Abstract
Previous findings from our laboratory and others indicate that two-dimensional gel electrophoresis (2-DE) can be used to study protein expression in defined brain regions, but mainly the proteins which are present in high abundance in glia are readily detected. The current study was undertaken to determine the protein profile in a synaptosomal subcellular fraction isolated from the cerebral cortex of the rat. Both 2-DE and liquid chromatography – tandem mass spectrometry (LC-MS/MS) procedures were used to isolate and identify proteins in the synaptosomal fraction and accordingly >900 proteins were detected using 2-DE; the 167 most intense gel spots were isolated and identified with matrix-assisted laser desorption/ionization – time of flight peptide mass fingerprinting or LC-MS/MS. In addition, over 200 proteins were separated and identified with the LC-MS/MS “shotgun proteomics” technique, some in post-translationally modified form. The following classes of proteins associated with synaptic function were detected: (a) proteins involved in synaptic vesicle trafficking-docking (e.g., SNAP-25, synapsin I and II, synaptotagmin I, II, and V, VAMP-2, syntaxin 1A and 1B, etc.); (b) proteins that function as transporters or receptors (e.g., excitatory amino acid transporters 1 and 2, GABA transporter 1); (c) proteins that are associated with the synaptic plasma membrane (e.g., post-synaptic density-95/synapse-associated protein-90 complex, neuromodulin (GAP-43), voltage-dependent anion-selective channel protein (VDACs), sodium-potassium ATPase subunits, alpha 2 spectrin, septin 7, etc.); and (d) proteins that mediate intracellular signaling cascades that modulate synaptic function (e.g., calmodulin, calcium-calmodulin-dependent protein kinase subunits, etc.). Other identified proteins are associated with mitochondrial or general cytosolic function. Of the two proteins identified as endoplasmic reticular, both interact with the synaptic SNARE complex to regulate vesicle trafficking. Taken together, these results suggest that the integrity of the synaptosomes was maintained during the isolation procedure and that this subcellular fractionation technique enables the enrichment of proteins associated with synaptic function. The results also suggest that this experimental approach can be used to study the differential expression of multiple proteins involved in alterations of synaptic function.
Keywords: Cerebral cortex, Mass spectrometry, Proteome, Rat, Synaptic proteins, Synaptosomes, Two-dimensional gel electrophoresis
1 Introduction
The advent of genomics, which includes the mapping of gene sequences and the development of functional genomics, has contributed insights to many physiological and pathophysiological conditions. Despite these contributions, however, genomics is limited in its ability to address such important issues as levels of protein expression. In this regard, the proteome is dictated by more factors than simply the level of mRNA, e.g., post-transcriptional events such as alternative splicing and PTMs of proteins. These deficiencies in genomics have led to an increased interest in proteomics, the analysis of the profile of proteins expressed and/or modified by an organism, tissue, cell type, or sub-cellular compartment. Recently, evolving technical advances have yielded the capability to perform such complex analyses.
One discipline in which proteomics promises to have significant impact is neuroscience. Many neurodegenerative diseases, such as Alzheimer’s, are thought to be due to altered functional levels of structural or metabolic proteins. Other conditions, such as addiction and mood disorders, are likely to be secondary to altered expression of proteins, which are involved in neurotransmission or neuroplasticity. Reference proteome databases have been constructed for whole rat brain [1], whole mouse brain [2], mouse cerebellum [3], human parietal cortex [4], and human hippocampus [5]. Our laboratories have recently demonstrated that the expressed proteome can vary in various brain regions based on genetic selection for alcohol preference, and, within these genetic lines, by functional nuclei [6]. Interesting as these documented changes in whole brain tissue are, we are aware that 90–95% of the cells in such tissue are not neurons but glia, which provide support or insulation for neurons [7], and that the majority of these glia are astrocytes [8]. It is likely, therefore, that many of the proteins previously identified by us and by others in whole brain tissue preparations are of glial, not neuronal origin.
We wished to improve our ability to resolve the proteome of neurons and in doing so turned to a well-established procedure for isolating the sub-cellular fraction containing the inter-cellular communication junction between nerves, the synapse [9, 10]. Preparations of these “synaptosome” fractions should be greatly enriched in proteins involved in synaptic transmission and reception, the genetic or pathologic alterations of which may underlie many neurologic and psychiatric disorders. There is precedence behind the assumption that sub-cellular fractionation can improve resolution of brain proteins. In rat brain, fractionation of whole tissue into cytosolic, mitochondrial, and microsomal fractions before 2-DE separation and MS identification has led to the identification of hundreds of additional proteins that were not identified in a high-speed supernatant of total rat forebrain [11]. Comprehensive studies on the synaptic proteome, however, have been scarce. This is due in part to the fact that many synaptic proteins, such as receptor, transporter, and channel proteins, are hydrophobic and membrane-bound, characteristics that can lead to poor protein resolution by 2-DE. Some studies have used limited versions of various proteomics approaches such as SDS-PAGE combined with MALDI-TOF MS [12], where 31 individual proteins were identified from resolved bands from post-synaptic densities of whole rat brain. Efforts have also been made to identify proteins from membrane-enriched fractions from pig cerebellum [13], and squid optic lobe synaptosomes [14]. Most recently, using LC/ESI-IT/MS, over a hundred proteins were identified from the tryptic digests of rat forebrain synaptic plasma membranes [15].
As suggested in the prior paragraph, the methods chosen for the analysis of synaptosomal preparations are of critical importance. Because our goals included both reliable quantitation of relative protein levels under different experimental conditions, and detection of PTM of detected proteins, we chose to analyze our synaptosome samples with several techniques. One of the most effective tools for differential protein expression analysis is 2-DE [16, 17]. When combined with MALDI-TOF MS, the electrophoretically separated proteins can be identified and characterized [18]. In-line HPLC separation followed by IT MS/MS, so-called “shotgun proteomics”, can also be used to detect individual proteins in the expressed proteome and is a valuable tool in detecting PTMs in detected proteins. Glycoproteins exhibit both functional and structural importance in the synapse [19] and can be concentrated using lectin affinity columns prior to analysis with tandem MS.
In summary, the current study was undertaken to focus on the more behaviorally and functionally relevant neuronal elements by determining the protein profile of synaptosomes isolated from the cerebral cortex of the rat. Techniques used to resolve the expressed proteome of synaptosomes included 2-DE and LC-MS/MS procedures, the latter with and without prior application of a lectin affinity column that binds glycoproteins. Proteins resolved by 2-DE were subsequently identified by MALDI-TOF and LC-MS/MS.
2 Materials and methods
2.1 Materials
Acrylamide for slab gels and IPG strips were purchased from Bio-Rad (Richmond, CA, USA). Other ultrapure electrophoretic reagents were obtained from Bio-Rad, Sigma (St. Louis, MO, USA), or BDH (Poole, UK). Sequence grade trypsin was obtained from Promega (Madison, WI, USA). Ammonium bicarbonate was purchased from Mallinckrodt (Paris, KY, USA). Proteomics grade trypsin, formic acid, iodoethanol, and triethylphosphine were obtained from Sigma-Aldrich (St. Louis, MO, USA). ACN and hydrochloric acid solution N/10 were obtained from Fisher Scientific (Fair Lawn, NJ, USA). Con A Sepharose was obtained from Amersham Biosciences (Piscataway, NJ, USA). All other chemicals used were of the highest grade obtainable.
2.2 Animals
Adult male Wistar rats (n = 3, for 2-DE and LC-MS/MS studies) were used in this study, and were singly housed in standard animal colony rooms under normal 12 h light cycle conditions (lights on at 700 h). Rats were sacrificed by decapitation, the brain rapidly removed, and placed on a chilled glass plate on ice. All subsequent procedures involved in the tissue preparation were performed at 4°C. Animals used in this study were maintained in facilities accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, and all experimental procedures were approved by the Institutional Animal Care and Use Committee and were in accordance with the guidelines of the Institutional Animal Care and Use Committee of the National Institute on Drug Abuse, and the Guide for the Care and Use of Laboratory Animals of the National Research Council, 1996.
