Abstract
Kaposi's sarcoma-associated herpesvirus encodes a protein, kaposin B, which is composed of multiple copies of 23-amino-acid direct repeats, termed DR2 and DR1. Kaposin B enhances the release of pathogenetically important proinflammatory cytokines by activating the p38 mitogen-activated protein kinase (MAPK)-MK2 kinase pathway and blocking cytokine mRNA decay. Here, we show that this mRNA stabilization function requires both the DR2 and DR1 elements of kaposin B; a monomeric form of the protein consisting of one copy of each repeat retains function. Furthermore, we show that p38 MAPK is capable of directly phosphorylating kaposin B in vitro and map the site of phosphorylation to a specific serine residue in DR1. Mutational ablation of this serine abolishes phosphorylation of the protein by p38 MAPK but does not affect kaposin B's ability to extend mRNA half-life.
Kaposi's sarcoma (KS) is a complex angioproliferative lesion that is the most common neoplasm of patients with untreated AIDS, although it can also arise in human immunodeficiency virus-negative hosts (9). Histologically, KS involves the proliferation of spindle-shaped endothelial cells, accompanied by local infiltration of inflammatory cells and striking neoangiogenesis. The development of all forms of KS is critically dependent upon infection by KS-associated herpesvirus (KSHV) (also called human herpesvirus 8) (2, 3). KSHV infection targets the endothelial (“spindle”) cells of the lesion, most of which are latently infected (1, 24). These latency genes are thought to extend the life span of infected cells, alter their morphology, and also contribute to the inflammatory character of KS lesions by promoting cytokine release from infected cells. The inflammatory process is also considered pathogenetically important (18), and considerable effort has been invested in uncovering the links between viral infection and proinflammatory cytokine production.
Recent studies have shown that one link between infection and cytokine release is the latent viral protein known as kaposin B (16, 20). Kaposin B consists largely of two sets of reiterated, proline-rich, 23-amino-acid direct repeats, known as DR2 and DR1 (20). The protein binds to a host cell protein kinase known as mitogen-activated protein kinase (MAPK)-associated protein kinase 2, or MK2 (16). MK2 is an important kinase in the proinflammatory p38 MAPK signaling pathway, which is designed to sense inflammatory and other stress signals (e.g., hyperosmolarity and oxidative stress) (10, 12, 13, 21). Activation of p38 MAPK results in its nuclear translocation, where it can bind and phosphorylate MK2 (8, 19). This phosphorylation leads to the export of both proteins to the cytosol, where MK2 can phosphorylate additional target proteins (7, 17, 27), including proteins that control the half-life of an important subpopulation of labile cytoplasmic mRNAs—those bearing AU-rich elements (AREs) in their 3′ untranslated regions (14). In the ground state, ARE-containing mRNAs are extremely unstable (4, 22, 23); when MK2 is activated, however, phosphorylation of its downstream cytosolic substrates results in marked stabilization of these messages (25, 28). This result is of interest because many cytokine and growth factor transcripts harbor AREs and are regulated in this fashion.
