Abstract
Sulfur (S) deprivation responses have been studied extensively in algae and land plants; however, little is known of the signals that link perception of S status to chloroplast gene expression. Here, we have compared the chloroplast S limitation response in WT vs. sac1 and sac3 sulfur acclimation mutants of the green alga Chlamydomonas reinhardtii. We provide evidence that in the WT, chloroplast transcriptional activity rapidly decreases after removal of S from the medium, leading to reduced transcript accumulation. This decrease correlates with reduced abundance of a σ70-like factor, Sig1, which is most likely the unique chloroplast transcription specificity factor. We further show that reduced transcription activity and diminished Sig1 accumulation are mediated by the SAC3 gene product, a putative Snf1-type Ser/Thr kinase previously shown to have both positive and negative effects on nuclear gene expression. Inclusion of the protein kinase inhibitor 6-dimethylaminopurine during S limitation yielded a pattern of expression that was largely similar to that seen in the sac3 mutant, lending support to the hypothesis that Sac3 kinase activation leads to transcriptional repression and Sig1 proteolysis. The finding that Sac3 regulates chloroplast gene expression suggests that it has a previously unknown role in integrating the S limitation response in multiple subcellular compartments.
Keywords: sigma factor, dimethylaminopurine, RNA polymerase
Sulfur (S) is a required macronutrient; it is a constituent of proteins, lipids, electron transport components, and many cell metabolites (1, 2). Most photosynthetic organisms have the capacity to assimilate S as a sulfate anion (SO42−) and translocate it to the plastid, where primary S metabolism takes place. In chloroplasts, S is first activated by ATP sulfurylase to form APS (5′-adenylyl sulfate), which is a branch-point intermediate. APS can be phosphorylated by APS kinase, and the product can be used in sulfation reactions or to synthesize cysteine and methionine (1). Chloroplasts are thus crucial in the assimilation of S, because ATP and photosynthetic reductant are needed for the first activation and reduction steps.
S insufficiency constitutes a serious stress situation for plants and algae, which respond with a series of metabolic adjustments (3, 4). Studies of S deprivation in the green alga Chlamydomonas reinhardtii have shown that limiting S leads to altered photosynthetic performance, reduced levels of photosynthetic proteins, and induction of genes encoding high-affinity S transporters (5, 6). S deprivation also leads to increased accumulation of arylsulfatase (ARS), which releases sulfate anion from esterified organic sulfates, allowing cells to access new sulfur stores (7). These acclimation responses are thought to prevent photodamage under conditions where the cell has limited capacity for metabolism of S-containing compounds, particularly proteins.
Several Chlamydomonas mutants that do not respond correctly to S deprivation have been identified based on their inability to regulate ARS1 expression. Among these mutants are sac1 and sac3 (in which “sac” indicates sulfur acclimation). The sac1 mutant is aberrant in most of the normal responses to S deprivation and dies rapidly under −S conditions. It is unable to synthesize ARS, exhibits abnormal S uptake, and does not down-regulate photosynthesis (8). The SAC1 gene encodes a protein with hydrophobic domains similar to Na+/SO42− ion transporters found in cell membranes of several organisms (8), but it has not been established whether it functions as an S transporter in Chlamydomonas. Unlike sac1, sac3 exhibits constitutive low-level expression of ARS under normal growth conditions, but, like WT cells, it increases ARS transcription under −S conditions. However, sac3 is unable to fully induce the high-affinity S transport system (9). Thus, SAC3 has both positive and negative effects on nuclear gene expression. It has been shown that SAC3 encodes a putative Ser/Thr kinase related to Snf1p of Saccharomyces cerevisiae (9); however, the substrates and/or activators of Sac3 are undefined.
Although S limitation has been correlated with altered chloroplast functions, little is known of the signals that link perception of S status to the chloroplast. Here, we have studied the chloroplast S stress response in Chlamydomonas, comparing the WT to sac1 and sac3. We provide evidence that chloroplast transcriptional activity decreases rapidly upon S limitation and that this response requires SAC3. Our data further suggest that Sac3 represses chloroplast transcription, at least in part through a proteolytic mechanism requiring phosphorylation activity. We conclude that Sac3 is a response regulator that integrates metabolism across cellular compartments.
Results
Abundance of Several Chloroplast mRNAs Decreases Rapidly Under S Starvation.
Studies in Chlamydomonas have shown that −S conditions induce a reduction in the rate of oxygen evolution, which correlates with a decrease in photosystem (PS) II active centers and reduced abundance of proteins of the photosynthetic apparatus (6, 8, 10). S deprivation was also linked to reduced accumulation of nuclear transcripts encoding proteins involved in PSI, PSII, light-harvesting complex, and photosynthetic electron transport machinery (11). Given the above observations, it was of interest to investigate whether under S limitation, chloroplast mRNA levels are adjusted as part of a global cellular response.