2.3 Preparation of synaptosomes
The cerebral cortex (frontal) was dissected and the adhering white matter was removed. Cortical samples were weighed and homogenized in 10 volumes of 0.32 m sucrose buffered to pH 7.4 with 20 mm HEPES, and containing 1 mm EDTA, 5 mm dithioerythritol, 1 mm PmsF, 0.2 mm sodium vanadate, and 1 mm sodium fluoride [11]. Standard homogenization and ultracentrifugation procedures were used to isolate synaptosomes [20, 21]. Homogenization was performed using a glass homogenizer and a teflon pestle. Homogenates were centrifuged at 1000 × g for 10 min to obtain the crude nuclear pellet (P1) and the S1 supernatant. The S1 fraction was centrifuged at 17 000 × g for 15 min to obtain the crude mitochondrial fraction (P2 pellet), which was used for the preparation of synaptosomal fractions. The P2 pellet was resuspended in the same homogenizing buffer used for initial homogenization of the tissue, and layered on top of a discontinuous sucrose density gradient consisting of 1.2 m sucrose and 0.8 m sucrose. The gradient was centrifuged at 54 000 × g for 90 min, and the synaptosomal fraction was removed from the 0.8 m sucrose and 1.2 m sucrose interface. This fraction was slowly diluted with 10 volumes of ice-cold 0.32 m sucrose, centrifuged at 20 000 × g for 15 min, and the resulting synaptosomal pellet frozen at −80°C until used for protein extraction.
2.4 2-DE and image analysis
Frozen synaptosomes were solubilized in 500 μL of a solution containing 9 m urea, 4% Igepal CA-630 ((octylphenoxy) polyethoxyethanol), 1% DTT, and 2% carrier ampholytes (pH 3–10). Each sample was sonicated with a Fisher® Sonic Dismembranator using 3 × 2 s bursts at instrument setting no. 3. Sonication was carried out every 15 min for 1 h at room temperature. The protein concentration of each sample was determined using the RC DC Protein Assay kit (Bio-Rad) according to the manufacturer’s protocol. After solubilization, the samples were stored at −45°C. 2-DE was performed on synaptosomal protein samples as follows. Aliquots (180 μL each) containing ~200 μg of protein from the solubilized synaptosomes were diluted with 320 μL of rehydration buffer (8 m urea, 2% CHAPS, 15 mm DTT, 0.2% carrier ampholytes pH 3–10, and 0.001% orange G). The resulting 500 μL protein dilutions were loaded onto IPG strips (24 cm, linear pH 3–10) by overnight, passive rehydration at room temperature. Iso-electric focusing was performed simultaneously on all IPG strips using the Protean IEF Cell (Bio-Rad), by a program of progressively increasing voltage (150 V for 2 h, 300 V for 4 h, 1500 V for 1 h, 5000 V for 5 h, 7000 V for 6 h, and 10 000 V for 3 h) for a total of 100 000 Vh. A computer-controlled gradient casting system was used to prepare second dimension SDS gradient slab gels (20 × 25 × 0.15 cm) in which the acrylamide concentration varied linearly from 11 to 17%T. First dimension IPG strips were loaded directly onto the slab gels following equilibration for 10 min in Equilibration Buffer I and 10 min in Equilibration Buffer II (Equilibration Buffer I: 6 m urea, 2% SDS, 0.375 m Tris-HCl pH 8.8, 20% glycerol, 130 mm DTT; Equilibration Buffer II: 6 m urea, 2% SDS, 0.375 m Tris-HCl pH 8.8, 20% glycerol, 135 mm iodoacetamide). Second dimension slab gels were run in parallel at 8°C for 18 h at 160 V. Slab gels were stained using a colloidal CBB G-250 procedure [22]. Gels were fixed in 1.5 L of 50% ethanol/2% phosphoric acid overnight followed by three 30 min washes in 2 L of deionized water. Gels were transferred to 1.5 L of 30% methanol/17% ammonium sulfate/3% phosphoric acid for 1 h followed by an addition of 1 g of powdered CBB G-250 stain. After 96 h, gels were washed several times with water and scanned at 95.3 μm per pixel resolution using a GS-800 Calibrated Imaging Densitometer (Bio-Rad). The resulting 12-bit images were analyzed using PDQuest™ software (Bio-Rad, v.7.1). Background was subtracted and peaks for the protein spots located and counted. The most abundant spots (190) were selected for MS identification.
2.5 In-gel tryptic digestion and PMF
Ninety-four protein spots with the highest intensity were cut from the gel by hand using a 1.5 mm gel cutting tool and placed in each of 94 wells of a 96-well plate, along with an grp78 standard and one gel blank, and processed using the Multiprobe II (Perkin-Elmer, Boston MA, USA). The remaining 96 gel cutouts were placed in a second 96-well plate and processed for LC-MS/MS analysis (see below). In this automated system, the 94 excised protein spots were first destained with 50 mm ammonium bicarbonate-50% ACN followed by 100% ACN. Reduction with 10 mm DTT and alkylation with 55 mm iodoacetamide was carried out prior to overnight tryptic digestion using modified trypsin at 6 ng·μL−1. The grp78 (StressGen, Victoria, BC Canada) calibrant and a gel blank were digested in the additional two wells using identical conditions. The resulting peptides were extracted by the addition of 25 μL 0.2% formic acid (aqueous) and 7 μL of ACN solution to the wells, and plates were shaken at 37°C for 1 h. The resulting solution was placed in a separate 96-well plate and dried using a Speed-Vac. The dehydrated peptides were then reconstituted in 5 μL of 0.2% formic acid and 1 μL of ACN with continuous shaking of the plate for 5 min. Aliquots from peptide extracts (in 3 μL volumes) were then placed onto a MALDI target plate, air dried, and the application repeated until all the extraction solution was used up. Just before the spots finished drying, 0.8 μL of matrix (2 mg·mL−1 CHCA in 50% ACN) was added to each peptide spot and allowed to dry completely.
Peptide masses were analyzed by MALDI-TOF MS using a Waters Micromass M@LDI SYSTEM (Micromass, Milford, MA, USA). Prior to data collection, the instrument was calibrated externally using a mixture of peptide standards, digested standard (grp78), and experiment artifact peaks based on tryptic autolysis. Twenty-five to thirty-five peaks were used in conjunction with a fifth-order curve to produce the external calibration plot. After data collection, each spectrum was processed (background subtracted, smoothed, and centroid determined), recalibrated (for MALDI plate topology using trypsin autolysis peaks 1045.56 or 2211.10 as internal calibrants), and the data exported to mass-only text files. Proteins were identified by manual ProFound™ (Proteometrics LLC) database searches using the mass lists obtained from exported MALDI spectra of the excised 94 spots. A Z-score of 1.30, corresponding to the 90th percentile, was the threshold for what was considered a positive identification.
2.6 In-gel tryptic digestion for LC-MS/MS analysis
The next 96 most abundant protein spots on the 2-D gel (95–190) were excised, placed in an Eppendorf tube, cut into smaller (less than 1 mm in each dimension) pieces, and destained with 200 μL of 200 mm ammonium bicarbonate in 40% ACN at 37°C for 30 min. This destaining step was repeated once and the gel pieces were completely dehydrated in a Vacufuge concentrator (Eppendorf, Westburg, NY, USA) for 20 min followed by rehydration with 20 μL of 20 g·mL−1 trypsin solution (in 36 mm ammonium bicarbonate, 8% ACN). An aliquot of 50 μL of 40 mm ammonium bicarbonate in 9% ACN was added to each sample before the digestion was carried out at 37°C for 18 h. The tryptic digests were extracted from the gel pieces, dried in a Vacufuge concentrator, and rehydrated with 10 μL of 1% formic acid. The extract solution was kept frozen until LC-MS/MS analysis.
2.7 In-solution tryptic digestion for LC-MS/MS analysis
Synaptosomal proteins were resuspended in water to produce 1 mg·mL−1 sample concentrations. Forty-five microgram of total synaptosomal protein were mixed with 5 μL of 1 m ammonium bicarbonate (final concentration 50 mm). Reduction and alkylation were carried out for 1 h at 37°C by adding an equal volume of a cocktail containing 2% iodoethanol, 0.5% triethylphosphine, and 97.5% ACN [23]. The reaction mixture was evaporated to dryness in a Vacufuge concentrator. The dried sample was digested in 20 μL of 10 mm, pH 7.85, ammonium bicarbonate containing 1 μg of trypsin for 18 h at 37°C. The digested protein mixture was subsequently subjected to LC-MS/MS analysis.
