Abstract
The murine cytomegalovirus (MCMV) protein m4/gp34 is unique among known viral genes that target the major histocompatibility complex (MHC) class I pathway of antigen presentation in the following two ways: it is found in association with class I MHC molecules at the cell surface, and it inhibits antigen presentation without reducing cell surface class I levels. The current study was undertaken to define more clearly the structural and cellular requirements for m4/gp34 association with the MHC class I molecule Kb. We first assessed the role of the peptide-loading complex in m4/gp34-Kb association, using cell lines lacking TAP, tapasin, or β2m. m4/gp34-Kb complexes formed in the absence of TAP or tapasin, although not as efficiently as in wild-type cells. The expression of full-length and truncation mutants of m4/gp34 in a gutless adenovirus vector revealed that the transmembrane region of m4/gp34 was required for efficient association with the Kb heavy chain. However, the peptide-loading complex was not absolutely required for the association, since m4/gp34 readily formed complexes with Kb in detergent lysates. The addition of Kb-binding peptide to the detergent lysates facilitated but was not essential for the formation of the complexes. The ease of complex formation in detergent lysates contrasted with the small fractions of m4/gp34 and Kb that form complexes in infected cells, suggesting that the endoplasmic reticulum (ER) environment restricts access of m4/gp34 to Kb. Finally, although m4/gp34-Kb complexes could form when m4 was carried either by MCMV or by the adenovirus vector, they were only efficiently exported from the ER in MCMV-infected cells, suggesting that MCMV provides additional factors needed for transport of the complexes.
Cytomegaloviruses (CMVs), including human CMV and murine CMV (MCMV), belong to the β subfamily of Herpesviridae, a family of large, double-stranded DNA viruses. CMVs cause life-long infections with little pathology in healthy hosts but cause severe disease when the immune system is compromised (15). The multitude of mechanisms by which CMVs actively modulate the host immune response enables them to establish lifelong infections with minimal impact on their hosts' well-being.
The major histocompatibility complex (MHC) class I pathway of antigen presentation is the means by which the internal contents of a cell are displayed to the immune system, and in consequence, it is a frequent target for virus-carried immune evasion genes (18, 23). We call genes that mediate this type of immune evasion VIPRs (viral genes that interfere with antigen presentation) (27). During normal assembly, the MHC class I heavy chain (HC) is cotranslationally translocated into the endoplasmic reticulum (ER), where it associates first with the chaperone calnexin and binds to the light chain, β2-microglobulin (β2m). The HC-β2m heterodimer then associates with a complex of proteins called the peptide-loading complex (PLC), which includes the chaperones calreticulin and tapasin, the thiol oxy-reductase ERp57, and the peptide transporter associated with antigen processing (TAP). TAP transports short peptides, generated by proteasomal degradation of cytosolic proteins, into the ER, where they are loaded onto empty class I molecules. The PLC facilitates the loading of peptides in a manner that remains incompletely understood. In addition to stabilizing TAP and linking class I molecules to the peptide-loading complex, tapasin is thought to function as a peptide editor, facilitating the exchange of low-quality peptides that have been loaded onto MHC I molecules with high-quality peptides (3, 6, 17). After binding of a peptide, the trimolecular complex of HC, β2m, and peptide dissociates from the assembly complex, leaves the ER, and travels through the Golgi and out to the cell surface.
MCMV carries three VIPRs that have been shown to interfere with the assembly/maturation of MHC class I molecules in infected cells. m152 encodes a glycoprotein, m152/gp40, which acts through an unknown mechanism to retain class I molecules in the ER-cis-Golgi intermediate compartment (28). m6 encodes a glycoprotein, m6/gp48, that binds to MHC class I molecules in the ER and redirects them to the lysosome for degradation (19). MCMV's third VIPR is m4, which encodes the glycoprotein m4/gp34.
m4/gp34 is involved in the prevention of antigen presentation to selected cytotoxic T-lymphocyte clones and T-cell lines, as evidenced by the ability to lyse cells infected with a virus lacking m4 but not cells infected with wild-type virus (10, 14). However, the mechanism by which m4/gp34 inhibits antigen presentation is not known. m4/gp34 is expressed abundantly in the ER, and the vast majority of m4/gp34 remains resident in the ER (13). Some of this m4/gp34 forms complexes with MHC class I molecules that are disrupted by the detergent NP-40; these detergent-labile complexes remain in the ER (12). A small amount of m4/gp34 forms complexes with class I that are stable in NP-40; these “detergent-stable” complexes leave the ER and travel to the cell surface, where they remain stably associated for many hours (12). Thus, in contrast to m6/gp48 and m152/gp40, m4/gp34 does not reduce cell surface expression of class I molecules; on the contrary, it may actually lead to higher cell surface levels of MHC class I molecules (25).
Little is known about the nature of the MHC molecules that are found in association with m4/gp34 or about the conditions that promote complex formation. We originally observed that the association between m4/gp34 and MHC class I molecules was impaired in the absence of TAP and/or β2m (13), which suggested that m4/gp34 may associate preferentially with well-conformed, peptide-loaded MHC class I molecules. However, it has not been shown that the MHC class I molecules that are found in association with m4/gp34 have actually bound peptide. Furthermore, despite a massive excess of m4/gp34 in the ER, only a small fraction of MHC class I molecules in the ER form the detergent-stable complexes with m4/gp34 that can be exported to the cell surface (12). This might indicate that these complexes are difficult to form and perhaps require assistance from chaperone molecules. Thus, an alternative explanation for the influence of TAP and β2m on the formation of the detergent-stable complexes could be that m4/gp34 needs the assistance of the chaperones associated with the PLC to form this association with MHC class I molecules.