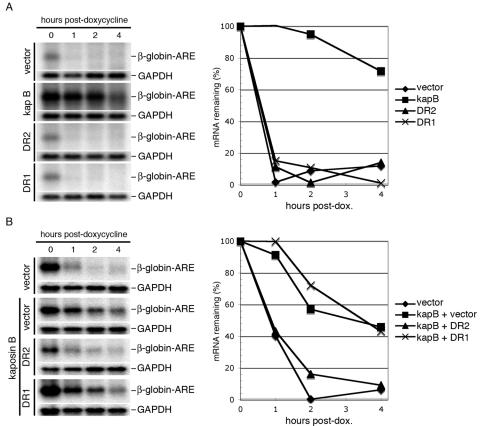
In kaposin B-expressing cells, the protein can be readily found bound to MK2, and MK2 activity is enhanced in this complex (16). Kaposin B-mediated activation of MK2 blocks the decay of several ARE-containing mRNAs, with a resulting net increase in the production of proinflammatory cytokines, such as interleukin-6 and granulocyte-macrophage colony-stimulating factor (GM-CSF) (16). We have previously shown that the binding to MK2 maps to the amino-terminal DR2 element of kaposin B (16). To determine whether the DR2 elements alone could mediate the downstream blockade in ARE-mRNA decay, we employed a previously described assay in which a β-globin reporter gene bearing a 3′ ARE (from GM-CSF) is placed under the control of a Tet operator in HeLa Tet-Off cells (5, 6, 16). These cells stably express a chimeric transcriptional activator whose activity is repressed by doxycycline. Transient transfection of these cells in the absence of the drug results in expression of the chimeric mRNA; the addition of doxycycline turns off new transcription, allowing assessment of the half-life of the transcript by Northern blotting of RNA harvested at serial time points thereafter (16). HeLa Tet-Off cells were transfected with either a control (empty) vector, a vector expressing wild-type (WT) kaposin B, a DR2 multimer, or a DR1 multimer; at 30 h posttransfection, doxycycline was added and RNA harvested at 0, 1, 2, and 4 h thereafter. As shown in Fig. 1A, the β-globin-ARE-mRNA was extremely unstable in the absence of kaposin B and was dramatically stabilized by expression of WT kaposin B. As expected, proteins composed of DR1 alone did not function in this assay. The construct containing DR2 alone was also inactive, indicating that the ability to bind MK2 in vitro is not sufficient for functional activation of MK2 in vivo. This implies that DR1 contributes an important function to kaposin B either by the presentation of DR2 to MK2 in an optimal manner or by the interaction of DR1 with other cellular components required for activation. Interestingly, expression of the DR2 elements dominantly inhibits the function of WT kaposin B in cotransfected cells (Fig. 1B) without affecting WT kaposin B protein levels. Conversely, the DR1 elements have little effect on WT kaposin B activity (Fig. 1B). Taken together, these findings suggest that each set of repeats contributes importantly to the function of the wild-type molecule.
FIG. 1.
Analysis of the kaposin B direct repeats in ARE-mRNA decay. (A) Kaposin B (kap B) DR2 and DR1 do not block ARE-mRNA decay. HeLa Tet-Off cells were cotransfected with a β-globin-based reporter (with the AU-rich element from GM-CSF inserted in its 3′ untranslated region) and test plasmids. After 30 h, doxycycline (dox.) was added to the media to stop transcription. RNA was harvested at 0, 1, 2, and 4 h after doxycycline addition; β-globin and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mRNAs were detected using 32P-labeled antisense riboprobes. (B) The DR2 elements block the function of WT kaposin B in ARE-mRNA decay. Cells were cotransfected with equal amounts of the indicated test plasmids and β-globin-ARE reporter and treated with doxycycline as described above. β-globin-ARE-mRNA levels were normalized to GAPDH-mRNA levels and expressed in terms of percent mRNA remaining.
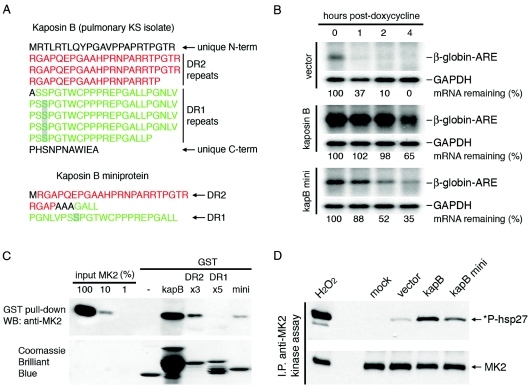