As a first step, the accumulation of several chloroplast mRNAs was measured under normal and −S conditions. To do so, WT cells were grown in S-replete medium, and then the culture was divided into two portions, one with S and one without. Samples were removed at various intervals, and, after 24 h, the starved culture was transferred back to S-containing medium for 2 h. Fig. 1 shows a representative RNA gel blot of the chloroplast genes atpB (encoding the β-subunit of ATP synthase), petD and petB (encoding subunit IV and cytochrome b6 of the cytochrome b6/f complex, respectively), and tufA (encoding elongation factor Tu). The results showed that although levels of chloroplast RNAs (cpRNAs) remained mainly unchanged under normal conditions, removal of S from the medium resulted in a decrease of 20–90% within 4–8 h and a decrease of 40–60% after 24 h. This decrease was reversible, and transcript abundance recovered to at or above the prestarvation levels after 2 h of S replenishment. This result contrasts with the accumulation of the nuclear ARS1 transcript, which is known to be induced under −S conditions (12). As shown in Fig. 1, ARS1 mRNA did not accumulate under +S conditions but increased rapidly under −S conditions, subsequently declining upon the replenishment of S. Thus, under conditions where the accumulation of some transcripts is increasing, several cpRNAs decrease in response to S stress, correlating with reduced photosynthesis.
Fig. 1.
cpRNA and ARS1 accumulation under normal and −S conditions. WT cells were grown to midlogarithmic phase in TAP medium, collected, resuspended in +S or −S medium, and incubated for 0, 4, 8, 12, or 24 h before harvesting. At the end, the S-starved culture was transferred back to S-containing medium for 2 h (SR). Five micrograms of total RNA isolated from the indicated samples was separated by electrophoresis, blotted onto a membrane, and hybridized initially with the probes shown on the left. The signals corresponding to cpRNAs were normalized to 25S rRNA, and the initial time point was set to 1.0. The numbers beneath each lane show the ratio of RNA accumulations for each track. Two filters were used, and the 25S reprobing of each is shown. The use of each of these filters is denoted by either one or two asterisks to the right of the experimental blots.
Sac3 Is Involved in Rapid Repression of Chloroplast Transcription Activity upon S Starvation.
The RNA gel blot data presented above revealed that cpRNA accumulation decreased under −S conditions. One way of assessing whether this phenomenon is a specific response to S limitation is to determine whether it is under genetic control. Therefore, cpRNA accumulation was explored after S deprivation in the sac1 and sac3 mutants, whose abnormal responses to S limitation were described above. Because cpRNA abundance decreases in WT cells after 4–8 h (Fig. 1), we focused on a relatively early part of the starvation response (i.e., the first 12 h). To confirm the S limitation condition, ARS1 mRNA was monitored. Consistent with previous reports, ARS1 was induced upon S deprivation in the WT and sac3 cells but not in sac1 cells (Fig. 2). In addition, low expression of ARS1 was detected in sac3 cells when grown in the presence of S (0-h time point), as previously reported.
Fig. 2.
Chloroplast and ARS1 RNA accumulation in WT cells, sac mutants, and complemented sac3 cells after S deprivation. Cells were grown to midlogarithmic phase in TAP medium, collected, resuspended in −S medium, and incubated for 0, 2, 4, 8, or 12 h before harvesting. RNA was isolated and analyzed as described in the legend of Fig. 1. Data were also analyzed collectively (see Fig. 7, which is published as supporting information on the PNAS web site).
When cpRNA accumulation was compared between strains, both the WT and sac1 cells exhibited decreases under −S conditions. The decrease appeared to be more rapid in sac1 cells, with 50–90% decreases in transcript levels at the earliest time point taken (2 h), contrasting with only minor changes in the WT. More striking was the constant or increasing cpRNA abundance in sac3 cells up to 12 h after starvation, suggesting that SAC3 function is required for depletion of cpRNA. To verify this observation, cpRNA levels were monitored in the same mutant that had been complemented (9) with the WT SAC3 gene (sac3::SAC3). This strain showed a WT pattern of ARS1 induction and decreased cpRNA accumulation under −S conditions, confirming the role of SAC3. Thus, chloroplast transcript reduction is SAC3-dependent and perhaps modulated directly or indirectly by SAC1.
Decreased cpRNA levels under −S conditions could result from changes in transcription rates and/or RNA stability. To assess the contribution of transcriptional changes, run-on assays were performed. In these experiments, chloroplast transcription activity was compared at time 0 (+S medium) and 8 h after starvation by using WT and sac3 cells permeabilized by freeze/thaw cycles, followed by a short incubation in the presence of [α-32P]UTP to extend previously initiated transcripts (13). RNAs were labeled and extracted identically from both strains in duplicate and then used as probes on DNA dot blots to estimate transcription rates of nine chloroplast genes encoding components of the photosynthesis and gene expression machineries.