For the current study, we used a combination of cell lines lacking components of the PLC and expression of m4/gp34 in a gutless adenovirus (Ad) vector to define more clearly the structural and cellular requirements for m4/gp34 association with the MHC class I molecule Kb.
MATERIALS AND METHODS
Cell culture and virus stocks.
Mouse embryo fibroblasts (MEFs) were grown from trypsin-digested 12- to 14-day mouse embryos from wild-type C57BL/6, TAP−/−, and β2m−/− mice purchased from Jackson Laboratories and were used between passages 2 and 8. Tapasin−/− cells were grown from ear biopsies from adult tapasin−/− mice and were the kind gift of Glen Grandea. KbDb−/− fibroblasts were grown from the skin of adult KbDb−/− mice (a kind gift from David Raulet) and transformed with simian virus 40 T antigen. BALB 3T3 cells were obtained from the American Type Culture Collection. All cell lines were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum and penicillin-streptomycin-glutamine.
Stocks of wild-type MCMV strain MW97.01 (26), Δm4 (MCMV with m4 deleted) (10), and Δm6+m152 (MCMV with m6 and m152 deleted) (25) were generated by infecting subconfluent MEFs with low-passage seed stocks at a multiplicity of infection (MOI) of 0.01. When the monolayer showed 100% cytopathic effect, cells were harvested and subjected to Dounce homogenization, and virus stocks were purified by centrifugation at 72,000 × g over a sucrose cushion. The titer (PFU) was determined by serial dilution and carboxymethyl cellulose overlay on BALB 3T3 cells.
Recombinant Ad-m4 constructs.
A replication-defective (E1−) adenovirus vector expressing MCMV m4 was constructed as follows. The m4 gene was amplified by PCR from a wild-type bacterial artificial chromosome (BAC) of MCMV (26) with a HindIII site flanking the 5′ terminus and a NotI site flanking the 3′ terminus. The cloned m4 gene was inserted into a shuttle plasmid, pAdtet7 (2), downstream of a human CMV immediate-early promoter element that is regulated by a tetracycline transactivator element (7). The pAdtet7 plasmid contains a loxP site for recombination with the helper virus ψ5, an adenovirus vector that contains loxP sites flanking the DNA packaging sequences. The m4-inserted pAdtet7 plasmid and ψ5 virus DNA were cotransfected into Cre4/293 cells (1, 9) with Fugene 6 (Roche, IN). Cre4/293 cells express Cre recombinase, which mediates recombination between the pAdtet7-derived shuttle plasmids and ψ5 DNA to produce recombinant adenovirus vectors (1). After 7 days, the cytopathic effect was visible; supernatant containing recombinant Ad-m4 was recovered and passaged twice to remove any ψ5 helper virus. The correct insert in Ad-m4 was confirmed by sequencing. To express the m4/gp34 protein, cells were coinfected with Ad-m4 and Ad-tet, an adenovirus vector that expresses the tetracycline transactivator protein (1, 9) (a kind gift from David Johnson), using an Ad-m4/Ad-tet ratio of 5:1.
To construct m4 carboxy-terminal truncation mutants, m4 expressed in pcDNA3 was amplified using the following primers: 5′, 5′-CCCAAGCTTGGGCACCATGTCTCTCGTATGTCGGCT-3′ (HindIII site is underlined); 3′ wild-type m4, 5′-ATAAGAATGCGGCCGCTAAACTATTTAGTTACTCTTAAGCGGTTT-3′ (NotI site is underlined); 3′ m4-ΔCT, 5′-ATAAGAATGCGGCCGCTTAGTATAATGAGGGTCCGTACAAG-3′ (NotI site is underlined); and 3′ m4-ΔCT/TM, 5′-ATAAGAATGCGGCCGCTTACGTGTTTGGTGACTCATTCTTG-3′ (NotI site is underlined). The m4 mutants were constructed by placing a stop codon after nucleotide 744 (m4-ΔCT) or nucleotide 657 (m4-ΔCT/TM). The PCR products were cloned into the shuttle plasmid pAdtet7, and the recombinant adenoviruses Ad-m4-ΔCT and Ad-m4-ΔCT/TM were generated as described above.
Antibodies.
Serum 8010 (rabbit serum specific for exon 8 in the cytoplasmic tail of H-2 Kb mouse MHC class I heavy chains; anti-p8) and sera 8139 and 8142 (anti-m4/gp34) are polyclonal rabbit antisera and have been described previously (10-12). Antitapasin antiserum was raised by immunizing rabbits with a peptide (SKEKATAASLTIPRNSKKSQ-OH) corresponding to the cytoplasmic tail of tapasin coupled to keyhole limpet hemocyanin and bovine albumin. The monoclonal antibody (MAb) Y3, which recognizes the α1 and α2 domains of properly folded Kb heavy chains (8), was purified from a hybridoma supernatant by using a protein A column.
Infection of cells with MCMV.
Cells were grown as an adherent monolayer and infected when ∼90% confluent. The cells were exposed to gamma interferon (IFN-γ) (50 U/ml; Sigma-Aldrich) for 24 to 48 h before infection. MCMV was added to the cells in a 6-cm dish containing 1 ml medium at the indicated MOI. For overnight infection, the cells were infected at 37°C for 1 h, with gentle rocking every 10 min. The inoculum was removed and replaced with medium containing 0.3 mg/ml phosphonoacetic acid to inhibit viral DNA replication and late protein synthesis. For short infections, the cells were infected at 37°C for the indicated times, without phosphonoacetic acid.
Biochemical analysis.
