Abstract
We show here that the high-molecular-weight protein (HMWP or pUL48; 253 kDa) of human cytomegalovirus (HCMV) is a functionally competent deubiquitinating protease (DUB). By using a suicide substrate probe specific for ubiquitin-binding cysteine proteases (DUB probe) to screen lysates of HCMV-infected cells, we found just one infected-cell-specific DUB. Characteristics of this protein, including its large size, expression at late times of infection, presence in extracellular virus particles, and reactivity with an antiserum to the HMWP, identified it as the HMWP. This was confirmed by constructing mutant viruses with substitutions in two of the putative active-site residues, Cys24Ile and His162Ala. HMWP with these mutations either failed to bind the DUB probe (C24I) or had significantly reduced reactivity with it (H162A). More compellingly, the deubiquitinating activity detected in wild-type virus particles was completely abolished in both the C24I and H162A mutants, thereby directly linking HMWP with deubiquitinating enzyme activity. Mutations in these active-site residues were not lethal to virus replication but slowed production of infectious virus relative to wild type and mutations of other conserved residues. Initial studies, by electron microscopy, of cells infected with the mutants revealed no obvious differences at late times of replication in the general appearance of the cells or in the distribution, relative numbers, or appearance of virus particles in the cytoplasm or nucleus.
Herpesviruses contain a large double-stranded DNA genome that codes for many of the enzymes required to produce new infectious virus. Identification of these proteins and their functions has helped delineate the biology of herpesvirus replication and provided new targets for development of antiviral drugs. Among the enzymes known to be encoded by herpesviruses are DNA polymerase (39), DNase (2), dUTPase (38), helicase/primase (10, 50), maturational protease (31, 47), phosphotransferase (20), protein kinase (11, 16), ribonucleotide reductase (1), terminase (41, 45), thymidine kinase (25), ubiquitin ligase (7, 9), and uracil deglycosidase (8). Most recently added to this list is a ubiquitin-specific cysteine protease (USP), also called deubiquitinating enzyme (DUB), discovered in human fibroblasts infected with herpes simplex virus type 1 (HSV) (23). Increasing awareness of DUBs as major regulators in biological systems focuses attention on this viral enzyme for the information that it can provide about unknown steps in virus replication and potential sites for pharmacological intervention, as well as for insights that it may provide into more general aspects of this class of enzymes.
The HSV DUB was identified by using a ubiquitin-derived active-site-directed probe (DUB probe) designed to selectively bind these enzymes by forming a covalent adduct with their active-site cysteine (5). Analysis of the isolated DUB (≈57 kDa with probe bound) established that its sequence is the same as the amino end of virion protein 1/2 (VP1/2, 336 kDa) encoded by open reading frame UL36 (33), and it was designated HSV UL36USP (23). UL36USP appears late in infection, but it is unknown whether it originates as a cleavage product of VP1/2 or by another mechanism. Attachment of the DUB probe was mapped to Cys65, implicating it as the catalytic nucleophile of the enzyme, and alignment of VP1/2 homologs revealed an absolute conservation of this and three other amino acids potentially involved in the catalytic mechanism of the protease. Enzymatic studies done with recombinant UL36USP, and even shorter recombinant forms of the corresponding domains from murine cytomegalovirus (MCMV285) and Epstein-Barr virus (EBV205), verified its deubiquitinating activity and established it as the prototype of a new family of viral DUBs (23, 42).
Although little is yet known about the function of HSV UL36USP, there is considerable information about the full-length gene product, VP1/2, and its homologs in other herpesviruses. It was first identified as a constituent of virions (43) and subsequently localized to the tegument (17, 18), where it appears to make direct contact with the capsid (44, 49). It is synthesized at late times of infection (21), is phosphorylated in some instances (29, 33) but apparently not others (40), and forms oligomers with a protein encoded by the adjacent open reading frame (4, 19, 26, 46). It is also an essential protein with critical functions at both early and late times of infection (3, 12-15, 27, 28, 35) and has been speculated to be involved with transport of infecting capsids to the nucleus as well as with tegumentation and envelopment of maturing cytoplasmic capsids. Discovery of a DUB activity associated with this protein raises questions as to whether and how it may modulate one or more of these processes.
The human cytomegalovirus (HCMV) homolog of HSV VP1/2 is the 253-kDa, high-molecular-weight protein (HMWP) encoded by UL48. Even though 83 kDa shorter than VP1/2, HMWP retains the putative catalytic residues of its HSV counterpart (i.e., Gln52, Cys65, Asp197, and His199)—each about 40 residues closer to the amino end (i.e., HCMV Gln10, Cys24, Asp159, and His162, respectively). In the work reported here we have applied approaches complementing those used to identify HSV UL36USP, including assays on unfractionated cell lysates and virus particles, to identify the 253-kDa HMWP of HCMV as a functional DUB. DUB probe binding by the counterpart 246-kDa HMWP of simian CMV (SCMV) and the 336-kDa VP1/2 of HSV indicates that this activity is intrinsic to the full-length HMWP counterparts of other, if not all, herpesviruses.
MATERIALS AND METHODS
Cells and viruses.
The AD169 strain of human cytomegalovirus (HCMV; VR-538; ATCC, Manassas, VA) and SCMV strain Colburn (17) were grown in human foreskin fibroblast (HFF) cell cultures maintained in Dulbecco’s modified Eagle medium (Invitrogen, Carlsbad, CA) containing high glucose, 100 U/ml of penicillin, 10 μg/ml of streptomycin sulfate, and 10% fetal bovine serum (HyClone, Logan, UT). AD169 bacmids encoding (AD169ΔUS2-11-EGFP-loxP) or not encoding (AD169RVHB5/pHB5) (6) a green fluorescent protein (GFP) marker were from U. Koszinowski, M. Messerle, and G. Hahn. HSV strain KOS (30) was from P. Desai.
Extracellular noninfectious enveloped particles (NIEPs), virions, and dense bodies (22) were recovered from the growth medium of virus-infected HFF cells, following clarification by centrifugation at 2,000 × g for 5 min at 4°C. Rate-velocity sedimentation (39,000 rpm at 4°C for 20 min in a Beckman SW41 rotor) of particles in the clarified medium, through gradients of sucrose (15 to 50%, wt/wt, in 0.04 M phosphate buffer containing 150 mM NaCl, pH 7.4), resulted in three well-separated bands—NIEPs, virions, and dense bodies (top to bottom, respectively). Each was collected by aspiration through the wall of the centrifuge tube, diluted 1:1 with the gradient buffer, concentrated by pelleting (35,000 rpm at 4°C for 2 h in an SW55 rotor), and stored tightly sealed at −80°C, after the supernatant was decanted and excess buffer was wiped from the tube. In preparation for analysis, the pellets were suspended and collected in 30 μl of phosphate-buffered saline (PBS; SH30264; HyClone, Logan, UT) with no calcium or magnesium (calcium- and magnesium-free PBS [CMF-PBS]), containing 0.5% zwitterionic detergent {3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate} (CHAPS; Sigma-Aldrich, St. Louis, MO). After a second rinse with the same buffer, residual pellet material was collected in 30 μl of protein sample buffer (three parts NuPAGE lithium dodecyl sulfate sample buffer [NP0007; Invitrogen] plus two parts 1 M dithiothreitol). The protein compositions of the respective rinses were qualitatively similar, but the first contained the greatest percentage of the total (e.g., wild-type NIEPs: 67%, 11%, and 22%, respectively). Samples were stored at −80°C.
Construction of mutant viruses and plasmids.
Mutant viruses were made using the counterselection BAC modification kit from Gene Bridges (Gene Bridges GmbH BioZ, Dresden, Germany), based on the Redα/β recombination method (36, 48). Mutations were introduced into open reading frame UL48 of the bacmid at Cys24 (C24I, codon TGC to ATA), Leu32 (L32A, codon CTA to GCA), Phe159 (F159A, codon TTT to GCA), Asp160 (D160A, codon GAT to GCA), and His162 (H162A, codon CAC to GCA; H162G, codon CAC to GGA).
Mutations were sequence confirmed in the bacmids, which were then transfected into HFF cells by using the amaxa system (amaxa, Cologne, Germany) with 100 μl of VPD-1001 solution (amaxa), 0.5 × 106 to 1.0 × 106 HFF cells, and 3 to 4 μg of DNA. Cytopathic effects (CPE) typical of HCMV were observed by 3 weeks in all cultures. Stocks of the viruses were prepared when extensive CPE were observed—usually within 4 to 5 weeks of transfection. The presence of the intended C24I and H162A mutations in the resulting viruses was confirmed by sequencing a 720-bp, inclusive PCR-amplified portion of DNA from gradient-purified virions.
