Abstract
Only five monoclonal antibodies (MAbs) neutralizing a broad range of primary isolates (PI) have been identified up to now. We have found that some MAbs with no neutralizing activities according to the “conventional” neutralization assay, involving phytohemagglutinin-stimulated peripheral blood mononuclear cells as targets, efficiently inhibit the replication of human immunodeficiency virus type 1 (HIV-1) PI in macrophages and immature dendritic cells (iDC). The mechanism of inhibition is distinct from the neutralization of infectivity occurring via Fab fragments and involves the interaction of the F portion with the FcγRs present on macrophages and iDC. We propose that, if such nonneutralizing inhibitory antibodies limit mucosal HIV transmission, they should be induced by vaccination.
It is now generally recognized that neutralizing antibodies (NAbs) constitute one of the elements of the adaptative immune response that must be induced by an effective vaccine against human immunodeficiency virus (HIV). Complete protection of macaques from experimental challenge was obtained after a passive transfer of NAbs (4, 12, 15). However, only five broadly NAbs have been identified and attempts to induce strong and broad NAb responses by immunization have failed. Broadly NAbs are also infrequently detected in sera from HIV type 1-infected individuals (13). NAbs are identified in vitro using “conventional” neutralization assays with phytohemagglutinin (PHA)-stimulated peripheral blood mononuclear cells (PBMC) as targets. This assay is based on the evaluation of HIV replication in CD4+ T lymphocytes. Nevertheless, macrophages and dendritic cells (DC) are also permissive for HIV. Macrophages are considered a major reservoir of HIV in vivo, and immature DC (iDC) are among the first cells infected by HIV after mucosal transmission (1, 9). Thus, antibodies induced by immunization should also prevent HIV infection of these target cells.
To study the ability of MAbs to inhibit viral growth in various cells, the activity of anti-HIV MAbs was analyzed in vitro using a neutralization assay that measures, by flow cytometry, the percentage of infected cells by detecting intracellular p24 in lymphocytes obtained from PHA-PBMC (6, 11) and in macrophages (6) or iDC (7) generated by the differentiation of CD14+ cells. Briefly, antibodies (Abs) were incubated for 1 h with viruses at concentrations of 2 to 10 μg/ml of p24, depending on the stain and the target cell (to reach 2 to 5% cells infected after a single round of infection) and then the mixture was added to target cells. This technique has the advantage of allowing the characterization of infected cells by phenotyping. For the five previously described broadly NAbs, we found higher inhibitory activity when iDC instead of PHA-stimulated PBMC were used as target cells and an even higher activity when macrophages were the HIV targets (Table 1). When two subtype B primary isolates (PI), HIV-1BaL (obtained from the NIH) and Bx08 (courtesy of H. Fleury), were tested with each of the NAbs, activities were increased by 16- to 12,000-fold when macrophages and iDC were used as target cells compared to activities measured with PBMC. With a subtype C PI TV1 (obtained from S. Engelbrecht), a virus described as relatively “resistant” to neutralization (10), no neutralization was noted with MAbs IgG1b12 or 447-52D, but with the other three MAbs, increases of 8- to 2,000-fold in activity were found with macrophages and iDC compared to those with PBMC. Contrary to the change in inhibitory concentrations noted with the MAbs, the 90% inhibitory concentrations (IC90) for soluble CD4 and the T20 fusion inhibitor were similar on the three different target cells (Table 1), confirming previously published data for T20 (8). These results suggest that the increased inhibitory activity detected in iDC and macrophages is not due to a lower density of CD4 or to a reduction in virus fusion.
TABLE 1.
Inhibition of HIV-1BaL, Bx08, and TV1 by neutralizing MAbs, sCD4, and T20 when PHA-stimulated PBMC, macrophages, or iDC were used as target cells
| MAb name | Epitope or target | IC90 (μg/ml)a
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PHA-stimulated cells
|
iDC
|
Macrophages
|
||||||||
| BaL | Bx08 | TV1 | BaL | Bx08 | TV1 | BaL | Bx08 | TV1 | ||
| 2F5 | gp41 (aa 662-667) | 30b | 40b | 50b | 1d | 2c | 6c | 0.0025f | 0.01 | 0.2c |
| IgG1 b12 | CD4 binding site | 25b | 50b | >100 | 1d | 2c | >50 | 0.5d | 5c | >40 |
| 447-52D | Crown of the V3 loop | 50b | 50b | >100 | 2c | 5c | >50 | 0.5d | 0.2e | >50 |
| 4E10 | gp41 (aa 671-676) | 50b | 60b | 100b | 5c | 3c | 1.5d | 0.06e | 0.2e | <0.1e |
| 2G12 | gp120 (carbohydrate) | 20b | 100b | >100 | 1d | 2c | 5c | 0.05e | 0.1e | 1d |
| sCD4 | gp120 protein | 15b | 40b | 10c | 10b | 40b | 10c | 5c | 20b | 5c |
| T20 | gp41 fusion domain | 1d | 1d | 1d | 0.5d | 2c | 1d | 0.5d | 1d | 1d |
IC90 correspond to the concentrations (μg/ml) of MAbs or recombinant proteins that lead to a 90% reduction in the percentage of infected cells.