Cells were incubated for 1 h in methionine- and cysteine-free DMEM and labeled with [35S]methionine-cysteine (up to 0.2 μCi/ml for long labeling periods and 0.5 μCi/ml for pulse labeling) (Perkin-Elmer, Boston, MA) for the times indicated in Results. If needed, the cells were chased with regular DMEM supplemented with nonradioactive methionine and cysteine to a final concentration of 1 mM. Cells were lysed in NP-40 lysis buffer (0.5% NP-40, 50 mM Tris-HCl, pH 6.5, 5 mM MgCl2) or digitonin lysis buffer (1% high-purity digitonin [Calbiochem, La Jolla, CA] in freshly prepared phosphate-buffered saline, pH 7.4) containing 1 mM phenylmethylsulfonyl fluoride. Nuclei and insoluble debris were removed by centrifugation (Savant SFA13K centrifuge; 13,000 rpm, 10 min). Supernatants were precleared with normal rabbit serum and formalin-fixed Staphylococcus aureus and then subjected to primary immunoprecipitation (IP) with the indicated antibodies and formalin-fixed S. aureus. Sequential IP was performed by clearing the supernatant from primary IP with formalin-fixed S. aureus for 0.5 h, followed by another round of IP with the indicated antibodies and formalin-fixed S. aureus. Digestion with endo-β-N-acetylglucosaminidase H (endo-H; New England Biolabs, Beverly, MA) was performed on immune complexes bound to formalin-fixed S. aureus according to the vendor's instructions. Re-IP was performed as follows. Immune complexes bound to formalin-fixed S. aureus were dissociated by boiling for 10 min in 150 μl of phosphate-buffered saline containing 1% sodium dodecyl sulfate (SDS) and then resuspended in 1 ml of NP-40 lysis buffer supplemented with 0.1% bovine serum albumin. After centrifugation to remove formalin-fixed S. aureus, antibody and formalin-fixed S. aureus were added to the supernatant to immunoprecipitate proteins from the dissociated complexes. Samples were resuspended, boiled at 95°C for 5 min in denaturing buffer, and separated by 12.5% SDS-polyacrylamide gel electrophoresis (SDS-PAGE). The gels were imaged using a Molecular Dynamics PhosphorImager (Sunnyvale, CA). Band intensities were quantified by using Adobe Photoshop software.
RESULTS
The peptide-loading complex facilitates m4/gp34 association with MHC class I molecules in MCMV-infected cells.
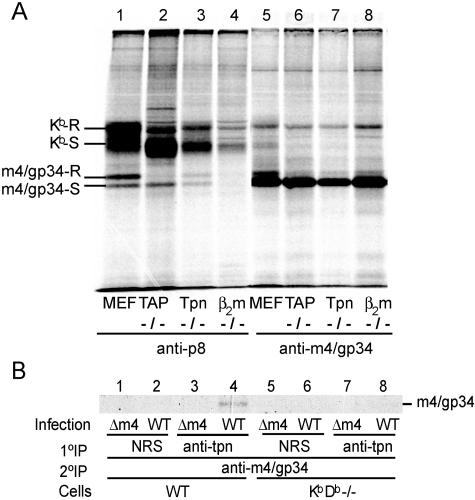
In our original paper describing m4/gp34, we observed that m4/gp34-Kb complexes formed poorly in the absence of TAP, and no complex formation was observed in the absence of β2m (13). At that time, the chaperone tapasin had not been discovered. In the present study, we extended these studies to include a cell line lacking tapasin. Fibroblast cell lines were grown from mice lacking TAP, tapasin, or β2m and infected with MCMV for 16 h in the presence of [35S]methionine-cysteine. Kb was precipitated using antiserum against its cytoplasmic tail (anti-p8), after which m4/gp34 was sequentially precipitated from the lysate and analyzed by SDS-PAGE (Fig. 1). The typical pattern of m4/gp34 coprecipitation with class I molecules in wild-type MCMV-infected fibroblasts is shown in Fig. 1A, lane 1: during the long labeling period, most coprecipitated m4/gp34 had acquired endo-H resistance in one of its three glycans, although some amount of endo-H-sensitive m4/gp34 also coprecipitated with class I molecules. Sequential IP of m4/gp34 from the same lysate revealed abundant residual m4/gp34, which was mostly endo-H sensitive (lane 5). Since m4/gp34 can generally only obtain endo-H resistance if it is associated with MHC class I molecules (13), the small amount of endo-H-resistant m4/gp34 seen in Fig. 1A, lane 5, likely represents m4/gp34 that was associated with Db. As previously reported (13), no m4/gp34 coprecipitated with the small amount of MHC class I molecules that could be recovered from the cells lacking β2m. Compared to the case for wild-type cells, less m4/gp34 coprecipitated with recovered Kb in TAP−/− cells, and the m4/gp34 that did coprecipitate remained endo-H sensitive, corresponding to the endo-H-susceptibility of MHC class I molecules in the absence of TAP (Fig. 1A, lane 2).
FIG. 1.
Role of the peptide-loading complex in m4/gp34-Kb association. (A) Effects of peptide-loading complex on the association of m4/gp34 with Kb molecules in the context of MCMV infection. TAP−/−, tapasin−/− (Tpn−/−), or β2m−/− fibroblasts or normal MEFs from C57BL/6 mice were infected with wild-type MCMV (MOI = 20) and labeled overnight with 35S. The cells were lysed in NP-40 lysis buffer and precleared with normal rabbit serum, followed by sequential IP with the polyclonal antiserum anti-p8 to precipitate Kb and its associated molecules and with antiserum 8139 to precipitate non-Kb-associated free m4/gp34. (B) m4/gp34-Kb complexes associate with tapasin. IFN-γ-treated B6 MEFs or KbDb−/− fibroblasts were infected with either wild-type or Δm4 MCMV for 3.5 h and then labeled with 35S for 2 h. The cells were lysed in digitonin lysis buffer. Tapasin was immunoprecipitated from the precleared lysates. A control IP was performed with normal rabbit serum (NRS). The immune complexes were dissociated by boiling in 1% SDS for 10 min, resuspended in 1% NP-40 lysis buffer, and subjected to secondary IP against m4/gp34. Samples were treated with endo-H and analyzed by SDS-PAGE.