Mutant bacmid DNAs were also used in a homologous recombination-based procedure (Gene Bridges) to transfer a 1,200-bp, 5′ sequence from each into plasmid pGEM-7Zf(+) (Promega, Madison, WI) for protein expression.
Microscopy.
Fluorescence microscopy was done with a Nikon Eclipse TE200 inverted microscope equipped with a Sony DKC5000 imaging system. Contrast of the photomicrographs was adjusted in Photoshop CS, v8.0 (Adobe, San Jose, CA).
For transmission electron microscopy, cells were fixed in a mixture of 2% paraformaldehyde-2% glutaraldehyde in HEPES buffer, stained with 1% OsO4 and 2% uranyl acetate, and embedded in Epon resin, all in situ on plastic dishes. Ultrathin sections (80 to 90 nm) were stained with 2% uranyl acetate and 0.3% lead citrate before being viewed with a Hitachi 7600 electron microscope equipped with a DVC1412M-FW digital camera system and AMTV542 software (Advanced Microscopy Techniques, Corp., Danvers, MA).
Assays for DUB probe binding.
Infected cells from one well of a 24-well culture plate (Becton Dickinson, Franklin Lakes, NJ) were dislodged into the medium by using the rubber plunger of a 1-μl hypodermic syringe (309602; Becton Dickinson) and collected by centrifugation for 2 min at 16,000 × g in a microcentrifuge at 4°C. The medium was aspirated by using a hypodermic needle; the small cell pellet was suspended in 30 to 50 μl of PBS or CMF-PBS as specified, transferred to a 500-μl tube, and dispersed by micropipetting either just before or after freezing at −80°C (whole-cell lysate). Where specified, CHAPS was added to a final concentration of 0.5%. This addition did not noticeably affect reactivity of the DUB probe in these assays (unpublished data).
Probe-binding assays were done by combining 2 to 6 μl of whole-cell lysate with 2 μl of 5× reaction buffer (5× = 250 mM Tris, pH 7.4, 750 mM NaCl, 2.5 mM EDTA, 25 mM MgCl2, 10 mM dithiothreitol, 10 mM ATP), adding 1 μl of the DUB probe (hemagglutinin [HA]-tagged Ub-vinylmethyl ester [HAUbVME] [5]; 0.083 mg/ml [23]), and incubating the mixture at room temperature for 30 to 60 min. Reactions were terminated by adding an equal volume of protein sample buffer (see above), and the samples were stored at −80°C until needed. Probe binding to cell lysate proteins was determined by Western immunoassays (see below).
In vitro protein synthesis, polyacrylamide gel electrophoresis (PAGE), and Western immunoassays.
[35S]methionine/cysteine-labeled proteins were synthesized from plasmids using a rabbit reticulocyte, coupled transcription/translation kit (L1170; Promega). Reaction mixtures containing 20 μl of lysate, 1 μl of [35S]Met/Cys (Translabel; 51006; MP Biomedicals, Irvine, CA), and 1 μl of plasmid (≈1 μg/μl), were incubated for 60 min at 30°C and used immediately or stored at −80°C until needed. Resulting lysates were reacted with the DUB probe, as described above, and analyzed following sodium dodecyl sulfate (SDS)-PAGE and phosphorimaging, as described below.
Samples were typically combined with an equal volume of protein sample buffer (see above). Proteins were separated electrophoretically in 4 to 12% polyacrylamide gradient gels (NP0323BOX; Invitrogen), using 2-(N-morpholino)ethanesulfonic acid (MES) buffer or 3-(N-morpholino)propanesulfonic acid (MOPS) buffer (Invitrogen), both containing sodium dodecyl sulfate (SDS-PAGE). Molecular weight markers were either Mark 12 (LC5677; Invitrogen) for gels to be stained or MultiMark (LC5725; Invitrogen) for Western immunoassays. Protein sizes in Western immunoassays were determined by overlaying the corresponding phosphorimage and membrane and comparing the distance moved by unknown proteins with a semilog10 plot of distance moved versus molecular weight for color-tagged, size-calibrated marker proteins (MultiMark; Invitrogen). Protein staining in gels was with SYPRO-Ruby (SYPRO-R; S12000; Molecular Probes, Eugene, OR); imaging and quantification of stained proteins were done by UV transillumination using a Kodak Gel Logic 200 imager and Kodak MI software v4.0.0. Figures were prepared in Photoshop CS, v8.0, from exported data files.
Western immunoassays were done essentially as described before (32). The HA-tagged DUB probe was detected by incubating the membrane with (i) biotinylated mouse or rat anti-HA monoclonal antibodies (MAbs to HA sequence N′-YPYDVPDYA-C′: 11-666-851-001 and 12-158-167-001, respectively; Roche Diagnostics, Indianapolis, IN), followed by (ii) 125I-streptavidin (IM236; GE-Healthcare, Piscataway, NJ) to reveal the HA-bound MAb. HCMV HMWP was detected with a primary rabbit antipeptide antiserum followed by 125I-protein A (NEX-146L; Perkin-Elmer, Boston, MA) as the secondary reagent. Processed membranes were incubated briefly in TN buffer (10 mM Tris, 0.9% NaCl, pH 7.4) containing 5% bovine serum albumin (TN+BSA) before being dried to facilitate subsequent reactions with other antibodies (24). Dry membranes to be assayed again were either (i) directly hydrated in TN+BSA and reassayed or (ii) heated at 70°C in a solution containing SDS and β-mercaptoethanol to remove or reduce the first set of reagents (24), incubated for 1 to 12 h in TN+BSA, and then reassayed.
Antipeptide antiserum anti-UL48c was prepared using a synthetic peptide representing a sequence near its carboxyl end (amino acids 2120 through 2135; ≤18% identity to sequences in counterpart HMWP of SCMV and VP1/2 of HSV). Anti-VP1/2 was a gift from Richard Courtney and was prepared using virion VP1/2 isolated by SDS-PAGE, as described and characterized before (34). Radioactivity was detected and quantified by phosphorimaging (Fuji BAS 1500 and ImageGauge v4.22; Fuji Film Medical Systems, Stamford, CT). The relative positions of the color-tagged molecular weight marker proteins were measured from the membrane and then annotated on the electronic phosphorimage.
Oligoubiquitin cleavage reaction.
Each reaction mixture contained (i) 3 μl of oligoubiquitin (preparations of Lys48 [UC-220]- or Lys63 [UC-320]-linked 3- to 7-mers; Boston Biochem, Boston, MA; dissolved, 2 μg/μl, in CMF-PBS); (ii) 2 μl of “5× reaction buffer” (described above) minus ATP; and (iii) 5 μl of NIEPs prepared, concentrated, and disrupted as described above. A substrate control containing 5 μl of CMF-PBS in place of a NIEP preparation was processed in parallel. The samples were incubated at 37°C for 15 h, combined with an equal volume of protein sample buffer, and analyzed following SDS-PAGE and protein staining with SYPRO-R.
RESULTS
Probe for deubiquitinating cysteine proteases binds within amino end of HCMV HMWP.
As a pilot experiment, we tested proteins encoded by the 5′ end of the HCMV UL48 open reading frame for their ability to bind the DUB probe used to identify UL36USP. We cloned the 5′ 1,200-bp sequence of UL48 from wild-type and mutant (C24I, D160A, and H162A) bacmids into a bacterial expression vector and then used each plasmid to make [35S]Met/Cys-labeled proteins in a coupled transcription/translation system.