Range, 11 to 100 μg/ml.
Range, 2 to 10 μg/ml.
Range, 0.3 to 1.9 μg/ml.
Range, 0.05 to 0.2 μg/ml.
Range, 0.005 to 0.04 μg/ml.
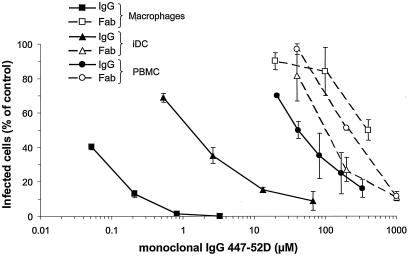
Next, the mechanism of HIV inhibition was investigated. The iDC and macrophages express FcγRs on their surfaces. These receptors are involved in immune complex capture, endocytosis, and degradation. To define their roles in HIV inhibition, we analyzed the inhibitory activity of the Fab fragments of MAb 447-52D (provided by R. Stanfield) (Fig. 1). Contrary to whole immunoglobulin G (IgG), 447-52D Fabs exhibit similar inhibition of HIV-1BaL infection in PBMC, macrophages, and iDC. This result strongly suggests a role for the Fc portion of the IgG in the increased inhibitory activity observed with macrophages and iDC.
FIG. 1.
Inhibition of HIV replication by MAb 447-52D (whole IgG) or its corresponding Fab fragment in PHA-stimulated PBMC, macrophages, and iDC. Values correspond to the percentage of infected cells in the presence of different dilutions of 447-52D versus infected cells without Ab (control infected cells). Mean and standard deviation of two independent wells obtained for one representative experiment are shown.
Given these observations, we extended the studies by determining the IC90 of additional anti-HIV MAbs (Table 2). Of the 45 MAbs to various epitopes of gp120 and gp41 tested, only two, MAbs F425B4e8 and 391-D directed against the V3 loop, were able to inhibit HIV-1BaL infection of PHA-stimulated PBMC. In contrast, 13 of the 45 MAbs tested inhibit HIV-1BaL replication in macrophages. These 13 MAbs mainly recognize either a linear epitope on the V3 loop of gp120 or the principal immunodominant domain (PID) of gp41. In addition, one MAb that was active in the macrophage-based assay recognizes a conformational epitope on gp120 (anti-C5 MAb 221) and one is directed against amino acids (aa) 731 to 752 of gp41 (MAb 1577) (Table 2). Most of these MAbs also inhibit the replication of Bx08, and the three MAbs directed against the PID inhibit the “neutralization-resistant” strain TV1 (Table 3). These latter results with MAbs to the PID are particularly notable since MAbs to this region have not previously been shown to exhibit potent neutralizing activity (3, 5, 17). These results indicate that some MAbs exhibiting low or no inhibitory activity on T lymphocytes are able to successfully inhibit the replication of three PI in macrophages. Moreover, six of nine MAbs tested were able to diminish HIV-1BaL replication in iDC (Table 3).
TABLE 2.