Fig. 3 shows that after S starvation, radioactive incorporation was reduced into all cpRNAs examined in WT cells, indicating that decreased cpRNA abundance during S limitation results at least in part from reduced transcription activity. In contrast and consistent with the lack of cpRNA reduction under −S conditions, no decrease was observed in chloroplast transcription rates for sac3. This finding suggests that the putative Sac3 kinase mediates repression of chloroplast transcription under −S conditions. It should be noted, however, that our results do not rule out a contributory role of decreased transcript stability under −S conditions, which we have not measured directly. We were able to discount the possibility that reduced cpDNA copy number limits transcription under −S conditions, because cpDNA abundance does not change under −S conditions in WT cells (S. Yehudai-Resheff and D.B.S., unpublished data).
Fig. 3.
Changes in transcription rates for chloroplast-encoded genes in WT cells and sac3 mutants after S deprivation. Cells were grown to midlogarithmic phase in TAP medium, collected, and resuspended in −S medium for 8 h. Cells (5 × 107) were removed at 0 and 8 h after starvation and permeabilized for run-on transcription. All of the 32P-labeled transcription products were hybridized to DNA dot blots (in duplicate) containing 0.2 μg (0.5×) or 0.4 μg (1×) of PCR products as shown. pBluescript plasmid DNA was used as a negative control. Dot blots were hybridized, washed, and exposed under identical conditions and visualized by using the Storm scanner.
The Chloroplast S Limitation Response Is Regulated by Phosphorylation.
Previous analysis of the sac3 mutant demonstrated that SAC3 has both positive and negative effects on nuclear gene expression. Here, our results indicate that SAC3 can regulate chloroplast transcription. Because SAC3 encodes a putative kinase, it can be envisaged that this regulation takes place by means of activation of the Sac3 kinase under −S conditions. As one approach to test this hypothesis, we carried out experiments in the presence of a general kinase inhibitor, 6-dimethylaminopurine (DMAP) (14), previously used in Chlamydomonas (15).
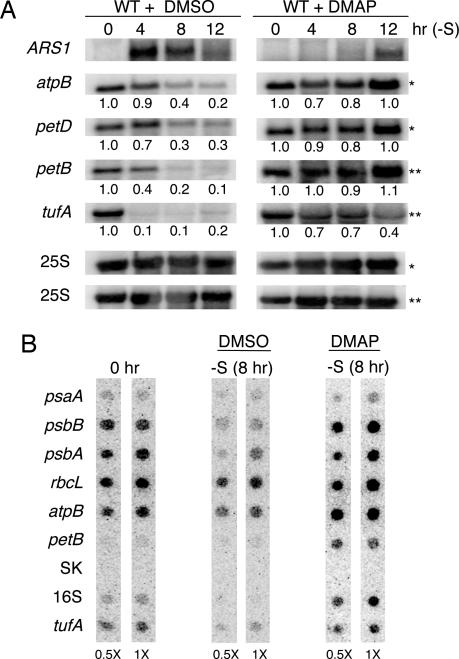
When cpRNA accumulation was examined under −S conditions, the addition of DMAP was found to prevent or mitigate the decrease in cpRNA levels during S starvation (Fig. 4A), consistent with the notion that Sac3 kinase activity represses transcription. On the contrary, cpRNA levels decreased 70–90% over 12 h in the control experiment, in which the solvent DMSO was used. To see whether DMAP affected chloroplast transcription activity, run-on assays were performed, and, as shown in Fig. 4B, −S conditions resulted in reduced transcription in the DMSO control but not in the presence of DMAP. Together, these results provide in vivo evidence that protein kinase activity is necessary to reduce cpRNA accumulation under −S conditions. It was also noted that in the presence of DMAP, ARS1 mRNA levels increased only after 12 h, in contrast to the increase seen after 4 h in the control experiment (Fig. 4A), suggesting that phosphorylation, although not necessarily mediated by Sac3, also regulates ARS1 derepression under S limitation.
Fig. 4.
Effects of DMAP on transcription and RNA accumulation. (A) Chloroplast and ARS1 RNA accumulation after S deprivation in the presence of DMAP. Cells were grown to midlogarithmic phase in TAP medium and then resuspended in TAP-S with 3 mM DMAP or an equal volume of DMSO as a control. Samples were taken at the time the kinase inhibitor was added (0) and 4, 8, and 12 h after starvation. Isolated RNA was analyzed as described in the legend of Fig. 1. Data were also analyzed collectively (Fig. 7). (B) Run-on transcription was performed as described in the legend of Fig. 3, except DMSO or 3 mM DMAP was added as shown.
Sac3 May Deactivate and/or Destabilize the Chloroplast Sigma Factor.