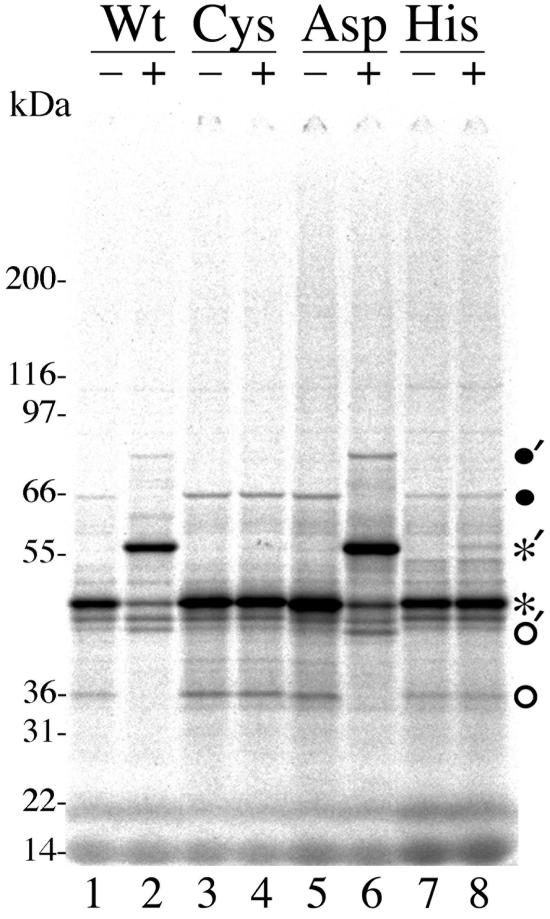
The resulting preparations were reacted with the DUB probe, or with no probe added, and subjected to SDS-PAGE. The stained and dried gel showed a predominant ≈48-kDa protein, whose size approximates that predicted for the first 400 amino acids of HMWP (i.e., 42.5 kDa), in the wild-type preparation (Fig. 1, lane 1, asterisk). When incubated with the DUB probe, approximately 70% of this protein was shifted to ≈56 kDa (Fig. 1, lane 2, asterisk), compatible with the addition of one molecule of the ≈10-kDa DUB probe. Two less intense bands at ≈39 kDa and ≈65 kDa also appeared to increase by ≈10 kDa in the probe-treated sample. All six of these bands were also present in the preparation from mutant D160A reacted with probe (Fig. 1, lane 6). In contrast, none of the three primary bands changed mobility when the preparation from mutant C24I was reacted with probe, and only a trace amount of the 48-kDa predominant translation product bound probe in the preparation from mutant H162A (Fig. 1, lane 4 and lane 8, see asterisk), indicating that substitution of these most critical putative catalytic-site residues abolished or severely reduced the reactivity of these mutant proteins.
FIG. 1.
DUB probe binding to amino-terminal sequence of wild-type, C24I, D160A, and H162A HMWPs. Plasmids encoding the first 400 amino acids of wild-type (Wt) HMWP and the C24I (Cys), D160A (Asp), and H162A (His) mutants were constructed, transcribed, and translated in vitro as described in Materials and Methods. The [35S]Met/Cys-labeled products were reacted with (+) or without (−) the DUB probe and subjected to SDS-PAGE in a 4 to 12% gradient gel using MOPS electrode buffer, and the Coomassie brilliant blue-stained gel was dried. Shown here is a phosphorimage of the dried gel. Symbols indicate the 48 (asterisk)-, 39 (open circle)-, and 65 (solid circle)-kDa bands referenced in Results; tick marks indicate presumptive probe-bound adducts of those same bands. Locations of Mark 12 (Invitrogen) marker proteins are indicated to the left of the figure.
Proteins in HCMV-infected cells react with DUB probe.
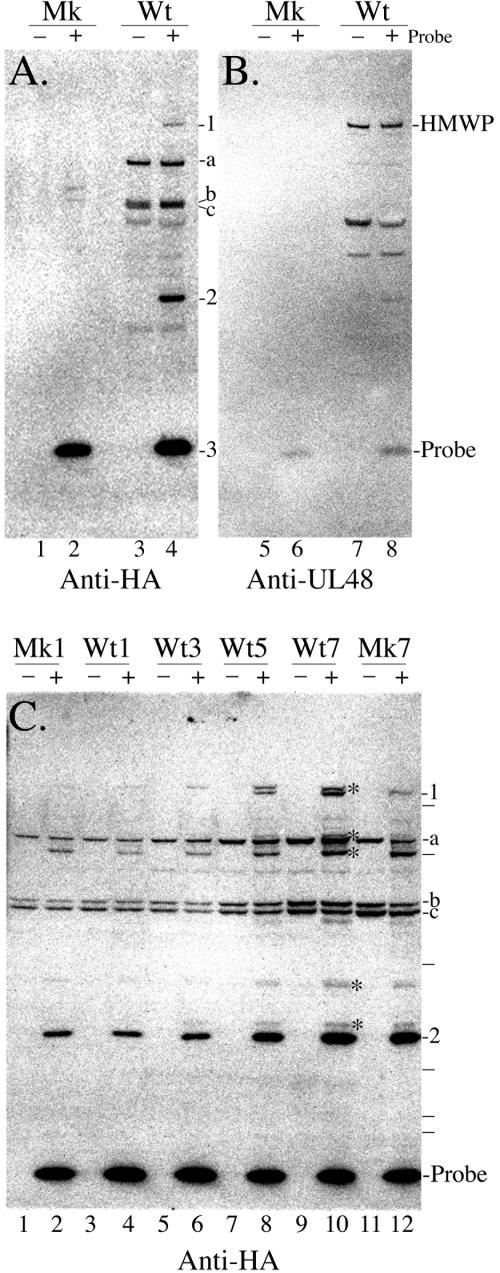
We next used the DUB probe to test for reactive proteins in HCMV-infected cells. Lysates of infected and noninfected cells were prepared and reacted with or without probe. Following SDS-PAGE and Western immunoassay to detect the HA-tagged probe, three bands stood out as more abundant in the infected-cell lysate incubated with probe than in any of the other preparations (Fig. 2A, lane 4, bands 1 to 3). The smallest and most intense of these is the ≈10-kDa probe itself, which migrated slightly slower than the 6-kDa aprotinin marker protein. The largest and least intense of the three migrated slower than the 200-kDa myosin marker protein, with an estimated size approximating that of the largest HCMV protein, HMWP (253 kDa). The third probe-specific band migrated just above the 31-kDa carbonic anhydrase marker protein with a size of ≈38 kDa. Other bands present in the infected-cell lysate were equally intense in the absence of probe and are therefore considered nonspecific (Fig. 2A, compare lanes 3 and 4). Rat anti-HA MAbs gave improved sensitivity and specificity in these assays (Fig. 2C, better detection of cellular DUBs and less cross-reactivity with “probe −” preparations; also see relative positions of molecular weight markers) and were used in all subsequent assays.
FIG. 2.
DUB probe-binding proteins in HCMV-infected and noninfected human fibroblasts. Samples of infected (Wt) or noninfected (Mk) cells were lysed by freezing at −80°C in PBS, incubated with (+) or without (−) DUB probe, and subjected to Western immunoassay, as described in Results and Materials and Methods. Panel A shows an image prepared from a membrane incubated with antibodies to the DUB probe (Anti-HA). Characters to the right of the image indicate bands referenced in Results. Estimated sizes of three prominent nonspecific bands are ≈115 kDa (a) and ≈72 to 75 kDa (b and c). Panel B shows the same membrane as in panel A after rehydration and reaction with antibodies to HMWP (Anti-UL48). Positions of HMWP and DUB probe are indicated to the right of each image. Panel C shows a time course of expression for DUB probe-binding proteins in infected or noninfected cells on days 1, 3, 5, and 7 after infection in a 24-well plate. Designations to the right of the image are as in panels A and B. Asterisks to the right of lane 10 indicate presumptive cellular DUBs tentatively identified by size and relative abundance as USP9X (>250 kDa, observed), USP7 (≈130 kDa, observed), USP15 (≈97 kDa, observed), UCH-L5 (≈48 kDa, observed), UXCH-L3 (≈40 kDa, observed), and UCH-L1 (≈38 kDa, observed), top to bottom, respectively. Dashes to the right of the image indicate positions of MultiMark (Invitrogen) proteins with the following sizes, from top to bottom: 188 kDa, 97 kDa, 52 kDa, 33 kDa, 21 kDa, and 19 kDa.
Time course of DUB expression in HCMV-infected cells.
We investigated the origin (i.e., host or viral) of the two probe-specific proteins identified in virus-infected cells (Fig. 2A, bands 1 and 2), by measuring their expression over the course of 1 week in virus-infected versus noninfected cells. On days 1, 3, 5, and 7, cells were separately collected from one infected culture and one noninfected culture and frozen at −80°C. At the end of the time course, the frozen cell pellets were thawed and suspended in CMF-PBS, incubated with or without the DUB probe in reaction buffer containing 0.5% CHAPS detergent, and subjected to Western immunoassay to detect bound probe.