Less m4/gp34 also coprecipitated with Kb in tapasin−/− cells (Fig. 1A, lane 3). Tapasin−/− cells also had fewer total class I molecules than either wild-type or TAP−/− cells, which could explain the lesser amount of m4/gp34. However, in contrast to the case in TAP−/− cells, about half of the m4/gp34 had acquired endo-H resistance in tapasin−/− cells. Tapasin plays a role in the ER retention of MHC class I molecules that have not bound a high-affinity peptide: in the absence of tapasin, unstable class I complexes travel to the cell surface and are rapidly lost (20, 22). The fact that m4/gp34 coprecipitating with Kb acquired endo-H resistance in tapasin−/− cells suggested that tapasin also plays a role in retaining m4/gp34-Kb complexes in the ER. If this is so, m4/gp34-Kb complexes should be found in association with tapasin in normal cells. To determine if this was the case, we immunoprecipitated tapasin from cells infected with either wild-type MCMV or MCMV lacking m4 (Δm4) and then reimmunoprecipitated m4/gp34 from the dissociated immune complexes. Figure 1B (lane 4) shows that m4/gp34 was recovered from the tapasin IP. This could imply either that m4/gp34 associated directly with tapasin or that the association of m4/gp34 with tapasin was mediated through MHC class I molecules. To distinguish these possibilities, we performed the same experiment with KbDb−/− cells (Fig. 1B, lanes 5 to 8). m4/gp34 was not recovered from the tapasin IP with KbDb−/− cells, indicating that m4/gp34's association with tapasin is mediated by classical MHC class I molecules.
Although m4/gp34-Kb complexes associate with tapasin, the results shown in Fig. 1A indicate that a chaperone function of tapasin is not necessary for m4/gp34-Kb complex formation. However, they do not answer the question of whether m4/gp34 associates with peptide-loaded MHC class I molecules. On the one hand, m4/gp34-Kb complex formation was decreased (but not abolished) in the absence of TAP, suggesting a preference for peptide-loaded class I molecules. On the other hand, the amount of exported m4/gp34 seen in tapasin−/− cells suggested that m4/gp34 could form complexes with class I molecules that were either empty or loaded with suboptimal peptides.
m4/gp34 expressed in the absence of other MCMV genes associates with Kb.
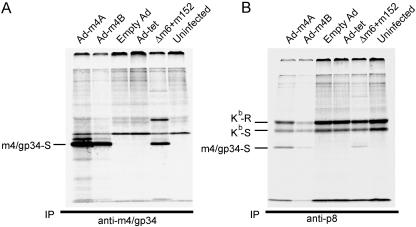
To further pursue the question of what is required for m4/gp34-Kb complex formation, we expressed m4/gp34 in a gutless adenovirus vector. The m4 gene was cloned from the MCMV genome expressed as a BAC and inserted into an adenovirus vector under the control of a tetracycline-responsive regulator (9). The expression of m4 requires the presence of the tetracycline transactivator, which is expressed by a second adenovirus (Ad-tet). In this system, after coinfection with Ad-m4 and Ad-tet, the expression of m4/gp34 does not occur for several hours, until sufficient tetracycline transactivator has been expressed. To confirm m4/gp34 expression in this system, cells were infected with Ad-tet and Ad-m4 for 16 h, followed by 3 h of labeling with [35S]methionine-cysteine. Control cells were infected with an MCMV mutant lacking MCMV's other VIPRs, Δm6+m152, for 5 h, followed by labeling for 3 h. Cell lysates were immunoprecipitated with anti-m4/gp34 (Fig. 2A) or anti-p8 (anti-Kb) (Fig. 2B), followed by endo-H digestion. Figure 2A shows that m4/gp34 expressed from two independent adenovirus constructs had the same molecular weight and endo-H susceptibility as MCMV-expressed m4/gp34. Figure 2B shows that m4/gp34 expressed from the adenovirus vectors was also coprecipitated with Kb. Thus, the formation of m4/gp34-Kb complexes can occur independently of the context of MCMV infection.
FIG. 2.
m4/gp34 expressed by an adenovirus vector associates with Kb molecules. B6 MEFs were infected for 16 h with the indicated adenovirus vectors and Ad-tet or with Δm6+m152 MCMV for 5 h and then labeled with [35S]Met for 3 h. Ad-m4A and Ad-m4B are independent Ad-m4 constructs, and empty-Ad is the vector without an insert. m4/gp34 (A) and Kb (B) were immunoprecipitated from equal aliquots of the labeled cell lysates. All samples were digested with endo-H. The identity of the band with the approximate molecular weight of MHC HC in the Δm6+m152 lane (A) has not been confirmed.
The transmembrane domain of m4/gp34 is critical for m4/gp34-Kb association.
