Abstract
We have previously shown that the addition of the cyclin-dependent kinase (cdk) inhibitor Roscovitine at the beginning of infection of cells with human cytomegalovirus (HCMV) significantly disrupts immediate-early gene expression and the progression of the infection. In the present study, we have examined the effects of cdk inhibition on late viral events by delaying addition of Roscovitine until 24 h postinfection. Although viral DNA replication was inhibited two- to threefold by treatment of infected cells with Roscovitine, the drop did not correspond to the 1- to 2-log-unit decrease in virus titer. Quantification of viral DNA in the supernatant from cells revealed that there was a significant reduction in the production or release of extracellular particles. We observed a lag in the expression of several viral proteins but there was a significant decrease in the steady-state levels of IE2-86. Likewise, the steady-state level of the essential tegument protein UL32 (pp150) was reduced. The levels of pp150 and IE2-86 mRNA were not greatly affected by treatment with Roscovitine and thus did not correlate with the reduced levels of protein. In contrast, the expression of the tegument protein ppUL69 was higher in drug-treated samples, and the protein accumulated in a hyperphosphorylated form. ppUL69 localized to intranuclear aggregates that did not overlap with viral replication centers in cells treated with Roscovitine. Taken together, these data indicate that cdk activity is required at multiple steps during HCMV infection, including the expression, modification, and localization of virus-encoded proteins.
Human cytomegalovirus (HCMV), the prototypical betaherpesvirus, is a common pathogen that remains the leading viral cause of birth defects. It is estimated that congenital CMV infection occurs in 0.2 to 2.2% of live births, which translates to approximately 40,000 cases annually (33). Of these, symptomatic infection appears in 10 to 15% of cases and presents itself as progressive hearing loss and in some cases, severe psychomotor retardation (33). HCMV also continues to cause problematic opportunistic infections in immunocompromised patients including transplant recipients. In addition, CMV infection has been implicated as a cofactor in atherosclerosis and restenosis (52), and infection may play a role in the development or persistence of some cancer cells (13, 20, 35). These observations motivate studies to understand the complex interactions between the virus and the host cell that contribute to viral pathogenesis.
HCMV has developed multiple mechanisms to hijack the host cell's regulatory systems in order to achieve cell cycle arrest and, at the same time, to maintain an active metabolic state conducive to productive infection (5, 7). The block to cellular DNA replication results from the lack of licensing of cellular DNA origins of replication (6, 48), but proteins involved in production of nucleotide intermediates used in the process of replication, such as dihydrofolate reductase and ornithine decarboxylase, are induced (22, 28). Cells arrest in a pseudo-G1 state with high levels of cyclin E mRNA, protein, and cyclin E-associated kinase activity (9, 16, 23). The mitotic cyclin-dependent kinase (cdk) complex, cdk1/cyclin B1, also accumulates in its active state as a result of cyclin B stabilization and stimulatory phosphorylation of cdk1 (23, 34, 37). In contrast, the G1-phase cyclin D1 and the S-phase cyclin A are inhibited by the infection, while the steady-state levels of their kinase partners, cdk4 and cdk2, respectively, are unchanged (9).
The up-regulation of cdk activity during the infection implies that the virus is dependent on these enzymes to create an environment favorable for viral transcription, replication, and/or assembly of virus particles. Several studies have addressed the effect of cdk inhibition on replication of herpesviruses. Early work by Bresnahan and colleagues demonstrated that treatment of HCMV-infected cells with the cdk inhibitor Roscovitine, a purine analog that reversibly inhibits the activity of cdk1, -2, -5, -7, and -9, resulted in decreases in viral DNA synthesis, late (L) antigen expression, and the production of infectious virus (8). From this study it became clear that the drug Roscovitine is a useful tool for investigating the impact of cdk activity on viral infection.
In cells infected with herpes simplex virus (HSV), Roscovitine treatment blocks accumulation of the mRNAs encoding specific viral immediate-early (IE) and early (E) genes and inhibits viral DNA synthesis (39-41). In addition, at least two virus-encoded proteins, ICP0 and ICP4 (and possibly UL42), are phosphorylated by Roscovitine-sensitive kinases (2, 3, 14, 15). In the case of ICP0, drug treatment alters the transactivating abilities of the protein (14) but not its ability to direct degradation of ND10-associated proteins (15). Whether the effects of Roscovitine on ICP0 function are directly related to differences in the phosphorylation state of the protein was not definitively determined; however, from these studies it appears that there is a requirement for cdk activity in HSV-infected cells. Roscovitine also inhibits viral replication in cells infected with Epstein-Barr virus (26) and varicella-zoster virus (44), suggesting that the manipulation of cdks for the purposes of making the host cell permissive to infection is a conserved feature of herpesviruses.
Our laboratory has also used Roscovitine as a tool to investigate the dependence of HCMV infection on cdk activity (38). Drug treatment had a dose-dependent effect on the production of extracellular virus, and the level of inhibition was dependent on whether Roscovitine was added before or after the onset of viral DNA replication. Addition of 15 μM Roscovitine at the time of infection altered the accumulation and processing of IE gene transcripts and inhibited the expression of selected E genes. Viral DNA replication was also inhibited. In addition, a significant decrease in virus titer was observed. Delaying the addition of the drug until 6 hours postinfection (h p.i.) abrogated the deleterious effects on IE and E gene expression and viral DNA synthesis, which begins at approximately 16 to 20 h p.i. The effects on titer, however, were still observed even if the addition of Roscovitine did not occur until 24 or 48 h p.i. These data indicated that cdks might be important for virus maturation. In the present work we have analyzed of the effects of cdk inhibition on the expression and localization of several HCMV-encoded proteins late in infection. Our data indicate that the expression, posttranslational modification, and localization of some HCMV proteins are altered in cells treated with Roscovitine.
MATERIALS AND METHODS
Cell culture and virus.
Human foreskin fibroblasts (HFF) were maintained in minimal essential medium with Earle's salts supplemented with 10% fetal bovine serum, penicillin, streptomycin, amphotericin B, and glutamine as described previously (37). Cells were synchronized in G0 by allowing them to grow to confluence and to arrest for 3 additional days before trypsinization and replating at a lower density. Infection at the time of release from confluence allows for synchronous expression of IE genes (19, 34). Cells were infected at a multiplicity of infection (MOI) of 5 with the Towne strain of HCMV at the time of release from confluence. Mock-infected cells were incubated with an equal volume of conditioned medium. Cells were plated at a density of 8 × 105 cells/10-cm dish and maintained in 8 ml of complete medium. At 24 h p.i., Roscovitine (Calbiochem or Sigma; final concentration of 20 μM) or dimethyl sulfoxide (DMSO) was added as indicated. Media and chemicals were replenished every 24 h thereafter. In some experiments, the proteasome inhibitor Lactacystin (Calbiochem; final concentration of 10 μM) was added to cultures at 42 h p.i., and cells were harvested at 48 h p.i.
Cytotoxicity assays.
The cytotoxic effect of 20 μM Roscovitine on HFF was determined using the LIVE/DEAD viability/cytotoxicity assay kit as previously described (38).
Determining the effects of Roscovitine on virus titer.