Bands 1 and 2 were again detected only in samples that had been reacted with the probe (Fig. 2C, even-numbered lanes). Band 2 was present in both infected and noninfected cells beginning on day 1, and its intensity increased 8 (noninfected cells) to 11 (infected cells) fold over the 7-day period (Fig. 2C, compare lane 2 with lane 12 and lane 4 with lane 10). Several other probe-specific (Fig. 2C, see asterisks) and nonspecific (Fig. 2A and C, see a, b, and c) bands in both infected and noninfected cells also increased in expression or accumulation with time (see legend to Fig. 2). Band 1 resolved in these MOPS-buffered gels into two proteins with markedly different kinetics of synthesis and accumulation. The larger was present in both noninfected and infected cells beginning on day 1 and gradually increased fourfold (noninfected cells) to eightfold (infected cells) during the 7-day period (Fig. 2C, compare lane 2 with lane 12 and lane 4 with lane 10). In contrast, the smaller of the two band 1 proteins was absent from noninfected cells; was detected only late in infected cells (e.g., 5 and 7 days); and increased abruptly, beginning on day 4 to 5, to ≥16-fold above background by day 7 (Fig. 2C, compare lane 2 with lane 12 and lane 4 with lane 10).
These data indicate that the upper band 1 protein and the band 2 protein are cellular and that the lower band 1 protein shares characteristics in common with the viral HMWP, including its >200-kDa size, late time of synthesis, and presence in only infected cells.
HMWP (pUL48) binds DUB probe.
Two experiments were done to determine whether the DUB probe-reactive, lower band 1 protein is the HCMV HMWP. In the first, the blot shown in Fig. 2A was rehydrated and incubated with anti-UL48, followed by 125I-protein A, to detect HMWP (Fig. 2B). An image of the resulting membrane shows that HMWP was present only in the infected-cell preparations, its detection was independent of the DUB probe, and its position coincides with that of DUB probe-specific band 1 (Fig. 2A and 2B, compare lanes 7 and 8 with lanes 3 and 4). The relationship of the smaller, infected-cell-specific bands detected in lanes 7 and 8 to HMWP is unknown.
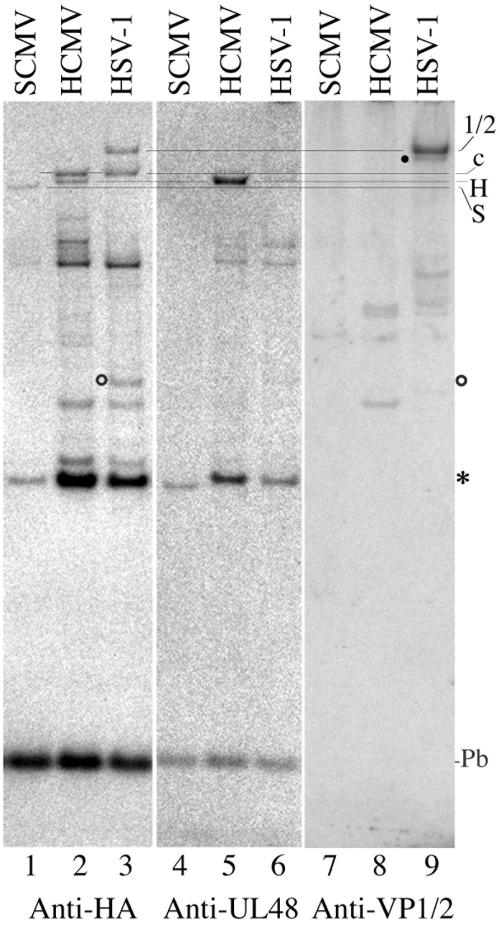
The second experiment included lysates prepared from cells infected with a different betaherpesvirus (SCMV) and with an alphaherpesvirus (HSV), as an additional test of specificity. All were compared by SDS-PAGE/Western immunoassays following reaction with the DUB probe. Results of the experiment were consistent with the different relative sizes of the SCMV (246 kDa), HCMV (253 kDa), and HSV (336 kDa) HMWP counterparts and the expectation that each would be unique to cells infected with that virus. As shown in Fig. 3, (i) the lower band 1 protein (HCMV HMWP, designated H) was present only in the HCMV lysate, (ii) a slightly smaller band (SCMV HMWP, designated S) was present only in the SCMV lysate, (iii) a much larger band (HSV HMWP counterpart VP1/2, designated 1/2) was present only in the HSV lysate, and (iv) the cellular upper band 1 protein (designated c) was present in all samples (Fig. 3, compare lane 1 to lane 3). The only other protein detected in either the SCMV or HSV preparation that was not also in the HCMV lysate was a ≈54-kDa band in the HSV preparation, corresponding in size with probe-bound UL36USP (≈57 kDa) (23) (Fig. 3, lane 3). We calculated its abundance in this experiment to be 70% of that of VP1/2.
FIG. 3.
DUB probe binds HCMV HMWP and counterparts in SCMV- and HSV-infected cells. Cells infected with HCMV or with SCMV or HSV-1 were collected when strong CPE was evident, lysed by freezing at −80°C in PBS, reacted with the DUB probe, subjected to SDS-PAGE in a 4 to 12% gradient gel with MES electrode buffer, and analyzed by Western immunoassay, all as described in Results and Materials and Methods. Shown here are phosphorimages prepared from a single membrane after incubating it sequentially with rat MAbs to detect DUB probe-binding proteins (Anti-HA), with antiserum anti-UL48c to detect HCMV HMWP (Anti-UL48), and with an antiserum to detect HSV-1 VP1/2 (Anti-VP1/2 [34]). Antibodies were stripped from the membrane only between the last two assays. Images were aligned, and the positions of HCMV HMWP (H) and its counterparts in SCMV (S) and HSV-1 (1/2) are indicated in the right-hand margin. Other designations indicate host cell band 1 (c) and band 2 (asterisk) proteins, the DUB probe (Pb), the position of the possible VP1/2 form (filled circle) mentioned in Results, and the position of UL36USP (empty circle) reported before (23). Lines are intended to help show the electrophoretic mobility differences between the closely spaced probe-binding proteins in the anti-HA panel and to emphasize the coincidence of the HCMV HMWP bands detected by anti-HA and anti-UL48 and the HSV VP1/2 bands detected by anti-HA and anti-VP1/2.
Incubating the same membrane sequentially with anti-UL48 (Fig. 3, lanes 4 to 6) and then anti-VP1/2 (Fig. 3, lanes 7 to 9) showed that (i) the HMWP identified in lane 5 coincides with the smaller of the two band 1 proteins in lane 2, supporting its identity as the HMWP; (ii) anti-UL48 failed to react with the counterpart HMWPs of SCMV and HSV in lanes 1 and 3, consistent with their limited amino acid sequence identity and expected lack of antigenic cross-reactivity (see Materials and Methods); and (iii) the largest and strongest-reacting protein identified by anti-VP1/2 coincides with the largest DUB probe-binding band in lane 3, substantiating its identity as HSV VP1/2 and making it the largest DUB so far reported. Anti-VP1/2 also identified a slightly smaller band (indicated by filled circle) that was not detected by the DUB probe (compare lane 9 with lane 3) and may be a modified form of VP1/2. Thus, the differing relative sizes and immunological reactivities of the DUB probe-binding proteins detected in lanes 1 to 3 support their identification as the HCMV and SCMV HMWPs and the HSV counterpart VP1/2.
Mutations of predicted active-site residues are not lethal.
Mutant viruses carrying substitutions in one of five conserved amino acids in the predicted active-site region of the protease were made as described in Materials and Methods. Four of the substitutions changed predicted catalytic residues (C24I, D160A, H162A, and H162G), and two changed nearby Leu and Phe residues (L32A and F159A).
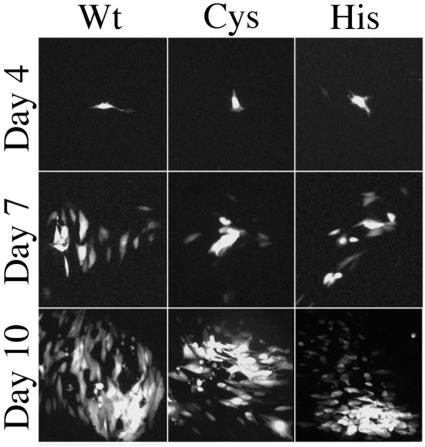
Following transfection of HFF cells with the respective mutant bacmids, the progression of CPE was similar in all cultures, showing expression of the GFP marker within 3 to 4 days, cell-to-cell spread by 1 week, and characteristic CPE of the virus within several weeks (Fig. 4). After approximately 4 weeks, stocks of each virus were prepared by infecting 10-cm petri dishes. In contrast to the similar progression of CPE following transfection, differences were found when cells were infected using the virus stocks. Spread and development of CPE by the L32A, F159A, and D160A mutants were similar to those of wild-type virus, but CPE developed more slowly in cultures infected with the C24I and H162A mutants and slowest in cultures infected with the H162G mutant.
