Abstract
Recent in vivo studies have revealed that the subgenomic promoter (sgp) in brome mosaic bromovirus (BMV) RNA3 supports frequent homologous recombination events (R. Wierzchoslawski, A. Dzianott, and J. Bujarski, J. Virol. 78:8552-8564, 2004). In this paper, we describe an sgp-driven in vitro system that supports efficient RNA3 crossovers. A 1:1 mixture of two (−)-sense RNA3 templates was copied with either a BMV replicase (RdRp) preparation or recombinant BMV protein 2a. The BMV replicase enzyme supported a lower recombination frequency than 2a, demonstrating a role of other viral and/or host factors. The described in vitro system will allow us to study the mechanism of homologous RNA recombination.
RNA recombination is one of the fundamental aspects of the life cycle and evolution of RNA viruses and contributes significantly to a high level of variation in viral RNAs, as demonstrated by sequencing of virus populations (6, 7, 32, 34, 45, 50, 58) and with experimental in vivo systems for animal viruses (19, 31, 44, 51, 54), plant viruses (1, 2, 12, 15, 25, 35, 41, 53, 55, 59), bacteriophages (38), and retroviruses (40). In vitro recombination assays (reviewed in reference 21) revealed either a nonreplicative (breakage-religation) (22) or replicative (template switch) (3, 28, 30, 36) mechanism of crossover. During the template switch, a premature dissociation of the replicating enzyme from the donor RNA molecule is evidently facilitated by double-stranded structures (9, 10, 30, 43, 51), homopolymeric tracts (11), AU-rich motifs (29), or subgenomic promoters (23). The detached replicase then reinitiates synthesis on the acceptor RNA, either de novo, usually in the vicinity of promoters or enhancers (13, 14, 49, 51), or via priming by the nascent 3′ end of the newly synthesized RNA (43).
Brome mosaic bromovirus (BMV) is a tripartite RNA virus with frequent homologous crossovers within the subgenomic promoter (sgp) region of the RNA3 segment (5, 20, 31, 47). The junctions cluster within the sgp poly(A) tract and the core, plausibly due to replicase detachment during copying of (−) strands (56, 57). Mutagenesis of the sgp demonstrated a partial overlap of recombination and transcription activities in vivo (56, 57), and end-to-end template switching events have been described for BMV RNA-dependent RNA synthesis in vitro (28). In this paper, we present the first sgp-mediated RNA virus homologous recombination system, where BMV RNA3 crossovers occur by copying with either BMV replicase isolated from virus-infected plants or Escherichia coli-expressed protein 2a, which is a catalytic subunit of the BMV replicase. The recombination frequencies and junction sites varied between the two enzymes, leading to assumptions about the mechanism of crossover.
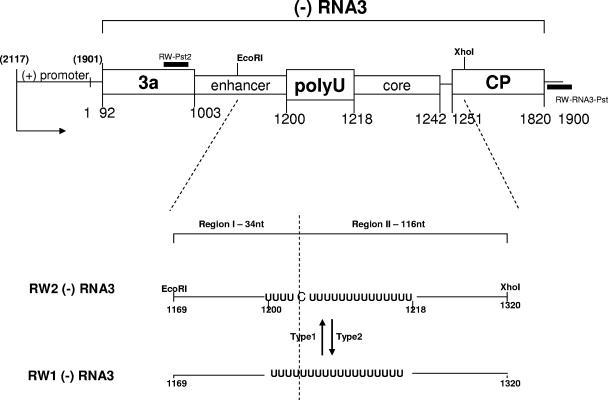
Plasmid pB3TP7 (27) was used to prepare two similar RW (−) RNA3 constructs (Fig. 1) by recombinant DNA technology. RW1 (−) RNA3 carried the (+)-sense 3′-noncoding region of BMV RNA3 (nucleotides [nt] 1901 to 2117) fused to the remaining RNA3 sequence of (−) polarity (nt 1 to 1900), such that after initiation at the (+)-sense 3′-terminal promoter, the enzymes copied the (−) sequence. RW2 (−) RNA3 carried EcoRI (A-to-G-1169 and T-to-A-1170 substitutions) and XhoI (A-to-C-1322 and G-to-A-1324 substitutions) sgp-flanking restriction markers and an insertion (C4/5) between U-1204 and U-1205 of the poly(U) tract (Fig. 1). The RNA templates were synthesized in vitro by T7 transcription from PvuII-linearized plasmids.
FIG. 1.