G0-synchronized HFF were infected and treated as described above. Supernatants were collected at 96, 120, and 144 h p.i. Virus titer (PFU) was determined by plaque assay (43).
Analysis of viral DNA replication by real-time PCR.
G0-synchronized cells were infected and treated with Roscovitine or DMSO beginning at 24 h p.i. as described above. Cells were harvested at 24, 48, 72, and 96 h p.i. and frozen. DNA was extracted from the pellets using the QIAGEN Mini-Blood kit per the manufacturer's instructions. Viral DNA replication was measured by quantitative real-time PCR using TaqMan One-Step PCR master mix reagent kit (Applied Biosystems), primers and TaqMan dual-labeled (5′ 6-carboxyfluorescein-3′ Black Hole quencher) probe (Integrated DNA Technologies) to HCMV UL77 as described previously (47) (Table 1). A standard curve was made using dilutions of the DNA from samples harvested at 24 h p.i. Differences (n-fold) were calculated relative to the level of viral DNA at 24 h p.i.
TABLE 1.
Primer and probe sequences for real-time PCR
| Gene | Primer sequencea | TaqMan probe sequence |
|---|---|---|
| G6PD | 5′-TCTACCGCATCGACCACTACC-3′ (F) | 5-′ATGGTGCTGAGATTTGCCAACAGGA-3′ |
| 5′-GCGATGTTGTCCCGGTTC-3′ (R) | ||
| pp150 | 5′-GGAGCGCAAATGTCTGGC-3′ (F) | 5′-CATTCAGGAGCGCTGCAAGCTGC-3′ |
| 5′-AAAGGCACATGCGCAGC-3′ (R) | ||
| IE2-86 | 5′-TGACCGAGGATTGCAACGA-3′ (F) | 5′-TGGCAGAACTCGGTGACATCCTCGCC-3′ |
| 5′-CGGCATGATTGACAGCCTG-3′ (R) | ||
| UL77 | 5′-CGTTGCCCGGGAACG-3′ (F) | 5′-ACCTAGCTACTTTGGAATCACGCAGAACGA-3′ |
| 5-GGTGTGAAAGCGGATAAAGGG-3′ (R) | ||
| MCMV IE1 | 5′-TGGATGAGAACCGTGTCTAC-3′ (F) | N/Ab |
| 5′-ATATCATCTTGCGTTGTCTT-3′ (R) |
Forward (F) and reverse (R) primers are indicated in parentheses.
N/A, not applicable.
Quantification of infectivity/production of extracellular particles.
Infected-cell supernatants were prepared as described above. Prior to isolation of the DNA in the supernatant, an aliquot was removed to determine virus titer by plaque assay. Supernatants were mixed with a known amount of murine cytomegalovirus (MCMV) as an internal control for recovery of DNA using a QIAGEN Mini-Blood kit per the manufacturer's instructions, and sonicated salmon sperm DNA was used as carrier. DNA was quantified and used as template for real-time PCR using SYBR green (SYBR green PCR master mix; Applied Biosystems) to quantify the amplification of the IE region of MCMV (25) (Table 1). These data were then used to normalize the amplification of the HCMV-specific UL77 region as described above.
Western blotting.
At the time points indicated, cells were trypsinized, counted, and frozen. Pellets were lysed in reducing sample buffer (RSB), sonicated, and boiled as previously described (36). Equivalent cell numbers were loaded into each lane of polyacrylamide gels, and proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were transferred to nitrocellulose. The following antibodies were used to probe filters: anti-UL122/UL123 (IE1/IE2), anti-UL44, anti-UL83, and anti-UL99 (the Goodwin Research Institute); anti-IE2 monoclonal antibody (Chemicon); anti-IE2 rabbit antiserum 1218 (Jay Nelson, Oregon Health Sciences University); anti-UL32, anti-UL69, anti-UL86, anti-UL85, and anti-UL55 (William Britt, University of Alabama at Birmingham); anti-beta actin (Sigma); and anti-UL80/UL80.5 C1 rabbit serum (Wade Gibson, Johns Hopkins University School of Medicine). Band intensity was quantified by measuring integrated pixel densities as determined by NIH Image or MetaMorph (Universal Imaging Corporation) software.
For phosphatase assays, proteins were extracted as previously reported (6). Protein extracted from equivalent numbers of cells was treated with 100 U of lambda phosphatase (New England Biolabs) for 30 min at 30°C. Reactions were stopped by adding 2× RSB to the samples, followed by boiling. Equivalent cell numbers were loaded onto polyacrylamide gels as described above.
Immunofluorescence.
Confluence-synchronized cells were seeded on glass coverslips and infected and treated above. In some experiments, 20 μM Roscovitine or DMSO was added starting at 72 h p.i. At 72, 75, and 96 h p.i., coverslips were fixed in 2% paraformaldehyde in phosphate-buffered saline for 15 min at room temperature. Bromodeoxyuridine (BrDU) labeling and immunofluorescence staining were performed as previously described (19). Images were captured with a DeltaVision Restoration microscope system (Applied Precision Inc., Issaquah, WA) using a Photometrics Sony Coolsnap HQ charge-coupled device camera system (10 MHz, 12 bit, 1392 × 1040) attached to an inverted, wide-field fluorescence microscope (Nikon TE-200). Optical sections were acquired using the 100× (numerical aperture of 1.4) oil immersion objective in 0.2-μm steps in the z axis using the attached Applied Precision Inc. motorized stage. Images were saved, processed, and analyzed on Silicon Graphics workstations (O2, Octane) using the DeltaVision software package SoftWorx (version 2.50). For some experiments, coverslips were viewed with a Nikon E800 epifluorescence microscope, and images were captured using MetaMorph software and processed using Adobe Photoshop.
RNA isolation and real-time RT-PCR.
Infections in the presence of 20 μM Roscovitine were performed as described above. At the designated time p.i., cells were harvested and cell pellets were frozen. Total RNA was isolated using the PARIS RNA isolation kit (Ambion) per the manufacturer's instructions. After the RNA was quantified, the samples were treated with Turbo DNase (Ambion) to remove residual DNA contamination. Samples were quantified and diluted to a concentration of 12.5 ng/μl. Real-time reverse transcriptase PCR (RT-PCR) was used to determine the relative quantities of transcripts for UL32 (pp150), IE2-86, and the cellular housekeeping gene glucose-6-phosphate dehydrogenase (G6PD) (Table 1) as previously described (38, 47). The amounts were normalized to the level of G6PD in each of the samples. As an additional control, equal volumes of RNase-treated template were used to test for the presence of contaminating DNA when using primers for unspliced transcripts.
RESULTS
Roscovitine treatment results in a reduction of virus titer that does not correlate with the decrease in viral replication.