FIG. 4.
HCMV bacmid viruses mutated at Cys24 and His162 of HMWP grow in cell culture. The wild-type parental HCMV bacmid (Wt) or mutants carrying the HMWP substitutions C24I (Cys) or H162A (His) were transfected into HFF cells growing in six-well culture plates, as described in Results and Materials and Methods. The transfected cultures were monitored for fluorescence of GFP expressed from a marker gene inserted into the wild-type bacmid. Fluorescence images of the cultures were recorded on days 4, 7, and 10 after transfection and assembled into the collage shown here.
Extracellular NIEPs, virions, and dense bodies (22) were recovered from each culture after 2 weeks. Yields from the L32A, F159A, and D160A mutants were comparable to that of wild-type virus; those from the C24I and H162A mutants were conspicuously lower; and that from the H162G mutant was negligible after 2 weeks. Because the L32A, F159A, and D160A mutants seemed essentially normal with respect to growth and the H162G mutant was impractically slow growing, we concentrated our efforts on the C24I and H162A mutations intended to eliminate the critical active-site residues of the ubiquitin-specific cysteine protease (23).
Protein patterns of mutant NIEPs and virions are similar to those of the wild type.
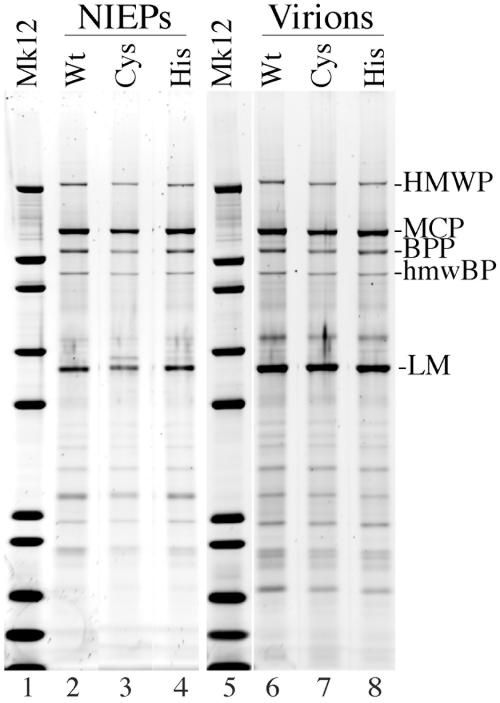
Both the C24I and H162A mutants produced extracellular NIEPs, virions, and dense bodies but at consistently lower yields than the wild type after 2 weeks. Yields more comparable to those of the wild type were obtained from the C24I and H162A mutants when their infections were allowed to proceed for an additional week or two (data not shown). NIEPs and virions from all three viruses were recovered by a single banding in sucrose gradients 4 weeks after infection, concentrated by pelleting, and compared by SDS-PAGE and protein staining with SYPRO-R (Fig. 5). The relative amounts of virions recovered from each preparation were 1 (wild type), 0.6 (C24I), and 0.9 (H162A), based on the amount of major capsid protein (Fig. 5). Otherwise, aside from minor preparation-to-preparation variations (e.g., band above LM in lane 3), no consistent qualitative or quantitative differences between the respective protein patterns were evident among the six preparations (Fig. 5).
FIG. 5.
Protein composition of wild-type virions and NIEPs compared with those of HMWP mutants C24I and H162A. Extracellular virions and NIEPs were recovered in parallel from 10-cm cultures of cells infected with wild-type HCMV (Wt) or with HMWP mutant C24I (Cys) or H162A (His) at approximately 4 weeks after infection as described in Materials and Methods. After concentration by pelleting and suspension in CMF-PBS containing 0.5% CHAPS detergent, samples of each preparation were subjected to SDS-PAGE (4 to 12% gradient gel using MES electrode buffer) and staining with SYPRO-R, all as described in Materials and Methods. Shown here is a collage prepared from an image of the stained gel. Abbreviations to the right of the image indicate positions of HMWP, major capsid protein (MCP, pUL86), basic phosphoprotein (BPP, pp150, pUL32), HMWP-binding protein (hmwBP, pUL47), and lower matrix protein (LM, pp65, pUL83). Lanes 1 and 5 contain Mark 12 marker proteins (Mk12). The band above LM in the Cys NIEP preparation is absent in the virion preparation and has not been noticed as a distinguishing characteristic in other preparations.
HMWP in HCMV NIEPs and virions binds DUB probe.
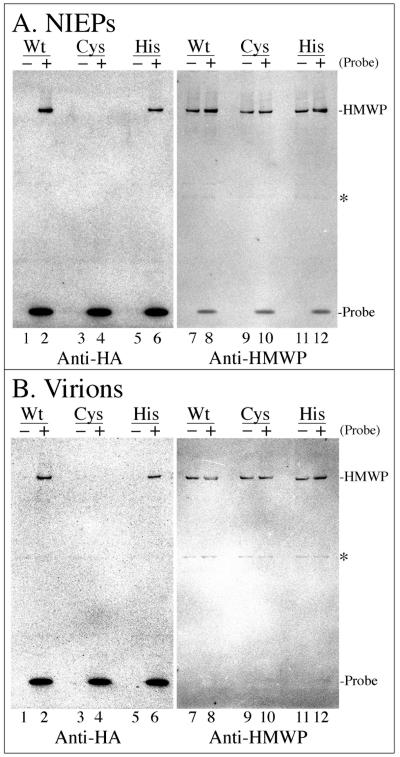
Data shown in Fig. 2 and 3 suggest that HMWP is a DUB. To substantiate this, we reacted the DUB probe with HCMV NIEPs and virions, which both contain HMWP as a characteristic tegument constituent (22). Particles recovered in the experiment described above were reacted with the probe and then tested for its binding by SDS-PAGE/Western immunoassay. A single band, corresponding in position to HMWP, was detected in wild-type NIEPs and virions (Fig. 6, lanes 2). H162A mutant HMWP also reacted with the DUB probe, indicating that this predicted catalytic-site residue is not essential for substrate binding (Fig. 6, lanes 6). In contrast, C24I mutant HMWP was nonreactive with the probe, consistent with Cys24 being the enzyme nucleophile and required to form a covalent adduct with the probe (Fig. 6, lanes 4). Parenthetically, and consistent with it being HMWP, there was no probe binding to the lower band 1 protein in lysates of cells infected with the C24I mutant virus (data not shown). None of the other probe-specific bands seen in Fig. 2 and 3 were apparent. Traces of a ≈63-kDa band in the virion preparations were independent of whether or not the sample had been reacted with the DUB probe and are considered nonspecific (Fig. 6, asterisks).
FIG. 6.
DUB probe binds to wild-type and H162A mutant HMWP, but not to C24I mutant HMWP, in virions and NIEPs. Samples of the virions and NIEPs shown in Fig. 5 were reacted with (+) or without (−) the DUB probe, subjected to SDS-PAGE (4 to 12% gel with MOPS electrode buffer), and analyzed by phosphorimaging following Western immunoassay with antibodies to detect the DUB probe (anti-HA) or to detect HMWP (anti-UL48). Shown here are images of one membrane with proteins from the NIEP preparations (A) and a second membrane with proteins from the virion preparations (B). Each membrane was probed first with anti-HA and imaged, rehydrated for 10 min in TN+BSA, and then probed with anti-UL48 and imaged again. Abbreviations to the right of images are as in Fig. 2; asterisks indicate the position of a ≈63-kDa cross-reacting band that is not probe specific. Amounts of DUB probe bound relative to the amount of HMWP present were calculated using measurements from the four phosphorimages shown. Values for wild-type particles were determined as the quotient of the HMWP intensities in lane 2 divided by that in lanes 7 plus 8; values for H162A particles were determined as the quotient of the HMWP intensities in lane 6 divided by that in lanes 11 plus 12.
