.19, P = 0.02]. Planned follow-up comparisons between women given AVP and saline showed that responses to neutral stimuli were significantly increased by AVP [F(1,34) = 4.94, P = 0.03; Fig. 3], and responses to happy faces were marginally increased [F(1,34) = 4.11, P = 0.051].
Fig. 3.
Mean and SEM of zygomaticus major EMG responses to faces in women on the second stimulus set, 50 min after drug administration (saline, n = 19; AVP, n = 17). AVP significantly increased zygomaticus responses to neutral faces (∗, P < 0.05).
Zygomaticus responsiveness did not change significantly across stimulus sets in control women. However, Levine tests revealed that variances for responses to angry [F(18,18) = 3.76, P = 0.004] and neutral [F(18,18) = 2.57, P = 0.03] faces were significantly higher on the first stimulus set than on the second and tended to be higher for responses to happy faces [F(18,18) = 1.93, P = 0.09], which could be why we were unable to detect AVP influences on the first stimulus set.
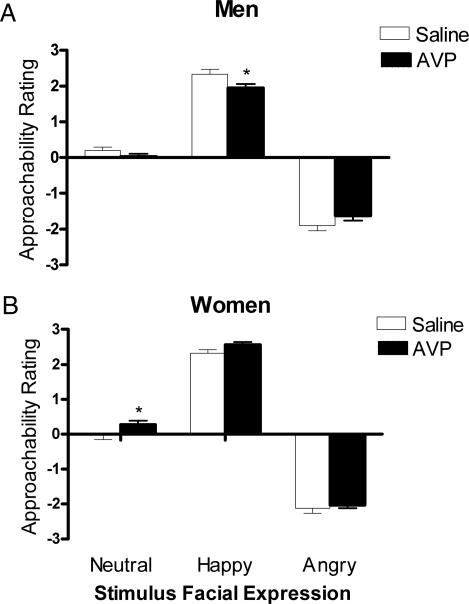
There was a significant Drug × Gender interaction [F(1,65) = 8.65, P = 0.005] and a significant Drug × Gender × Face interaction [F(2,130) = 3.15, P = 0.046], for perceptual ratings of the friendliness/approachability of faces on the first stimulus set, indicating that AVP differentially affected the ratings of particular types of emotional expressions in men and women. We therefore analyzed the sexes separately. In men, AVP uniquely affected responses to different facial expressions [Drug × Face interaction: F(2,70) = 3.7, P = 0.03], significantly reducing approachability ratings of happy faces [F(1,35) = 4.42, P = 0.04; Fig. 4A). In women, there was a significant Drug main effect [F(1,30) = 13.68, P = 0.001]. Planned comparisons indicated that the effect was strongest toward neutral expressions, which were the only ones rated as significantly more friendly/approachable by women given AVP than by women given saline [F(1,30) = 9.8, P = 0.004; Fig. 4B], although AVP also tended to increase ratings of happy expressions [F(1,30) = 3.6, P = 0.07]. On the second stimulus set, a similar (but not significant) Drug × Gender × Face interaction was observed [F(2,122) = 2.74, P = 0.085]. There were no significant changes in ratings across stimulus sets in control men or women.
Fig. 4.
Mean and SEM of approachability ratings of faces on set 1 in men (A; saline, n = 18; AVP, n = 19) and women (B; saline, n = 16: AVP, n = 16). AVP significantly reduced ratings of happy faces in men and significantly increased ratings of neutral faces in women (∗, P < 0.05).
AVP marginally increased SC responses to faces on the first set of stimulus presentations [Drug main effect: F(1,70) = 3.81, P = 0.055]. Because there was not a significant Drug × Gender interaction and we had no a priori expectations of sex-specific effects of AVP on SC responsiveness, men and women were not analyzed separately. Planned follow-up tests revealed that AVP significantly increased SC responses to angry faces [F(1,70) = 4.18, P = 0.045; Fig. 5A] and tended to increase SC responses to neutral faces [F(1,70) = 3.18, P = 0.06]. There were no main or interactive effects of AVP on the second stimulus set, perhaps because repeated testing changed SC responsiveness. SC responses to angry expressions, which were the only responses affected by AVP on the first set, decreased significantly across sets in control subjects [t(36) = 2.71, P = 0.01]. Responses to neutral and happy expressions did not.