DR2 is reiterated three to five times in known strains of KSHV and is followed by longer expanses of DR1 elements; the structure of the kaposin B protein used in the present study is depicted in Fig. 2A. To determine whether multimerization of DR2 and DR1 is important for kaposin B function, we constructed a monomeric form of kaposin B containing only a single copy of DR2 and DR1. The miniprotein eliminates the short unique sequences at the N and C termini of authentic kaposin B and contains a single copy of DR2 linked by a short bridge of alanines to a single copy of DR1 (Fig. 2A). In the ARE-mRNA decay assay, the kaposin B miniprotein substantially enhanced the stability of this mRNA, though its activity was discernably less than that of the full-length isoform (Fig. 2B). The ability of the monomeric kaposin B to stabilize ARE-mRNAs predicts that this isoform should also be able to bind and activate MK2, the central regulator of ARE-mRNA decay in the cell. To address this, we tested the ability of a glutathione S-transferase (GST)-kaposin B miniprotein purified from Escherichia coli to interact in vitro with purified recombinant MK2, as judged by the ability of the complex to be precipitated by glutathione-agarose beads. Figure 2C shows that efficient MK2 binding occurs with GST-kaposin B, while none is observed with GST alone. Parallel experiments with GST-DR1 and GST-DR2 proteins mapped the interaction domain to the region encoded by DR2, consistent with previous findings (16). The kaposin B miniprotein retains the ability to bind to MK2 in vitro, although this binding is weaker than that observed for full-length kaposin B. To document that MK2 kinase activity was also enhanced by monomeric kaposin B, we transfected HEK 293T cells with full-length kaposin B or the kaposin B miniprotein for 48 h, immunoprecipitated MK2, and examined MK2 kinase activity in the precipitate (as assayed by its ability to phosphorylate a well-known substrate, Hsp27 [26]; method described in reference 16). The positive control in these kinase assays is hydrogen peroxide (H2O2), an oxidative stress and activator of p38 MAPK and MK2 (11, 16). Figure 2D shows that MK2 kinase activity was indeed augmented by expression of the kaposin B monomer. We conclude that oligomerization of the kaposin B direct repeats is not absolutely required for activity, though it clearly greatly amplifies it. These experiments also demonstrate that the unique amino- and carboxy-terminal regions of kaposin B are dispensable for MK2 activation. However, there is no question that the multimeric form of kaposin B is more active in MK2 activation; this may be because docking of multiple MK2 proteins on the scaffold increases the local concentration of MK2 chains around p38 MAPK that are also present in the complex (16). However, other models are possible, for example, multimerization of the repeats may change the tertiary structure of one or more repeats, making it more accessible to MK2 or more efficient in provoking an activating conformational change in that kinase. Further work will be necessary to distinguish among these possibilities.
FIG. 2.
The kaposin B miniprotein activates MK2 and blocks ARE-mRNA decay. (A) Primary amino acid sequence of kaposin B from a pulmonary KS isolate and the monomeric kaposin B miniprotein. Sequences are expressed in one-letter codes and aligned to highlight the 23-amino-acid direct repeats. DR2 elements are labeled in red, and DR1 elements are labeled in green. The putative p38 MAPK phosphorylation site in the DR1 element is shaded. (B) The kaposin B miniprotein blocks ARE-mediated mRNA decay. HeLa Tet-Off cells were cotransfected with a β-globin-based reporter and test plasmids. After 30 h, doxycycline was added to the media to stop transcription. RNA was harvested at 0, 1, 2, and 4 h after doxycycline addition; β-globin and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mRNAs were detected using 32P-labeled antisense riboprobes. β-Globin-ARE-mRNA levels were normalized to GAPDH mRNA levels and expressed in terms of percent mRNA remaining. (C) Kaposin B (kapB) miniprotein interacts with MK2. GST fusion proteins were incubated with purified, recombinant MK2. Complexes were SDS-PAGE purified and Western blotted (WB) with an anti-MK2 antibody (Cell Signaling Technology). (D) Kaposin B miniprotein activates MK2. MK2 was immunoprecipitated (I.P.) from cell lysates and incubated with GST-hsp27 fusion protein and ATP for 1 h at 30°C. Reaction products were immunoblotted with anti-phospho-hsp27 and anti-MK2 antibodies (Cell Signaling Technology).