Incubating the two membranes with anti-UL48 showed similar amounts of HMWP in all samples and demonstrated its coincidence with the DUB-binding band in the wild-type and H162A preparations (Fig. 6, compare lanes 7 to 12 with lanes 2 and 6). Calculations explained in the legend to Fig. 6 indicate that H162A HMWP bound ≈60% less probe than the wild type. The faint nonspecific bands in lanes 1 to 6 of Fig. 6B also appear weakly reactive with anti-UL48 (Fig. 6, lanes 7 to 12).
HMWP in NIEPs has Cys24- and His162-dependent ubiquitinase activity.
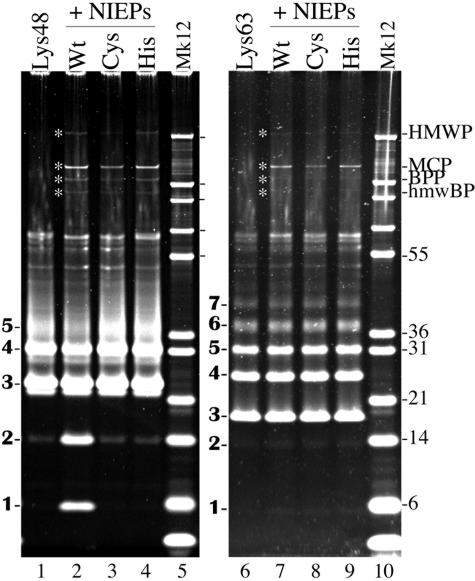
Since the relatively broad-spectrum HAUbVME probe (5) detected just HMWP in NIEPs and virions (Fig. 6), indicating that it may be the only DUB present in these particles, we used NIEPs as a source of HMWP to test for its enzymatic activity. Briefly, NIEPs from the preparations shown in Fig. 5 and 6 were combined with Lys48- or Lys63-linked oligomeric ubiquitin substrate and incubated overnight at 37°C. The reaction products were then separated by SDS-PAGE and the proteins stained with SYPRO-R.
In the absence of added NIEPs, the Lys48-linked oligoubiquitin substrate contained prominent bands of ubiquitin trimers and tetramers, a less-discrete band of pentamers, and trace amounts of dimers and monomers (Fig. 7, lane 1). Following incubation with NIEPs containing wild-type HMWP, ubiquitin dimers and monomers were produced (Fig. 7, lane 2). In contrast, NIEPs containing the C24I or H162A mutation of HMWP produced no increase in the amount of ubiquitin monomers or dimers, compared with the substrate control (Fig. 7, compare lanes 3 and 4 with lane 1). Based on the calculated sensitivity of the assay and accounting for the lower amount of mutant particles, we estimate that if the mutant NIEPs contain any DUB activity, it is at least 95% lower than in the wild type.
FIG. 7.
HMWP cleaves Lys48-linked oligoubiquitin. Ubiquitin oligomers linked through Lys48 or Lys63 were incubated alone (lanes 1 and 6, respectively) or with CHAPS-disrupted NIEPs recovered from cells infected with wild-type (Wt, lanes 2 and 7) HCMV or with HMWP mutant C24I (Cys, lanes 3 and 8) or H162A (His, lanes 4 and 9), all as described in Materials and Methods. Separate gels (4 to 12% gradient gels with MES electrode buffer) were used to analyze the reaction products of the two substrates. Shown here are images of the gels following protein staining with SYPRO-R. Abbreviations for viral proteins shown at top right are as in Fig. 5; asterisks between lanes 1 and 2 and lanes 6 and 7 indicate those proteins in the gel. Lanes 5 and 10 contain Mark 12 marker proteins (Mk12), some of which are indicated by size (kilodaltons) in the right-hand margin. Boldface numbers to the left of each image indicate the number of ubiquitin subunits per oligomer.
The Lys63-linked oligoubiquitin substrate resolved into more evenly distributed amounts of 3-, 4-, and 5-mers, with traces of monomers and dimers (Fig. 7, lane 1). We detected no cleavage of this substrate (i.e., production of monomers and dimers) following incubation with the same NIEP preparations (Fig. 7, lanes 6 to 9). These results indicate that HMWP cleaves Lys48- but not Lys63-linked oligoubiquitin chains and demonstrate that both the C24I and H162A mutations of HMWP eliminate this enzymatic activity. The absence of substrate cleavage by either mutant supports HMWP being the predominant if not only DUB with this activity present in NIEPs.
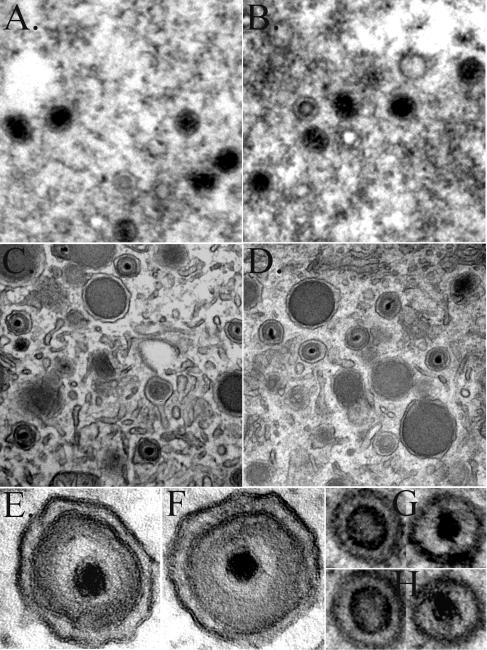
Electron microscopy of cells infected with wild-type virus or C24I mutant virus.
Cells infected with either wild-type or C24I mutant virus were processed and examined by electron microscopy. No conspicuous differences were evident between the two preparations. The general appearance, distribution, and relative numbers of particles and particle types were similar in the respective nuclear and cytoplasmic compartments. Typical capsids with a darkly stained “concentric ring” or a “central button” or with a more completely “filled”-looking interior were present in nuclei of cells infected with either virus (Fig. 8A and G [wild type] and B and H [C24I]). Virions (enveloped and tegumented with densely stained centers) and dense bodies (large, uniformly stained, enveloped spheres) within vesicles or tubules were also present and similar looking in the cytoplasm of cells infected with either virus (Fig. 8C and E [wild type] and D and F [C24I]).
FIG. 8.
Electron micrographs of cells infected with wild-type HCMV or the C24I HMWP mutant virus. Cells infected with wild-type virus or with the C24I HMWP mutant virus were processed and examined by electron microscopy as described in Materials and Methods. Shown here are nuclear (A and B) and cytoplasmic (C and D) regions of cells infected with the wild-type (A and C) or C24I mutant (B and D) virus, recorded at a magnification of ×30,000; panels A and B are enlarged twofold relative to panels C and D. Images of wild-type (E) and C24I (F) virions (≈200-nm outside diameter) within cytoplasmic vesicles or tubules were recorded at a magnification of ×200,000; those of wild-type (G) and C24I (H) nuclear capsids (≈100-nm outside diameter, with “concentric ring” [left] or “central button” [right] interiors) were recorded at a magnification of ×80,000 and enlarged 2.5-fold, approximating the magnification in panels E and F.
DISCUSSION
Discovery of a novel, virus-encoded ubiquitin-binding cysteine protease in HSV-infected cells (23) attracted interest in its function during virus replication and its potential as a new antiviral target. In the work reported here, we used a similar approach to identify a viral ubiquitin-specific protease in cells infected with HCMV. Our main conclusions are that the full-length 253-kDa HMWP is a functional deubiquitinase and that this enzyme activity is not absolutely essential for the production of infectious virus.
Our finding that full-length HMWP binds the DUB probe and is enzymatically competent to cleave oligoubiquitin demonstrates that its DUB activity is intrinsic to the protein and does not require release of the catalytic domain for activation. We also found that HSV VP1/2 binds the DUB probe, which is a difference from the original study, where it was not detected. We attribute this to procedural differences including using crude cell lysates in the work reported here, where such large particle-associated proteins may be less susceptible to proteolytic attack and differential partitioning that could occur during more involved procedures. It is worth noting that more HMWP interacted with the probe when the initial cell lysates were further disrupted by cycles of freezing and thawing or by addition of the zwitterionic detergent CHAPS, suggesting that at least some of it may be sequestered within virus particles or otherwise less accessible to the probe under milder conditions. Considering that the HCMV, SCMV, and HSV counterparts of HMWP all react with the DUB probe (Fig. 3), we anticipate that this activity is intrinsic to the full-length protein homologs of all herpesviruses with the conserved catalytic sites.