RNA templates used for in vitro recombination assays with BMV replicase or 2a protein. (Top) Schematic representation of template RNAs used in the experiments. A portion of (−) RNA3 between nt 1 and 1900 [the nucleotide positions correspond to those in wt genomic (+) RNA3 sequence] was fused immediately downstream of the (+) RNA3 promoter (nt 1901 to 2117 [shown in brackets]). The replication initiation site is marked by an arrow, while the locations of the RT-PCR primers RW-Pst2 and RW-RNA3-Pst are represented by thick black lines. The BMV RNA3 open reading frames are represented by shaded boxes, while the elements of the sgp (not to scale) are shown in open boxes with corresponding nucleotide position numbers. The positions of flanking EcoRI and XhoI marker restriction sites are indicated. (Bottom) Magnified image showing the crossover portion of the sgp. The template variant RW2 (−) RNA3 carries both marker restriction sites and the C4/5 insertion between U-1204 and U-1205, while the variant RW1 (−) RNA3 has the wild-type sequence. The C4/5 insertion divides the poly(A) tract into two parts (indicated by a broken line), so the crossovers can be mapped separately for region I (34-nt sequence between EcoRI-1169 and C4/5) and region II (116-nt sequence between C4/5 and XhoI-1320). The template-switching events occurring from the RW1 (−) RNA3 (donor) to RW2 (−) RNA3 (acceptor) are defined as type 1, while their reciprocals are defined as type 2 (see text).
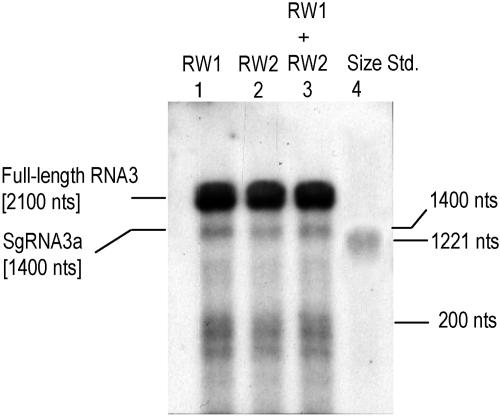
To demonstrate in vitro the RW (−) RNA3 copying by BMV replicase, a reaction mixture was set up containing 1 μg of RW1 (−) RNA3 or RW2 (−) RNA3 template (separately or as a 1:1 mixture), 15 μl BMV replicase enzyme (extracted as described in reference 18), 50 mM Tris-HCl, pH 8.0, 10 mM MgCl2, 8 mM dithiothreitol, 0.6% Triton X-100, 8 U RNasin, 0.4 mCi [α-32P]CTP, 0.01 mM CTP, and a 1 mM concentration (each) of GTP, ATP, and UTP (18). After a 90-min incubation at 30°C, the radioactive RNA products were extracted with phenol-chloroform and resolved in a denaturing agarose gel, followed by autoradiography. Figure 2 (a reproducible gel from two independent experiments) confirms the equal template competencies for copying of both RW (−) RNA3s (compare lanes 1 through 3). Both the full-length (+)-sense RNA3 product and the short genomic RNA (sgRNA) 3a [prematurely terminated at the poly(U) tract of the (−) RNA template] (unpublished data) as well as short RNAs were detected.
FIG. 2.
Products of copying reaction of RW (−) RNA3 templates with BMV replicase preparation. The copying reaction contained 2 μg of RW1 (−) RNA3 (lane 1), 2 μg RW2 (−) RNA3 (lane 2), or 1 μg each of RW1 (−) RNA3 and RW2 (−) RNA3 (lane 3) and all other components described in reference 18. After 90 min of incubation at 37°C, the radioactive reaction products were purified with phenol-chloroform and resolved in a 6% polyacrylamide-urea gel, followed by direct exposure to X-ray film. In addition to the full-length (+)-sense RNA3, the other RNA3-related products are also visible, including sgRNA 3a and shorter RNAs of about 200 nt. sgRNA 3a most likely arises due to premature termination on the poly(U) tract of the (−) RNA template (unpublished). The positions and sizes of the RNA products are indicated. Lane 4 shows the migration of an in vitro-transcribed radioactive (−)-sense RNA size marker of 1,221 nt (transcript corresponding to nucleotide positions 1 to 1221 of the wild-type RNA3 sequence).
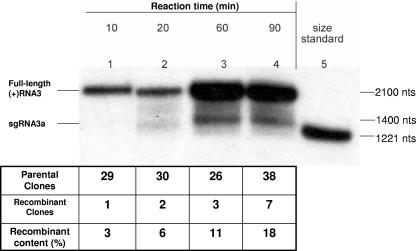
Figure 3 shows the kinetics of the copying reaction for the 1:1 template mixture (see above) containing BMV replicase. Fifteen-microliter aliquots were analyzed electrophoretically after 10, 20, 60, and 90 min of reaction time, as described above, and they demonstrated an increase with time in the copied full-length RNA which was paralleled by increasing quantities of sgRNA 3a, thus confirming the RNA copying activity.