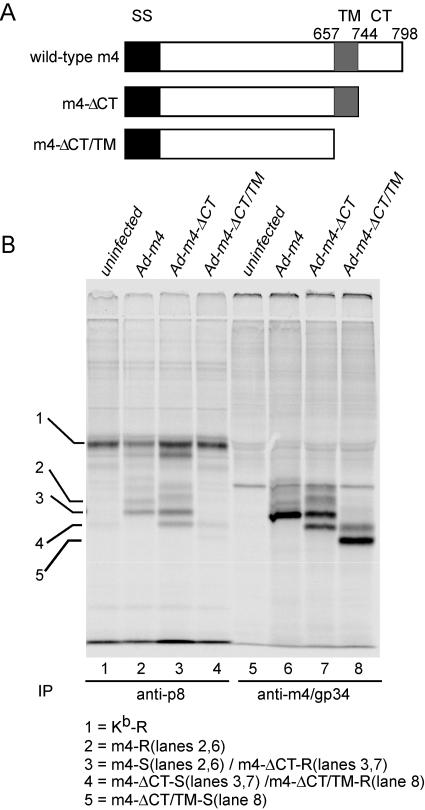
Both m4/gp34 and MHC class I molecules are type I transmembrane proteins. To determine which domains of m4/gp34 are required for the formation of m4/gp34-Kb complexes, we constructed m4/gp34 mutants that lacked the cytoplasmic tail (Ad-m4-ΔCT) or both the cytoplasmic tail and transmembrane region (Ad-m4-ΔCT/TM) (Fig. 3A). After overnight infection and labeling, Kb and m4/gp34 were sequentially immunoprecipitated (Fig. 3B). The truncated forms of m4/gp34 were expressed at equivalent levels to wild-type m4/gp34 and had the expected molecular weights (lanes 6 to 8). The absence of the cytoplasmic tail did not impair the association of m4/gp34 with Kb molecules (Fig. 3B, lane 3). However, the absence of both the cytoplasmic tail and the transmembrane domain dramatically decreased the formation of m4/gp34-Kb complexes (Fig. 3B, lane 4). Impaired complex formation was not due to rapid secretion of the soluble m4/gp34, since most m4-ΔCT/TM remained endo-H sensitive (lane 8). These data suggest that the transmembrane domain of m4/gp34 plays an important role in the formation of m4/gp34-Kb complexes. In addition, note that some m4/gp34 lacking its cytoplasmic tail acquired endo-H resistance, apparently in the absence of complexes with MHC class I molecules (Fig. 3B, lanes 7 and 8). This suggests that an ER retention motif in the cytoplasmic tail may be involved in retaining free m4/gp34 in the ER.
FIG. 3.
The transmembrane domain of m4/gp34 is needed for optimal association with Kb. (A) Schematic illustration of m4/gp34 mutants constructed in adenovirus vectors. SS, signal sequence; CT, cytoplasmic tail; TM, transmembrane region. (B) IFN-γ-treated B6 MEFs were infected with the indicated viruses and labeled overnight. Kb was immunoprecipitated from cleared cell lysates by using anti-p8, and then m4/gp34 was sequentially immunoprecipitated from the same lysates. All samples were treated with endo-H and analyzed by 12.5% SDS-PAGE.
m4/gp34 associates with MHC class I molecules in detergent lysates, and this association is facilitated by proper peptide loading.
The experiment whose results are shown in Fig. 2 indicated that m4/gp34-Kb complex formation did not require the presence of other MCMV-carried genes. However, it was still not clear whether ER chaperones are necessary for complex formation or whether m4/gp34 associates with peptide-loaded MHC class I molecules. To determine the minimal molecular requirements for m4/gp34 and Kb association and to assess the role of peptides in complex formation, we wanted to investigate whether m4/gp34 and Kb complexes could form in vitro, outside the context of a living cell. Because the experiment described for Fig. 3 indicated that the transmembrane domain of m4/gp34 was involved in complex formation, we needed a system in which m4/gp34 could be solubilized while retaining its transmembrane domain. We decided to adapt an assay originally developed to assess MHC class I assembly in detergent lysates of labeled cells (5, 21). In dilute detergent lysates, class I heavy chain and β2m derived from TAP−/− cells dissociate unless stabilized by the addition of a peptide able to bind to the class I molecule. Stabilization of MHC class I complexes is dependent on the affinity of the peptide for MHC class I molecules, and this assay is a sensitive indicator of peptide binding to MHC class I molecules (4, 24). We decided to determine whether m4/gp34 could bind to MHC class I molecules under these conditions and whether m4/gp34 binding would be affected by the addition of peptide.
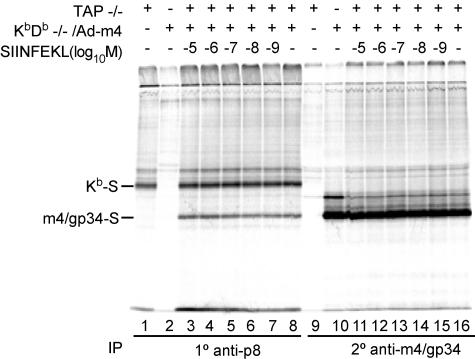
As a source of m4/gp34, Kb/Db−/− fibroblasts were infected with Ad-m4 and labeled overnight. As a source of peptide-empty Kb dissociated from the PLC, we labeled TAP−/− fibroblasts. Both sets of cells were lysed in NP-40 lysis buffer, which causes dissociation of the PLC (16). Aliquots of the mixed lysates were incubated overnight in the presence of a range of concentrations of the Kb-binding peptide, SIINFEKL. Kb was then immunoprecipitated from the lysates by the conformation-independent antiserum anti-p8, after which residual m4/gp34 was precipitated from the same lysates. Figure 4 shows that m4/gp34 coprecipitated with Kb, indicating that m4/gp34 could form complexes with Kb in detergent lysates. Furthermore, m4/gp34-Kb complexes formed over the entire range of peptide concentrations, although a little bit more m4/gp34 coprecipitated at higher concentrations of SIINFEKL than in its absence (compare lanes 3 and 4 to lane 8).
FIG. 4.