2.8 Isolation of glycoproteins for LC-MS/MS analysis
Frozen synaptosomes were diluted with 150 μL of the binding buffer consisting of 50 mm Tris, 500 mm NaCl, pH 6.5. The sample was loaded onto a Con A Sepharose column (1 mL bed volume) and unbound proteins were eluted with 5 bed-volumes of binding buffer. The glycoproteins, which were expected to bind to the Con A Sepharose column, were eluted with 5 bed-volumes of elution buffer that was identical to the binding buffer but contained 300 mm 1-O-methyl-β-d-glucopyranoside. The fraction enriched in glycoprotein was desalted overnight using 1000 MW cut-off dialysis membrane. The dialyzed sample was concentrated to dryness using a Vacufuge concentrator, and the dried sample was resuspended in 50 μL of 1 m ammonium bicarbonate and subjected to trypsin digestion as described above.
2.9 LC-MS/MS and “shotgun” proteomic analysis
The nano-LC separations were performed using an LC Packings system (Dionex, Sunnyvale, CA, USA) consisting of a Famos™ autosampler, Switchos™ switching valve and pump (used for sample trapping and washing), and UltiMate gradient pump. Aliquots of the tryptic digests (3 μL for solution digestion and 6 μL for in-gel digestion) were loaded onto a trapping column (15 mm × 100 mm) in-house packed with 5 μm, 200 Å Magic C18AQ packing media. The trapping column was then washed to remove any salts and unretainable materials prior to elution and separation of the retained peptides on a pulled-tip capillary column (150 mm × 75 mm) in-house packed with the same packing materials used for the trapping column, but with 100 Å pore size. In-gel digested peptide samples were separated by a gradient in which solvent B was increased linearly from 10 to 35% in 15 min at a flow rate of 250 nL·min−1. Solvent B consisted of ACN with 0.1% formic acid, while solvent A consisted of 3% ACN and 97% water with 0.1% formic acid. A much longer gradient was used for the separation of the tryptic digests of the total proteome or isolated glycoproteins. In this case, a 3 h gradient was utilized in which solvent B was first increased linearly from 6 to 20% in 120 min, followed by another linear increase from 20 to 40% in 45 min, both at a flow rate of 250 nL·min−1. The ions were directly sprayed from the separation column into an LCQ Deca XP ion-trap mass spectrometer (Thermo-Finnigan, San Jose, CA, USA). The mass spectra of the separated peptide ions and data-dependent tandem mass spectra of product ions from precursor ions were recorded. The acquired MS/MS spectra were searched against protein sequences for Rattus in the Swiss-Prot database using MASCOT for peptide recognition and consequent protein identification.
Except for glycosylation, PTM identification was based on the use of MASCOT with selecting the identified PTMs as variable modifications. MS/MS data with an ion score of >35 were then manually inspected to confirm the identified PTM. For glycosylation, the identification was based on the LC-MS/MS analysis of the tryptic digest of the lectin bound fraction. The amino acid sequence of the identified proteins was then checked to account for the presence or absence of the N-glycan motif.
2.10 Bioinformatic analysis of identified proteins
Functions and sub-cellular locations of identified proteins were analyzed by both manual and automated methods. PubMed was used to search for abstracts pertaining to synapse-specific proteins; the remaining proteins were categorized by Pandora [24] (http://www.pandora.cs.huji.ac.il/) according to the gene ontology (GO) sub-cellular location schema [25].
3 Results
3.1 2-DE and MS
Figure 1 illustrates a representative image of the synaptosomal fraction separated by 2-DE and stained with colloidal CBB. Distinct spots identified by PMF or LC-MS/MS have been assigned a number ranging from 1 to 163 for the convenience of the reader. These proteins are listed in Table 1 where they are accompanied by their respective unique PDQuest spot numbers, which were assigned automatically during creation of the matchset. A total of 968 protein spots were detected and matched by PDQuest; the 190 most abundant spots were cut from the gel and subjected to tryptic digestion. The resulting peptides were analyzed by one of the two mass spectrometric methods.
Figure 1.
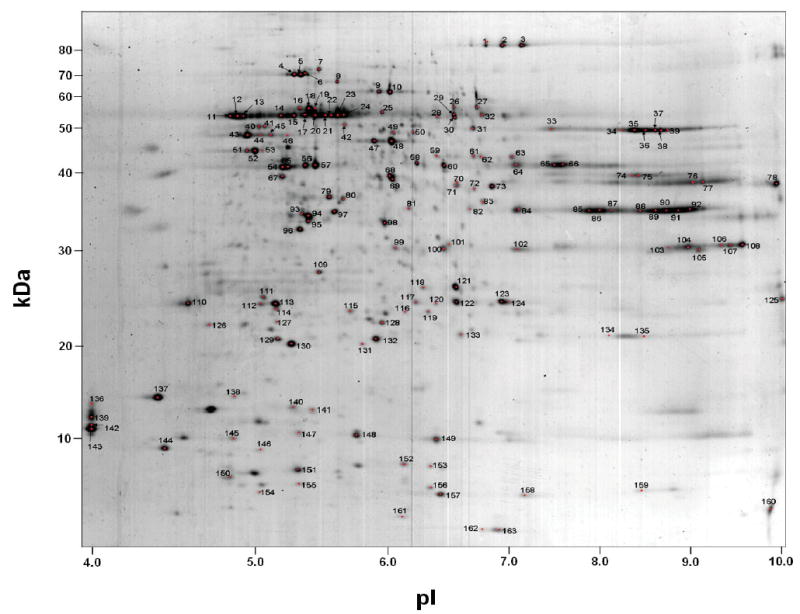
Representative 2-DE pattern of synaptosomal proteins stained with colloidal CBB. Proteins (200 μg) were focused on 24 cm IPG strips pH 3–10, followed by SDS-PAGE in a linear acrylamide gradient. Protein spots were cut from the gel, tryptically digested, and identified either by MALDI-MS or LC-MS/MS. These are numbered arbitrarily 1–163 and appear in Table 1 along with their PDQuest spot number assignments and other pertinent information. Axes were calibrated based on calculated pI and mass from identified proteins, using the Compute pI/Mw Tool (<http://us.expasy.org/tools/pi_tool.html>).
Table 1.