FIG. 3.
Accumulation of recombinants over reaction time with BMV replicase. The copying reaction mixture with a 1:1 mixture of RW1 (−) RNA3 and RW2 (−) RNA3 and with BMV replicase was set up as described in the legend to Fig. 2. Fifteen-microliter aliquots were taken out after 10, 20, 60, and 90 min, purified, and analyzed as described in the legend to Fig. 2. The aliquots were RT-PCR amplified with oligonucleotides RW-Pst2 and RW-RNA3-Pst, followed by cloning and sequencing. The top panel shows the accumulation of full-length (+) RNA3 and sgRNA 3a (positions marked on the left) over time (lanes 1 to 4), with the corresponding molecular sizes given on the right. Lane 5 displays the 1,221-nt RNA size marker. The table below shows the recombination frequencies based on the ratios of parental and recombinant clones.
To determine the sgp-supported recombination frequency, the full-length products were excised from the gel and amplified by reverse transcription-PCR (RT-PCR) with oligonucleotides RW-Pst2 (5′-AAAACTGCAGCCAAGTCTGTATCCGCGTCATCTGTCG-3′), covering (+)-strand RNA3 nt 940 to 962, and RW-RNA3-Pst (5′-AAAACTGCAGAACCTTAGCCAAAGTGTCCTAC-3′), covering (−)-strand RNA3 nt 1893 to 1914 (PstI sites are underlined), and the 974-nt cDNA products were cloned into the pUC19 vector and sequenced. The recombination frequency was determined as the percentage of clones with nonparental distributions of markers. The analysis of 123 clones revealed an increased accumulation of recombinants to 3%, 6%, 11%, and 18% of total clones after 10, 20, 60, and 90 min of incubation (see the table in Fig. 3). These in vitro data confirmed previous in vivo results on the sgp recombination hot spot (56, 57). The time-dependent correlation between full-length RNA synthesis and the number of recombinants suggested successful reinitiation on the new RNA template. The progressive accumulation of recombinant RNAs likely reflects copying and further recombining of previous recombinants. However, the effects on replicase detachment with increased pyrophosphate by-products or depletion of nucleotide substrates cannot be excluded. The possibility that the polymerase ternary complex vacates the original template during recombination could be confirmed by using heparin, which efficiently inhibits unbound polymerase (48).
A control RT-PCR with RW1 and RW2 (−)-strand RNA3 templates (1:1) and with primers RW-Pst2 and RW-RNA3-Pst did not give recombinant cDNA clones (not shown). Similar results were obtained with RT-PCR on a mixture of both RW1 (−) and RW2 (−) RNA3s and their (+) RNA counterparts; such a mixture mimicked the RNA-dependent RNA polymerase (RdRp)-induced products (not shown). These results reduced the possibility of false recombinants generated by RT-PCR. Also, different ratios between parental and recombinant RNAs and different poly(A) length profiles for BMV replicase and the 2a protein (Table 1) speak against RT-PCR-generated recombinants and RT-PCR-induced poly(A) heterogeneity, respectively.
TABLE 1.
Characterization of cDNA clones representing recombinant and parental (nonrecombined substrate) BMV RNA3 (−) sequences within the sgp region after in vitro copying of RW1 (−) RNA3 and RW2 (−) RNA3 templates either with BMV replicase or with protein 2a
| Enzyme prepn and type of RNA producta | Clone no. | Crossover regionb | Crossover typec | Length (nt) (SD) of poly(A) tractd |
|---|---|---|---|---|
| BMV replicase | ||||
| Recombinant sequence | 1 | II | 1 | 18 (0.43) |
| 2 | II | 1 | 18 (0.43) | |
| 3 | II | 2 | 19 (0.43) | |
| 4 | II | 2 | 19 (0.43) | |
| 5 | II | 1 | 18 (0.43) | |
| 6 | II | 1 | 18 (0.43) | |
| 7 | II | 2 | 17 (0.43) | |
| Parental sequence | RW1 (−) RNA3 | NA | NA | 18 (0.25) |
| RW1 (−) RNA3 | NA | NA | 18 (0.25) | |
| RW2 (−) RNA3 | NA | NA | 18 (0.25) | |
| RW2 (−) RNA3 | NA | NA | 19 (0.25) | |
| 2a | ||||
| Recombinant sequence | 1 | I | 1 | 16 (2) |
| 2 | I | 2 | 20 (2) | |
| 3 | I | 2 | 18 (2) | |
| 4 | I | 2 | 19 (2) | |
| 5 | II | 2 | 19 (2) | |
| 6 | II | 2 | 17 (2) | |
| 7 | II | 1 | 19 (2) | |
| 8 | II | 1 | 19 (2) | |
| 9 | II | 1 | 22 (2) | |
| 10 | II | 2 | 16 (2) | |
| 11 | II | 2 | 17 (2) | |
| 12 | II | 1 | 21 (2) | |
| 13 | II | 1 | 25 (2) | |
| Parental sequence | RW1 (−) RNA3 | NA | NA | 17 (1.8) |
| RW1 (−) RNA3 | NA | NA | 20 (1.8) | |
| RW1 (−) RNA3 | NA | NA | 16 (1.8) | |
| RW2 (−) RNA3 | NA | NA | 15 (1.8) | |
| RW2 (−) RNA3 | NA | NA | 19 (1.8) |
Types of RNA products were determined by sequencing of RT-PCR-generated cDNAs from copying reaction products of a 1:1 mixture of RW1 (−) and RW2(−) RNA3 templates (reaction time, 90 min). The sequenced sgp regions carried flanking markers derived from either parental [RW1 (−) or RW2 (−)] or recombined (between two RNA templates) (−) RNA3 sequences (see Fig. 1 for more details).