Association of m4/gp34 with Kb in detergent lysates. KbDb−/− cells were infected with Ad-m4 for 4 h, followed by overnight labeling with 35S. TAP−/− cells were labeled overnight without infection. The next morning, the cells were lysed in NP-40 lysis buffer supplemented with 1 mM phenylmethylsulfonyl fluoride. After the lysates were precleared with normal rabbit serum, equal volumes of TAP−/− lysate and Ad-m4-infected KbDb−/− lysate (KbDb−/−/Ad-m4) were mixed and incubated overnight at 4°C with the indicated concentration of the SIINFEKL peptide. The mixed lysates were then sequentially immunoprecipitated with the antibody anti-p8 and the m4/gp34-specific antiserum 8139. All samples were treated with endo-H.
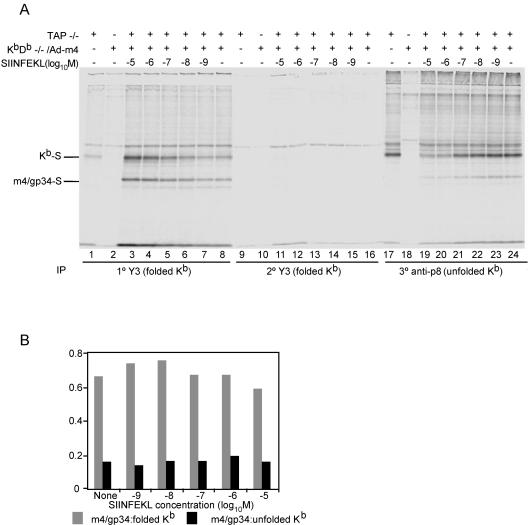
To probe the question of m4/gp34's preference for peptide-loaded Kb more clearly, we repeated this experiment, but first we immunoprecipitated Kb with the conformation-dependent MAb Y3. Residual nonfolded Kb was then immunoprecipitated with anti-p8 antiserum. As expected, the recovery of conformed Kb correlated with the concentration of exogenously added SIINFEKL peptide. Consequently, the amount of residual nonfolded Kb was inversely correlated with the SIINFEKL concentration. m4/gp34 coprecipitation was seen with both the folded class I and residual nonfolded heavy chain. However, more m4/gp34 coprecipitated with folded than unfolded Kb. The intensities of the bands in Fig. 5A were quantified by a PhosphorImager. The ratio of m4/gp34 band intensity to Kb-heavy-chain band intensity was calculated for both Y3 and p8 IPs; the results are graphed in Fig. 5B. At each peptide concentration, the ratio of m4/gp34 to Kb was about fourfold higher with folded than with unfolded Kb. This experiment demonstrated for the first time that m4/gp34 associates with peptide-loaded Kb. However, it suggested that although m4/gp34 preferentially formed complexes with peptide-loaded Kb, it was also able to form complexes with peptide-empty Kb.
FIG. 5.
Influence of Kb conformation on formation of m4/gp34-Kb complexes in detergent lysates. (A) Cell lysates containing labeled m4/gp34 and Kb were prepared, mixed, and incubated overnight with the SIINFEKL peptide as described in the legend to Fig. 4. Kb was then immunoprecipitated using the conformation-dependent MAb Y3. After a second round of Y3 immunoprecipitation to ensure the removal of all the folded Kb molecules, residual unfolded Kb was immunoprecipitated with anti-p8. All samples were treated with endo-H and run in a 12.5% SDS-PAGE gel. (B) Band intensities of folded (Y3-reactive) Kb, residual unfolded Kb, and coprecipitated m4/gp34 were quantified by a PhosphorImager. The ratios of m4/gp34 to Kb intensities are shown in gray for Y3-reactive Kb and in black for residual unfolded Kb for each SIINFEKL concentration.
Impact of β2m and the PLC on m4/gp34-Kb complex formation in Ad-m4-infected cells.
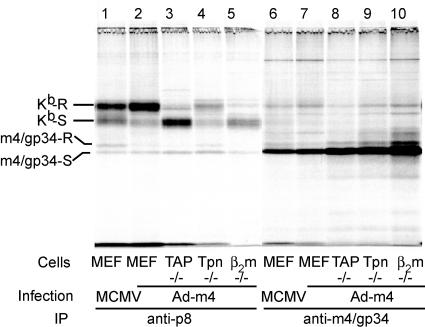
We also examined the formation of m4/gp34-Kb complexes in cells lacking TAP, tapasin, or β2m that were infected with Ad-m4 (Fig. 6). We were surprised to observe some m4/gp34 coprecipitation with Kb in β2m−/− cells (lane 5), something that we have never seen in MCMV-infected cells, despite similar expression levels of m4/gp34 (compare Fig. 6 with Fig. 1). In addition, m4/gp34 expressed by Ad-m4 formed complexes with Kb in cells lacking TAP and tapasin as efficiently as in wild-type cells (Fig. 6, compare lanes 2, 3, and 4). Again, this is different from the results for MCMV-infected cells (13). However, because of the difficulty in ensuring identical quantities of m4/gp34 from Ad-m4 and MCMV infections, the significance of this rather small difference is uncertain. Finally, we observed that whereas m4/gp34 that coprecipitated with Kb from MCMV-infected cells mostly acquired endo-H resistance during the 16-h labeling period, most Kb-associated m4/gp34 in Ad-m4-infected cells remained endo-H sensitive under the same conditions (compare lanes 1 and 2). This was not due to a general impairment of glycoprotein transport, because during the same period, the opposite pattern was observed for MHC class I molecules: most of the Kb became endo-H resistant in Ad-m4-infected cells (Fig. 6, lane 2), whereas some Kb remained endo-H sensitive in MCMV-infected cells (Fig. 6, lane 1). These results suggest that there are significant differences in the ER environment of MCMV-infected cells compared to that in Ad-m4-infected cells that impact m4/gp34-Kb transport and, possibly, the formation of the complexes.
FIG. 6.