Fig. 5.
Mean and SEM of SC responses to faces on set 1 (A; saline, n = 37; AVP, n = 37) and of minimum HR responses to faces on set 2 (B; saline, n = 30; AVP, n = 33) in men and women combined. AVP significantly increased SC responses to angry faces and significantly reduced HR decelerations to angry faces (∗, P < 0.05).
There were no main or interactive effects of AVP on minimum HR responses on the first stimulus set, but on the second, 50 min after drug administration, there was a marginal effect of AVP [Drug main effect: F(1,59) = 3.87, P = 0.054]. Again, the Drug × Gender interaction was not significant, and we had not predicted differential effects of AVP on HR in men and women, so both sexes were analyzed together. Planned follow-up comparisons revealed that AVP significantly reduced the magnitude of the deceleration response to angry faces [F(1,59) = 5.07, P = 0.03; Fig. 5B] but did not significantly affect responses to neutral or happy faces. There were no significant changes in minimum HR responses to any of the facial expressions in control subjects or in the variances of the responses across stimulus sets that could explain why AVP effects on HR decelerations were detected only on the second stimulus set. There were no main or interactive effects of AVP on mean or maximum (max) HR responses on either set.
On a test given after subjects viewed both stimulus sets, AVP significantly increased state anxiety [Drug main effect: F(1,70) = 6.18, P = 0.01; mean and SEM for control subjects, 29.87 ± 0.92; mean and SEM for AVP subjects, 33.89 ± 1.3]. Men and women were not analyzed separately because the Drug × Gender interaction was not significant and we had not predicted differential effects of AVP on anxiety in the sexes. AVP did not affect resting blood pressure 30 or 60 min after drug administration.
Discussion
Intranasally delivered AVP differentially affected social communication processes in men and women, including facial motor patterns associated with emotional communication and cognitive social evaluations. Specifically, AVP promoted agonistic responses in men and affiliative responses in women toward the faces of same-sex models. AVP also affected autonomic responsiveness to threatening social stimuli and increased anxiety, which may underlie the peptide’s sex-specific effects on social communication by promoting different social strategies in response to stress in the sexes. Because intranasal AVP administration crosses the blood–brain barrier (12) and, at the dose we used, directly affects central processes, whereas peripheral elevations do not (15), we argue that the effects we observed were likely centrally mediated, either through CSF-signaling mechanisms or by means of diffusion into discrete brain areas. Thus, our results support the hypothesis that central AVP’s ability to influence social communication processes, a conserved trait of AVT and AVP in vertebrates, has been retained in humans.
Sex-Specific Effects on Social Communication.
As we had previously shown (10), in men, AVP enhanced EMG responses of the corrugator supercilii, a facial muscle above the brow whose activity is related to anger and threat (9), to emotionally neutral/ambiguous facial expressions. Here, we also show that AVP simultaneously affects cognitive social perceptions, decreasing friendliness/approachability ratings of unfamiliar men displaying happy, affiliative expressions. Men with high levels of endogenous AVP may therefore be unlikely to engage in prosocial interactions with other men and likely to generate threatening expressions in the interactions in which they do engage. Because facial muscle activity can feed back to influence emotional states (16), such increased corrugator EMG activity could elevate anger in such individuals during social interactions and thereby increase the likelihood of initiating aggressive behavior. It might also increase the likelihood of eliciting aggressive responses from others as a result of having sent such an agonistic signal. Such mechanisms could, at least in part, explain the positive correlation between levels of AVP in the CSF of men with personality disorders and aggressive life histories (2).