Synaptosomal proteins cut from the 2-D gel and identified by either PMF or peptide sequencing via LC-MS/MS
| # | SSP | NCBI accession | Swiss-Prot entry name | Protein ID | Z- score | pI | Mass (kDa) | %c | MS/MS (sequence data) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 6813 | NP_077374.1 | Q99KI0 | Mitochondrial aconitase (nuclear aco2 gene) | 1.07 | 8.2 | 86.2 | 13 | – |
| 2 | 6815 | NP_077374.1 | Q99KI0 | Mitochondrial aconitase (nuclear aco2 gene) | 2.4 | 8.2 | 86.2 | 20 | – |
| 3 | 7801 | NP_077374.1 | Q99KI0 | Mitochondrial aconitase (nuclear aco2 gene) | 2.39 | 8.2 | 86.2 | 23 | – |
| 4 | 2807 | S31716 | BQ078983* | DNAk-type molecular chaperone hsp72-ps1 | 2.43 | 5.4 | 71.1 | 34 | – |
| 5 | 3801 | S31716 | BQ078983* | DNAk-type molecular chaperone hsp72-ps1 | 2.43 | 5.4 | 71.1 | 38 | – |
| 6 | 3802 | P08109 | HS7C_ MOUSE | Heat shock cognatete 71 kDa protein | – | 5.4 | 70.8 | – | LLQDFFNGK; FEELNADLFR; IINEPTAAAIAYGLDK |
| 7 | 3811 | P48721 | GR75_RAT | DNAk-type molecular chaperone grp75 precursor | 2.43 | 5.9 | 74.0 | 35 | – |
| 8 | 3815 | P08461 | ODP2_RAT | Dihydrolipoamide acetyl-transferase component of pyruvate dehydrogenase | – | −5.7 | 58.7 | – | ISVNDFIIK; YLEKPVTMLL + oxidation (M) |
| 9 | 4813 | P47942 | DPY2_RAT | Dihydropyrimidinase related protein-2 (DRP-2) (collapsin response mediator protein 2) | 2.3 | 6.0 | 62.7 | 20 | – |
| 10 | 4815 | P47942 | DPY2_RAT | DRP-2 (collapsin response mediator protein°2) | 2.37 | 6.0 | 62.7 | 24 | – |
| 11 | 1704 | P04691 | TBB1_RAT | Tubulin beta chain 15 | 2.43 | 4.8 | 50.4 | 31 | – |
| 12 | 1705 | P05218 | TBB5_HUMAN | Tubulin, beta 5 | 2.39 | 4.8 | 50.1 | 31 | – |
| 13 | 1708 | P04691 | TBB1_RAT | Tubulin beta chain | – | 4.8 | 49.9 | – | FPGQLNADLR; INVYYNEAAGNK; NSSFBYVEWIPNNVK + 8 additional peptides |
| 14 | 2713 | P04691 | TBB1_RAT | Tubulin beta chain | – | 4.8 | 49.9 | – | AIL VDLEPGTMDSVR + oxidation (M); NSSYFVEWIPNNVK; GHYTEGAELVDSVLDVVR + 2 additional peptides |
| 15 | 2717 | P05218 | TB B5_ HUMAN | Tubulin, beta 5 | 2.43 | 4.8 | 50.1 | 23 | – |
| 16 | 2718 | P19226 | CH60_MOUSE | 60 kDa heat shock protein | – | 5.9 | 60.9 | – | DIGNIISDAMK + oxidation (M); GYISPBYFINTSK; TLNDELE TIEGMK + oxidation (M); TAL LDAAGVASLLTTAEAVVTEIPK |
| 17 | 3703 | P02571 | ACTG_HUMAN | Actin, cytoplasmic 2, gamma | – | 5.3 | 41.8 | – | EITALAPSTMK; DSYVGDEAQSK; SYELPDGQVITIGNER; VAPEEHPVLLTEALNPK + 1 additional peptide |
| 18 | 3704 | P05218 | TBB5_HUMAN | Tubulin, beta 5 | 2.43 | 4.8 | 50.1 | 27 | – |
| 19 | 3705 | P05218 | TBB5_HUMAN | Tubulin, beta 5 | 2.43 | 4.8 | 50.1 | 27 | – |
| 20 | 3708 | P04691 | TBB1_RAT | Tubulin beta chain | – | 4.8 | 49.9 | – | FPGQLNADLR; NSSFYFVEWIPN NK; ALTVPELTQQMFDSK + oxidation (M) + 3 additional peptides |
| 21 | 3712 | P04691 | TBB1_RAT | Tubulin beta chain 15 | 2.43 | 4.8 | 50.4 | 36 | – |
| 22 | 3716 | P04691 | TBB1_RAT | Tubulin beta chain 15 | 2.43 | 4.8 | 50.4 | 36 | – |
| 23 | 3719 | P04691 | TBB1_RAT | Tubulin beta chain 15 | 2.43 | 4.8 | 50.4 | 36 | – |
| 24 | 3720 | P04691 | TBB1_RAT | Tubulin beta chain 15 | 2.43 | 4.8 | 50.4 | 36 | – |
| 25 | 4716 | P21707 | SYT1_RAT | Synaptotagmin I | – | – | 8.4 | 47.4 | TLNPVFNEQFTFK |
| 26 | 6703 | Q63537 | SYN2_RAT | Synapsin II | – | 8.7 | 63.4 | – | MNQLLSR + oxidation (M); ILGDYDIK; QLITDLVISK; EMLTLPTFPVVVK + 2 additional peptides |
| 27 | 6709 | NP_445749.1 | KPY2_MOUSE | Pyruvate kinase 3 | 2.39 | 6.6 | 58.3 | 20 | – |
| 28 | 5716 | P15999 | ATPA_RAT | ATP synthase alpha chain | – | 9.2 | 58.8 | – | IMNVIGEPIDER + oxidation (M); VALTGLTVAEYFR; EGNDLYHE MIESGVINLK + oxidation (HW) |
| 29 | 6702 | Q63537 | SYN2_RAT | Synapsin II | – | 8.7 | 63.4 | – | TPALSPQR; ILGDYDIK; QLITDL VISK; SFRPDFVLIR; FPLIEQ TYYPNHR; TNTGSAMLE QIAMSDR + 2 oxidation (M) |
| 30 | 6704 | NP_476554.1 | Q8TAU2 | Pancreatic lipase-related protein 2 | 2.43 | 6.0 | 54.8 | 10 | – |
| 31 | 6605 | AAA61256 | PYR5_HUMAN | Orotidine 5′-monophosphate decarboxylase (EC 4.1.1.23) | 2.43 | 6.6 | 51.5 | 15 | – |
| 32 | 6710 | Q63537 | SYN2_RAT | Synapsin II | – | 8.7 | 63.4 | – | ILGDYDIK; SFRPDFVLIR; EMLTLPTFPVVVK + oxidation (M); VLLVVDEPHTDWAK |
| 33 | 7601 | P15999 | ATPA_RAT | ATP synthase alpha chain | – | 9.2 | 58.8 | – | QAVAYR; HALIIYDDSK; ILGADTSVDLEETGR; TGAIVDVPVGDELLGR |
| 34 | 8601 | P15999 | ATPA_RAT | ATP synthase alpha chain | – | 9.2 | 58.8 | – | QAVAYR; LTELLK; APGIIPR; ELIIGDR; VLSIGDGIAR; AVDSLVPIGR; HALIIYDDLSK + 6 additional peptides |
| 35 | 8603 | 1MAB | – | Chain A, rat liver F-1 Atpase | 2.23 | 8.4 | 55.4 | 22 | – |
| 36 | 8604 | 1MAB | – | Chain A, rat liver F1-Atpase | 2.23 | 8.4 | 55.4 | 22 | – |
| 37 | 8605 | P1599 | ATPA_RAT | ATP synthase alpha chain | – | 9.2 | 58.8 | – | QAVAYR; APGIIPR; VGSAAQTR; STVAQLVK; FNDGTDEK; VLSIGDGIAR; AVDSLV PIGR + 9 additional peptides |
| 38 | 8606 | P15999 | ATPA_RAT | ATP synthase alpha chain | – | 9.2 | 58.8 | – | QAVAYR; LTELLK; QMSLLLR + oxidation (M); FNDGTDEK; VLSIGDGIAR; AVDSLVPIGR + 7 additional peptides |
| 39 | 8608 | P15999 | ATPA_RAT | ATP synthase alpha chain | – | 9.2 | 58.8 | – | LTELLK; APGIIPR; ELIIGDR; STVAQLVK; OMSLLLR + oxidation (M); EPMQTGIK; VLSIGDGIAR + 8 additional peptides |
| 40 | 2701 | Q9Z0W5 | PAC1_RAT | Protein kinase C and casein kinase substrate in neurones protein 1 | – | 5.2 | 50.4 | – | QLIEK; VLEDVGK; ELEQAIR; GSVSSYDR; GADAQEDLR + 9 additional peptides |