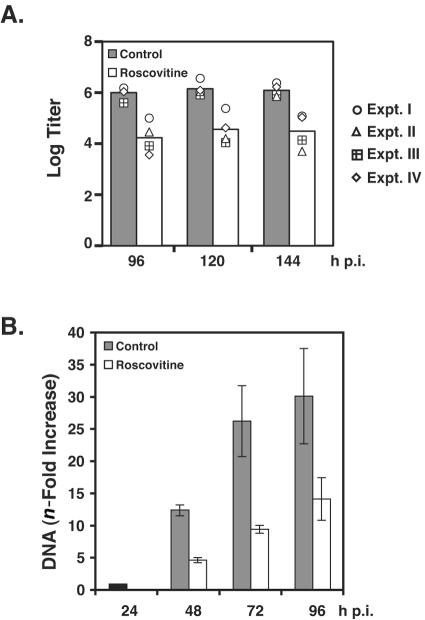
Our previous results indicated that treatment of cells with Roscovitine at a final concentration of 15 μM resulted in an approximately sixfold drop in virus titer when the drug was added beginning at 24 h p.i (38). In order to increase the effect on titer, we raised the concentration to 20 μM, which did not increase cytotoxicity (data not shown). At this concentration, addition of Roscovitine at 24 h p.i. resulted in a 15- to 57-fold drop in peak titers. Figure 1A illustrates the data from four independent experiments expressed as log titers.
FIG. 1.
Addition of Roscovitine beginning at 24 h p.i. causes a reduction in HCMV titer and a modest inhibition of viral DNA replication. Human fibroblasts were infected at an MOI of 5 with HCMV Towne. At 24 h p.i., infected cells were treated with 20 μM Roscovitine or DMSO as vehicle control. Growth medium was changed every 24 h as the drug was replenished. (A) Tissue culture supernatants were collected every 24 h beginning at 96 h p.i. through 144 h p.i. Viral titer was determined by standard plaque assay. The results from four independent experiments (Expt. I to Expt. IV) are shown. The bars represent the average log titers for each time point. (B) At the time points indicated, infected cells were collected and pellets were frozen. Viral DNA was extracted as described in Materials and Methods and used as a template for real-time PCR to quantify the level of viral DNA replication. The bars represent the average differences (n-fold) from two independent experiments. In each data set, the level of viral DNA at 24 h p.i. was set at 1 and the increase was calculated relative to the value at 24 h p.i. The error bars indicate ranges of the values from the two experiments.
We then determined whether the drug inhibited viral DNA replication. Viral DNA synthesis begins at 16 to 20 h p.i., and the quantity of DNA steadily increases as the infection progresses. Using quantitative real-time PCR, we measured the amount of viral DNA at 48, 72, and 96 h p.i. in treated and untreated cells relative to the quantity of DNA present at 24 h p.i. (Fig. 1B). The graph shows the average values from two experiments, and results are presented on a linear scale. At 48 h p.i., there was an approximately 10- to 15-fold increase in viral DNA in the control cells relative to the level of DNA at 24 h p.i., while the increase in viral DNA was 3- to 4-fold in Roscovitine-treated cells. At 72 h p.i. and 96 h p.i., the level of viral DNA in cells treated with the drug continued to lag behind the DMSO-treated cells, but the difference was about threefold. Taken together, the results of the plaque and replication assays suggested that a step in addition to viral DNA synthesis was regulated by cdk activity, resulting in the 1- to 2-log-unit drop in virus titer observed when infected cells were treated with Roscovitine beginning at 24 h p.i.
The decrease in titer observed upon treatment with cdk inhibitor correlates with a reduction in the production of extracellular virions.
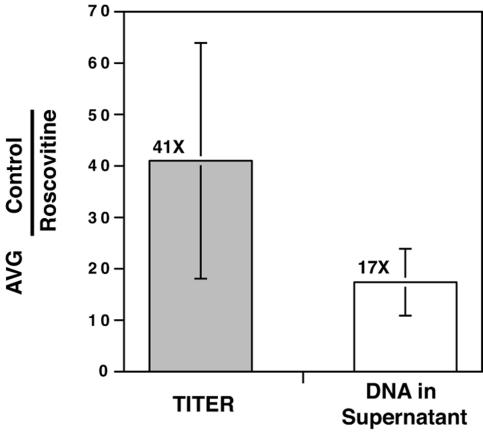
One possible explanation for the reduced titer observed upon treatment of virus-infected cells with Roscovitine was that cdk inhibition somehow altered viral infectivity and thus the ratio of particles to PFU (particle-to-PFU ratio). If this were the case, the number of extracellular particles that was required for the formation of a plaque might significantly differ in the presence or absence of drug. To test this possibility, quantitative real-time PCR was used to measure the relative amounts of viral DNA that were released into the supernatant over time when cells were treated with Roscovitine starting at 24 h p.i. The data show the severalfold increase in titer and viral DNA released from control cells relative to that released from Roscovitine-treated cells (Fig. 2). For simplicity, only the average differences (n-fold) in titer and extracellular viral DNA between control and treated samples containing peak titers from three separate experiments are shown. In these experiments there was 15- to 57-fold fewer PFU in supernatants from Roscovitine-treated cells, with an average difference of 41-fold. Our results indicated that this reduction in titer was correlated with a 10- to 22-fold decrease in viral DNA in these samples, with an average difference of 17-fold (Fig. 2). Comparison of the differences in titer and DNA suggested that there was not a significant change in infectivity caused by treatment of infected cells with the cdk inhibitor. The results instead indicated that the maturation and/or release of virus particles was affected.
FIG. 2.
Roscovitine treatment of HCMV-infected cells reduces the production of extracellular virus particles and has little effect on infectivity. Tissue culture supernatants from infected cells treated with 20 μM Roscovitine or DMSO were used to determine the titer and amount of viral DNA released into the media over time. DNA was isolated from extracellular virus particles as described in Materials and Methods and used as template for real-time PCR to determine the quantity of extracellular virions released from DMSO- or Roscovitine-treated cells. For simplicity, only values for days in which the peak titer was achieved for three independent experiments were used to determine the average (AVG) difference (n-fold) in titer and extracellular DNA between controls and drug-treated samples. Error bars represent the standard deviations between experiments.
Roscovitine added at 24 h p.i. affects the accumulation of specific virus-encoded proteins.
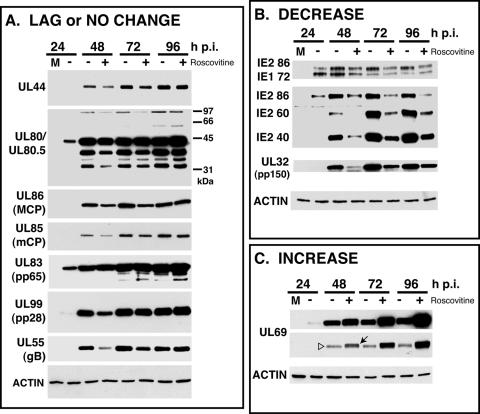
Although DNA replication was reduced at most threefold upon Roscovitine treatment, the drop in virus titer of 1 to 2 log units suggested that cdk inhibition might exert some effects on the expression of viral genes, especially those involved in assembly of the virus. To examine these changes, we performed Western blotting for several virus-encoded proteins of the IE, E, and L classes of genes. The expression levels fell in three general classes: proteins that showed a lag but were comparable by 96 h p.i. or that showed no change (Fig. 3A); proteins that were consistently lower at all time points (Fig. 3B); and one protein that increased in cells treated with Roscovitine (Fig. 3C). Most of the proteins examined fell into the group exhibiting a lag or little change in expression (Fig. 3A). These included the polymerase accessory protein UL44 and the capsid proteins UL80/UL80.5, UL85 (minor capsid protein), and UL86 (major capsid protein). We also observed little or no change in the expression of UL83 (pp65) and delays in the accumulation of UL99 (pp28) and envelope glycoprotein UL55 (gB).