In contrast, in women, intranasal AVP inhibited corrugator responses to the faces of unfamiliar women, stimulated zygomaticus responses associated with smiling, and enhanced perceptions of the friendliness/approachability of the pictured women. Although the time at which AVP affected zygomaticus responses (50 min) differed from that at which it affected corrugator and perceptual responses (15 min; see further discussion below), the overall pattern is consistent; in women, AVP seems to promote affiliative responses toward other women. Similarly, AVP stimulates affiliative responses toward females and pups in male prairie voles (17, 18) and male-directed affiliative behaviors in female prairie voles (19).
Potential Mechanisms.
Although AVP increased SC responses and reduced cardiac decelerations to angry faces at different time points after drug administration, together these effects suggest that AVP increases autonomic responsiveness to threatening social stimuli. Also, either in response to feedback from those peripheral changes and/or the result of direct actions within the brain, AVP increased state anxiety. Central AVP likewise has been implicated in anxiogenic processes in rodents (20, 21). It is therefore possible that AVP’s effects on human social communication were indirect consequences of primary actions on autonomic responsiveness to social stimuli and/or anxiety. In fact, such indirect behavioral mechanisms could be common in vertebrates; Porges (22) has argued that an ancestral mechanism through which AVP and AVT affect social behavior is by stimulating sympathetic outflow associated with “fight or flight” responses. The peripheral changes associated with increased sympathetic activation in response to social stimuli could feed back to increase a central state of anxiety that promotes aggression or withdrawal, depending on the social context, or direct anxiogenic actions of AVT/AVP within the brain could be part of the same, potentially ancestral mechanism.
But why would increased anxiety promote affiliative responses in women? Taylor et al. (13) have hypothesized that different selective pressures associated with reproduction and group living on men and women may have led to the evolution of different stress-response strategies in the sexes. Accordingly, fight or flight responses, as discussed above, evolved in men to aid survival in the event of predatory or social attack, whereas an alternative “tend and befriend” strategy evolved in women caregivers for whom the risks to offspring and self associated with fight or flight responses were too great to be maintained. Taylor argues that in women the tendency to affiliate with other women in times of stress therefore evolved, because such alliances could aid in childcare and/or provide shelter from attack. Using Taylor’s evolutionary framework, we propose that AVP may have induced a central state associated with stress, anxiety, which prompted men to display agonistic response patterns toward other men consistent with fight or flight tendencies and women to display affiliative response patterns toward other women consistent with tend and befriend tendencies
It is also possible that AVP’s effects on autonomic, motor, and psychological responses each resulted from independent peptide actions in different brain regions. If so, the orthogonal effects of AVP on social communication patterns in men and women may be the result of sex differences in receptor distributions in the brain, such that AVP directly activates specific fight or flight circuits in men and tend and befriend circuits in women. Alternatively, AVP may influence both types of circuit in men and women, but the observable manifestations of those influences depend upon the sex of the stimulus person. Future studies could address this possibility by testing the effects of AVP on responses to opposite-sex models.
Such AVP “social circuits” have been identified in several animal models; AVP modulates aggression through actions in the septum, anterior hypothalamus, and central gray (23–25), and, at least in male prairie voles, it modulates affiliative responses related to parental and pair-bonding behaviors by means of actions in the septum (18, 26) and ventral pallidum (27). Although AVP actions in some areas like the septum may be associated with influences on aggressive and affiliative behaviors, it is probable that unique patterns of AVP receptor activation across several brain regions are associated with the modulation of each. Additionally, AVP promotes affiliative behaviors in female prairie voles (19), although the site(s) of action for this effect has not been determined, nor has it been shown that AVP can stimulate females to exhibit affiliative behaviors toward other adult females in that species. Nonetheless, it is possible that AVP circuits modulating agonistic and affiliative responses in humans are the same as those associated with the regulation of related behaviors in other vertebrates. In fact, humans have an extended promoter region on the AVP receptor gene that is similar to one that has been linked to expression patterns of the AVP receptor and sociality in prairie voles (28). If future experiments demonstrate that AVP increases affiliative responses in men toward women, then it would support this hypothesis by showing that, as in male prairie voles, AVP simultaneously enhances agonistic responses toward other males and affiliative responses toward females.