Inhibition of HIV-1BaL replication in PHA-stimulated PBMC and macrophages by MAbs directed against various HIV epitopesa
| Protein | Reference no. | MAb name | Ig | Epitope | IC90 (μg/ml or dilution)b
|
|
|---|---|---|---|---|---|---|
| PBMC | Macrophages | |||||
| gp120 | ARP 301 | 221 | IgG1 | gp160/gp120 (aa 482-495) C term of gp120 | >50 | 20c |
| NIH 857 | F105 | IgG1k | Conformational gp120 | >100 | >50 | |
| NIH 7369 | 654-30D | IgG1I | Tertary gp120 | ND | Ni | |
| ARP 3119 | CA13 | IgG1 | Cross reactive to env | ND | Ni | |
| ARP 390 | ICR39.13 | IgG2b | Conformational (gp120) | Ni | Ni | |
| ARP 3041 | 11/68b | IgG1 | gp120 (V1, V2+C4) | Ni | Ni | |
| V3 loop | ARP 3036 | 8/64b | IgM | V3 (aa 300-315) | ND | Ni |
| ARP 3038 | 10/540.w | IgG1 | V3 (aa 311-321) | Ni | Ni | |
| ARP 3039 | 8/38 | IgG2a | V3 (aa 300-315) | ND | Ni | |
| EVA 331 | 178.1.1 | IgG2ak | V3 (to KSIRI sequence) | Ni | Ni | |
| EVA 3047 | IIIB-V3-13 | IgG1 | V3 (IRIQRGPGRAFTIGC sequence) | >50 | 3c | |
| ARP 3023 | 257-D IV | IgG1λ | V3 (KRIHI sequence) | >9.4 | 0.05c | |
| ARP 3024 | 268-D IV | IgG1λ | V3 (HIGPGR sequence) | >14 | 1c | |
| EVA 3056 | MN215 | IgG1 | V3 KS/GIHIGPGKAFYTTGEI sequence) | >125 | 10c | |
| NIH 7625 | F425B4a1 | IgG1λ | V3 | >33 | 0.4c | |
| 391-D | IgG1k | V3 | 20c | 1c | ||
| NIH 7626 | F425B4e8 | IgG1k | Base of V3 loop | 25c | 1c | |
| NIH 2534 | 4G10 | IgG1 | V3 (RIQRGPGRAFVTGK) | ND | Ni | |
| V2 | ARP 324 | CRA3 | IgG2a | Conformational (V2 and C1) | Ni | Ni |
| ARP 325 | CRA4 | IgG1 | Conformational (V2) | Ni | Ni | |
| ARP 3075 | 62c | IgG1 | Conformational (V2) | Ni | Ni | |
| ARP 3218 | 697D | IgG1λ | Conformational (V2, region 164-194) | >43 | >20 | |
| C2, C4, C5 | 847-30 | IgG1λ | C2 | ND | >50 | |
| ARP 388 | ICR38 | IgG2b | C4 (aa 427-436) | Ni | Ni | |
| 858-D | IgG3λ | C5 (aa 495-516) | >100 | >50 | ||
| 1331A | IgG3λ | C5 (aa 495-516) | >100 | >50 | ||
| 450-D | IgG1λ | C5 (aa 503-509) | >100 | >50 | ||
| 722-D | IgG1k | C5 (aa 503-509) | ND | >50 | ||
| ARP 3221 | 670D | IgG1λ | C5 (aa 503-509) | >100 | >50 | |
| CD4b5 | EVA 3055 | GP68 | IgG1 | CD4 binding site | Ni | Ni |
| ARP 3220 | 654D | IgG1λ | CD4 binding site (discontinious) | >25 | >20 | |
| 570-D | IgG1λ | CD4 binding site | >100 | >50 | ||
| 654-D | IgG1λ | CD4 binding site | >100 | >50 | ||
| ARP 3078 | 1.7B | IgG1 | CD4 induced | >50 | >50 | |
| ARP 3079 | 4.8D | IgG1 | CD4 induced | >50 | >50 | |
| gp41 | NIH 6882 | 5F3 | IgG1λ | gp41 (526-543) | ND | >100 |
| 181-D | IgG1k | gp141 (I)d | >100 | >50 | ||
| 240-D | IgG1k | gp41 (aa 579-604) (I) | >35 | 1.8c | ||
| 246-D | IgG1k | gp41 (aa 579-604) (I) | >100 | 0.8c | ||
| 50-69 | IgG1λ | gp41 (aa 579-613 conformational) (I) | >100 | 0.4c | ||
| NIH 7623 | F240 | IgG1k | gp41 (aa 592-606) | >100 | 0.5c | |
| 98-6D | IgG1k | gp41 (aa 644-663) (II)e | >100 | >50 | ||
| 126-6 | IgG1k | gp41 (aa 644-663) (II) | >100 | >50 | ||
| 167-D | IgG1λ | gp41 (aa 644-663) (II) | >100 | >50 | ||
| NIH 1172 | 1577 | IgG3 | gp41 (731-752) | >100 | 25c | |
MAbs, selected because they either bind to native env or exhibit a restricted neutralizing activity, were obtained through the NIBSC, the NIH, or our laboratory (SZP).