FIG. 3.
The steady-state levels of specific proteins are affected by treatment of infected cells with 20 μM Roscovitine. Human fibroblasts were infected with HCMV Towne at an MOI of 5 and treated with 20 μM Roscovitine (+) or DMSO as a control (−) as described in Materials and Methods. At the time points indicated, infected cells were harvested. Samples were processed for Western blotting using equivalent cell numbers in each lane. Filters were reacted with the antibodies recognizing proteins of the three kinetic classes. Panels A, B, and C show the results from representative experiments. In panel C, both a long exposure (top) and a short exposure (bottom) of the Western blot for ppUL69 are shown. The arrow denotes the slower-migrating form(s) of ppUL69, and the open triangle denotes the faster-migrating form of ppUL69. M, mock-infected cells.
Two proteins were consistently reduced in cells treated with Roscovitine, IE2-86 and the major tegument phosphoprotein encoded by UL32, also referred to as pp150. As shown in Fig. 3B, addition of Roscovitine altered the ratio of IE2-86 and IE1-72 relative to the untreated samples. At each of the time points, the level of IE2-86 was lower in the Roscovitine-treated cells, while the level of IE1-72 was comparable or slightly higher. Throughout the infection, a significant reduction was also observed in the levels of the p60 and p40 forms of IE2 in drug-treated cells relative to the controls. In contrast, the most significant difference in pp150 levels, about eightfold, was observed at 48 h p.i. At 72 and 96 h p.i., the reduction was not as marked, but pp150 levels were nevertheless two- to threefold lower in samples treated with the cdk inhibitor.
The only protein observed to increase in drug-treated cells was ppUL69, which was higher at all of the time points (Fig. 3C). The second panel is a lighter exposure of the same Western blot to demonstrate that the increased signal arose from the accumulation of one or two slower-migrating species of the protein. Quantification of the bands on the blot showed that there was an approximately fourfold increase in ppUL69 in cells treated with Roscovitine.
Taken together with the data presented in Fig. 1 and 2, the delayed or altered expression of a number of virion-associated proteins and the reduced accumulation of pp150 and the full-length and shorter forms of IE2 in cells treated with Roscovitine could affect virion maturation.
The levels of IE2-86 and pp150 mRNAs do not correlate with the steady-state levels of their proteins in cells treated with the cdk inhibitor.
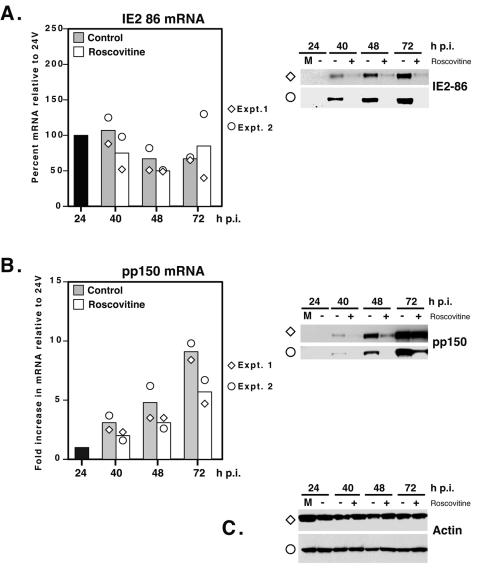
To further characterize the decrease in IE2-86 and pp150, we analyzed RNA levels in Roscovitine-treated samples. The results from two independent experiments are presented (Fig. 4). The RNA quantities are calculated relative to the level of RNA at the 24-h time point, and the bars in the graph represent the average quantities of the two experiments. The corresponding Western blot for each experiment is denoted by the appropriate symbol adjacent to the panel.
FIG. 4.
Cdk inhibition by Roscovitine affects the expression of viral proteins but not their mRNAs. At the time points indicated, infected cells were harvested and processed for RNA analysis and Western blotting. The levels of viral transcripts were determined by quantitative real-time RT-PCR as described in Materials and Methods using probes specific for IE2-86 (A) and pp150 (B). For each experiment, values were normalized to the level of the internal control (G6PD). Bars represent the averaged value from two independent experiments, while the diamond and circle symbols correspond to the values from each individual time course (experiments [Expt.] 1 and 2). Protein levels were determined by Western blotting using antibodies to IE2 (A) and pp150 (B). (C) Actin loading control for each experiment. M, mock-infected cells. The diamond and circle symbols denote the corresponding Western blot for the individual RNA experiments shown in the graphs.
In the case of IE2-86 mRNA, on average there were small differences (less than twofold) between experimental and control samples at all time points (Fig. 4A). While IE2-86 mRNA decreased at 72 h p.i. in Roscovitine-treated cells in one experiment, the mRNA increased in the other experiment; however, regardless of the level of mRNA, the steady-state level of IE2-86 protein was lower in both experiments at all time points. At 72 h p.i. in experiment 1, there was an approximately 11-fold reduction in IE2-86 protein, but RNA levels differed by approximately twofold. In experiment 2, IE2-86 was either at or below the limit of detection in the Western blot at 72 h p.i. in Roscovitine-treated samples, but the level of mRNA was higher than in untreated controls.
As was observed for IE2-86, the differences in the levels of pp150 mRNA between Roscovitine-treated and control samples was twofold or less at all time points (Fig. 4B); yet at 48 h p.i., there was at least sevenfold more pp150 protein in the control samples. At 72 h p.i., there was some variability between the experiments. In experiment 1, there was approximately twofold-less protein in Roscovitine-treated samples than in the controls, which correlated with the difference in mRNA. In experiment 2, there was three- to fourfold-less pp150 in drug-treated cells, but the difference in the corresponding mRNA was less than twofold.
These results suggested that the expression of the pp150 and IE2-86 proteins was inhibited at a step after transcription, such as export or translation of the mRNAs or protein stability. To determine whether IE2-86 and pp150 were degraded by the proteasome in Roscovitine-treated cells, we added Roscovitine to the cultures at 24 h p.i. and then added the proteasome inhibitor Lactacystin for 6 h prior to harvest of the cells at 48 h p.i. Figure 5A shows the results of RNA analyses from two experiments. Quantities of mRNA were calculated relative to the level at 48 h in the untreated control sample. The bars represent the average values of the two experiments. The Western blots corresponding to individual experiments are denoted by the appropriate symbol adjacent to the panel.
FIG. 5.
Inhibition of the proteasome partially restores IE2-86 protein levels. Human fibroblasts were infected with HCMV Towne at an MOI of 5. At 24 h p.i., Roscovitine or DMSO was added to the cultures. At 42 h p.i., growth medium was replenished and Roscovitine (20 μM) (+), Lactacysin (10 μM) (+), or DMSO (−) was added. At 48 h p.i., cells were harvested and samples were processed for RNA analysis and Western blotting as described in Materials and Methods. (A) The levels of viral transcripts were determined by quantitative real-time RT-PCR as described in Materials and Methods using probes specific for IE2-86 and pp150. The percentage of mRNA was calculated relative to the quantity in untreated, infected cells at 48 h p.i. (48V), which was set to 100%. Bars represent the average values of two independent experiments, while the diamond and circle symbols correspond to the values from each experiment (experiments [Expt.] 1 and 2). (B) Protein levels were determined by Western blotting using antibodies to IE2-86, pp150, and actin. The diamond and circle symbols denote the corresponding Western blot for the RNA analyses shown in panel A.