| 41 | 2707 | Q9Z0W5 | PAC1_RAT | Protein kinase C and casein kinase substrate in neurons protein 2 | – | 5.2 | 50.4 | – | VLEDVGK; VSELHQEVK; NSLLNEDLEK; TEQSVTPEQQK + 3 additional peptides |
| 42 | 4702 | P08461 | ODP2_RAT | Dihydroliponamide succinyltransferase component of 2-oxoglutarate dehydrogenase | – | 8.2 | 47.4 | – | EAVTFLR; GLVVPVIR; TINELGEK |
| 43 | 1610 | P10719 | ATPB_RAT | ATP synthase beta subunit | 2.43 | 4.9 | 51.2 | 28 | – |
| 44 | 2601 | P10719 | ATPB_RAT | ATP synthase beta chain | – | 5.2 | 56.3 | – | VLDSGAPIK; IGLFGGAGVGK; IPVGPETLGR; VVDLLAPYAK + 7 additional peptides |
| 45 | 2604 | P10719 | ATPB_RAT | Chain B, rat liver F1-ATPase | 2.43 | 4.9 | 51.3 | 19 | – |
| 46 | 2608 | P10719 | ATPB_RAT | ATP synthase beta chain | – | 5.2 | 56.3 | – | VVDLLAPYAK; VALTGLTVAEYFR; DQEGQDVLLFIDNIFR + 2 additional peptides |
| 47 | 4607 | P04764 | ENOA_RAT | Alpha enolase (2-phospho-d-glycerate hydrolyase) (non-neural enolase, NNE | 2.43 | 5.8 | 47.5 | 23 | – |
| 48 | 4613 | P04764 | ENOA_RAT | Alpha enolase (2-phospho-d-glycerate hydrolyase) NNE (enolase 1) | 2.43 | 6.2 | 47.4 | 50 | – |
| 49 | 5602 | P04764 | ENOA_RAT | Alpha enolase (2-phospho-d-glycerate hydrolyase) NNE (enolase 1) | – | 5.8 | 47.0 | – | EALELLK; IEEELGSK; LNVVEQEK; KLNVVEQEK; GNPTVEVDLY TAK + 3 additional peptides |
| 50 | 5605 | P04764 | ENOA_RAT | Alpha enolase (2-phospho-d-glycerate hydrolyase) NNE (enolase 1) | – | 5.8 | 47.0 | – | YITPDQLADLYK; AAVPSGAST GIYEALELR; LAMQEFMILPV GASSFR + 2oxidation (M) |
| 51 | 1611 | NP_647541 | Q922A0 | Enolase 2, gamma; enolase 2, gamma, neuronal | 2.43 | 5.0 | 47.5 | 25 | – |
| 52 | 1612 | NP_647541 | Q922A0 | Enolase 2, gamma; enolase 2, gamma, neuronal | 2.43 | 5.0 | 47.5 | 25 | – |
| 53 | 2602 | P07323 | ENOG_RAT | Enolase, gamma | – | 5.0 | 47.0 | – | LGAEVYHTLK; GNPTVEDLHTAK; AAVPSGASTGIYEALELR; AVD HINSTIAPALISSGLSWEQEK |
| 54 | 2508 | NP_112406 | ACTB_RAT | Cytoplasmic beta-actin | 2.43 | 5.3 | 42.1 | 28 | – |
| 55 | 2513 | P02570 | ACTB_HUMAN | Actin beta | 2.43 | 5.3 | 42.1 | 32 | – |
| 56 | 3502 | P07335 | KCRB_RAT | Creatine kinase-B | 2.43 | 5.3 | 40.9 | 29 | – |
| 57 | 3504 | P07335 | KCRB_RAT | Creatine kinase, brain | 2.43 | 5.3 | 43.0 | 48 | – |
| 58 | 5505 | NP_653134.2 | – | Tribbles homolog 2 | 2.43 | 5.8 | 39.4 | 11 | – |
| 59 | 5508 | P02551 | TBA1_MOUSE | Tubulin alpha-1 chain | – | 4.9 | 50.1 | – | LIGQIVSSITASLR |
| 60 | 6502 | 1717354A | GL-_RAT | Glutamine synthetase | 2.43 | 6.4 | 41.2 | 19 | – |
| 61 | 6507 | P26284 | ODPA_RAT | Pyruvate dehydrogenase E1 component alpha subunit | – | 8.5 | 43.2 | – | SDPIMLLK + oxidation (M); AAASTDYYK; RGDFIPGLR; LEEGPPVTTVLTR + 3 additional peptides |
| 62 | 6509 | P14408 | FUMH_RAT | Fumarate hydratase | – | 9.1 | 54.4 | – | LHDALSAK; IEYDTFGELK; VAALTGLPFVTAPNK |
| 63 | 7501 | NP_080215.1 | PUR6_MOUSE | Phosphoribosylaminoimidazole carboxylase [Mus musculus] | 2.43 | 7.0 | 47.7 | 17 | – |
| 64 | 7502 | P25809 | KCRU_RAT | Creatine kinase | – | 8.9 | 47.3 | – | LPLLSK; SGYFDER; HTTDLDASK; VVVDALSGLK; GWEFMWNER + oxidation (M) + 2 additional peptides |
| 65 | 7505 | XP_215806.1 | KCRU_RAT | Creatine kinase, mitochondrial 1, ubiquitous | 2.38 | 8.9 | 47.3 | – | – |
| 66 | 7508 | XP_215806.1 | KCRU_RAT | Creatine kinase, mitochondrial 1, ubiquitous | 2.26 | 8.9 | 47.3 | 29 | – |
| 67 | 2511 | Q99963 | SH33_HUMAN | SH3-domain GRB2-like protein 2 | 2.43 | 5.3 | 40.1 | 37 | – |
| 68 | 4511 | NP_446383.1 | – | Glycoprotein lb (platelet), beta polypeptide | 2.43 | 6.3 | 44.4 | 40 | – |
| 69 | 4512 | NP_446383.1 | – | Glycoprotein lb (platelet), beta polypeptide | 2.43 | 6.3 | 44.4 | 15 | – |
| 70 | 6403 | P09117 | ALFC_RAT | Fructose-bisphosphate aldolase C | – | 6.8 | 39.1 | – | DNAGAATEEFIK; GILAA DESVGSMAK + oxidation (M); LSQIGVENTEENR; YSPEEIA MATVTALR + oxidation (M) |
| 71 | 6404 | P09117 | ALFC_RAT | Fructose-bisphosphate aldolase C | – | 6.8 | 39.1 | – | QVLFSADDR; TPSALAILENAN-VLAR; YSPEEIAMATV-TALR + oxidation (M) |
| 72 | 6406 | P09117 | ALFC_RAT | Fructose-bisphosphate aldotase C | – | 6.8 | 39.1 | – | TPSALAILENANVLAR; YSPEEIA MATVTALR + oxidation (M) |
| 73 | 6409 | CAA30044.1 | ALFC_RAT | Brain-specific rat aldolase C | 2.43 | 6.8 | 39.6 | 23 | – |
| 74 | 8506 | P16617 | PGK2_RAT | Phosphoglycerate kinase | – | 7.5 | 44.4 | – | YSLEPVAAELK |
| 75 | 8508 | P16617 | PGK2_RAT | Phosphoglycerate kinase | – | 7.5 | 44.4 | – | DVLFLK, YAEAVAR; KYAEAVAR; YSLEPVAAELK; LGDVYVN DAFGTAHR + 2 additional peptides |
| 76 | 8418 | AAL99984.1 | Q8R4B4 | Down syndrome cell adhesion molecule-like protein | 2.08 | 9.6 | 40.7 | 22 | – |
| 77 | 8419 | P05065 | ALFA_RAT | Fructose-bisphosphate aldolase A | – | 8.4 | 39.2 | – | PFPQVIK, ELADIAHR; AAQEEYIK; QLLLTADDR; GILAA DESTGSIAK; LQSIGTEN TEENR + 2 additional peptides |
| 78 | 9408 | NP_037309.1 | AATM_RAT | Glutamate oxaloacetate transaminase 2 | 2.43 | 9.4 | 47.7 | 33 | – |
| 79 | 3408 | NP_446090 | AB047541* | Isocitrate dehydrogenase 3(-D+) alpha | 2.43 | 6.5 | 40.1 | 33 | – |
| 80 | 4401 | NP_620266 | Y15068* | Stress-induced phosphoprotein 1 | 1.56 | 6.1 | 40.7 | 31 | – |
| 81 | 5402 | P26284 | ODPA_RAT | Pyruvate dehydrogenase E1 component alpha subunit | – | 8.5 | 43.2 | – | EEIQEVR; AAASTDYYK; LEEGPPVTTVLTR; YGMGTS VER + oxidation (M) + 2 additional peptides |