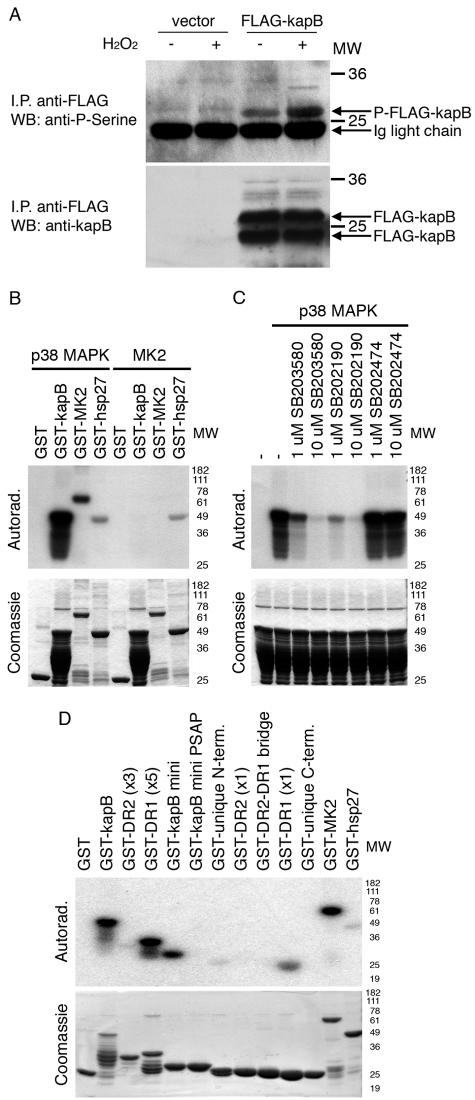
The close association of kaposin B and a host cell kinase raises the possibility that kaposin B may be a target for phosphorylation. To investigate kaposin B phosphorylation, FLAG-tagged kaposin B was immunoprecipitated from cells treated with H2O2 and immunoblotted with antiphosphoserine antibodies. Figure 3A reveals that H2O2 treatment induces a serine-phosphorylated species that comigrates with FLAG-tagged kaposin B. A review of the kaposin B sequence predicted a single major candidate site for phosphorylation by p38 (PSSP), indicated by shading in Fig. 2A (15). This site is located in the DR1 elements of the protein, a result that was of special interest given that (i) kaposin B is found in a complex with p38 in cells (16) and (ii) the DR1 elements are essential for kaposin B activity, though they appear to be dispensable for MK2 binding (Fig. 2C). Accordingly, we wondered whether phosphorylation of this site by p38 could be demonstrated and whether it was required for function in ARE-mRNA stabilization. To address this issue, in vitro p38 and MK2 kinase assays were performed using purified GST-kaposin B as a substrate. Figure 3B shows that GST-kaposin B is efficiently phosphorylated by p38 MAPK but not by MK2. This phosphorylation is not due to contaminating kinases, because the selective p38 MAPK inhibitors SB203580 and SB202190 are able to efficiently block phosphorylation of GST-kaposin B (Fig. 3C).
FIG. 3.
Kaposin B DR1 elements are phosphorylated by p38 MAPK. (A) Kaposin B (kapB) is serine phosphorylated in response to oxidative stress. HEK 293T cells were transfected with empty vector or FLAG-tagged kaposin B for 48 h and treated with 5 mM H2O2 for 15 min and lysed. FLAG-tagged kaposin B was immunoprecipitated (I.P.) with anti-FLAG antibody (Sigma) and Western blotted (WB) with rabbit antiphosphoserine antibody (Zymed) (top) and anti-kaposin B monoclonal antibody (bottom). The kaposin B monoclonal antibody is described in reference 20. (B) Kaposin B is phosphorylated by p38 MAPK but not by MK2. A total of 10 μg of the indicated fusion proteins was incubated with active p38 MAPK or active MK2 (Upstate) and [γ-32P]ATP for 30 min at 30°C in kinase buffer. (C) Selective p38 MAPK inhibitors block phosphorylation of kaposin B. GST-kaposin B was incubated with active p38 MAPK, [γ-32P]ATP, and the selective p38 MAPK inhibitor SB203580 or SB202190 or the inactive analog SB202474 (Calbiochem). (D) Mapping of the p38 MAPK phosphorylation site in kaposin B. GST fusion proteins were generated containing full-length kaposin B, the kaposin B miniprotein, and indicated kaposin protein fragments. A total of 10 μg of fusion protein was incubated with active p38 MAPK and [γ-32P]ATP for 30 min at 30°C in kinase buffer. Portions of these reaction mixtures were electrophoresed, stained with Coomassie brilliant blue (bottom), and exposed to X-ray film (top). Molecular weight standards are indicated to the right of each panel. Ig, immunoglobulin; Autorad., autoradiography.