Region of crossovers within the tested sgp sequence (see Fig. 1), as follows: region I, 116-nt sequence between C-4/5 and XhoI-1320; and region II, 34-nt sequence between EcoRI-1169 and C-4/5. NA, not applicable.
Direction of crossovers, as follows: type 1, RW1 (−) RNA3 (donor)→RW2 (−) RNA3 (acceptor); and type 2, RW2 (−) RNA3 (donor)→RW1 (−) RNA3 (acceptor).
Standard deviations for the lengths of poly(A) tracts were calculated based on the 18-adenine wild-type length.
Protein 2a, the catalytic subunit of BMV replicase, was used for comparative in vitro analysis of recombination at the sgp region. To express 2a, a cDNA fragment representing the 2a open reading frame (BMV RNA2; nt 107 to 2571) was ligated between EcoRI and BamHI sites in the JBWA expression vector (prepared by subcloning the NcoI-BamHI fragment of vector pPROEX1 into pET15b [both from Promega Corp.]), creating an N-terminal six-His-rTEV-2a fusion expressed from the T7 promoter (2a-JBWA). 2a-JBWA-transformed E. coli cells (Tuner-Novagen) were induced with 0.4 mM IPTG (isopropyl-β-d-thiogalactopyranoside), and the bacterial pellet was collected from 250 ml culture by centrifugation. The pellet was resuspended in 12 ml of lysis cocktail (0.1 M HEPES, pH 8.0, 0.1 M KCl, 1.2 mM phenylmethylsulfonyl fluoride, 1 mM EDTA, 120 μM EGTA, 2 mM CaCl2, 120 mg DNase I, 500 mg lysozyme, 0.035% β-mercaptoethanol, 10% glycerol, 0.1% IG-PAL), the lysate was incubated for 30 min at 4°C and ultracentrifuged for 1 h at 90,000 × g, and the supernatant was passed through a column (Poly-Prep chromatography column; Bio-Rad) with 0.7 ml of BD Talon resin (BD Biosciences). The column was washed four times by centrifugation with 1 ml of washing buffer (50 mM Na3PO4, 0.3 M NaCl, 10 mM imidazole, pH 7.0), and the protein was eluted with 0.5 ml of 50 mM Na3PO4, 0.3 M NaCl, and 170 mM imidazole directly into 50% glycerol and dialyzed overnight at 4°C against a storage buffer (50 mM Tris, pH 8.2, 50 mM KCl, 50 mM MgCl2, 2 mM dithiothreitol, 0.75% Triton X-100, and 50% glycerol).
The 2a copying reaction mixture included the same ingredients as the mixture for BMV replicase (see above) plus 1 μg of an additional in vitro-transcribed RNA complementary to the 3′-terminal 150 nt of RW1 (−) RNA3 or RW2 (−) RNA3. The latter served to prime the 2a copying reaction; unlike BMV replicase, 2a was found to be incapable of de novo initiation from the 3′ replication promoter. The reaction mixture was incubated under the same conditions as those described above, and the products were amplified, cloned, and sequenced exactly as described for reactions with BMV replicase. This yielded a 36% recombination frequency after 90 min of incubation (reproduced in two separate experiments). Six clones represented the type 1 recombinant RNA3 (Fig. 1), whereas seven clones were of the reciprocal type 2, demonstrating a lack of template preference (Table 1). Also, both parental (substrate) RNAs were represented at comparable frequencies [three clones of RW1 (−) RNA3 versus two clones of RW2 (−) RNA3]. The junction sites were distributed within the sgp poly(A) tract and the core regions (nine clones) (Fig. 1, region II) as well as within the enhancer sequences (four clones in region I), demonstrating a lack of a clear preference for crossover sites. In contrast to protein 2a, but consistent with previous in vivo results (56, 57), BMV replicase generated crossovers at a lower frequency, but site specifically, within region II (Table 1), supporting the suggested participation of the poly(A)/core modules in homologous recombination (56, 57).