Inhibition of the proteasome in cells also treated with Roscovitine partially restored IE2-86 expression in both experiments. IE2-86 protein levels in cells treated with both drugs were approximately fourfold higher than in cells treated with Roscovitine alone (Fig. 5B), while RNA levels were unchanged (Fig. 5A). We did not detect an increase in IE2-86 in cells treated with Lactacystin alone.
In the case of pp150, treatment of cells with cdk inhibitor resulted in approximately fourfold-less protein at the 48-h time point in experiment 1 (Fig. 5B). There were small increases in the amount of pp150 protein in both samples treated with the proteasome inhibitor in this experiment, suggesting that increased proteasome-mediated degradation did not contribute to the lower level of pp150 in cells treated with Roscovitine. In experiment 2, the levels of pp150 were at least sixfold lower in the Roscovitine-treated samples, and proteasome inhibition led to a small increase in protein levels in cells treated with both inhibitors (Fig. 5B), although the increased levels could have resulted from the higher levels of pp150 mRNA observed in this sample (Fig. 5A). Addition of an inhibitor of the cysteine protease calpain, previously shown to be active in HCMV-infected cells (12), did not increase the level of IE2-86 or pp150 protein (data not shown).
These experiments suggested that enhanced proteasome-mediated degradation of IE2-86 might play a role in the lower levels of protein observed in cells treated with Roscovitine. We could not definitively determine whether the proteasome was involved in regulation of pp150 levels in cells treated with cdk inhibitor.
Roscovitine alters the phosphorylation state and localization of ppUL69.
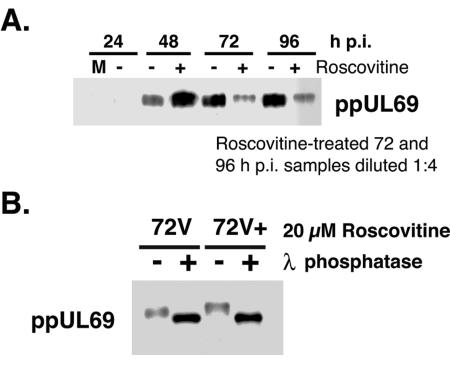
ppUL69 is the homolog of HSV ICP27, a protein known to play a role in transport and translation of virion-encoded RNAs in cells infected with herpes simplex virus. Our observations regarding IE2-86 and pp150 RNA and protein levels prompted us to examine the accumulation of UL69 in cells treated with Roscovitine more carefully. Because the phosphorylation states of ppUL69 can be resolved by one-dimensional electrophoresis (49), we attempted to separate the phosphorylated forms of the protein. The accumulation of ppUL69 that we previously observed in whole-cell lysates was due to an increase in a slower-migrating form of ppUL69 when Roscovitine was added to the cultures beginning at 24 h p.i. (Fig. 6A). It should be noted that the Roscovitine-treated samples from 72 and 96 h p.i. were diluted to show that the increased signal corresponded to the slower-migrating species of ppUL69. We show that the mobility difference was due to phosphorylation, as treatment of NP-40 extracts with lambda phosphatase increased the mobility of the protein in both drug-treated and control samples (Fig. 6B). These data suggested that inhibition of cdk activity induced hyperphosphorylation of the protein. We could not determine whether ppUL69 was also induced at the transcriptional level due to overlapping transcripts from this region.
FIG. 6.
ppUL69 accumulates in a hyperphosphorylated form in the presence of Roscovitine. (A) Samples were processed for Western blotting as described in the legend to Fig. 3. To distinguish the phosphorylated forms of ppUL69, lysates were separated by SDS-PAGE in gels containing 4.2% cross-linking. Lanes were loaded by equivalent cell numbers except for drug-treated samples from 72 and 96 h p.i., which were diluted to prevent saturation of the chemiluminescent signal. +, treated with Roscovitine; −, treated with DMSO as a control; M, mock-infected cells. (B) Phosphatase treatment of infected-cell proteins. Cell pellets were extracted as described in Materials and Methods, and lysates were treated with lambda phosphatase for 30 min (+). The reaction was stopped by adding an equivalent volume of 2× RSB followed by boiling of the samples. Control samples (−) were processed in parallel. Proteins were separated by SDS-PAGE as described above. The filter was reacted with antibody specific for ppUL69. 72V, untreated viral samples from 72 h p.i.; 72V+, roscovitine-treated viral samples from 72 h p.i.
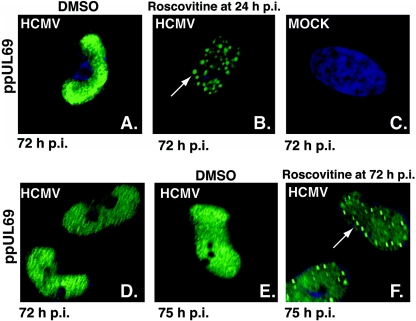
Since phosphorylation of proteins often alters their subcellular localization, we examined the effect of Roscovitine treatment on the localization of ppUL69 (Fig. 7). In cells treated with the drug at 24 h p.i., ppUL69 was found in small, round intranuclear aggregates at 72 h p.i. (Fig. 7B), while in untreated cells, ppUL69 was diffusely distributed in the nucleus and in the viral replication centers (Fig. 7A and D). The aggregates observed in cells treated with cdk inhibitor varied in size and were visible by phase microscopy (data not shown). The formation of these ppUL69-positive structures was not a result of the extended incubation of the infected cells with Roscovitine but a rapid response to treatment with the drug. Figure 7D and E show the distribution of ppUL69 in untreated cells at 72 and 75 h p.i., respectively. As shown in Fig. 7F, we observed the formation of ppUL69 aggregates in cells treated with Roscovitine for as little as 3 h between 72 and 75 h p.i. As expected, ppUL69 was not present in mock-infected cells (Fig. 7C).
FIG. 7.
cdk inhibition leads to accumulation of ppUL69 in intranuclear aggregates. Human fibroblasts were infected at an MOI of 5 with HCMV Towne and seeded onto glass coverslips. At 24 (A and B) or 72 (E and F) h p.i., 20 μM Roscovitine or DMSO was added to cultures. At 72 h or 75 h p.i., cells were fixed in 2% paraformaldehyde and processed for immunofluorescent staining with antibody against ppUL69 and Hoechst stain to visualize nuclei. (C) Mock-infected control. (D) ppUL69 distribution before addition of drug or vehicle control to cultures at 72 h p.i. Images were captured with a Nikon E800 epifluorescence microscope as described in Materials and Methods. Original magnification, ×1,000.