The above data support the concept that Sac3-mediated chloroplast transcriptional repression is stimulated under −S conditions. In land plants, chloroplast transcription is carried out by two distinct types of RNA polymerase (16). One is a bacterial-type enzyme whose catalytic subunits are encoded in the chloroplast (plastid-encoded polymerase, PEP), and the other is a phage-type enzyme encoded in the nucleus (nuclear-encoded polymerase, NEP). Chlamydomonas chloroplasts appear to possess only PEP (17), whose catalytic core consists of α-, β-, β′-, and β″-subunits. PEP additionally requires a sigma factor for promoter recognition, which is encoded by a gene family in land plants (18) but by a single gene in Chlamydomonas. This gene, RPOD, encodes the protein termed Sig1 or CrRpoD (19, 20). The fact that Chlamydomonas chloroplasts possess PEP but not NEP and that Sig1 apparently acts as the only specificity factor would appear to facilitate global control of chloroplast transcription activity.
Given the above observations, it was reasonable to hypothesize that under −S conditions, Sac3 kinase might alter RPOD expression, Sig1 stability, and/or Sig1 activity. To test the first possibility, RT-PCR analysis was performed to examine the accumulation of mRNAs encoding PEP subunits, including Sig1, after S deprivation. Fig. 5 shows that RPOD mRNA abundance exhibited only small variations both in WT and sac3 cells under these conditions. RT-PCR analysis was also performed for the chloroplast rpoA and rpoB1 genes, which encode the α- and β-subunits of PEP, respectively. This analysis revealed decreasing abundance of both transcripts in WT but not sac3 cells, an observation that is consistent with the notion that Sac3 mediates general chloroplast transcriptional repression under −S conditions.
Fig. 5.
RT-PCR analysis for genes encoding PEP subunits under −S conditions. rpoA and rpoB1 cDNAs were amplified with 30 PCR cycles, and RPOD cDNA was amplified with 35 cycles. Actin mRNA was used as an internal standard, using 30 PCR cycles.
Because chloroplast transcription appeared to be regulated independently of RPOD transcript abundance, it was of interest to determine whether Sig1 might be a target of regulation. To do so, immunoblot analysis was performed by using an anti-peptide antibody that recognizes a single polypeptide in total protein and chloroplast isolates of ≈85 kDa, corresponding in size to Sig1 (20). Fig. 6A shows immunoblot analysis of total protein collected at various intervals after S deprivation, revealing that at 12 h after starvation Sig1 abundance declines to between 20% and 50% of its initial level in WT cells with α-tubulin as a reference. The sac3 mutant, however, retained, on average, the initial amount of Sig1 even after 12 h of starvation. Furthermore, as shown in Fig. 6B, addition of DMAP to WT cells, when transferred to −S, prevented the decline in Sig1 as compared with the tubulin control. In contrast, Sig1 was reduced upon S limitation in the control experiment, in which DMAP was omitted. These results indicate that increased proteolysis and/or reduced synthesis of Sig1 occurs after S limitation and that Sac3 may be involved in a signaling cascade targeting Sig1 for degradation.
Fig. 6.
Immunoblot analysis of Sig1 under −S conditions. Total proteins (7.5 μg) from times 0, 4, 8, and 12 h after starvation from WT and sac3 cells (A) and WT cells treated with DMAP or DMSO (B) were separated in SDS-polyacrylamide gels and transferred to nitrocellulose. Blots were probed first with anti-Sig1 and then with anti-α-tubulin. Densitometric quantification of Sig1 and tubulin levels is shown. The relative values (from time 0) represent the mean ± SD of two independent experiments.
Discussion
Photosynthetic organisms use different strategies to manage S limitation. Although several aspects of Chlamydomonas S deprivation have been studied (10, 21), little has been learned of the molecular or genetic links between perception of S limitation and chloroplast responses. The results shown here indicate rapid repression of plastid transcription activity after removal of S from the culture medium and provide evidence that a SAC3-encoded protein kinase is involved in this process. Reduced chloroplast transcription correlated with reduced abundance of Sig1, the single Chlamydomonas PEP sigma factor, a phenomenon that also requires kinase activity. In sum, the present study assigns an unanticipated role for the Sac3 Ser/Thr kinase in regulating S limitation responses.
Upon S deprivation, the abundance of at least six cpRNAs decreases, with a 40–90% reduction observed, depending on the gene and experiment. Gel blot analysis also showed that the decreased abundance of cpRNA during S limitation requires SAC3 function but that SAC1 has, at most, a minor role. cpRNA levels declined more abruptly for some genes in S-deprived sac1 cells, mirroring what occurs for nucleus-encoded transcripts encoding photosynthesis components (11). It should be taken into consideration, however, that sac1 is very sensitive to S limitation (8), failing to survive when grown under −S conditions in the light. Thus, any conclusions about a specific role of SAC1 in regulating chloroplast transcription should be made with caution. Double mutant analysis suggests that SAC1 and SAC3 act in parallel (22), which would argue against one regulating the other directly.