The 2a-generated RNA products displayed greater variation in the poly(A) length (standard deviation, 2.0 nt and 1.8 nt for recombinant and nonrecombinant clones, respectively) than the products of BMV replicase (standard deviation, 0.43 and 0.25 nt, respectively). The lower copying fidelity displayed by 2a could be attributed to enzyme slippage, as demonstrated for other replicases (4, 24, 33, 60).
Inefficient RNA crossovers during in vitro RNA synthesis have been demonstrated previously with flaviviral RdRps and with the poliovirus RdRp (in a cell-free system with a HeLa S10 cytoplasmic extract) (17, 42), whereas this work describes the first in vitro system for studying homologous crossovers within the sgp region and compares the frequencies of crossover from the replicase and from its recombinant RdRp component. The observed high frequencies of recombination crossovers could reflect an absence of fitness selection that eliminates less fit recombinants but also the mechanism at the sgp region. The detachment of the enzyme complex could occur at sgp, especially via destabilization (slippage) on the poly(U) tract, followed by reattachment to new templates (the likely absence of RNA ligases in both enzyme preparations does not suggest a breakage-religation mechanism). For 2a alone, the detachment could be easier, as possibly reflected by the increased recombination frequency (Table 1). Further work is required to elucidate the mechanistic aspects of the template switch.
The elevated recombination activity by 2a (36%) vis-à-vis the BMV replicase preparation (18%) and the increased length variability among product poly(A) tracts (Table 1) are likely due to the absence of BMV protein 1a and/or accessory host factors rather than because of differences between recombinant and natural 2a proteins. The expressed 2a carried an additional N-terminal six-His tag, but it has been demonstrated that the N-terminal 150 amino acids are dispensable for 2a copying activity (46). Several host factors affecting BMV replication and recombination have been identified in Saccharomyces cerevisiae (26, 37, 39, 52). In this case, the accessory proteins may control the distribution of junction sites.
In contrast to the in vitro results, the recombination frequencies in natural BMV infections are low (between 20 and 25%) (56, 57), which suggests a plateau (possibly controlled by accessory proteins) that prevents reduction of the pool of best-fit genomes (8, 16). The kinetics of recombinant formation in vivo is poorly understood. The even distribution of substrate and recombinant genotypes speaks against the generation of recombinants at a high frequency with subsequent elimination of less-fit variants. In summary, we have developed an in vitro system for studying the mechanism of template switches within the subgenomic promoter region.
Acknowledgments
Rafal Wierzchoslawski is on leave from the Institute of Plant Breeding and Acclimatization, Bydgoszcz, Poland.
We thank Margaret and Kathy Bujarska for excellent technical assistance.
This work was supported by grants from the National Science Foundation (MCB-0317039), the National Institutes of Health (G1A62203), and the Plant Molecular Biology Center at Northern Illinois University and by the Polish Government through grants (6 P04C 046 19 and 3 P04 A 039 25) from the State Committee for Scientific Studies, awarded to J.J.B.