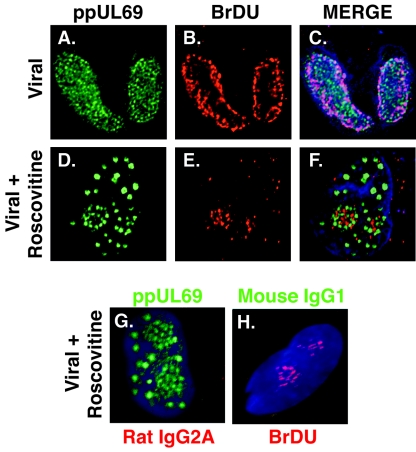
Because it was previously reported that ppUL69 localized to viral replication centers (50), we proceeded to determine whether the sites of viral DNA replication also changed in the presence of Roscovitine (Fig. 8). Infected cells were treated with cdk inhibitor from 24 h p.i. Just prior to fixation at 72 h p.i., infected-cell cultures were treated with BrDU, which incorporated into the newly synthesized viral DNA (19). The replication centers were then stained with anti-BrDU antibody. Unlike the diffuse distribution of ppUL69 within replication centers in control cells (Fig. 8A through C), the ppUL69 structures formed in the presence of the drug appeared to overlap and surround viral replication centers (Fig. 8D through F). These data showed that unlike ppUL69, there was not an alteration in the localization of active sites of viral DNA replication in the presence of Roscovitine; however, the viral replication centers were smaller relative to those of the controls. Figure 8G and H represent nonspecific antibody controls for the immunofluorescence staining.
FIG. 8.
ppUL69 is localized in aggregates around the viral replication center upon cdk inhibition. Virus-infected cells were treated with DMSO or 20 μM Roscovitine beginning at 24 h p.i. One hour prior to fixation, cells were labeled with 10 μM bromodeoxyuridine for 30 min. Cells were washed twice and refed. At 72 h p.i., cells were fixed in 2% paraformaldehyde and processed for immunofluorescent staining with antibodies against ppUL69 and BrDU (A through F). Images were captured by confocal microscopy as described in Materials and Methods. Original magnification, ×1,000. (G and H) Control stains with nonspecific rat immunoglobulin G2A (IgG2A) and mouse IgG1 antibodies, respectively. These images were captured with a Nikon E800 epifluorescence microscope as described in Materials and Methods. Original magnification, ×1,000.
DISCUSSION
In this report we present the results of our continuing studies on the roles of cyclin-dependent kinase activity during HCMV infection. The importance of cdk activity in the life cycle of the herpesviruses has been well documented, especially in the case of HSV (1, 4, 14, 15, 40, 41), although it is necessary to note that the modulation of specific cdks in cells infected with the alpha-, beta-, and gammaherpesviruses is quite different. These differences most likely reflect the metabolic state of the cells infected by these viruses, the cell type, and the length of the infectious cycle of the viruses themselves. Viruses such as HCMV with prolonged replication cycles have evolved mechanisms for short-circuiting the host cell cycle machinery very early in the infection to effect cell cycle arrest and prevent competition from cellular processes for factors used later in viral replication. Our observations indicate that cdks are required at multiple levels during HCMV infection for regulating the expression of viral genes (38), the posttranslational modification and localization of viral proteins, and the maturation and release of extracellular particles.
Quantification of viral DNA replication, the production of infectious, extracellular virus by plaque titer, and the accumulation of viral DNA in the supernatant demonstrated that the most significant defect caused by inhibition of cdk activity by Roscovitine treatment late in HCMV infection was a reduction in extracellular particles. Two possible explanations for this defect are that the assembly of particles is affected or that the release of particles from the cells is inhibited. Our data do not differentiate between these possibilities, but a defect in maturation of the virion is supported. The decrease in the steady-state levels of pp150 alone could explain the reduction in extracellular virus. This tegument protein was previously shown to be essential for infection (17), and it comprises ∼9% of the virion (46). At 48 h p.i, levels of pp150 were generally 4- to 10-fold lower in cells treated with Roscovitine. The differences in the levels of pp150 were smaller at the 72- and 96-h time points but were typically between two- to threefold higher in control cells in a number of experiments. A limiting quantity of pp150 could result in inefficient or altered tegumentation and/or envelopment of the nucleocapsid (32). In addition, the retention of pp65 in the nuclei of cells treated with Roscovitine (unpublished data) could enhance a defect in virion tegumentation, although pp65 has been reported to be dispensable for infection (17, 42). In addition, pp150, like pp65, ppUL69, ppUL97, and pp28, is a phosphoprotein, and its posttranslational modification could also be affected by cdk inhibition, resulting in inefficient cytoplasmic assembly. In fact, we detected changes in the phosphorylation states of ppUL69 and pp65 upon inhibition of cdk activity (data not shown). Thus, it is possible that pp150 phosphorylation could be affected like that of pp65 and ppUL69 and that these differences could alter the assembly of virion particles in cells treated with Roscovitine. These hypotheses refer only to the incorporation of pp150 and the other proteins into the tegument of the virus particle and do not address other as yet undetermined functions of these proteins during infection.
The altered localization of ppUL69 upon treatment of infected cells with the cdk inhibitor is also an intriguing observation. There is limited information on the function of ppUL69 during HCMV infection. As part of the tegument, ppUL69 is introduced into the cell upon infection and has been shown to contribute to the cell cycle arrest observed in infected cells (21, 31). In addition, ppUL69 plays a role in early gene expression (50). In contrast to pp150, however, ppUL69 is not essential, but recombinant viruses with deletion of ppUL69 do exhibit a severe growth defect (17, 21).
ppUL69 is the HCMV homolog of HSV ICP27, a protein that has been well studied with respect to its functions as an exporter of intronless viral RNAs through the TAP export pathway (10, 11) and as an activator of transcription through interactions with RNA polymerase II (51). Similar studies have been undertaken with regard to the role of ppUL69 in export. Lischka and colleagues (29) reported that like ICP27, ppUL69 shuttles between the nucleus and cytoplasm in a CRM-1-independent manner (11, 24). In addition, this group recently reported that ppUL69 binds RNA (30, 45), and thus, it may play a role similar to HSV ICP27 in promoting export and translation of specific viral RNAs (10, 11, 18, 27). We speculate that the change in the phosphorylation state of ppUL69 in cells treated with Roscovitine has a functional consequence with regard to viral gene expression. In support of this hypothesis, we observed that the steady-state levels of pp150 did not correlate with the accumulation of pp150 mRNA at 48 h p.i., suggesting a defect in mRNA export or translation. Many additional studies will be necessary to explore this hypothesis.
Finally, the results presented in this study support a role for IE2 late in infection. We previously reported that deletion of amino acids 136 to 290 of IE2-86 (ΔSX) resulted in the production of a virus that was viable but impaired at the late stages of infection (36). This mutant did not produce the p60 and p40 forms of IE2. The ΔSX mutant virus also showed lags in the expression of two tegument proteins, pp65 and pp28, and exhibited delayed kinetics in the generation of infectious virus compared to the parent and rescue viruses. Additionally, the relocalization of pp65 from the nucleus to the cytoplasm was delayed in cells infected with ΔSX, suggesting that there was a delay in the transition to the late phase of infection. Inhibition of cdk activity resulted in a dramatic reduction in the expression of IE2-86 and in retention of pp65 in the nuclei of Roscovitine-treated cells (unpublished data), suggesting that there is a relationship between IE2 function and export of pp65.