In order to rigorously map the p38 MAPK phosphorylation site on kaposin B, we tested a series of GST fusion proteins in the p38 kinase assay, including (i) native (multimeric) kaposin B, (ii) kaposin B miniprotein, (iii) multimers of DR2, (iv) monomers of DR2, (v) multimers of DR1, and (vi) monomers of DR1. Figure 3D (bottom) indicates that all of these polypeptides were expressed comparably well in E. coli, though several of the larger multimeric versions underwent limited proteolysis during extraction. Each of these polypeptides, bound to glutathione-Sepharose beads, was then admixed with 10 ng of purified recombinant active p38 and [γ-32P]ATP. After we washed away unincorporated isotope, the radiolabeled GST fusion proteins were examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiography (Fig. 3D, top). This revealed efficient phosphorylation of native and monomeric kaposin B, as well as the multimeric and monomeric DR1 fusions. As expected, no phosphorylation was observed on either the multimeric or monomeric DR2 fusions or on GST alone, excluding the possibility of adventitious phosphorylation of irrelevant vector sequences. We also tested a short peptide (GAAHPRNPARRTPASSPGTWCPPPREP) from the junction of DR1 and DR2 that does not possess the putative PSSP phosphorylation site; this too was not phosphorylated. To map the phosphorylation site, we then mutated the putative target serine in DR1 to alanine (PSSP → PSAP), in the context of the monomeric kaposin protein. As shown in Fig. 3D, this mutation completely ablated phosphorylation by p38, consistent with the prediction that this residue is the sole site of p38 phosphorylation in the chain.
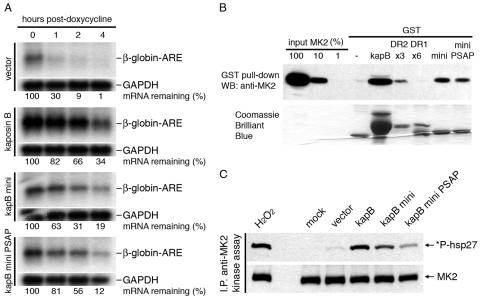
To ask whether phosphorylation at this site can have functional importance in cells, the PSAP mutant described above was introduced into a kaposin B monomer in the pCR3.1-based eukaryotic expression vector. This construct and its wild-type monomeric counterpart were tested for the ability to stabilize globin-ARE transcripts in HeLa Tet-Off cells, as described above. Figure 4A shows that the PSAP mutant miniprotein and the wild-type miniprotein possess identical ARE-mRNA-stabilizing activities. Furthermore, the p38 site mutant miniprotein retained the ability to bind to MK2 in vitro (Fig. 4B) and activate cellular MK2 kinase activity (Fig. 4C), though the level of this activation was somewhat reduced compared to those of full-length kaposin B and the kaposin B miniprotein. Thus, phosphorylation of kaposin B DR1 by p38 MAPK is dispensable for ARE-mRNA stabilization.
FIG. 4.
Mutation of the p38 phosphorylation site does not impair kaposin B activity. (A) The kaposin B (kapB) miniprotein p38 site mutant blocks ARE-mRNA decay. HeLa Tet-Off cells were cotransfected with a β-globin-based reporter and test plasmids. After 30 h, doxycycline was added to the media to stop transcription. RNA was harvested at 0, 1, 2, and 4 h after doxycycline addition; β-globin and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mRNAs were detected using 32P-labeled antisense riboprobes. β-Globin-ARE-mRNA levels were normalized to GAPDH mRNA levels and expressed in terms of percent mRNA remaining. (B) Kaposin B miniprotein p38 site mutant interacts with MK2. GST fusion proteins were incubated with purified, recombinant MK2. Complexes were SDS-PAGE purified and Western blotted (WB) with an anti-MK2 antibody. (C) Kaposin B miniprotein p38 site mutant activates MK2. MK2 was immunoprecipitated (I.P.) from cell lysates and incubated with GST-hsp27 fusion protein and ATP for 1 h at 30°C. Reaction products were immunoblotted with anti-phospho-hsp27 and anti-MK2 antibodies.
While our results clearly show that p38 phosphorylation within DR1 does not affect mRNA stabilization (and, by inference, cytokine release), we emphasize that this by no means implies that phosphorylation at this site can play no biological role. While DR1 has not yet been assigned a binding partner or any specific function in vitro or in vivo, it is always conserved in KSHV isolates, and deletion of DR1 cripples the RNA-stabilizing activity of kaposin B. We think it highly likely that DR1 has specific partners; if such partners mediate activities other than mRNA stabilization, then the lesions or modifications affecting them would have no phenotype in our present functional assay. Finally, our demonstration that monomeric kaposin B retains significant functional activity makes possible a detailed mutational analysis of the protein to map residues important for ARE-mRNA stabilization. Such an analysis is currently under way and should further inform our understanding of this remarkable polypeptide.