Not as clear, however, is whether all herpesviruses have a counterpart of the smaller HSV DUB, UL36USP, whose ≈47-kDa sequence is the same as the amino end of VP1/2 (23). In assays that showed the relative abundance of UL36USP to be ≈70% of that of VP1/2, we found no evidence of a potential counterpart in cells infected with HCMV or SCMV (Fig. 3, compare lanes 1 to 3). Although absence of the smaller protein would indicate an expression or processing difference between HSV VP1/2 and CMV HMWP, possibly reflecting an additional DUB function in HSV or a VP1/2 cleavage not required in CMV, we cannot exclude the possibility that a CMV counterpart may have escaped detection because its ability to bind probe and be recognized is rapidly lost, e.g., by proteolytic degradation or other posttranslational modification.
We concentrated and enriched for HMWP, relative to cellular proteins, by recovering extracellular virions and NIEPs, which contain that protein as an integral tegument constituent. The absence of other proteins reactive with the broad-spectrum HAUbVME DUB probe, in these particles, prompted their use as a source of HMWP for initial enzyme assays, results of which showed a selectivity for Lys48-linked ubiquitin and a requirement for Cys24 and His162. Recombinant UL36USP was also selective for Lys48-linked substrate. The complete loss of deubiquitinating activity observed with particles containing HMWP mutated at Cys24 supports its assignment as the enzyme nucleophile (23). It also validates the assay by demonstrating that there is no substrate cleavage in the absence of active HMWP and directly links deubiquitinating activity with HMWP.
The H162A mutant also abolished deubiquitinating activity, supporting its critical involvement in the catalytic triad (23). The reduced DUB probe binding by this mutant may be due to weakening the reactivity of Cys24 as a nucleophile or to a required direct interaction between H162 and substrate, or both. We infer from the much better probe binding by full-length H162A HMWP than by the first 400 amino acids of its sequence (compare Fig. 1 and 6) that regions of the protein beyond those first 400 residues are involved directly (they interact with substrate) or indirectly (they contribute to folding of the enzyme domain) in substrate recognition and binding. We also note that while there are potential advantages to evaluating the DUB activity of HMWP in the context of virus particles (e.g., minimal disruption of native intermolecular interactions and obviation of the challenge of expressing and purifying 253-kDa protein), optimization of reaction conditions and limitations of this approach remain to be established.
At least six cellular DUBs were detected in both infected and noninfected cells (indicated by asterisks in Fig. 2C). Two of these are the upper band 1 protein and the band 2 protein seen in Fig. 2 and are tentatively identified as USP9X (FAM, 293 kDa, predicted) and UCH-L1 (25 kDa, predicted), respectively, based on their relative abundance and size. USP9X is one of the largest DUBs reported, and UCH-L1 is one of the smallest, most abundant, and potentially most responsive to conditions of physiological stress (37). Other cellular DUBs detected in these lysates are in the size ranges of UCH-L3 (26 kDa, predicted; low abundance), UCH-L5 (UCH37, 37 kDa, predicted; low abundance), USP15 (114 kDa, predicted; moderate abundance), and USP7 (HAUSP, 129 kDa, predicted; low abundance). Their relative amounts were little affected by infection with HCMV.
Virus mutants altered in the DUB activity of HMWP were made primarily to uncover the function of this enzyme during virus replication. Although synthesized late during virus replication, mutants of VP1/2 and the pseudorabies virus homolog have been shown to affect both early and late stages of infection (12-14, 28, 35). At late times, possible sequestering of HMWP and its counterparts into maturing particles could focus, if not restrict, their DUB activity to the nearest neighbors within the particle. At early times of infection, VP1/2 may remain with the capsid and its DUB activity may help direct movement to the nuclear pore complex. Our initial observations by electron microscopy revealed no obvious differences, at late times of infection, between cells infected with the C24I HMWP mutant and those infected with wild-type virus. And yet, yields of extracellular particles, dense bodies in particular, appear to be reduced (data not presented). This has directed our attention to the possibility that the defect may be manifest during early stages of infection where HMWP DUB activity may modulate interactions with cellular proteins or where effects of improperly modified proteins of the mutant virion may be more apparent.
Acknowledgments
The experiments that prompted and expedited this study were done by Mary-Elizabeth Harmon as part of her thesis work on HCMV pUL48, in the pharmacology graduate program at JHMI. We thank Jian Zhang for expert help with the electron microscopy presented in Fig. 8, Richard Courtney for generously providing the anti-VP1/2 antiserum used to identify HSV-1 VP1/2 in Fig. 3, Ronald Schnaar for providing us with easy access to the inverted fluorescence microscope, Peter Pedersen for suggesting CHAPS as a solubilizing agent, and Cecile Pickart for valuable advice.
L.M.K. is in the virology graduate program at Harvard Medical School and is supported by NIH training grant T32 AI07638. This work was aided by USPHS research grant AI13718 to W.G.
Footnotes
We dedicate this article to the memory of Cecile M. Pickart, a leading investigator of the structure and regulatory involvements of ubiquitin and a generous colleague.
REFERENCES
- 1.Bacchetti, S., M. J. Evelegh, and B. Muirhead. 1986. Identification and separation of the two subunits of the herpes simplex virus ribonucleotide reductase. J. Virol. 57:1177-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banks, L. M., I. W. Halliburton, D. J. Purifoy, R. A. Killington, and K. L. Powell. 1985. Studies on the herpes simplex virus alkaline nuclease: detection of type-common and type-specific epitopes on the enzyme. J. Gen. Virol. 66:1-14. [DOI] [PubMed] [Google Scholar]
- 3.Batterson, W., D. Furlong, and B. Roizman. 1983. Molecular genetics of herpes simplex virus. VIII. Further characterization of a temperature-sensitive mutant defective in release of viral DNA and in other stages of viral reproductive cycle. J. Virol. 45:397-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bechtel, J. T., and T. Shenk. 2002. Human cytomegalovirus UL47 tegument protein functions after entry and before immediate-early gene expression. J. Virol. 76:1043-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borodovsky, A., H. Ovaa, N. Kolli, T. Gan-Erdene, K. D. Wilkinson, H. L. Ploegh, and B. M. Kessler. 2002. Chemistry-based functional proteomics reveals novel members of the deubiquitinating enzyme family. Chem. Biol. 9:1149-1159. [DOI] [PubMed] [Google Scholar]
- 6.Borst, E., and M. Messerle. 2000. Development of a cytomegalovirus vector for somatic gene therapy. Bone Marrow Transplant. 25(Suppl. 2):S80-S82. [DOI] [PubMed] [Google Scholar]
- 7.Boutell, C., S. Sadis, and R. D. Everett. 2002. Herpes simplex virus type 1 immediate-early protein ICP0 and its isolated RING finger domain act as ubiquitin E3 ligases in vitro. J. Virol. 76:841-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caradonna, S., D. Worrad, and R. Lirette. 1987. Isolation of a herpes simplex virus cDNA encoding the DNA repair enzyme uracil-DNA glycosylase. J. Virol. 61:3040-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coscoy, L., D. J. Sanchez, and D. Ganem. 2001. A novel class of herpesvirus-encoded membrane-bound E3 ubiquitin ligases regulates endocytosis of proteins involved in immune recognition. J. Cell Biol. 155:1265-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crute, J. J., T. Tsurumi, L. A. Zhu, S. K. Weller, P. D. Olivo, M. D. Challberg, E. S. Mocarski, and I. R. Lehman. 1989. Herpes simplex virus 1 helicase-primase: a complex of three herpes-encoded gene products. Proc. Natl. Acad. Sci. USA 86:2186-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cunningham, C., A. J. Davison, A. Dolan, M. C. Frame, D. J. McGeoch, D. M. Meredith, H. W. Moss, and A. C. Orr. 1992. The UL13 virion protein of herpes simplex virus type 1 is phosphorylated by a novel virus-induced protein kinase. J. Gen. Virol. 73:303-311. [DOI] [PubMed] [Google Scholar]
- 12.Desai, P. 2000. A null mutation in the UL36 gene of herpes simplex virus type 1 results in the accumulation of unenveloped DNA-filled capsids in the cytoplasm of infected cells. J. Virol. 74:11608-11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desai, P., J. C. Akpa, and S. Person. 2003. Residues of VP26 of herpes simplex virus type 1 that are required for its interaction with capsids. J. Virol. 77:391-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desai, P., G. Sexton, J. McCaffery, and S. Person. 2001. A null mutation in the gene encoding the UL37 polypeptide of herpes simplex virus type 1 abrogates virus maturation. J. Virol. 75:10259-10271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunn, W., C. Chou, H. Li, R. Hai, D. Patterson, V. Stolc, H. Zhu, and F. Liu. 2003. Functional profiling of a human cytomegalovirus genome. Proc. Natl. Acad. Sci. USA 100:14223-14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frame, M. C., F. C. Purves, D. J. McGeoch, H. S. Marsden, and D. P. Leader. 1987. Identification of the herpes simplex virus protein kinase as the product of viral gene US3. J. Gen. Virol. 68:2699-2704. [DOI] [PubMed] [Google Scholar]
- 17.Gibson, W. 1981. Structural and nonstructural proteins of strain Colburn cytomegalovirus. Virology 111:516-537. [DOI] [PubMed] [Google Scholar]
- 18.Gibson, W., and B. Roizman. 1972. Proteins specified by herpes simplex virus. VIII. Characterization and composition of multiple capsid forms of subtypes 1 and 2. J. Virol. 10:1044-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harmon, M.-E., and W. Gibson. 1996. High molecular weight virion protein of human cytomegalovirus forms complex with product of adjacent open reading frame, abstr. W35-4, p. 144. Proc. Am. Soc. Virol.