In summary, we report that the decreased titer observed when HCMV-infected fibroblasts were treated with Roscovitine during the early-late phases of infection resulted from decreased extracellular particle production, most likely due to down-regulation of IE2-86 and pp150 expression and mislocalization of ppUL69. Our results indicate that cdk activity is required for proper expression, modification, and localization of virion-encoded proteins important in maturation.
Acknowledgments
We thank William Britt, Wade Gibson, and Jay Nelson for antibodies. We also extend our thanks to other members of the laboratory, especially Chris Morello for assistance with reagents to murine cytomegalovirus and Elizabeth White for assistance with RNA analysis. We are grateful to Monika Szeszel and Kersi Pestonjamasp of the UCSD Cancer Center Shared Imaging Resource for assistance with confocal microscopy.
This work was funded by NIH grants CA073490 and CA034729 to D.H.S. and CA102094 to V.S.
REFERENCES
- 1.Advani, S. J., R. Brandimarti, R. R. Weichselbaum, and B. Roizman. 2000. The disappearance of cyclins A and B and the increase in activity of the G2/M-phase cellular kinase cdc2 in herpes simplex virus 1-infected cells require expression of the α22/US1.5 and UL13 viral genes. J. Virol. 74:8-15. [PMC free article] [PubMed] [Google Scholar]
- 2.Advani, S. J., R. Hagglund, R. R. Weichselbaum, and B. Roizman. 2001. Posttranslational processing of infected cell proteins 0 and 4 of herpes simplex virus 1 is sequential and reflects the subcellular compartment in which the proteins localize. J. Virol. 75:7904-7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Advani, S. J., R. R. Weichselbaum, and B. Roizman. 2001. cdc2 cyclin-dependent kinase binds and phosphorylates herpes simplex virus 1 UL42 DNA synthesis processivity factor. J. Virol. 75:10326-10333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Advani, S. J., R. R. Weichselbaum, and B. Roizman. 2000. The role of cdc2 in the expression of herpes simplex virus genes. Proc. Natl. Acad. Sci. USA 97:10996-11001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albrecht, T., M. P. Fons, I. Boldogh, S. AbuBakar, C. Z. Deng, and D. Millinoff. 1991. Metabolic and cellular effects of human cytomegalovirus infection. Transplant. Proc. 23:48-55. [PubMed] [Google Scholar]
- 6.Biswas, N., V. Sanchez, and D. H. Spector. 2003. Human cytomegalovirus infection leads to accumulation of geminin and inhibition of the licensing of cellular DNA replication. J. Virol. 77:2369-2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boldogh, L., S. AbuBakar, C. Z. Deng, and T. Albrecht. 1991. Transcriptional activation of cellular oncogenes fos, jun, and myc by human cytomegalovirus. J. Virol. 65:1568-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bresnahan, W. A., I. Boldogh, P. Chi, E. A. Thompson, and T. Albrecht. 1997. Inhibition of cellular Cdk2 activity blocks human cytomegalovirus replication. Virology 12:239-247. [DOI] [PubMed] [Google Scholar]
- 9.Bresnahan, W. A., I. Boldogh, E. A. Thompson, and T. Albrecht. 1996. Human cytomegalovirus inhibits cellular DNA synthesis and arrests productively infected cells in late G1. Virology 224:156-160. [DOI] [PubMed] [Google Scholar]
- 10.Chen, I.-H., L. Li, L. Silva, and R. Sandri-Goldin. 2005. ICP27 recruits Aly/REF but not TAP/NXF1 to herpes simplex virus type 1 transcription sites although TAP/NXF1 is required for ICP27 export. J. Virol. 79:3949-3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, I.-H., K. Sciabica, and R. Sandri-Goldin. 2002. ICP27 interacts with the RNA export factor Aly/REF to direct herpes simplex virus type 1 intronless mRNAs to the TAP export pathway. J. Virol. 76:12877-12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, Z., E. Knutson, A. Kurosky, and T. Albrecht. 2001. Degradation of p21cip1 in cells productively infected with human cytomegalovirus. J. Virol. 75:3613-3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cobbs, C. S., L. Harkins, M. Samanta, G. Y. Gillespie, S. Bharara, P. H. King, L. B. Nabors, C. G. Cobbs, and W. J. Britt. 2002. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res. 62:3347-3350. [PubMed] [Google Scholar]
- 14.Davido, D. J., D. A. Leib, and P. A. Schaffer. 2002. The cyclin-dependent kinase inhibitor Roscovitine inhibits the transactivating activity and alters the posttranslational modification of herpes simplex virus type 1 ICP0. J. Virol. 76:1077-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davido, D. J., W. F. von Zagorski, G. G. Maul, and P. A. Schaffer. 2003. The differential requirement for cyclin-dependent kinase activities distinguishes two functions of herpes simplex virus type 1 ICP0. J. Virol. 77:12603-12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dittmer, D., and E. S. Mocarski. 1997. Human cytomegalovirus infection inhibits G1/S transition. J. Virol. 71:1629-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunn, W., C. Chou, H. Li, R. Hai, D. Patterson, V. Stoic, H. Zhu, and F. Liu. 2003. Functional profiling of a human cytomegalovirus genome. Proc. Natl. Acad. Sci. USA 100:14223-14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ellison, K., R. Maranchuk, K. Mottet, and J. Smiley. 2005. Control of VP16 translation by the herpes simplex virus type 1 immediate-early protein ICP27. J. Virol. 79:4120-4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fortunato, E. A., V. Sanchez, J. Y. Yen, and D. H. Spector. 2002. Infection of cells with human cytomegalovirus during S phase results in a blockade to immediate-early gene expression that can be overcome by inhibition of the proteasome. J. Virol. 76:5369-5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harkins, L., A. L. Volk, M. Samanta, I. Mikolaenko, W. J. Britt, K. I. Bland, and C. S. Cobbs. 2002. Specific localisation of human cytomegalovirus nucleic acids and proteins in colorectal cancer. Lancet 360:1557-1563. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi, M. L., C. Blankenship, and T. Shenk. 2000. Human cytomegalovirus UL69 is required for efficient accumulation of infected cells in the G1 phase of the cell cycle. Proc. Natl. Acad. Sci. USA 97:2692-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isom, H. C. 1979. Stimulation of ornithine carboxylase by human cytomegalovirus. J. Gen. Virol. 42:265-278. [DOI] [PubMed] [Google Scholar]