Finally, it could be argued that AVP’s effects in men reflect the activation of AVP “agonistic circuits,” but that the peptides effects on affiliative responses in women were the result of pharmacological oxytocin (OT) receptor activation, because OT stimulates affiliative behaviors in females in other mammals (29). However, this explanation seems unlikely because OT has anxiolytic effects in humans (30), and AVP administration elevated anxiety in the present study.
Time Course Considerations.
AVP affected autonomic, motor and perceptual responses as early as 15 min after drug administration, which is consistent with the speed at which elevations of the peptide are observed in the CSF after intranasal administration (12). Unexpectedly, none of the responses affected by AVP on the first stimulus set were affected on the second, 50 min after drug administration, although elevations of the peptide in CSF remain for at least 80 min after intranasal delivery. We were especially surprised that AVP did not influence corrugator responses to neutral faces in men on the second stimulus set, 50 min after drug administration, because AVP did enhance those responses at that time point in our previous study (10). However, in our previous study, subjects were tested only once, and, in the present study, control men’s corrugator responses to neutral faces increased across stimulus sets, mirroring the effects of exogenous AVP on the first stimulus set. One interpretation of this finding is that exposure to faces, most likely angry/threatening ones, stimulated endogenous AVP release within men’s brains, and that additional, exogenous AVP therefore produced no further effects on the second stimulus set. Consistent with this hypothesis, agonistic social stimuli can stimulate AVP release within the brains of male rats (31). If true, and if elevated AVP does increase the likelihood of initiating aggressive behavior, such socially induced release could lead to escalations of aggression during social challenges in men.
We also observed changes in SC responsiveness across stimulus sets that could have made it difficult to detect AVP influences on the second stimulus set, 50 min after drug administration; SC responses to angry faces, the only ones affected by AVP on set 1, habituated across stimulus sets. Although we did not observe changes across stimulus sets in corrugator responsiveness in women or perceptual ratings in either sex, it remains possible that some undetected effect of repeated testing may have also prevented us from observing AVP influences on those responses on the second stimulus set.
On the other hand, AVP influenced zygomaticus and cardiac responses on the second set, but not the first. The inability to detect influences on women’s zygomaticus responses on the first stimulus set may have been because occasional laughter or smiling on that set, before subjects became familiar with the stimuli, some of which were obviously outdated and reported to be humorous by subjects after the experiment, increased response variability and thus obscured the effects of AVP. Additionally, AVP may have had stronger effects 50 min after delivery than 15 min after delivery, at least on responses for which a masking effect of repeated testing was not present, because AVP levels in CSF do increase slightly across that time span after intranasal administration (12). Additional time could have also allowed greater diffusion into discrete brain areas where AVP may induce its effects. Increasing efficacy across time may also explain why we detected AVP influences on HR decelerations only on the second stimulus set. In sum, changes in stimulus responsiveness as a result of repeated testing and/or changes in drug efficacy as a function of time likely account for AVP’s mixed pattern of effects across stimulus sets.
It should also be noted that AVP affected SC and HR in the present study, whereas it did not in our previous study (10). We believe we did not observe the same effects in our previous study because subjects in that study performed a Stroop task while looking at the pictures, which likely induced cognitive demands that independently influenced these variables and thus obscured AVP’s effects, which seem very sensitive to the testing parameters.
Conclusions.
We have shown that, in humans as in numerous other vertebrates, AVP has the ability to influence social processes, particularly those related to emotional social communication. Furthermore, AVP’s effects are sex-specific, promoting agonistic and affiliative types of responses toward same-sex faces in men and women, respectively. Our results thus support the possibility that variations in the AVP system could be related to individual differences in human sociality, particularly aggressive and affiliative tendencies, and possibly even to the abnormalities in emotional communication observed in some cases of autism. Finally, our results indicate that any psychiatric interventions targeting the AVP system should take into account the possibility of different behavioral outcomes in men and women.
Methods
Subjects.