Analysis of chloroplast transcription rates under −S conditions revealed reduced cpRNA synthesis in WT cells but not in sac3 cells. Because all cpRNAs that were examined showed the same trend, S deprivation appears to globally affect transcription, consistent with the single type of RNA polymerase found in Chlamydomonas chloroplasts. Plastid transcription activity can also be globally regulated in plants, generally correlating with the developmental state (23–25). Although the mechanisms controlling general plastid transcriptional regulation are not well understood, in the case studied here, we have hypothesized that Sac3 kinase activity triggers transcriptional repression. This conclusion is supported by the fact that addition of a general kinase inhibitor prevented repression under −S conditions (Fig. 4).
Sac3-mediated transcriptional repression may have some parallels with PEP regulation in mustard, where in vitro studies identified two forms of PEP, A and B, which could be interconverted by (de)phosphorylation (26). PEP-B occurs in etioplasts and is associated with phosphorylated sigma factor, which is postulated to inhibit its release from the catalytic core. Light triggers dephosphorylation, activating transcription as chloroplasts mature (27). It was subsequently shown that sigma phosphorylation is carried out by a plastid transcription kinase (PTK) related to the α-subunit of nucleocytoplasmic casein kinase 2 (28). Another example of PEP regulation by phosphorylation is in barley, where in vivo experiments showed that protein kinase and extraplastidic Ser/Thr phosphatases regulate plastid transcription in response to light (29).
SAC3 encodes a putative Ser/Thr kinase of the SnRK2 (SNF1-related kinase 2) subfamily featuring a canonical kinase domain at the N terminus and a C-terminal acidic domain (30), which may confer specificity. The emerging concept from yeast and plant studies is that members of the SNF1 family protect cells against nutritional and environmental stress (31, 32). Several SnRK2 family members from Arabidopsis and rice have been identified as stress-activated kinases involved in response to drought and humidity (33, 34). Evidence from wheat showed that the SnRK2 member PKABA1 is activated by abscisic acid (ABA) and phosphorylates a downstream transcription factor (35). Similarly, the ABA-responsive transcription factor TRAB1 is activated by SAPK10 (stress-activated protein kinase 10) in rice protoplasts (36). Another example is AAPK (ABA-activated protein kinase) from Vicia faba, which regulates stomatal closure by targeting an RNA-binding protein (37). Thus, SnRK2s appear to act both on the transcriptional and posttranscriptional levels. However, so far, no relationship had been established between SnRK2 proteins and chloroplast transcriptional activity.
Targeting prediction programs do not predict a chloroplast localization for Sac3 (data not shown). This indication leads to a model in which Sac3 is activated in the cytosol under −S conditions, initiating a cascade of events leading to chloroplast transcriptional repression. How this cascade is transduced to the chloroplast, and whether it involves PTK, is an open question. The Chlamydomonas nuclear genome encodes two PTK homologues (gene models C_670029 and C_200187), and one could envisage transduction of the Sac3 signal to PTK with subsequent phosphorylation of Sig1. This modification would lead to transcriptional arrest, as in the mustard PEP-based mechanism, and/or to degradation of Sig1, perhaps stimulated by a limitation of PEP core subunits. Although PTK has not been tested with Sig1, recombinant Sig1 can be phosphorylated in vitro by human casein kinase 2 (CK2) (data not shown), consistent with Sig1 containing multiple consensus CK2 recognition sites (as noted in ref. 19). We also note that alternative models for Sac3 function exist, including one where it is phosphorylated under +S conditions and dephosphorylated when activated (38). However, our data are most consistent with activation through phosphorylation.
It was recently proposed that in Chlamydomonas, circadian rhythms of chloroplast transcription are regulated by transcriptional oscillations of RPOD (19). In contrast, the data presented here show a constant level of RPOD mRNA (Fig. 5) but a 60–80% decrease in the Sig1 protein level under −S conditions (Fig. 6). Further analysis showed that Sig1 degradation occurred in a SAC3-dependent manner and required phosphorylation, with the latter conclusion based on prevention of Sig1 disappearance by the addition of DMAP. Regulation by degradation or stabilization of sigma factors is known and perhaps best studied for Escherichia coli RpoS, which is stress-induced. The level of RpoS remains low under normal conditions because of degradation by the ClpXP protease (reviewed in ref. 39). The degradation pathway depends on RssB, a response regulator that delivers RpoS to ClpXP through a still-undefined mechanism (40).