Values correspond to the ID90 (μg/ml). Ni, no inhibitory activity detected at the dilution half of the supernatant provided; ND, not done.
Boldface type indicates detection of inhibitory activity.
Immunodominant domain of gp41 (amino acids 598 to 604, cluster I).
Immunodominant domain of gp41 (amino acids 644 to 663, cluster II).
TABLE 3.
Inhibitory activity of MAbs against HIV-1BaL, Bx08, and TV1 replication in macrophages
| Protein | MAb name | Epitope (sequence or domain) | IC90 (μg/ml)a
|
|||||
|---|---|---|---|---|---|---|---|---|
| PBMC
|
iDC | Macrophages
|
||||||
| BaL | Bx08 | BaL | BaL | Bx08 | TV1 | |||
| gp120 | 221 | gp160/gp120 (aa 482-495) | >50 | >100 | ND | 20b | 50b | >100 |
| V3 loop | IIIB-V3-13 | V3 (IRIQRGPGRAFTIGC) | >50 | >100 | ND | 3 | ND | ND |
| 257-D IV | V3 (KRIHI sequence) | >9.4 | ND | 10c | 0.05e | 0.3d | >9 | |
| 268-D IV | V3 (HIGPGR sequence) | >14 | >14 | 15b | 1d | 0.5d | >15 | |
| MN215 | V3 (KS/GIHIGPGKAFYTTGEI) | >125 | >125 | >50 | 10c | 40b | 20b | |
| 391-D | V3 | 20b | 50b | ND | 1d | 0.8d | 20b | |
| F425B4a1 | V3 | >33 | ND | ND | 0.4d | ND | ND | |
| F425B4e8 | Base of V3 loop | 25b | 100b | 4c | 1d | 5c | >30 | |
| gp41 | 240-D | gp41 (aa 579-604) (I)f | >35 | >35 | 20b | 1.8d | 1.9d | 0.5d |
| 246-D | gp41 (aa 579-604) (I) | >100 | >100 | 45b | 0.8d | 0.5d | 0.06e | |
| 50-69 | gp41 (aa 579-613 conformational) | >100 | >100 | >50 | 0.4d | 0.8d | 0.6d | |
| F240 | gp41 (aa 592-606) | >100 | >100 | 50b | 0.5d | 0.6d | 15b | |
| 1577 | gp41 (aa 731-752) | >100 | >100 | >100 | 25b | 100b | >100 | |
Values correspond to the ID90 (μg/ml) determined when PHA-stimulated PBMC, macrophages, or iDC were used as target cells. ND, not done.
Range, 11 to 100 μg/ml.
Range, 2 to 10 μg/ml.
Range, 0.3 to 1.9 μg/ml.
Range, 0.05 to 0.2 μg/ml.
Immunodominant domain of gp 41 (cluster I).
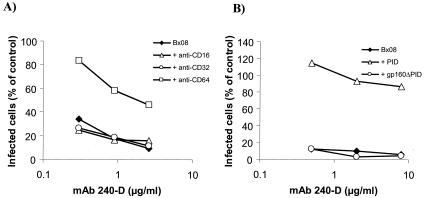
To determine whether the Fc portions of these IgGs bind to the FcγRs on the surface of macrophages and iDC, FcγRs were blocked by incubation for 30 min with 10 μg/ml of anti-FcγRI, anti-FcγRII, or anti-FcγRIII (BD-Pharmingen, San Diego, CA). The anti-FcγR Abs were added to cells before the addition of virus and MAb mixtures using conditions similar to those for neutralization assay. The inhibitory activity of MAb 240 on Bx08 replication was reversed after blockage of FcγRI in macrophages (Fig. 2A) and FcγRII in iDC (data not shown), demonstrating that binding of the Fc portion of IgG MAb 240-D to FcγR participates in HIV inhibition in macrophages and iDC. Moreover, the inhibitory activity of MAb 240-D targeting the PID was abolished in macrophages (Fig. 2B) and iDC (not shown) when 25 μg/ml of peptide corresponding to the gp41 PID (aa 593 to 616, ARP7022; obtained through the NIBSC) was added to the Ab-Bx08 mixture 1 h prior to incubation with target cells, whereas the addition of rgp160ΔPID (gp160 of Bx08 deleted from PID; kind gift from R. ElHabib, Aventis Pasteur) at 12.5 μg/ml had no effect. This competition experiment shows that the binding of the Fab portion of the MAb is necessary for HIV inhibition.