Participants were men and women 17–25 years old. Potential subjects were prescreened for medical conditions and prescription or recreational drug use. A urine drug test verified the absence of drugs. Subjects were medically cleared for participation by a physician, and females were given pregnancy tests to make sure they were not pregnant.
The experiment was conducted in the health center at Bowdoin College. Participants were provided with an informed consent form and told that they could leave the study at any time. All procedures complied with guidelines for the ethical use of human subjects set by Bowdoin College and the National Institutes of Health.
Drug Administration.
Under double-blind conditions, a 1-ml solution containing 20 units of AVP (American Reagent Laboratories, Shirley, NY), which is ≈50 μg (n = 37), or sterile saline (n = 37) was self-administered by means of intranasal sprays over a 2-min period in the presence of the experimenter. This dose leads to elevations in plasma equivalent to those produced by an i.v. dose of only 0.025 units, which does not produce central effects (15). There were no reports of dried nasal passages or increased water retention during the test.
Stimulus Presentation.
Participants were seated in a dimly lit room, and images were projected onto a computer monitor by using a customized version of human measurement system 7 (Coulborn Instruments, Lehigh Valley, PA). Ten minutes after inhaling saline or AVP, subjects were shown four practice images (opposite sex neutral faces). During the experiment, subjects viewed two sets of pictures, each consisting of 27 faces depicting various emotions. Faces were taken from Ekman and Friesen (32), Ekman and Matsumoto (33), Gur et al. (34), and a set of images produced and validated in our laboratory. Of the 27 faces constituting each picture set, 9 were emotionally neutral, 9 were angry, and 9 were happy. Images were displayed in random order, and a different set was shown on the first and second stimulus sets, in counterbalanced order across subjects. Men viewed only pictures of male faces, and women viewed only female faces. Pictures were displayed for 8 s, and each image was separated by a variable 25- to 30-s interval of blank computer screen. Prestimulus baseline physiological measurements were obtained during the 5 s preceding each picture. During the picture display, data were collected for 5 s beginning 500 ms after picture onset. After each image, subjects rated its perceived approachability/friendliness on a scale from −3 (threatening, very unapproachable) to +3 (friendly, very approachable). The first set of faces was shown 15 min after drug administration, and the second set was shown 50 min after drug administration. Participants viewed part of a nature film with an emotionally neutral content between stimulus sets. After viewing both sets, subjects filled out the State portion of the State/Trait Anxiety Inventory (STAI; Consulting Psychologists Press, Palo Alto, CA).
Physiological Measures.
Physiological measurements included EMG activity of the left corrugator supercilii and zygomaticus major facial muscles, SC, and HR. Facial electromyograms were recorded by using 4-mm surface electrodes, and EMG activity was amplified with a bandpass filter (90–1,000 Hz). EMG activity was integrated by a multifunction integrator with a time constant set to 200 ms. SC electrodes were placed on the palm of the nondominant hand. SC measurements were recorded directly by using an isolated skin conductance coupler, with a constant of 0.5 V. HR was measured through standard ECG leads connected to an isolated bioamplifier with bandpass filter. Physiological signals were digitized by a general purpose Lablinc Port (Coulborn Instruments) and processed by using a Cobalt computer. Blood pressure and body temperature were taken before drug administration and 30 and 60 min after drug administration.
Physiological Response Scores.
EMG and SC responses to the faces were calculated by subtracting the mean score during the 5-s prestimulus baseline from the peak score during the first 5 s of the stimulus presentation. HR responses were calculated by subtracting the mean score during the 5-s prestimulus baseline from the mean, maximum, and minimum scores during the first 5 sec of stimulus presentation. Mean responses for each stimulus set were calculated by averaging responses to all faces within each emotional category. Individual EMG responses >3 SDs from the mean of a given subject’s responses across all stimuli on both stimulus sets were removed. Because of positive skew, EMG and SC responses were square root transformed. Perceptual rating scores for one woman were dropped because her average rating score of neutral faces fell 4 SDs from the overall mean rating score of neutral faces for all subjects across both trials.
Data Analysis.