The regulatory model proposed here presumes that transcriptional repression under −S conditions is at least partly due to destabilization and/or reduced synthesis of Sig1 triggered by activated Sac3. Whether Sig1 is degraded inside or outside the chloroplast, and whether Sig1 phosphorylation initially represses transcription initiation and then stimulates decay, remains to be established. The decrease in Sig1 activity and/or abundance might represent an adjustment of the chloroplast transcription apparatus under −S conditions, coincident with reduced expression of catalytic component rpo genes, and a general reduction of photosynthetic activity. This regulation highlights the importance of integrating the chloroplast genetically into nutrient stress response.
Materials and Methods
Strains and Culture Conditions.
C. reinhardtii WT strain CC-125, the mutant strains sac1 and sac3, and the complemented sac3 strain (are10-12-C1), were grown in nutrient-replete Tris/acetate/phosphate (TAP) medium (41). For starvation experiments, cells were grown under continuous light at 25°C on a rotary shaker (125 rpm) to midlogarithmic phase (1–5 × 106 cells per ml). Cultures were then harvested by centrifugation, washed in sulfate-deficient TAP (TAP-S) (22), and resuspended in TAP-S. Repletion was achieved by harvesting cells, washing with TAP, and resuspending in TAP medium for 2 h. In some cases, DMAP (dissolved in DMSO) was added to cells transferred to TAP-S to a final concentration of 3 mM, or the same volume of DMSO was added as a control.
RNA Isolation and Gel Blot Analysis.
For RNA gel blot analysis, total RNA was isolated by using TRI Reagent (Molecular Research Center, Cincinnati). Five micrograms of RNA was resolved in 1% agarose/6% formaldehyde gels. Electrophoresis, blotting, and hybridization were performed as described in ref. 42. The DNA probes were PCR products for atpB, petD, petB, tufA, ARS1, and 25S ribosomal DNA labeled with [α-32P]dCTP. The radioactive bands were visualized by using a Storm scanner (Amersham Pharmacia Biosciences). Quantification of bands was performed by using imagequant software (Amersham Pharmacia Biotech).
Run-On Transcription in Permeabilized Cells.
For run-on transcription assays, WT and sac3 cells were grown in TAP medium, harvested, adjusted to 1 × 106 cells per ml, and transferred to TAP-S. Permeabilized cells for the assay were prepared by the freeze/thaw method (13), and aliquots were stored at −80°C until use. Of the pelleted permeabilized cells, 30 μl (≈5 × 107 cells) were mixed with 15 μl of 4× run-on transcription buffer (1 M sucrose/60 mM MgCl2/15 mM DTT/50 mM NaF/50 mM Hepes, pH 7.5), 2 μl of rRNasin RNase inhibitor (Promega), 0.25 mM GTP, 0.5 mM ATP, 0.25 CTP, and 125 μCi (1 Ci = 37 GBq) of [α-32P]UTP (43). The mixture was incubated on ice for 1 min and then at room temperature for 15 min. The reactions were terminated by addition of 10 μl of 20% SDS, and radiolabeled RNAs were extracted by using TRI Reagent. Pellets were dissolved in 100 μl of H2O and used for DNA dot blot hybridization. The DNA probes were PCR products for the chloroplast genes as shown, which were blotted on GeneScreen nylon filters by using the Bio-Dot microfiltration apparatus (Bio-Rad).
RT-PCR Analysis.
For RT-PCR, 2.5 μg of total RNA was treated with 1 unit of RQI RNase-free DNase I (Promega) for 30 min at 37°C in a total volume of 10 μl. Then DNase was inactivated by heating to 65°C for 10 min, and 2-μl aliquots were used for 50-μl single-tube RT-PCR (Access RT-PCR System; Promega), according to the manufacturer's instructions. The primers used were as follows: for RPOD, 5′-ACTACAAGCTGGTCATGACGGTGT-3′ and 5′-TTACGTTCAGCGCCTCTCCAATCT-3′; for rpoA, 5′-ATGACAATTTATCCAAATTTAAAAAAAATC-3′ and 5′-TTAAAATTTTCTTGCATATTCAAAAGTAAG-3′; for rpoB1, 5′-GATAACGTTAATGTATCCCAAATTGAT-3′ and 5′-TCAACGGGACAAATACGACCAT-3′; and for actin, 5′-AATCGTGCGCGACATCAAGGAGAA-3′ and 5′-TTGGCGATCCACATTTGCTGGAAGGT-3′. The first step was reverse transcription at 48°C for 45 min, followed by 30 or 35 PCR cycles. PCR products were sequenced to confirm their identities.
Immunoblot Analysis.