REFERENCES
- 1.Aaziz, R., and M. Tepfer. 1999. Recombination between genomic RNAs of two cucumoviruses under conditions of minimal selection pressure. Virology 263:282-289. [DOI] [PubMed] [Google Scholar]
- 2.Adair, T. L., and C. M. Kearney. 2000. Recombination between a 3-kilobase tobacco mosaic virus transgene and a homologous viral construct in the restoration of viral and nonviral genes. Arch. Virol. 145:1867-1883. [DOI] [PubMed] [Google Scholar]
- 3.Arnold, J. J., and C. E. Cameron. 1999. Poliovirus RNA-dependent RNA polymerase (3Dpol) is sufficient for template switching in vitro. J. Biol. Chem. 274:2706-2716. [DOI] [PubMed] [Google Scholar]
- 4.Barr, J. N., and G. W. Wertz. 2001. Polymerase slippage at vesicular stomatitis virus gene junctions to generate poly(A) is regulated by the upstream 3′-AUAC-5′ tetranucleotide: implications for the mechanism of transcription termination. J. Virol. 75:6901-6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baumstark, T., and P. Ahlquist. 2001. The brome mosaic virus RNA3 intergenic replication enhancer folds to mimic a tRNA TpsiC-stem loop and is modified in vivo. RNA 7:1652-1670. [PMC free article] [PubMed] [Google Scholar]
- 6.Becher, P., M. Orlich, and H. J. Thiel. 2001. RNA recombination between persisting pestivirus and a vaccine strain: generation of cytopathogenic virus and induction of lethal disease. J. Virol. 75:6256-6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bousalem, M., E. J. Douzery, and D. Fargette. 2000. High genetic diversity, distant phylogenetic relationships and intraspecies recombination events among natural populations of yam mosaic virus: a contribution to understanding potyvirus evolution. J. Gen. Virol. 81:243-255. [DOI] [PubMed] [Google Scholar]
- 8.Bretscher, M. T., C. L. Althaus, V. Muller, and S. Bonhoeffer. 2004. Recombination in HIV and the evolution of drug resistance: for better or for worse? Bioessays 26:180-188. [DOI] [PubMed] [Google Scholar]
- 9.Bujarski, J. J., and A. M. Dzianott. 1991. Generation and analysis of nonhomologous RNA-RNA recombinants in brome mosaic virus: sequence complementarities at crossover sites. J. Virol. 65:4153-4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bujarski, J. J. 1997. Experimental systems of genetic recombination and defective RNA formation in RNA viruses: part II. Semin. Virol. 8:75-76. [Google Scholar]
- 11.Bunch, T., E. Rieder, and P. Mason. 1994. Sequence of the S fragment of foot-and-mouth disease virus type A12. Virus Genes 8:173-175. [DOI] [PubMed] [Google Scholar]
- 12.Canto, T., S. K. Choi, and P. Palukaitis. 2001. A subpopulation of RNA 1 of cucumber mosaic virus contains 3′ termini originating from RNAs 2 or 3. J. Gen. Virol. 82:941-945. [DOI] [PubMed] [Google Scholar]
- 13.Cascone, P. J., T. F. Haydar, and A. E. Simon. 1993. Sequences and structures required for recombination between virus-associated RNAs. Science 260:801-805. [DOI] [PubMed] [Google Scholar]
- 14.Cheng, C. P., and P. D. Nagy. 2003. Mechanism of RNA recombination in carmo- and tombusviruses: evidence for template switching by the RNA-dependent RNA polymerase in vitro. J. Virol. 77:12033-12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dawson, W. O., D. J. Lewandowski, M. E. Hilf, P. Bubrick, A. J. Raffo, J. J. Shaw, G. L. Grantham, and P. R. Desjardins. 1989. A tobacco mosaic virus-hybrid expresses and loses an added gene. Virology 172:285-292. [DOI] [PubMed] [Google Scholar]
- 16.Duarte, E. A., I. S. Novella, S. C. Weaver, E. Domingo, S. Wain-Hobson, D. K. Clarke, A. Moya, S. F. Elena, J. C. de la Torre, and J. J. Holland. 1994. RNA virus quasispecies: significance for viral disease and epidemiology. Infect. Agents Dis. 3:201-214. [PubMed] [Google Scholar]
- 17.Duggal, R., A. Cuconati, M. Gromeier, and E. Wimmer. 1997. Genetic recombination of poliovirus in a cell-free system. Proc. Natl. Acad. Sci. USA 94:13786-13791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dzianott, A., N. Rauffer-Bruyere, and J. Bujarski. 2001. Studies on functional interaction between brome mosaic virus replicase proteins during RNA recombination, using combined mutants in vivo and in vitro. Virology 289:137-149. [DOI] [PubMed] [Google Scholar]
- 19.Emini, E. A., J. Leibowitz, D. C. Diamond, J. Bonin, and E. Wimmer. 1984. Recombinants of Mahoney and Sabin strain poliovirus type 1: analysis of in vitro phenotypic markers and evidence that resistance to guanidine maps in the nonstructural proteins. Virology 137:74-85. [DOI] [PubMed] [Google Scholar]