| 82 | 6405 | P04797 | G3P_RAT | Glyceraldehyde-3-phosphate dehydrogenase | – | 8.4 | 35.7 | – | GAAQNIIPASTGAAK; VPTPNVS VVDLTCR + carbamidomethyl (C); LISWYDNEYGYSNR + 1 additional peptide |
| 83 | 6408 | P51635 | AKA1_RAT | Alcohol dehydrogenase | – | 6.8 | 36.4 | – | YIVPMITVDGK + oxidation (M); QIDDVLSVASVR; GLEVTAYS PLGSSDR; HPDEPVLLEEPVV LALAEK |
| 84 | 7403 | NP_058704.1 | Q8K4T7 | Glyceraldehyde-3-phosphate dehydrogenase | 1.45 | 8.4 | 36.1 | 20 | – |
| 85 | 7409 | NP_034342.1 | Q8VDP9 | Four and a half LIM domains 2 | 2.43 | 7.8 | 34.1 | 40 | – |
| 86 | 7410 | XP_214333.1 | G3P_RAT | Glyceraldehyde-3-phosphate dehydrogenase | 2.43 | 7.8 | 36.1 | 29 | – |
| 87 | 7413 | P04797 | G3P_RAT | Glyceraldehyde-3-phosphate dehydrogenase | – | 8.4 | 35.7 | – | LVINGK; PITIFQER; VVDLMAY MASK + 2 oxidation (M); GAAQNIIPASTGAAK; LISWYDNEYGYSNR + 1 additional peptide |
| 88 | 8407 | NP_058704.1 | Q8K4T7 | Glyceraldehyde-3-phosphate dehydrogenase | 2.4 | 8.4 | 36.1 | 44 | – |
| 89 | 8410 | NP_058704.1 | Q8K4T7 | Glyceraldehyde-3-phosphate dehydrogenase | 2.4 | 8.4 | 36.1 | 44 | – |
| 90 | 8412 | NP_058704.1 | Q8K4T7 | Glyceraldehyde-3-phosphate dehydrogenase | 2.4 | 8.4 | 36.1 | 44 | – |
| 91 | 8415 | NP_058704.1 | Q8K4T7 | Glyceraldehyde-3-phosphate dehydrogenase | 2.4 | 8.4 | 36.1 | 44 | – |
| 92 | 8417 | NP_058704.1 | Q8K4T7 | Glyceraldehyde-3-phosphate dehydrogenase | 2.4 | 8.4 | 36.1 | 44 | – |
| 93 | 3303 | P54313 | GBB2_RAT | Guanine nucleotide-binding protein G(1)/G(S)/G(T) | – | 5.6 | 37.5 | – | LIIWDSYTTNK |
| 94 | 3306 | P54311 | GBB1_RAT | Transducin beta (guanine nucleotide-binding protein beta subunit 1) | 2.4 | 5.5 | 38.2 | 24 | – |
| 95 | 3305 | P54313 | GBB2_RAT | Guanine nucleotide-binding protein G(I)/G(S)/G(T) | – | 5.6 | 37.5 | – | LIIWDSYTTNK |
| 96 | 3301 | ODPB_RAT | ODPB_RAT | Pyruvate dehydrogenase E1 component beta subunit, mitochondrial precursor (PDHE1B) | 2.43 | 5.9 | 39.3 | 34 | – |
| 97 | 3312 | P42123 | LDHB_RAT | Lactate dehydrogenase B | 2.29 | 5.7 | 36.9 | 25 | – |
| 98 | 4309 | NP_150238 | O88989 | Malate dehydrogenase 1; malate dehydrogenase, soluble | 2.21 | 6.2 | 36.6 | 28 | – |
| 99 | 5301 | P81155 | POR2_RAT | Voltage-dependent anion-selective channel protein 2 | – | 7.4 | 31.7 | – | YQLDPTASISAK; LTFDTTFSPNTGK |
| 100 | 6301 | NP_058927.1 | TPIS_RAT | Phosphatidylinositol transfer protein | 2.43 | 6.0 | 32.2 | 22 | – |
| 101 | 6304 | Q9Z2L0 | POR1_RAT | Voltage-dependent anion-selective channel protein 1 | – | 8.4 | 32.4 | – | DVFTK; VTQSNFAVGYK; LTFDSSFSPNTGK |
| 102 | 7301 | P81155 | POR2_RAT | Voltage-dependent anion-selective channel protein 2 | – | 7.4 | 31.7 | – | GFGFGLVK; LTLSALVDGK; YQLDPTASISAK; LTFDTTFSPNIGK; TGDFQLHTNVNNGTEFGG SIYQK + 1 additional |
| 103 | 8306 | Q9Z2L0 | POR1_RAT | Voltage-dependent anion-selective channel protein 1 | – | 8.4 | 32.4 | – | WTEYGLTFTEK; LTFDSSFSPNTGK |
| 104 | 8309 | NP_112643.1 | POR1_RAT | Voltage-dependent anion channel 1 | 2.43 | 8.8 | 30.9 | 24 | – |
| 105 | 8311 | NP_112643.1 | POR1_RAT | Voltage-dependent anion channel 1 | 2.43 | 8.8 | 30.9 | 24 | – |
| 106 | 8312 | Q9Z2L0 | POR1_RAT | Voltage-dependent anion-selective channel protein 1 | – | 8.4 | 32.4 | – | DVFTK; LTLSALLDGK; VTQSNFAVGYK; WTEYGLTF TEK; LTFDSSFSPNTGK + 2 additional peptides |
| 107 | 9301 | NP_112643.1 | POR1_RAT | Voltage-dependent anion channel 1 | 2.43 | 8.8 | 30.9 | 43 | – |
| 108 | 9304 | NP_112643.1 | POR1_RAT | Voltage-dependent anion channel 1 | 2.43 | 8.8 | 30.9 | 43 | – |
| 109 | 3204 | P24142 | PHB_MOUSE | Prohibitin | 2.43 | 5.4 | 27.8 | 30 | – |
| 110 | 210 | NP_112253 | SN25_RAT | SNAP 25 synaptosomal-associated protein, 25 kDa | 2.43 | 4.7 | 23.5 | 33 | – |
| 111 | 2202 | P54313 | GBB2_RAT | Guanine nucleotide-binding protein G(I)/G(S)/G(T) beta subunit 2 | – | 6.5 | 37.5 | – | GHLAK; AGVLAGHDNR; TFVSGACDASIK + carbamidomethyl (C); LIIWDSYTTNK |
| 112 | 2201 | Q00981 | UBL1_RAT | Ubiquitin carboxyl-terminal hydrolase isozyme L1 | – | 5.1 | 24.8 | – | QIEELK; QFLSETEK; MPFPVNHGASSEDSLLQ DAAK + oxidation + P29(M) |
| 113 | 2207 | Q00981 | UBL1_RAT | Ubiquitin carboxy-terminal hydrolase L1 (cerebral protein-6) | 2.43 | 5.1 | 25.1 | 51 | – |
| 114 | 2208 | P19234 | NUHM_RAT | NADH-ubiquinone oxidoreductase 24 kDa subunit | – | 6.0 | 26.5 | – | DSDSILETLQR; AAAVLPVLDLAQR |
| 115 | 4201 | O35244 | PDX6_RAT | Peroxiredoxin 6 | 2.43 | 5.6 | 24.9 | 23 | – |
| 116 | 5204 | P22062 | PIMT_RAT | Protein-l-isoaspartate (d-aspartate) O-methyltransferase | – | 7.3 | 24.5 | – | LVVGDGR; VFEVMLATDR + oxidation (M); ELVDDSITNVK; SGGASHSELIHNLR + 1 additional peptide |
| 117 | 5208 | P48500 | TPIS_RAT | Triosephosphate isomerase | – | 6.5 | 26.8 | – | VVFEQTK; FFVGGNWK; TATPQQAQEVHEK; HIFGESDELIGQK + 2 additional peptides |
| 118 | 5210 | P25113 | PMG1_RAT | Phosphoglycerate mutase 1 | – | 6.2 | 28.5 | – | FSGWYDADLSPAGHEEAK |
| 119 | 5211 | Q9Z2L0 | POR1_RAT | Voltage-dependent anion-selective channel protein 1 | – | 8.4 | 32.4 | – | DVFTK; GYGFGLIK; VTQSNFAV GYK; WTEYGLTFTEK; LTFDSSFSPNTGK + 2 additional peptides |
| 120 | 5213 | P48500 | TPIS_RAT | Triosephosphate isomerase | – | 6.5 | 26.8 | – | TATPQQAQEVHEK; HIFGE SDELIGQK; VVLAYEPV WAIGTGK + 3 additional peptides |