For protein extraction, cells were grown as described above. Ten-milliliter samples were pelleted and washed with water, and total proteins were extracted by acetone precipitation. Protein concentration was determined by using Bradford reagent (Bio-Rad), and equivalent masses of protein were resolved in 12% SDS-polyacrylamide gels and transferred to nitrocellulose membranes with a TransBlot semidry cell (Bio-Rad). Immunoblotting was performed by using a peptide antibody directed against Sig1 (kindly provided by David Herrin, University of Texas, Austin) and a monoclonal anti-sea urchin α-tubulin antibody (Sigma). The Sig1 antibody was against the peptide LADELERLLGPTTSC, to which a nonencoded terminal cysteine was added for coupling. Secondary antibodies were horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (for Sig1 detection) or HRP-conjugated anti-mouse (for tubulin detection), and enhanced chemiluminescence (Amersham Pharmacia Biotech) was used. Quantification of bands was performed by using imagequant software after scanning of x-ray film and importation as TIFF files.
Supplementary Material
Acknowledgments
We thank Arthur Grossman (Carnegie Institution, Stanford, CA) for helpful suggestions and for providing strains carrying sac mutations, David Herrin for the anti-Sig1 antibody, and Katia Wostrikoff for a critical reading of the manuscript. This work was supported by National Science Foundation Award DBI-0211935.
Abbreviations
- ARS
arylsulfatase
- cpRNA
chloroplast RNA
- DMAP
6-dimethylaminopurine
- PEP
plastid-encoded polymerase
- PTK
plastid transcription kinase
- SnRK2
SNF1-related kinase 2
- TAP
Tris/acetate/phosphate
- TAP-S
sulfate-deficient TAP
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Leustek T., Saito K. Plant Physiol. 1999;120:637–644. doi: 10.1104/pp.120.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leustek T., Martin M. N., Bick J. A., Davies J. P. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000;51:141–165. doi: 10.1146/annurev.arplant.51.1.141. [DOI] [PubMed] [Google Scholar]
- 3.Hirai M. Y., Fujiwara T., Awazuhara M., Kimura T., Noji M., Saito K. Plant J. 2003;33:651–663. doi: 10.1046/j.1365-313x.2003.01658.x. [DOI] [PubMed] [Google Scholar]
- 4.Nikiforova V., Freitag J., Kempa S., Adamik M., Hesse H., Hoefgen R. Plant J. 2003;33:633–650. doi: 10.1046/j.1365-313x.2003.01657.x. [DOI] [PubMed] [Google Scholar]
- 5.Yildiz F. H., Davies J. P., Grossman A. R. Plant Physiol. 1994;104:981–987. doi: 10.1104/pp.104.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang L., Happe T., Melis A. Planta. 2002;214:552–561. doi: 10.1007/s004250100660. [DOI] [PubMed] [Google Scholar]
- 7.de Hostos E. L., Schilling J., Grossman A. R. Mol. Gen. Genet. 1989;218:229–239. doi: 10.1007/BF00331273. [DOI] [PubMed] [Google Scholar]
- 8.Davies J. P., Yildiz F. H., Grossman A. EMBO J. 1996;15:2150–2159. [PMC free article] [PubMed] [Google Scholar]
- 9.Davies J. P., Yildiz F. H., Grossman A. R. Plant Cell. 1999;11:1179–1190. doi: 10.1105/tpc.11.6.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wykoff D. D., Davies J. P., Melis A., Grossman A. R. Plant Physiol. 1998;117:129–139. doi: 10.1104/pp.117.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Z., Shrager J., Jain M., Chang C. W., Vallon O., Grossman A. R. Eukaryotic Cell. 2004;3:1331–1348. doi: 10.1128/EC.3.5.1331-1348.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Hostos E. L., Togasaki R. K., Grossman A. J. Cell Biol. 1988;106:29–37. doi: 10.1083/jcb.106.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gagne G., Guertin M. Plant Mol. Biol. 1992;18:429–445. doi: 10.1007/BF00040659. [DOI] [PubMed] [Google Scholar]
- 14.Néant I., Guerrier P. Exp. Cell Res. 1988;176:68–79. doi: 10.1016/0014-4827(88)90121-8. [DOI] [PubMed] [Google Scholar]
- 15.Reisdorph N. A., Small G. D. Plant Physiol. 2004;134:1546–1554. doi: 10.1104/pp.103.031930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cahoon A. B., Stern D. B. Trends Plant Sci. 2001;6:45–46. doi: 10.1016/s1360-1385(00)01858-6. [DOI] [PubMed] [Google Scholar]