- 20.He, Z., Y. S. He, Y. Kim, L. Chu, C. Ohmstede, K. K. Biron, and D. M. Coen. 1997. The human cytomegalovirus UL97 protein is a protein kinase that autophosphorylates on serines and threonines. J. Virol. 71:405-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Honess, R. W., and B. Roizman. 1974. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J. Virol. 14:8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irmiere, A., and W. Gibson. 1983. Isolation and characterization of a noninfectious virion-like particle released from cells infected with human strains of cytomegalovirus. Virology 130:118-133. [DOI] [PubMed] [Google Scholar]
- 23.Kattenhorn, L. M., G. A. Korbel, B. M. Kessler, E. Spooner, and H. L. Ploegh. 2005. A deubiquitinating enzyme encoded by HSV-1 belongs to a family of cysteine proteases that is conserved across the family Herpesviridae. Mol. Cell 19:547-557. [DOI] [PubMed] [Google Scholar]
- 24.Kaufmann, S. H., C. M. Ewing, and J. H. Shaper. 1987. The erasable western blot. Anal. Biochem. 161:89-95. [DOI] [PubMed] [Google Scholar]
- 25.Kit, S., and D. R. Dubbs. 1965. Properties of deoxythymidine kinase partially purified from noninfected mouse fibroblast cells. Virology 26:16-27. [DOI] [PubMed] [Google Scholar]
- 26.Klupp, B. G., W. Fuchs, H. Granzow, R. Nixdorf, and T. C. Mettenleiter. 2002. Pseudorabies virus UL36 tegument protein physically interacts with the UL37 protein. J. Virol. 76:3065-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klupp, B. G., H. Granzow, E. Mundt, and T. C. Mettenleiter. 2001. Pseudorabies virus UL37 gene product is involved in secondary envelopment. J. Virol. 75:8927-8936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knipe, D. M., W. Batterson, C. Nosal, B. Roizman, and A. Buchan. 1981. Molecular genetics of herpes simplex virus. VI. Characterization of a temperature-sensitive mutant defective in the expression of all early viral gene products. J. Virol. 38:539-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemaster, S., and B. Roizman. 1980. Herpes simplex virus phosphoproteins. II. Characterization of the virion protein kinase and of the polypeptides phosphorylated in the virion. J. Virol. 35:798-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Little, S. P., and P. A. Schaffer. 1981. Expression of the syncytial (syn) phenotype in HSV-1, strain KOS: genetic and phenotypic studies of mutants in two syn loci. Virology 112:686-702. [DOI] [PubMed] [Google Scholar]
- 31.Liu, F., and B. Roizman. 1991. The herpes simplex virus 1 gene encoding a protease also contains within its coding domain the gene encoding the more abundant substrate. J. Virol. 65:5149-5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loveland, A. N., C. K. Chan, E. J. Brignole, and W. Gibson. 2005. Cleavage of human cytomegalovirus protease pUL80a at internal and cryptic sites is not essential but enhances infectivity. J. Virol. 79:12961-12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McNabb, D., and R. Courtney. 1992. Characterization of the large protein (ICP1/2) of herpes simplex virus type 1. Virology 190:221-232. [DOI] [PubMed] [Google Scholar]
- 34.McNabb, D. S., and R. J. Courtney. 1992. Analysis of the UL36 open reading frame encoding the large tegument protein (ICP1/2) of herpes simplex virus type 1. J. Virol. 66:7581-7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mettenleiter, T. C. 2002. Herpesvirus assembly and egress. J. Virol. 76:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muyrers, J. P., Y. Zhang, V. Benes, G. Testa, W. Ansorge, and A. F. Stewart. 2000. Point mutation of bacterial artificial chromosomes by ET recombination. EMBO Rep. 1:239-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ovaa, H., B. M. Kessler, U. Rolen, P. J. Galardy, H. L. Ploegh, and M. G. Masucci. 2004. Activity-based ubiquitin-specific protease (USP) profiling of virus-infected and malignant human cells. Proc. Natl. Acad. Sci. USA 101:2253-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Preston, V. G., and F. B. Fisher. 1984. Identification of the herpes simplex virus type 1 gene encoding the dUTPase. Virology 138:58-68. [DOI] [PubMed] [Google Scholar]
- 39.Purifoy, D. J., R. B. Lewis, and K. L. Powell. 1977. Identification of the herpes simplex virus DNA polymerase gene. Nature 269:621-623. [DOI] [PubMed] [Google Scholar]
- 40.Roby, C., and W. Gibson. 1986. Characterization of phosphoproteins and protein kinase activity of virions, noninfectious enveloped particles, and dense bodies of human cytomegalovirus. J. Virol. 59:714-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scheffczik, H., C. G. Savva, A. Holzenburg, L. Kolesnikova, and E. Bogner. 2002. The terminase subunits pUL56 and pUL89 of human cytomegalovirus are DNA-metabolizing proteins with toroidal structure. Nucleic Acids Res. 30:1695-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schlieker, C., G. A. Korbel, L. M. Kattenhorn, and H. L. Ploegh. 2005. A deubiquitinating activity is conserved in the large tegument protein of the herpesviridae. J. Virol. 79:15582-15585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spear, P. G., and B. Roizman. 1972. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J. Virol. 9:143-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trus, B. L., W. Gibson, N. Cheng, and A. C. Steven. 1999. Capsid structure of simian cytomegalovirus from cryoelectron microscopy: evidence for tegument attachment sites. J. Virol. 73:2181-2192. (Erratum, 73:4530.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Underwood, M. R., R. J. Harvey, S. C. Stanat, M. L. Hemphill, T. Miller, J. C. Drach, L. B. Townsend, and K. K. Biron. 1998. Inhibition of human cytomegalovirus DNA maturation by a benzimidazole ribonucleoside is mediated through the UL89 gene product. J. Virol. 72:717-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vittone, V., E. Diefenbach, D. Triffett, M. W. Douglas, A. L. Cunningham, and R. J. Diefenbach. 2005. Determination of interactions between tegument proteins of herpes simplex virus type 1. J. Virol. 79:9566-9571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Welch, A. R., A. S. Woods, L. M. McNally, R. J. Cotter, and W. Gibson. 1991. A herpesvirus maturational protease, assemblin: identification of its gene, putative active site domain, and cleavage site. Proc. Natl. Acad. Sci. USA 88:10792-10796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang, Y., F. Buchholz, J. P. Muyrers, and A. F. Stewart. 1998. A new logic for DNA engineering using recombination in Escherichia coli. Nat. Genet. 20:123-128. [DOI] [PubMed] [Google Scholar]
- 49.Zhou, Z. H., D. H. Chen, J. Jakana, F. J. Rixon, and W. Chiu. 1999. Visualization of tegument-capsid interactions and DNA in intact herpes simplex virus type 1 virions. J. Virol. 73:3210-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu, L. A., and S. K. Weller. 1992. The six conserved helicase motifs of the UL5 gene product, a component of the herpes simplex virus type 1 helicase-primase, are essential for its function. J. Virol. 66:469-479. [DOI] [PMC free article] [PubMed] [Google Scholar]