Initial mixed-model ANOVAs were run for all variables with Drug and Gender as between-groups factors and Face (neutral, happy, angry) as a within-subjects factor. Separate, repeated-measures ANOVAs for men and women were done in cases where significant Drug × Gender interactions were present in the original mixed model or there was an a priori prediction in males or females, given that interaction effects are statistically more difficult to observe than main effects (14). Planned follow-up comparisons of responses to different facial expressions were performed between subjects given AVP and saline when significant main effects of Drug were observed, even when significant Drug × Face interactions were not evident, for the same statistical reason mentioned above. These tests also determined whether we replicated specific findings from our previous study (10). Paired t tests were used to compare responses across the trials to the different stimuli in each control group to assess whether there were changes in responsiveness, that occurred as a result of repeated testing, that were independent of AVP administration.
Acknowledgments
We thank E. Adkins-Regan and S. Lovett for helpful comments on an earlier version of this manuscript and Kathy Miller for pharmaceutical assistance. This work was supported by National Institutes of Health Grant R03MH063996-01A
Abbreviations
- AVP
arginine vasopressin
- AVT
arginine vasotocin
- EMG
electromyographic
- CSF
cerebrospinal fluid
- HR
heart rate
- SC
skin conductance.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Goodson J. L., Bass A. H. Brain Res. Brain Res. Rev. 2001;35:246–265. doi: 10.1016/s0165-0173(01)00043-1. [DOI] [PubMed] [Google Scholar]
- 2.Coccaro E. F., Kavoussi R. J., Hauger R. L., Cooper T. B., Ferris C. F. Arch. Gen. Psychiatry. 1998;55:708–714. doi: 10.1001/archpsyc.55.8.708. [DOI] [PubMed] [Google Scholar]
- 3.Insel T. R., Winslow J. T., Wang Z., Young L. J. Adv. Exp. Med. Biol. 1998;449:215–224. doi: 10.1007/978-1-4615-4871-3_28. [DOI] [PubMed] [Google Scholar]
- 4.Young L. J., Wang Z., Insel T. R. Trends Neurosci. 1998;21:71–75. doi: 10.1016/s0166-2236(97)01167-3. [DOI] [PubMed] [Google Scholar]
- 5.Insel T. R., O’Brien D. J., Leckman J. F. Biol. Psychiatry. 1999;45:145–157. doi: 10.1016/s0006-3223(98)00142-5. [DOI] [PubMed] [Google Scholar]
- 6.Wassink T. H., Piven J., Wassink T. H., Piven J., Vieland V. J., Pietila J., Goedken R. J., Folstein S. E., Sheffield V. C. Mol. Psychiatry. 2004;9:968–972. doi: 10.1038/sj.mp.4001503. [DOI] [PubMed] [Google Scholar]
- 7.Lim M. M., Bielsky L. F., Young L. J., Wassink T. H., Piven J., Vieland V. J., Pietila J., Goedken R. J., Folstein S. E., Sheffield V. C., et al. Int. J. Dev. Neurosci. 2005;23:235–243. [Google Scholar]
- 8.Ekman P. Am. Psychol. 1993;48:384–392. doi: 10.1037//0003-066x.48.4.384. [DOI] [PubMed] [Google Scholar]
- 9.Jancke L. Int. J. Psychophysiol. 1996;23:207–214. doi: 10.1016/s0167-8760(96)00062-1. [DOI] [PubMed] [Google Scholar]
- 10.Thompson R., Gupta S., Miller K., Mill S., Orr S. Psychoneuroendocrinology. 2004;29:35–48. doi: 10.1016/s0306-4530(02)00133-6. [DOI] [PubMed] [Google Scholar]
- 11.Fliers E., Guldenaar S. E., van de Wal N., Swaab D. F. Brain Res. 1986;375:363–367. doi: 10.1016/0006-8993(86)90759-6. [DOI] [PubMed] [Google Scholar]