Effect of peptide-loading complex on the formation of m4/gp34-Kb complexes during Ad-m4 infection. B6 MEFs or TAP−/−, tapasin−/− (Tpn−/−), or β2m−/− cells were infected with Ad-m4 and labeled overnight with 35S. Additional B6 MEFs were infected with MCMV to confirm the presence of comparable expression levels of m4/gp34 between MCMV infection and Ad-m4 infection. Sequential IPs were performed with anti-p8 and anti-m4/gp34.
DISCUSSION
In the current study, we examined the association of m4/gp34 with the MHC class I molecule Kb in three different contexts, i.e., in normal MCMV infection, when expressed from an adenovirus vector, and in detergent lysates. These experiments showed that m4/gp34 and Kb efficiently formed complexes in detergent lysates, indicating that no special contribution from enzymes or chaperones in the ER or from other MCMV-carried proteins is required for complex formation.
The association of m4/gp34 with Kb in detergent lysates was facilitated by the addition of Kb-binding peptide. This result demonstrated for the first time that m4/gp34 does associate with peptide-loaded MHC class I molecules. However, these experiments also showed that m4/gp34 could associate with MHC class I molecules in the absence of added peptide, albeit less efficiently (Fig. 5). This does not necessarily indicate that m4/gp34 could associate with peptide-empty MHC class I molecules, since the peptide-binding grooves of class I molecules in TAP−/− cells may be occupied by low-affinity peptides. However, any such peptides do not pass the “quality control” mechanisms of the PLC, since class I molecules from TAP−/− cells largely remain endo-H sensitive. Thus, m4/gp34's requirement for peptide-loaded class I molecules is either not absolute or else can be satisfied by lower-quality peptides than are necessary to permit the release of MHC class I molecules from the PLC and their export from the ER. This conclusion is in accord with our finding that m4/gp34-Kb complex formation was only modestly impaired by the absence of TAP or tapasin. In fact, when m4/gp34 was supplied in vast excess from an Ad-m4 vector, complex formation was even observed in β2m−/− cells.
Our experiments do not permit a precise quantitative comparison of the efficiencies of m4/gp34-Kb complex formation between the ER and dilute detergent lysates. However, given that only a minority of Kb and m4/gp34 seemed able to form complexes in infected cells, we were surprised at how readily the complexes formed in detergent lysates. This suggests that the ER environment is not completely permissive for complex formation. Possibly, chaperones normally function to keep the molecules apart and only allow m4/gp34 access to MHC class I molecules at a certain stage of their maturation. The finding that m4/gp34-Kb complexes associate with tapasin might suggest a role for the PLC in this process.
Our experiments also indicated that the ER environment in MCMV-infected cells has an additional impact on m4/gp34-Kb complexes. Although complex formation occurred efficiently in the absence of other MCMV gene products, export from the ER did not. This suggests that having achieved complex formation, the virus specifically promotes the export of complexes to the cell surface. We are currently pursuing the identity of the factor(s) provided by MCMV infection that permits this efficient export.
m4 was identified as a VIPR by the fact that some MCMV-specific CD8 T cells can lyse cells infected with MCMV lacking m4 but not those infected with wild-type MCMV (10, 14; A. K. Pinto, unpublished data). m4 is unique among known VIPRs in that it does not reduce cell surface MHC class I levels and is found bound to class I molecules at the cell surface (10, 13, 25). At present, it is not known whether m4's impact on antigen presentation occurs in the ER or at the cell surface. It is also unknown whether the presence of m4/gp34-MHC class I complexes at the cell surface may have other consequences, such as NK-cell inhibition. The results presented here indicate that outside the cell, m4/gp34 readily forms complexes with Kb, most efficiently when Kb is loaded with optimal peptides, but also when it is not. The ER environment of the MCMV-infected cells may serve to constrain these associations. However, once m4/gp34-Kb complexes are formed, the ER environment of MCMV-infected cells provides an additional function that actively promotes their export. Further dissection of these phenomena should help us to understand how m4/gp34 inhibits antigen presentation and whether the presence of m4/gp34-Kb complexes at the cell surface has any other immunological consequences.
Acknowledgments
This work was supported by NIH grant AI 50099.
We are grateful to Glen Grandea for the gift of tissues from tapasin−/− mice and to Todd Wisner for the gift of adenovirus vectors and advice on their use.