FIG. 2.
Involvement of MAb 240-D paratope and Fc domains in its inhibitory activity of HIV replication in macrophages. (A) FcγRs were blocked by the addition of 10 μg of anti-FcγRI (CD64), anti-FcγRII (CD32), or anti-FcγRIII (CD16) to macrophages for 30 min before the addition of the virus-MAb 240 mixture using the conditions of the neutralization assay. (B) Competition experiments were performed by mixing, in conditions of neutralization assays, MAb 240-D and Bx08 with peptide PID (gp41, aa 593 to 616) at 25 μg/ml or rgp160ΔPID at 12.5 μg/ml. Then, 1 h later, the mixture was added to macrophages. The percentages of infected cells were determined by flow cytometry.
We have demonstrated that for macrophages and iDC, the inhibition of HIV replication by Abs can occur by two distinct mechanisms, the first consisting of the neutralization of infectivity (which involves only the Fab part of the IgG) and the second depending on IgG-FcγR interaction, probably leading to endocytosis and the degradation of opsonized HIV particles. Abs that act only via the second mechanism in macrophages and iDC could be referred as nonneutralizing inhibitory Abs (NNiAbs) to distinguish them from NAbs displaying both mechanism of inhibition and neutralizing PBMC infection. The NNiAbs are directed against epitopes distinct from those recognized by NAbs and will not impair virus entry into cells. These Abs may not necessarily recognize functional envelope spikes, but they will link infectious virus particles to the target cell by efficient binding of the Fc region of IgG to FcγR and by binding of Fab regions to the HIV envelope. Binding of Ab to HIV is, however, apparently not sufficient for the inhibition of virus replication, as anti-C5 MAb 670D, for example, which binds to virus particles (16), was not able to inhibit HIV replication in macrophages (Table 2). The inhibition of HIV by Ab may also depend on the affinity of the Fc part of the Ab for FcγR and on an occupancy threshold below which MAb binds but does not inhibit. These parameters may also be involved in virus inhibition. We showed that NNiAbs were mainly directed against the V3 loop and the PID. These two regions are accessible and highly immunogenic domains of HIV. Indeed, Abs directed to V3 loop and PID are detected in sera from HIV-infected individuals (2, 14) and the inhibition of HIV replication in macrophages (6) and iDC (7) has been evidenced with such sera.
We propose that NNiAbs that are not detected by using conventional assays using PBMC as target cells could participate in vivo in the protection of mucosal HIV transmission by preventing the infection of macrophages and iDC. These results, demonstrating new categories of protective Abs, may open new perspectives in the development and design of vaccines.
Acknowledgments
This work was supported by grants from the Agence Nationale de Recherches sur le SIDA, the European Union (QLK2-CT-199-01321 “Eurovac”), and the NIH (AI36085, HL59725, and AI 27742) and funds from the U.S. Department of Veterans Affairs.
REFERENCES
- 1.Aquaro, S., R. Calio, J. Balzarini, M. C. Bellocchi, E. Garaci, and C. F. Perno. 2002. Macrophages and HIV infection: therapeutical approaches toward this strategic virus reservoir. Antivir. Res. 55:209-225. [DOI] [PubMed] [Google Scholar]
- 2.Burrer, R., S. Haessig-Einius, A. M. Aubertin, and C. Moog. 2005. Neutralizing as well as non-neutralizing polyclonal immunoglobulin (Ig)G from infected patients capture HIV-1 via antibodies directed against the principal immunodominant domain of gp41. Virology 333:102-113. [DOI] [PubMed] [Google Scholar]
- 3.Cavacini, L. A., C. L. Emes, A. V. Wisnewski, J. Power, G. Lewis, D. Montefiori, and M. R. Posner. 1998. Functional and molecular characterization of human monoclonal antibody reactive with the immunodominant region of HIV type 1 glycoprotein 41. AIDS Res. Hum. Retrovir. 14:1271-1280. [DOI] [PubMed] [Google Scholar]