- 12.Born J., Lange T., Kern W., McGregor G. P., Bickel U., Fehm H. L. Nat. Neurosci. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- 13.Taylor S. E., Klein L. C., Lewis B. P., Gruenewald T. L., Gurung R. A., Updegraff J. A. Psychol. Rev. 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- 14.McClelland G. H., Judd C. M. Psych. Bull. 1993;114:276–390. doi: 10.1037/0033-2909.114.2.376. [DOI] [PubMed] [Google Scholar]
- 15.Pietrowsky R., Struben C., Molle M., Fehm H. L., Born J. Biol. Psychiatry. 1996;39:332–340. doi: 10.1016/0006-3223(95)00180-8. [DOI] [PubMed] [Google Scholar]
- 16.Soussignan R. Emotion. 2002;2:52–74. doi: 10.1037/1528-3542.2.1.52. [DOI] [PubMed] [Google Scholar]
- 17.Winslow J. T., Hastings N., Carter C. S., Harbaugh C. R., Insel T. R. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z., Ferris C. F., De Vries G. J. Proc. Natl. Acad. Sci. USA. 1994;91:400–404. doi: 10.1073/pnas.91.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho M. M., DeVries A. C., Williams J. R., Carter C. S. Behav. Neurosci. 1999;113:1071–1079. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- 20.Landgraf R., Gerstberger R., Montkowski A., Probst J. C., Wotjak C. T., Holsboer F., Engelmann M. J. Neurosci. 1995;15:4250–4258. doi: 10.1523/JNEUROSCI.15-06-04250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bielsky I. F., Hu S. B., Szegda K. L., Westphal H., Young L. J. Neuropsychopharmacology. 2004;29:483–493. doi: 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- 22.Porges S. W. Int. J. Psychophysiol. 2001;42:123–146. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- 23.Ferris C. F., Delville Y., Irvin R. W., Potegal M. Physiol. Behav. 1994;55:755–759. doi: 10.1016/0031-9384(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 24.Albers H. E., Cooper T. T. Peptides. 1995;16:269–273. doi: 10.1016/0196-9781(94)00188-x. [DOI] [PubMed] [Google Scholar]
- 25.Goodson J. L., Adkins-Regan E. J. Neuroendocrinol. 1999;11:19–25. doi: 10.1046/j.1365-2826.1999.00284.x. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y., Curtis J. T., Wang Z. Behav. Neurosci. 2001;115:910–919. doi: 10.1037//0735-7044.115.4.910. [DOI] [PubMed] [Google Scholar]
- 27.Pitkow L. J., Sharer C. A., Ren X., Insel T. R., Terwilliger E. F., Young L. J. J. Neurosci. 2001;21:7392–7396. doi: 10.1523/JNEUROSCI.21-18-07392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammock E. A, Young L. J. Science. 2005;308:1630–1634. doi: 10.1126/science.1111427. [DOI] [PubMed] [Google Scholar]
- 29.Carter C. S., Williams J. R., Witt D. M., Insel T. R. Ann. N.Y. Acad. Sci. 1992;652:204–211. doi: 10.1111/j.1749-6632.1992.tb34356.x. [DOI] [PubMed] [Google Scholar]
- 30.Heinrichs M., Baumgartner T., Kirschbaum C., Ehlert U. Biol. Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- 31.Ebner K., Wojak C. T., Landgraf R., Engelmann M. Horm. Behav. 2005;47:14–21. doi: 10.1016/j.yhbeh.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Ekman P., Friesen W. V. Pictures of Facial Affect. Palo Alto, CA: Consulting Psychologists Press; 1976. [Google Scholar]
- 33.Ekman P., Matsumoto . Japanese and Caucasian Facial Expressions of Emotion (JACFEE)/Japanese and Caucasian Neutral Faces (JACNeuF) Oakland, CA: Paul Ekman; 1993. available at www.paulekman.com, CD-ROM. [Google Scholar]
- 34.Gur R. C., Sara R., Hagerndoorn M., Maron O., Hughett P., Marcy L., Turner T., Bejesy R., Posner A., Gur R. E. J. Neurosci. Methods. 2002;115:137–143. doi: 10.1016/s0165-0270(02)00006-7. [DOI] [PubMed] [Google Scholar]