REFERENCES
- 1.Chen, L., M. Anton, and F. L. Graham. 1996. Production and characterization of human 293 cell lines expressing the site-specific recombinase Cre. Somat. Cell Mol. Genet. 22:477-488. [DOI] [PubMed] [Google Scholar]
- 2.Chevalier, M. S., G. M. Daniels, and D. C. Johnson. 2002. Binding of human cytomegalovirus US2 to major histocompatibility complex class I and II proteins is not sufficient for their degradation. J. Virol. 76:8265-8275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cresswell, P. 2005. Antigen processing and presentation. Immunol. Rev. 207:5-7. [DOI] [PubMed] [Google Scholar]
- 4.Elliott, T., V. Cerundolo, J. Elvin, and A. Townsend. 1991. Peptide-induced conformational change of the class I heavy chain. Nature 351:402-406. [DOI] [PubMed] [Google Scholar]
- 5.Elliott, T., V. Cerundolo, and A. Townsend. 1992. Short peptides assist the folding of free class I heavy chains in solution. Eur. J. Immunol. 22:3121-3125. [DOI] [PubMed] [Google Scholar]
- 6.Elliott, T., and A. Williams. 2005. The optimization of peptide cargo bound to MHC class I molecules by the peptide-loading complex. Immunol. Rev. 207:89-99. [DOI] [PubMed] [Google Scholar]
- 7.Gossen, M., and H. Bujard. 1992. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. USA 89:5547-5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammerling, G. J., E. Rusch, N. Tada, S. Kimura, and U. Hammerling. 1982. Localization of allodeterminants on H-2Kb antigens determined with monoclonal antibodies and H-2 mutant mice. Proc. Natl. Acad. Sci. USA 79:4737-4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hardy, S., M. Kitamura, T. Harris-Stansil, Y. Dai, and M. L. Phipps. 1997. Construction of adenovirus vectors through Cre-lox recombination. J. Virol. 71:1842-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kavanagh, D. G., M. C. Gold, M. Wagner, U. H. Koszinowski, and A. B. Hill. 2001. The multiple immune-evasion genes of murine cytomegalovirus are not redundant: m4 and m152 inhibit antigen presentation in a complementary and cooperative fashion. J. Exp. Med. 194:967-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kavanagh, D. G., and A. B. Hill. 2001. Evasion of cytotoxic T lymphocytes by murine cytomegalovirus. Semin. Immunol. 13:19-26. [DOI] [PubMed] [Google Scholar]
- 12.Kavanagh, D. G., U. H. Koszinowski, and A. B. Hill. 2001. The murine cytomegalovirus immune evasion protein m4/gp34 forms biochemically distinct complexes with class I MHC at the cell surface and in a pre-Golgi compartment. J. Immunol. 167:3894-3902. [DOI] [PubMed] [Google Scholar]
- 13.Kleijnen, M. F., J. B. Huppa, P. Lucin, S. Mukherjee, H. Farrell, A. E. Campbell, U. H. Koszinowski, A. B. Hill, and H. L. Ploegh. 1997. A mouse cytomegalovirus glycoprotein, gp34, forms a complex with folded class I MHC molecules in the ER which is not retained but is transported to the cell surface. EMBO J. 16:685-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LoPiccolo, D. M., M. C. Gold, D. G. Kavanagh, M. Wagner, U. H. Koszinowski, and A. B. Hill. 2003. Effective inhibition of K(b)- and D(b)-restricted antigen presentation in primary macrophages by murine cytomegalovirus. J. Virol. 77:301-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mocarski, E. S., and C. T. Courcelle. 2001. Cytomegaloviruses and their replication, p. 2629-2674. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 16.Owen, B. A., and L. R. Pease. 2001. Thermal stability of MHC class I-beta 2-microglobulin peptide complexes in the endoplasmic reticulum is determined by the peptide occupancy of the transporter associated with antigen processing complex. J. Immunol. 166:1740-1747. [DOI] [PubMed] [Google Scholar]
- 17.Paulsson, K. M., and P. Wang. 2004. Quality control of MHC class I maturation. FASEB J. 18:31-38. [DOI] [PubMed] [Google Scholar]
- 18.Reddehase, M. J. 2002. Antigens and immunoevasins: opponents in cytomegalovirus immune surveillance. Nat. Rev. Immunol. 2:831-844. [DOI] [PubMed] [Google Scholar]
- 19.Reusch, U., W. Muranyi, P. Lucin, H. G. Burgert, H. Hengel, and U. H. Koszinowski. 1999. A cytomegalovirus glycoprotein re-routes MHC class I complexes to lysosomes for degradation. EMBO J. 18:1081-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schoenhals, G. J., R. M. Krishna, A. G. Grandea III, T. Spies, P. A. Peterson, Y. Yang, and K. Fruh. 1999. Retention of empty MHC class I molecules by tapasin is essential to reconstitute antigen presentation in invertebrate cells. EMBO J. 18:743-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schumacher, T. N., M. T. Heemels, J. J. Neefjes, W. M. Kast, C. J. Melief, and H. L. Ploegh. 1990. Direct binding of peptide to empty MHC class I molecules on intact cells and in vitro. Cell 62:563-567. [DOI] [PubMed] [Google Scholar]
- 22.Suh, W. K., M. A. Derby, M. F. Cohen-Doyle, G. J. Schoenhals, K. Fruh, J. A. Berzofsky, and D. B. Williams. 1999. Interaction of murine MHC class I molecules with tapasin and TAP enhances peptide loading and involves the heavy chain alpha3 domain. J. Immunol. 162:1530-1540. [PubMed] [Google Scholar]
- 23.Tortorella, D., B. E. Gewurz, M. H. Furman, D. J. Schust, and H. L. Ploegh. 2000. Viral subversion of the immune system. Annu. Rev. Immunol. 18:861-926. [DOI] [PubMed] [Google Scholar]
- 24.Townsend, A., T. Elliott, V. Cerundolo, L. Foster, B. Barber, and A. Tse. 1990. Assembly of MHC class I molecules analyzed in vitro. Cell 62:285-295. [DOI] [PubMed] [Google Scholar]
- 25.Wagner, M., A. Gutermann, J. Podlech, M. J. Reddehase, and U. H. Koszinowski. 2002. Major histocompatibility complex class I allele-specific cooperative and competitive interactions between immune evasion proteins of cytomegalovirus. J. Exp. Med. 196:805-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagner, M., S. Jonjic, U. H. Koszinowski, and M. Messerle. 1999. Systematic excision of vector sequences from the BAC-cloned herpesvirus genome during virus reconstitution. J. Virol. 73:7056-7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yewdell, J. W., and A. B. Hill. 2002. Viral interference with antigen presentation. Nat. Immunol. 3:1019-1025. [DOI] [PubMed] [Google Scholar]
- 28.Ziegler, H., R. Thale, P. Lucin, W. Muranyi, T. Flohr, H. Hengel, H. Farrell, W. Rawlinson, and U. H. Koszinowski. 1997. A mouse cytomegalovirus glycoprotein retains MHC class I complexes in the ERGIC/cis-Golgi compartments. Immunity 6:57-66. [DOI] [PubMed] [Google Scholar]