Acknowledgments
This study was supported in part by the Howard Hughes Medical Institute and by a grant from the National Cancer Institute, CA073506.
REFERENCES
- 1.Boshoff, C., T. F. Schulz, M. M. Kennedy, A. K. Graham, C. Fisher, A. Thomas, J. O. McGee, R. A. Weiss, and J. J. O'Leary. 1995. Kaposi's sarcoma-associated herpesvirus infects endothelial and spindle cells. Nat. Med. 1:1274-1278. [DOI] [PubMed] [Google Scholar]
- 2.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 3.Chang, Y., and P. S. Moore. 1996. Kaposi's Sarcoma (KS)-associated herpesvirus and its role in KS. Infect. Agents Dis. 5:215-222. [PubMed] [Google Scholar]
- 4.Chen, C. Y., and A. B. Shyu. 1995. AU-rich elements: characterization and importance in mRNA degradation. Trends Biochem. Sci. 20:465-470. [DOI] [PubMed] [Google Scholar]
- 5.Chen, C.-Y., and A.-B. Shyu. 1994. Selective degradation of early-response-gene mRNAs: functional analyses of sequence features of the AU-rich elements. Mol. Cell. Biol. 14:8471-8482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, C.-Y., N. Xu, and A.-B. Shyu. 1995. mRNA decay mediated by two distinct AU-rich elements from c-fos and granulocyte-macrophage colony-stimulating factor transcripts: different deadenylation kinetics and uncoupling from translation. Mol. Cell. Biol. 15:5777-5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engel, K., A. Kotlyarov, and M. Gaestel. 1998. Leptomycin B-sensitive nuclear export of MAPKAP kinase 2 is regulated by phosphorylation. EMBO J. 17:3363-3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freshney, N. W., L. Rawlinson, F. Guesdon, E. Jones, S. Cowley, J. Hsuan, and J. Saklatvala. 1994. Interleukin-1 activates a novel protein kinase cascade that results in the phosphorylation of Hsp27. Cell 78:1039-1049. [DOI] [PubMed] [Google Scholar]
- 9.Ganem, D. 1997. KSHV and Kaposi's sarcoma: the end of the beginning? Cell 91:157-160. [DOI] [PubMed] [Google Scholar]
- 10.Han, J., J. D. Lee, L. Bibbs, and R. J. Ulevitch. 1994. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 265:808-811. [DOI] [PubMed] [Google Scholar]
- 11.Huot, J., H. Lambert, J. N. Lavoie, A. Guimond, F. Houle, and J. Landry. 1995. Characterization of 45-kDa/54-kDa HSP27 kinase, a stress-sensitive kinase which may activate the phosphorylation-dependent protective function of mammalian 27-kDa heat-shock protein HSP27. Eur. J. Biochem. 227:416-427. [DOI] [PubMed] [Google Scholar]
- 12.Kyriakis, J. M., and J. Avruch. 1996. Sounding the alarm: protein kinase cascades activated by stress and inflammation. J. Biol. Chem. 271:24313-24316. [DOI] [PubMed] [Google Scholar]
- 13.Lee, J. C., and P. R. Young. 1996. Role of CSB/p38/RK stress response kinase in LPS and cytokine signaling mechanisms. J. Leukoc. Biol. 59:152-157. [DOI] [PubMed] [Google Scholar]
- 14.Mahtani, K. R., M. Brook, J. L. Dean, G. Sully, J. Saklatvala, and A. R. Clark. 2001. Mitogen-activated protein kinase p38 controls the expression and posttranslational modification of tristetraprolin, a regulator of tumor necrosis factor alpha mRNA stability. Mol. Cell. Biol. 21:6461-6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manke, I. A., A. Nguyen, D. Lim, M. Q. Stewart, A. E. Elia, and M. B. Yaffe. 2005. MAPKAP kinase-2 is a cell cycle checkpoint kinase that regulates the G2/M transition and S phase progression in response to UV irradiation. Mol. Cell 17:37-48. [DOI] [PubMed] [Google Scholar]