- 20.French, R., and P. Ahlquist. 1987. Intercistronic as well as terminal sequences are required for efficient amplification of brome mosaic virus RNA3. J. Virol. 61:1457-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gmyl, A. P., and V. I. Agol. 2005. Diverse mechanisms of RNA recombination. Mol. Biol. 4:529-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gmyl, A. P., E. V. Belousov, S. V. Maslova, E. V. Khitrina, A. B. Chetverin, and V. I. Agol. 1999. Nonreplicative RNA recombination in poliovirus. J. Virol. 73:8958-8965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guillot, S., V. Caro, N. Cuervo, E. Korotkova, M. Combiescu, A. Persu, A. Aubert-Combiescu, F. Delpeyroux, and R. Crainic. 2000. Natural genetic exchanges between vaccine and wild poliovirus strains in humans. J. Virol. 74:8434-8443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hausmann, S., D. Garcin, C. Delenda, and D. Kolakofsky. 1999. The versatility of paramyxovirus RNA polymerase stuttering. J. Virol. 73:5568-5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huisman, M. J., B. J. C. Cornelissen, C. F. M. Groenendijk, J. F. Bol, and L. van Vloten-Doting. 1989. Alfalfa mosaic virus temperature-sensitive mutants. V. The nucleotide sequence of TBS 7 RNA 3 shows limited nucleotide changes and evidence for heterologous recombination. Virology 171:409-416. [DOI] [PubMed] [Google Scholar]
- 26.Ishikawa, M., J. Díez, M. Restrepo-Hartwig, and P. Ahlquist. 1997. Yeast mutations in multiple complementation groups inhibit brome mosaic virus RNA replication and transcription and perturb regulated expression of the viral polymerase-like gene. Proc. Natl. Acad. Sci. USA 94:13810-13815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janda, M., R. French, and P. Ahlquist. 1987. High efficiency T7 polymerase synthesis of infectious RNA from cloned brome mosaic virus cDNA and effects of 5′ extensions of transcript infectivity. Virology 158:259-262. [DOI] [PubMed] [Google Scholar]
- 28.Kim, M. J., and C. Kao. 2001. Factors regulating template switch in vitro by viral RNA-dependent RNA polymerases: implications for RNA-RNA recombination. Proc. Natl. Acad. Sci. USA 98:4972-4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.King, A. M. 1988. Preferred sites of recombination in poliovirus RNA: an analysis of 40 intertypic crossover sequences. Nucleic Acids Res. 16:1705-1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirkegaard, K., and D. Baltimore. 1986. The mechanism of RNA recombination in poliovirus. Cell 47:433-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lai, M. C. M. 1992. RNA recombination in animal and plant viruses. Microbiol. Rev. 56:61-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li, Y., and L. A. Ball. 1993. Nonhomologous RNA recombination during negative-strand synthesis of flock house virus RNA. J. Virol. 67:3854-3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo, G. X., W. Luytjes, M. Enami, and P. Palese. 1991. The polyadenylation signal of influenza virus RNA involves a stretch of uridines followed by the RNA duplex of the panhandle structure. J. Virol. 65:2861-2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moonan, F., J. Molina, and T. E. Mirkov. 2000. Sugarcane yellow leaf virus: an emerging virus that has evolved by recombination between luteoviral and poleroviral ancestors. Virology 269:156-171. [DOI] [PubMed] [Google Scholar]
- 35.Nagy, P. D., and A. E. Simon. 1997. New insights into the mechanisms of RNA recombination. Virology 235:1-9. [DOI] [PubMed] [Google Scholar]
- 36.Negroni, M., M. Riccheti, P. Nouvel, and H. Buc. 1995. Homologous recombination promoted by reverse transcriptase during copying of two distinct RNA templates. Proc. Natl. Acad. Sci. USA 92:6971-6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noueiry, A. O., J. Chen, and P. Ahlquist. 2000. A mutant allele of essential, general translation initiation factor DED1 selectively inhibits translation of a viral mRNA. Proc. Natl. Acad. Sci. USA 97:12985-12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palasingam, K., and P. N. Shaklee. 1992. Reversion of Qβ RNA phage mutants by homologous RNA recombination. J. Virol. 66:2435-2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Panavas, T., E. Serviene, J. Brasher, and P. D. Nagy. 2005. Yeast genome-wide screen reveals dissimilar sets of host genes affecting replication of RNA viruses. Proc. Natl. Acad. Sci. USA 102:7326-7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peliska, J., and S. Benkovic. 1992. Mechanism of DNA strand transfer catalyzed by HIV-1 reverse transcriptase. Science 258:1112-1118. [DOI] [PubMed] [Google Scholar]
- 41.Rabindran, S., and W. O. Dawson. 2001. Assessment of recombinants that arise from the use of a TMV-based transient expression vector. Virology 284:182-189. [DOI] [PubMed] [Google Scholar]