| 121 | 6202 | JC1132 | PMG2_RAT | Phosphoglycerate mutase (EC 5.4.2.1) B chain | 2.43 | 6.7 | 28.9 | 65 | – |
| 122 | 6204 | NP_075211.1 | TPIS_RAT | Triosephosphate isomerase 1 | 2.43 | 6.5 | 27.4 | 62 | – |
| 123 | 6212 | NP_075211.1 | TPIS_RAT | Triosephosphate isomerase 1 | 2.43 | 6.5 | 27.4 | 39 | – |
| 124 | 6213 | NP_075211.1 | TPIS_RAT | Triosephosphate isomerase 1 | 2.43 | 6.5 | 27.4 | 43 | – |
| 125 | 9204 | NP_032996.1 | PTHR_RAT | Parathyroid hormone-related protein; PTH-related peptide | 2.43 | 10.7 | 20.1 | 61 | – |
| 126 | 1101 | P14701 | TCTP_MOUSE | Translationally controlled tumor protein | – | 4.8 | 19.5 | – | DLISHDELFSDIYK |
| 127 | 2106 | P14701 | TCTP_MOUSE | Translationally controlled tumor protein | – | 4.8 | 19.5 | – | DLISHDELFSDIYK |
| 128 | 4110 | NP_476484 | AF157511* | SP22 (fertility protein) | 2.43 | 6.3 | 20.2 | 39 | – |
| 129 | 2107 | P35704 | PDX2_RAT | Peroxiredoxin 2; thioredoxin peroxidase 1 | 2.43 | 5.3 | 21.9 | 34 | – |
| 130 | 2111 | P31044 | PEBP_RAT | Phosphatidylethanolamine binding protein; hippocampal cholinergic neurostimulating peptide | 2.4 | 5.5 | 20.9 | 60 | – |
| 131 | 4106 | P04631 | S10B_RAT | S-100 protein, beta chain | – | 4.5 | 10.6 | – | AMVALIDVFHQYSGR + oxidation (M) |
| 132 | 4109 | P31399 | ATPQ_RAT | ATP synthase subunit d | 2.3 | 6.2 | 18.8 | 42 | – |
| 133 | 6101 | P37805 | NP25_RAT | Neuronal protein NP25 | – | 6.5 | 24.7 | – | DMAAVQR; GPSYGLSR; AAE VYGVR; GFSEEQLR; YDAD LENK + 7 additional peptides |
| 134 | 7108 | P07895 | SODM_RAT | Superoxide dismutase | – | 9.0 | 24.7 | – | DFGSFEEK; YHEALAK; GELLEAIK; NVRPDYLK; GDVTTQVALQ PALK; AIWNVINWENVSQR |
| 135 | 8106 | P07895 | SODM_RAT | Superoxide dismutase | – | 9.0 | 24.7 | – | GELLEAIK; NVRPDYLK; GDVTTQVALQPALK |
| 136 | 107 | P02593 | CALM_HUMAN | Calmodulin | – | 4.1 | 16.7 | – | ELGTVMR + oxidation (M); DTDSEEEIR |
| 137 | 111 | Q63754 | SYUB_RAT | Synuclein, beta | 1.51 | 4.5 | 14.5 | 26 | – |
| 138 | 1108 | P01946 | HBA_RAT | Hemoglobin alpha-1 and alpha-2 chains | – | 7.9 | 15.2 | – | FLASVSTVLTSK |
| 139 | 105 | P02593 | CALM_HUMAN | Calmodulin | – | 4.1 | 16.7 | – | ELGTVMR + oxidation (M); EAFSLFDK; DTDSEEEIR; DGNGYISAAELR; EAFSLFDKDGDGTITTK |
| 140 | 2112 | Q63228 | GLMB_RAT | Glia maturation factor beta | – | 5.3 | 16.6 | – | LVQTAELTK; LVVLDEE LEGVSPDELK |
| 140 | 2112 | P13668 | STN1_RAT | Stathmin | – | 5.8 | 17.1 | – | SHEAVLK; DLSLEEIQK; ASGQA FELILSPR |
| 141 | 3104 | P13668 | STN1_RAT | Stathmin | – | 5.8 | 17.1 | – | ASGQAFELILSPR |
| 142 | 104 | P02593 | CALM_HUMAN | Calmodulin | – | 4.1 | 16.7 | – | EAFSLFDK |
| 143 | 103 | NP_036645 | Q9QWC5 | Calmodulin, Ca(2+)-dependent ganglioside-binding protein (fragment) | 1.34 | 4.0 | 11.7 | 47 | – |
| 144 | 6 | P10639 | THIO_MOUSE | Thioredoxin 1; thioredoxin | 1.43 | 4.8 | 12.0 | 40 | – |
| 145 | 1107 | Q04758 | IPKB_MOUSE | cAMP-dependent protein kinase inhibitor beta | 2.43 | 4.7 | 9.7 | 53 | – |
| 146 | 2001 | Q9CQI6 | COAC_MOUSE | Coactosin-like protein | – | 5.3 | 15.9 | – | EVVQNFAK |
| 147 | 2114 | Q64271 | VAM3_MOUSE | Vesicle-associated membrane protein 3 | – | 8.7 | 11.5 | – | LSELDDR; ADALQAGAS QFETSAAK |
| 148 | 4103 | P13795 | SN25_HUMAN | Chain B of complex between N-terminus of SNAP25 and SNARE region of syntaxin 1a | 2.38 | 5.9 | 9.1 | 19 | – |
| 149 | 5109 | 1SFC | – | Chain B, neuronal synaptic fusion complex | 2.43 | 5.1 | 9.6 | 35 | – |
| 150 | 1005 | P11232 | THIO_RAT | Thioredoxin | – | 4.8 | 11.5 | – | VGEFSGANK; EAFQEALAAAGDK |
| 151 | 2005 | NP_067710 | Q9Z2N6 | CaM-KII inhibitory protein | 2.43 | 5.3 | 8.7 | 38 | – |
| 152 | 5004 | XP_220432.1 | HNT1_MOUSE | Similar to histidine triad nucleotide binding protein | 2.43 | 6.2 | 11.6 | 28 | – |
| 153 | 5013 | Q63362 | NUFM_RAT | NADH-ubiquinone oxidoreductase 13 kDa-B subunit | – | 7.1 | 13.3 | – | KYTEQITSEK; TTGLVGLAVCDT PHER + carbamidomethyl (C); KLENLLQGGEVEEVILQAEK |
| 154 | 2002 | P80144 | MTPN_MOUSE | Myotropin | – | 5.3 | 12.7 | – | GPDGLTALEATDNQAIK |
| 155 | 2006 | P50408 | VATF_RAT | Vacuolar ATP synthase subunit F | – | 5.5 | 13.4 | – | SIPAVLEIPSK; DTTINEIEDTFR |
| 156 | 5012 | Q63362 | NUFM_RAT | NADH-ubiquinone oxidoreductase 13 kDa-B subunit | – | 7.1 | 13.3 | – | ILDLLK; YTEQITSEK; KYTEQITSEK; PWEPLVEEPPANQWK + 3 additional peptides |
| 157 | 5014 | 1JTH | – | Chain B of complex between N-terminus of SNAP25 and SNARE region of syntaxin 1a | 2.43 | 5.9 | 9.1 | 30 | – |
| 158 | 7010 | P30904 | MIF_RAT | Macrophage migration inhibitory factor | – | 7.3 | 12.3 | – | LLCGLLSDR + carbamidomethyl (C); PMFIVNTNVPR + oxidation (M) |
| 159 | 8001 | Q62658 | FKB1_RAT | FK506-binding protein 1A | – | 8.1 | 11.8 | – | GVQVETISSGDGR |
| 160 | 9006 | P26772 | CH10_RAT | 10 kDa heat shock protein, mitochondrial (Hsp 10) (10 kDa chaperonin) (CPN10) | – | 9.3 | 8.1 | – | GGEIQPVSVK; VLLPEYGGTK; VLQATVVAVGSGGLK; VVLDDKDYFLFR |
| 161 | 5003 | P17074 | RS19_RAT | 40S ribosomal protein S19 | – | 10.4 | 15.9 | – | IAGQVAAANK |
| 162 | 6002 | P02248 | UBIQ_HUMAN | Ubiquitin | – | 6.6 | 8.6 | – | TITLEVEPSDTIENVK |
| 163 | 6003 | NP_006189.1 | PE19_MOUSE | Purkinje cell protein 4; brain specific polypeptide PEP-19 | 1.81 | 6.2 | 6.8 | 44 | – |
Protein spot number (arbitrarily assigned) from Fig. 1; SSP, PDQuest assigned spot number;%C, percent sequence coverage by measured masses; Z-score from ProFound database.