- 17.Eberhard S., Drapier D., Wollman F. A. Plant J. 2002;31:149–160. doi: 10.1046/j.1365-313x.2002.01340.x. [DOI] [PubMed] [Google Scholar]
- 18.Allison L. A. Biochimie. 2000;82:537–548. doi: 10.1016/s0300-9084(00)00611-8. [DOI] [PubMed] [Google Scholar]
- 19.Carter M. L., Smith A. C., Kobayashi H., Purton S., Herrin D. L. Photosynth. Res. 2004;82:339–349. doi: 10.1007/s11120-004-4213-6. [DOI] [PubMed] [Google Scholar]
- 20.Bohne A. V., Irihimovitch V., Weihe A., Stern D. B. Curr. Genet. February 2 2006 doi: 10.1007/s00294-006-0060-7. [DOI] [PubMed] [Google Scholar]
- 21.Grossman A. Protist. 2000;151:201–224. doi: 10.1078/1434-4610-00020. [DOI] [PubMed] [Google Scholar]
- 22.Davies J. P., Yildiz F., Grossman A. R. Plant Cell. 1994;6:53–63. doi: 10.1105/tpc.6.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng X. W., Gruissem W. Cell. 1987;49:379–387. doi: 10.1016/0092-8674(87)90290-x. [DOI] [PubMed] [Google Scholar]
- 24.Mullet J. E., Klein R. R. EMBO J. 1987;6:1571–1579. doi: 10.1002/j.1460-2075.1987.tb02402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cahoon A. B., Harris F. M., Stern D. B. EMBO Rep. 2004;5:801–806. doi: 10.1038/sj.embor.7400202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfannschmidt T., Ogrzewalla K., Baginsky S., Sickmann A., Meyer H. E., Link G. Eur. J. Biochem. 2000;267:253–261. doi: 10.1046/j.1432-1327.2000.00991.x. [DOI] [PubMed] [Google Scholar]
- 27.Tiller K., Link G. EMBO J. 1993;12:1745–1753. doi: 10.1002/j.1460-2075.1993.tb05822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogrzewalla K., Piotrowski M., Reinbothe S., Link G. Eur. J. Biochem. 2002;269:3329–3337. [PubMed] [Google Scholar]
- 29.Christopher D. A., Li X., Kim M., Mullet J. E. Plant Physiol. 1997;113:1273–1282. doi: 10.1104/pp.113.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hardie D. G., Carling D., Carlson M. Annu. Rev. Biochem. 1998;67:821–855. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]
- 31.Halford N. G., Hardie D. G. Plant Mol. Biol. 1998;37:735–748. doi: 10.1023/a:1006024231305. [DOI] [PubMed] [Google Scholar]
- 32.Sanz P. Biochem. Soc. Trans. 2003;31:178–181. doi: 10.1042/bst0310178. [DOI] [PubMed] [Google Scholar]
- 33.Umezawa T., Yoshida R., Maruyama K., Yamaguchi-Shinozaki K., Shinozaki K. Proc. Natl. Acad. Sci. USA. 2004;101:17306–17311. doi: 10.1073/pnas.0407758101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kobayashi Y., Yamamoto S., Minami H., Kagaya Y., Hattori T. Plant Cell. 2004;16:1163–1177. doi: 10.1105/tpc.019943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson R. R., Wagner R. L., Verhey S. D., Walker-Simmons M. K. Plant Physiol. 2002;130:837–846. doi: 10.1104/pp.001354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kobayashi Y., Murata M., Minami H., Yamamoto S., Kagaya Y., Hobo T., Yamamoto A., Hattori T. Plant J. 2005;44:939–949. doi: 10.1111/j.1365-313X.2005.02583.x. [DOI] [PubMed] [Google Scholar]
- 37.Li J., Kinoshita T., Pandey S., Ng C. K., Gygi S. P., Shimazaki K., Assmann S. M. Nature. 2002;418:793–797. doi: 10.1038/nature00936. [DOI] [PubMed] [Google Scholar]
- 38.Pollock S., Pootakham W., Shibagaki N., Moseley J., Grossman A. Photosynth. Res. 2005;86:475–489. doi: 10.1007/s11120-005-4048-9. [DOI] [PubMed] [Google Scholar]
- 39.Peterson C. N., Mandel M. J., Silhavy T. J. J. Bacteriol. 2005;187:7549–7553. doi: 10.1128/JB.187.22.7549-7553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peterson C. N., Ruiz N., Silhavy T. J. J. Bacteriol. 2004;186:7403–7410. doi: 10.1128/JB.186.21.7403-7410.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harris E. H. The Chlamydomonas Sourcebook: A Comprehensive Guide to Biology and Laboratory Use. San Diego: Academic; 1989. [DOI] [PubMed] [Google Scholar]
- 42.Drager R. G., Stern D. B. In: The Molecular Biology of Chloroplasts and Mitochondria in Chlamydomonas. Rochaix J.-D., Goldschmidt-Clermont M., Merchant S., editors. Vol. 7. Dordrecht, The Netherlands: Kluwer; 1998. pp. 165–181. [Google Scholar]
- 43.Sakamoto W., Kindle K. L., Stern D. B. Proc. Natl. Acad. Sci. USA. 1993;90:497–501. doi: 10.1073/pnas.90.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.