- 16.McCormick, C., and D. Ganem. 2005. The kaposin B protein of KSHV activates the p38/MK2 pathway and stabilizes cytokine mRNAs. Science 307:739-741. [DOI] [PubMed] [Google Scholar]
- 17.Meng, W., L. L. Swenson, M. J. Fitzgibbon, K. Hayakawa, E. Ter Haar, A. E. Behrens, J. R. Fulghum, and J. A. Lippke. 2002. Structure of mitogen-activated protein kinase-activated protein (MAPKAP) kinase 2 suggests a bifunctional switch that couples kinase activation with nuclear export. J. Biol. Chem. 277:37401-37405. [DOI] [PubMed] [Google Scholar]
- 18.Miles, S. A., A. R. Rezai, J. F. Salazar-Gonzalez, M. Vander Meyden, R. H. Stevens, D. M. Logan, R. T. Mitsuyasu, T. Taga, T. Hirano, T. Kishimoto, et al. 1990. AIDS Kaposi sarcoma-derived cells produce and respond to interleukin 6. Proc. Natl. Acad. Sci. USA 87:4068-4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rouse, J., P. Cohen, S. Trigon, M. Morange, A. Alonso-Llamazares, D. Zamanillo, T. Hunt, and A. R. Nebreda. 1994. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell 78:1027-1037. [DOI] [PubMed] [Google Scholar]
- 20.Sadler, R., L. Wu, B. Forghani, R. Renne, W. Zhong, B. Herndier, and D. Ganem. 1999. A complex translational program generates multiple novel proteins from the latently expressed kaposin (K12) locus of Kaposi's sarcoma-associated herpesvirus. J. Virol. 73:5722-5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saklatvala, J., W. Davis, and F. Guesdon. 1996. Interleukin 1 (IL1) and tumour necrosis factor (TNF) signal transduction. Philos. Trans. R. Soc. Lond. B 351:151-157. [DOI] [PubMed] [Google Scholar]
- 22.Shaw, G., and R. Kamen. 1986. A conserved AU sequence from the 3′ untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell 46:659-667. [DOI] [PubMed] [Google Scholar]
- 23.Shyu, A. B., M. E. Greenberg, and J. G. Belasco. 1989. The c-fos transcript is targeted for rapid decay by two distinct mRNA degradation pathways. Genes Dev. 3:60-72. [DOI] [PubMed] [Google Scholar]
- 24.Staskus, K. A., W. Zhong, K. Gebhard, B. Herndier, H. Wang, R. Renne, J. Beneke, J. Pudney, D. J. Anderson, D. Ganem, and A. T. Haase. 1997. Kaposi's sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J. Virol. 71:715-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stoecklin, G., T. Stubbs, N. Kedersha, S. Wax, W. F. Rigby, T. K. Blackwell, and P. Anderson. 2004. MK2-induced tristetraprolin: 14-3-3 complexes prevent stress granule association and ARE-mRNA decay. EMBO J. 23:1313-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stokoe, D., K. Engel, D. G. Campbell, P. Cohen, and M. Gaestel. 1992. Identification of MAPKAP kinase 2 as a major enzyme responsible for the phosphorylation of the small mammalian heat shock proteins. FEBS Lett. 313:307-313. [DOI] [PubMed] [Google Scholar]
- 27.Underwood, K. W., K. D. Parris, E. Federico, L. Mosyak, R. M. Czerwinski, T. Shane, M. Taylor, K. Svenson, Y. Liu, C. L. Hsiao, S. Wolfrom, M. Maguire, K. Malakian, J. B. Telliez, L. L. Lin, R. W. Kriz, J. Seehra, W. S. Somers, and M. L. Stahl. 2003. Catalytically active MAP KAP kinase 2 structures in complex with staurosporine and ADP reveal differences with the autoinhibited enzyme. Structure (Cambridge) 11:627-636. [DOI] [PubMed] [Google Scholar]
- 28.Winzen, R., M. Kracht, B. Ritter, A. Wilhelm, C. Y. Chen, A. B. Shyu, M. Muller, M. Gaestel, K. Resch, and H. Holtmann. 1999. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. EMBO J. 18:4969-4980. [DOI] [PMC free article] [PubMed] [Google Scholar]