- 23.Jault, F. M., J.-M. Jault, F. Ruchti, E. A. Fortunato, C. Clark, J. Corbeil, D. D. Richman, and D. H. Spector. 1995. Cytomegalovirus infection induces high levels of cyclins, phosphorylated Rb, and p53, leading to cell cycle arrest. J. Virol. 69:6697-6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koffa, M., J. Clements, E. Izaurralde, S. Wadd, S. Wilson, I. Mattaj, and S. Kuersten. 2001. Herpes simplex virus ICP27 protein provides viral mRNAs with access to the cellular mRNA export pathway. EMBO J. 20:5769-5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koffron, A. J., M. Hummel, B. K. Patterson, S. Yan, D. B. Kaufman, J. P. Fryer, F. P. Stuart, and M. I. Abecassis. 1998. Cellular localization of latent murine cytomegalovirus. J. Virol. 72:95-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kudoh, A., T. Daikoku, Y. Sugaya, H. Isomura, M. Fujita, T. Kiyono, Y. Nishiyama, and T. Tsurumi. 2004. Inhibition of S-phase cyclin-dependent kinase activity blocks expression of Epstein-Barr virus immediate-early and early genes, preventing viral lytic replication. J. Virol. 78:104-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larralde, O., R. Smith, G. Wilkie, P. Malik, N. Gray, and J. Clements. 2006. Direct stimulation of translation by the multifunctional herpesvirus ICP27 protein. J. Virol. 80:1588-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lembo, D., G. Gribaudo, R. Cavallo, R. Riera, A. Angeretti, L. Hertel, and S. Landolfo. 1999. Human cytomegalovirus stimulates cellular dihydrofolate reductase activity in quiescent cells. Intervirology 42:30-36. [DOI] [PubMed] [Google Scholar]
- 29.Lischka, P., O. Rosorius, E. Trommer, and T. Stamminger. 2001. A novel transferable nuclear export signal mediates CRM1-independent nucleocytoplasmic shuttling of the human cytomegalovirus transactivator protein pUL69. EMBO J. 20:7271-7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lischka, P., Z. Toth, M. Thomas, R. Mueller, and T. Stamminger. 2006. The UL69 transactivator protein of human cytomegalovirus interacts with the DEXD/H-box RNA helicase UAP56 to promote cytoplasmic accumulation of unspliced RNA. Mol. Cell. Biol. 26:1631-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu, M., and T. Shenk. 1999. Human cytomegalovirus UL69 protein induces cells to accumulate in G1 phase of the cell cycle. J. Virol. 73:676-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyer, H., A. Ripalti, M. Landini, K. Radsak, H. Kern, and G. Hensel. 1997. Human cytomegalovirus late-phase maturation is blocked by stably expressed UL32 antisense mRNA in astrocytoma cells. J. Gen. Virol. 78:2621-2631. [DOI] [PubMed] [Google Scholar]
- 33.Pass, R. P. 2001. Cytomegalovirus, p. 2675-2705. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 34.Salvant, B. S., E. A. Fortunato, and D. H. Spector. 1998. Cell cycle dysregulation by human cytomegalovirus: influence of the cell cycle at the time of infection and effects on cyclin transcription. J. Virol. 72:3729-3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samanta, M., L. Harkins, K. Klemm, W. J. Britt, and C. S. Cobbs. 2003. High prevalence of human cytomegalovirus in prostatic intraepithelial neoplasia and prostatic carcinoma. J. Urol. 170:998-1002. [DOI] [PubMed] [Google Scholar]
- 36.Sanchez, V., C. L. Clark, J. Y. Yen, R. Dwarakanath, and D. H. Spector. 2002. Viable human cytomegalovirus recombinant virus with an internal deletion of the IE2 86 gene affects late stages of viral replication. J. Virol. 76:2973-2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanchez, V., A. K. McElroy, and D. H. Spector. 2003. Mechanisms governing maintenance of Cdk1/cyclin B1 kinase activity in cells infected with human cytomegalovirus. J. Virol. 77:13214-13224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanchez, V., A. K. McElroy, J. Yen, S. Tamrakar, C. L. Clark, R. A. Schwartz, and D. H. Spector. 2004. Cyclin-dependent kinase activity is required at early times for accurate processing and accumulation of the human cytomegalovirus UL122-123 and UL37 immediate-early transcripts and at later times for virus production. J. Virol. 78:11219-11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schang, L. M., J. Phillips, and P. A. Schaffer. 1998. Requirement for cellular cyclin-dependent kinases in herpes simplex virus replication and transcription. J. Virol. 72:5626-5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schang, L. M., A. Rosenberg, and P. A. Schaffer. 2000. Roscovitine, a specific inhibitor of cellular cyclin-dependent kinases, inhibits herpes simplex virus DNA synthesis in the presence of viral early proteins. J. Virol. 74:2107-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schang, L. M., A. Rosenberg, and P. A. Schaffer. 1999. Transcription of herpes simplex virus immediate-early and early genes is inhibited by Roscovitine, an inhibitor specific for cellular cyclin-dependent kinases. J. Virol. 73:2161-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmolke, S., H. F. Kern, P. Drescher, G. Jahn, and B. Plachter. 1995. The dominant phosphoprotein pp65 (UL83) of human cytomegalovirus is dispensable for growth in cell culture. J. Virol. 69:5959-5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tamashiro, J. C., L. J. Hock, and D. H. Spector. 1982. Construction of a cloned library of the EcoRI fragments from the human cytomegalovirus genome (strain AD169). J. Virol. 42:547-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor, S., P. Kinchington, A. Brooks, and J. Moffat. 2004. Roscovitine, a cyclin-dependent kinase inhibitor, prevents replication of varicella-zoster virus. J. Virol. 78:2853-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toth, Z., P. Lischka, and T. Stamminger. 2006. RNA-binding of the human cytomegalovirus transactivator UL69, mediated by arginine-rich motifs, is not required for nuclear export of unspliced RNA. Nucleic Acids Res. 34:1237-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Varnum, S. M., D. N. Streblow, M. E. Monroe, P. Smith, K. J. Auberry, L. Paša-Tolić, D. Wang, D. G. Camp II, K. Rodland, S. Wiley, W. Britt, T. Shenk, R. D. Smith, and J. A. Nelson. 2004. Identification of proteins in human cytomegalovirus (HCMV) particles: the HCMV proteome. J. Virol. 78:10960-10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.White, E. A., C. L. Clark, V. Sanchez, and D. H. Spector. 2004. Small internal deletions in the human cytomegalovirus IE2 gene result in nonviable recombinant viruses with differential defects in viral gene expression. J. Virol. 78:1817-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wiebusch, L., R. Uecker, and C. Hagemeier. 2003. Human cytomegalovirus prevents replication licensing by inhibiting MCM loading onto chromatin. EMBO Rep. 4:42-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Winkler, M., and T. Stamminger. 1996. A specific subform of the human cytomegalovirus transactivator protein pUL69 is contained within the tegument of virus particles. J. Virol. 70:8984-8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winkler, M. S., S. A. Rice, and T. Stamminger. 1994. UL69 of human cytomegalovirus, an open reading frame with homology to ICP27 of herpes simplex virus, encodes a transactivator of gene expression. J. Virol. 68:3943-3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou, C., and D. Knipe. 2002. Association of herpes simplex virus type 1 ICP8 and ICP27 proteins with cellular RNA polymerase II holoenzyme. J. Virol. 76:5893-5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou, Y. F., M. B. Leon, M. A. Waclawiw, J. J. Popma, Z. X. Yu, T. Finkel, and S. E. Epstein. 1996. Association between prior cytomegalovirus infection and risk of restenosis after coronary atherectomy. N. Engl. J. Med. 335:624-630. [DOI] [PubMed] [Google Scholar]