- 42.Ranjith-Kumar, C. T., R. T. Sarisky, L. Gutshall, M. Thomson, and C. C. Kao. 2004. De novo initiation pocket mutations have multiple effects on hepatitis C virus RNA-dependent RNA polymerase activities. J. Virol. 78:12207-12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romanova, L. I., V. M. Blinov, E. A. Tolskaya, E. G. Viktorova, M. S. Kolesnikowa, E. A. Guseva, and V. I. Agol. 1986. The primary structure of crossover regions of intertypic poliovirus recombinants: a model of recombination between RNA genomes. Virology 155:202-213. [DOI] [PubMed] [Google Scholar]
- 44.Saunders, K., A. M. Q. King, D. McCahon, J. W. I. Newman, W. R. Slade, and S. Forss. 1985. Recombination and oligonucleotide analysis of guanidine-resistant foot-and-mouth disease mutants. J. Virol. 56:921-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sivakumaran, K., Y. Bao, M. J. Roossinck, and C. C. Kao. 2000. Recognition of the core RNA promoter for minus-strand RNA synthesis by the replicases of brome mosaic virus and cucumber mosaic virus. J. Virol. 74:10323-10331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smirnyagina, E., N. S. Lin, and P. Ahlquist. 1996. The polymerase-like core of brome mosaic virus 2a protein, lacking a region interacting with viral 1a protein in vitro, maintains activity and 1a selectivity in RNA replication. J. Virol. 70:4729-4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sullivan, M. L., and P. Ahlquist. 1999. A brome mosaic virus intergenic RNA3 replication signal functions with viral replication protein 1a to dramatically stabilize RNA in vivo. J. Virol. 73:2622-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun, J. H., and C. C. Kao. 1997. RNA synthesis by the brome mosaic virus RNA-dependent RNA polymerase: transition from initiation to elongation. Virology 233:63-73. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki, M., T. Hibi, and C. Masuta. 2003. RNA recombination between cucumoviruses: possible role of predicted stem-loop structures and an internal subgenomic promoter-like motif. Virology 306:77-86. [DOI] [PubMed] [Google Scholar]
- 50.Tolou, H. J., P. Couissinier-Paris, J. P. Durand, V. Mercier, J. J. de Pina, P. de Micco, F. Billoir, R. N. Charrel, and X. de Lamballerie. 2001. Evidence for recombination in natural populations of dengue virus type 1 based on the analysis of complete genome sequences. J. Gen. Virol. 82:1283-1290. [DOI] [PubMed] [Google Scholar]
- 51.Tolskaya, E. A., L. A. Romanova, V. M. Blinow, E. G. Virtorova, A. N. Sinyakov, M. S. Kolesnikova, and V. I. Agol. 1987. Studies on the recombination between RNA genomes of poliovirus: the primary structure and nonrandom distribution of crossover regions in the genomes of intertypic poliovirus recombinants. Virology 161:54-61. [DOI] [PubMed] [Google Scholar]
- 52.Tomita, Y., T. Mizuno, J. Díez, S. Naito, P. Ahlquist, and M. Ishikawa. 2003. Mutation of host dnaJ homolog inhibits brome mosaic virus negative-strand RNA synthesis. J. Virol. 77:2990-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Varrelmann, M., L. Palkovics, and E. Maiss. 2000. Transgenic or plant expression vector-mediated recombination of plum pox virus. J. Virol. 74:7462-7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weiss, B. G., and S. Schlesinger. 1991. Recombination between Sindbis virus RNAs. J. Virol. 65:4017-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.White, K. A., and T. J. Morris. 1994. Recombination between defective tombusvirus RNAs generates functional hybrid genomes. Proc. Natl. Acad. Sci. USA 91:3642-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wierzchoslawski, R., A. Dzianott, and J. Bujarski. 2004. Dissecting the requirement for subgenomic promoter sequences by RNA recombination of brome mosaic virus in vivo: evidence for functional separation of transcription and recombination. J. Virol. 78:8552-8564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wierzchoslawski, R., A. Dzianott, S. Kunimalayan, and J. Bujarski. 2003. A transcriptionally active subgenomic promoter supports homologous crossovers in a plus-strand RNA virus. J. Virol. 77:6769-6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu, J. C., T. Y. Chiang, W. K. Shiue, S. Y. Wang, I. J. Sheen, Y. H. Huang, and W. J. Syu. 1999. Recombination of hepatitis D virus RNA sequences and its implications. Mol. Biol. Evol. 16:1622-1632. [DOI] [PubMed] [Google Scholar]
- 59.Zhao, Y., J. Hammond, M. E. Tousignant, and R. W. Hammond. 2000. Development and evaluation of a complementation-dependent gene delivery system based on cucumber mosaic virus. Arch. Virol. 145:2285-2295. [DOI] [PubMed] [Google Scholar]
- 60.Zheng, H., H. A. Lee, P. Palese, and A. Garcia-Sastre. 1999. Influenza A virus RNA polymerase has the ability to stutter at the polyadenylation site of a viral RNA template during RNA replication. J. Virol. 73:5240-5243. [DOI] [PMC free article] [PubMed] [Google Scholar]