- 4.Ferrantelli, F., and R. M. Ruprecht. 2002. Neutralizing antibodies against HIV—back in the major leagues? Curr. Opin. Immunol. 14:495-502. [DOI] [PubMed] [Google Scholar]
- 5.Hioe, C. E., S. Xu, P. Chigurupati, S. Burda, C. Williams, M. K. Gorny, and S. Zolla-Pazner. 1997. Neutralization of HIV-1 primary isolates by polyclonal and monoclonal human antibodies. Int. Immunol. 9:1281-1290. [DOI] [PubMed] [Google Scholar]
- 6.Holl, V., S. Hemmerter, R. Burrer, S. Schmidt, A. Bohbot, A. M. Aubertin, and C. Moog. 2004. Involvement of Fc gamma RI (CD64) in the mechanism of HIV-1 inhibition by polyclonal IgG purified from infected patients in cultured monocyte-derived macrophages. J. Immunol. 173:6274-6283. [DOI] [PubMed] [Google Scholar]
- 7.Holl, V., M. Peressin, S. Schmidt, Decoville, T., S. Zolla-Pazner, A. M. Aubertin, and C. Moog. Efficient inhibition of HIV-1 replication in human immature monocyte derived dendritic cells by purified anti-HIV IgG without induction of maturation. Blood, in press. [DOI] [PMC free article] [PubMed]
- 8.Ketas, T. J., P. J. Klasse, C. Spenlehauer, M. Nesin, I. Frank, M. Pope, J. M. Strizki, G. R. Reyes, B. M. Baroudy, and J. P. Moore. 2003. Entry inhibitors SCH-C, RANTES, and T-20 block HIV type 1 replication in multiple cell types. AIDS Res. Hum. Retrovir. 19:177-186. [DOI] [PubMed] [Google Scholar]
- 9.Larsson, M. 2005. HIV-1 and the hijacking of dendritic cells: a tug of war. Springer Semin. Immunopathol. 26:309-328. [DOI] [PubMed] [Google Scholar]
- 10.Lian, Y., I. Srivastava, V. R. Gomez-Roman, M. J. zur, Y. Sun, E. Kan, S. Hilt, S. Engelbrecht, S. Himathongkham, P. A. Luciw, G. Otten, J. B. Ulmer, J. J. Donnelly, D. Rabussay, D. Montefiori, E. J. van Rensburg, and S. W. Barnett. 2005. Evaluation of envelope vaccines derived from the South African subtype C human immunodeficiency virus type 1 TV1 strain. J. Virol. 79:13338-13349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mascola, J. R. 2004. Neutralizing antibody quantification by flow cytometry. Methods Cell Biol. 75:709-716. [DOI] [PubMed] [Google Scholar]
- 12.Mascola, J. R., G. Stiegler, T. C. VanCott, H. Katinger, C. B. Carpenter, C. E. Hanson, H. Beary, D. Hayes, S. S. Frankel, D. L. Birx, and M. G. Lewis. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6:207-210. [DOI] [PubMed] [Google Scholar]
- 13.Moog, C., H. J. Fleury, I. Pellegrin, A. Kirn, and A. M. Aubertin. 1997. Autologous and heterologous neutralizing antibody responses following initial seroconversion in human immunodeficiency virus type 1-infected individuals. J. Virol. 71:3734-3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore, J. P., Y. Cao, D. D. Ho, and R. A. Koup. 1994. Development of the anti-gp120 antibody response during seroconversion to human immunodeficiency virus type 1. J. Virol. 68:5142-5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishimura, Y., T. Igarashi, N. L. Haigwood, R. Sadjadpour, O. K. Donau, C. Buckler, R. J. Plishka, A. Buckler-White, and M. A. Martin. 2003. Transfer of neutralizing IgG to macaques 6 h but not 24 h after SHIV infection confers sterilizing protection: implications for HIV-1 vaccine development. Proc. Natl. Acad. Sci. USA 100:15131-15136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nyambi, P. N., A. Nadas, H. A. Mbah, S. Burda, C. Williams, M. K. Gorny, and S. Zolla-Pazner. 2000. Immunoreactivity of intact virions of human immunodeficiency virus type 1 (HIV-1) reveals the existence of fewer HIV-1 immunotypes than genotypes. J. Virol. 74:10670-10680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu, J. Y., M. K. Gorny, T. Palker, S. Karwowska, and S. Zolla-Pazner. 1991. Epitope mapping of two immunodominant domains of gp41, the transmembrane protein of human immunodeficiency virus type 1, using ten human monoclonal antibodies. J. Virol. 65:4832-4838. [DOI] [PMC free article] [PubMed] [Google Scholar]