The 94 most abundant spots were subjected to MALDI-based PMF resulting in the identification of 85 spots, representing 61 unique proteins. The peptides from the remaining 96 spot digests were analyzed by LC-MS/MS, which yielded 79 identifications, representing 46 unique proteins. The proteins identified using the combination of 2-DE and the two MS techniques derived from synapse-specific structures such as synaptic vesicles and the synaptic membrane as well as from the cytoplasmic and mitochondrial compartments. Identified proteins that are specific to the synapse and function in neurotransmission are of particular interest; these include: calmodulin (CaM), Ca(2+)-dependent ganglio-side-binding protein (fragment), cAMP-dependent protein kinase inhibitor beta, chain B of complex between N-terminus of SNAP25 and SNARE region of Syntaxin 1a, chain B, neuronal synaptic fusion complex, SNAP 25 synaptosomal-associated protein, 25 kDa, synapsin II, synaptotagmin I, transducin beta, and synaptobrevin 3.
3.2 Shotgun proteomics and post-translational modification analysis
Although 2-DE coupled with MS or LC-MS is a powerful approach to differential expression analysis, the number of proteins that can be resolved and identified in 2-DE gel is limited. Highly hydrophobic proteins and those with extremes of pI, particularly basic proteins, are poorly resolved by this technique. In addition, 2-DEs relatively high concentration threshold for detection makes the analysis of low abundance proteins, many of which are physiologically relevant, a major challenge. To augment our 2-DE approach, proteins from synaptosomal fractions were analyzed directly after solution tryptic digestion using an LC-MS/MS approach.
Using this approach, 201 distinct proteins were identified (Table 2). Of these, ~20–30 proteins are known to be involved in synaptic vesicle trafficking/docking (e.g., Syntaxin 1A, Synapsin I, II, Synaptophysin, and Synaptotagmin I, II, V, protein kinase C and kinase substrate (PACSIN1), and calcium/CaM-dependent protein kinase type II) and synaptic plasma membrane structure and function (e.g., sodium-potassium ATPase, clathrin, channel-associated protein of synapse-110, pre-synaptic density protein-95, Dynamin 1–3, glutamate-aspartate transporter 2, neural cell adhesive molecule 1, GAP-43, opioid binding protein B, regulating synaptic membrane exocytosis protein 1, GABAB transporter, Septin 7, and Synaptojanin 1).
Table 2.
Synaptosomal proteins identified by shotgun LC-MS/MS Analysis
| No. | Abbreviation | NCBI Accession | Protein | 1 coverage [%]a) | 2 coverage [%]a) | 3 coverage [%]a) | PTM |
|---|---|---|---|---|---|---|---|
| 1 | 143B | P35213 | 14-3-3 protein beta/alpha(Protein kinase C inhibitor protein-1) | 18.40 | 14.80 | 19.60 | g |
| 2 | A180 | Q05140 | Clathrin coat assembly protein AP180 | 3.97 | 2.69 | 3.97 | |
| 3 | A1A1 | P06685 | Sodium/potassium-transporting ATPase alpha-1 chain | 10.57 | 11.82 | 11.05 | G |
| 4 | A1A2 | P06686 | Sodium/potassium-transporting ATPase alpha-2 chain | 10.32 | 10.70 | 10.03 | |
| 5 | A1A3 | P06687 | Sodium/potassium-transporting ATPase alpha-3 chain | 11.36 | 9.61 | 10.10 | G |
| 6 | A1A4 | Q64541 | Sodium/potassium-transporting ATPase alpha-4 chain | 4.02 | 2.58 | ||
| 7 | A1B1 | P52303 | Adapter-related protein complex 1 beta 1 subunit | 2.8 | 2.80 | 2.8 | |
| 8 | A2A2 | P18484 | Adaptor-related protein complex 2 alpha 2 subunit | 2.94 | 9.86 | 8.5 | G |
| 9 | AATC | P13221 | Aspartate aminotransferase, cytoplasmic | 20.05 | 20.05 | 20.05 | |
| 10 | AATM | P00507 | Aspartate aminotransferase, mitochondrial precursor | 25.34 | 13.24 | 13.24 | |
| 11 | ACLY | P16638 | ATP-citrate synthase (EC 2.3.3.8) | 1.7 | |||
| 12 | ADT1 | Q05962 | ADP,ATP carrier protein, heart/skeletal muscle isoform | 19.47 | 20.46 | 11.55 | A, g |
| 13 | ADT2 | Q09073 | ADP,ATP carrier protein, fibroblast isoform | 15.18 | 12.54 | 10.23 | A, G |
| 14 | ALFA | P05065 | Fructose-bisphosphate aldolase A (EC 4.1.2.13) | 35.68 | 27.57 | 21.98 | G |
| 15 | ALFC | P09117 | Fructose-bisphosphate aldolase C (EC 4.1.2.13) | 15.72 | 26.02 | 18.16 | |
| 16 | AMPH | O08838 | Amphiphysin | 3.45 | 2.88 | 2.88 | G |
| 17 | ANX5 | P14668 | Annexin A5 (Annexin V) (Lipocortin V) (Endonexin I) | 4.94 | 4.94 | ||
| 18 | ANX6 | P48037 | Annexin A6 (Annexin VI) (Lipocortin VI) (P68) (P70) | 2.34 | |||
| 19 | AOFA | P21396 | Amine oxidase [flavin-containing] A (EC 1.4.3.4) | 2.62 | |||
| 20 | ATB1 | P11505 | Plasma membrane calcium-transporting ATPase 1 | 2.74 | |||
| 21 | ATB2 | P11506 | Plasma membrane calcium-transporting ATPase 2 | 1.34 | 2.77 | ||
| 22 | ATB3 | Q64568 | Plasma membrane calcium-transporting ATPase 3 | 1.33 | 1.33 | ||
| 23 | ATB4 | Q64542 | Plasma membrane calcium-transporting ATPase 4 | 1.39 | 2.86 | 2.86 | |
| 24 | ATHA | P09626 | Potassium-transporting ATPase alpha chain 1 | 2.67 | |||
| 25 | ATHL | P54708 | Potassium-transporting ATPase alpha chain 2 | 0.85 | |||
| 26 | ATNB | P07340 | Sodium/potassium-transporting ATPase beta-1 chain | 8.71 | 8.71 | 4.19 | |
| 27 | ATPA | P15999 | ATP synthase alpha chain, mitochondrial precursor | 34.54 | 35.26 | 36.35 | G |
| 28 | ATPB | P10719 | ATP synthase beta chain, mitochondrial precursor | 43.49 | 49.81 | 56.51 | g |
| 29 | ATPD | P35434 | ATP synthase delta chain, mitochondrial precursor | 5.26 | |||
| 30 | ATPF | P19511 | ATP synthase B chain, mitochondrial precursor | 5.75 | 5.75 | ||
| 31 | ATPG | P35435 | ATP synthase gamma chain, mitochondrial | 4.32 | 3.60 | G | |
| 32 | ATPJ | P29419 | ATP synthase e chain, mitochondrial (EC 3.6.3.14) | 16.67 | 16.67 | 31.94 | |
| 33 | ATPO | Q06647 | ATP synthase oligomycin sensitivity conferral protein | 5.07 | 17.98 | 17.97 | |
| 34 | ATPQ | P31399 | ATP synthase D chain, mitochondrial (EC 3.6.3.14) | 30.06 | 33.13 | 23.31 | |
| 35 | ATPR | P21571 | ATP synthase coupling factor 6, mitochondrial precursor | 17.27 | |||
| 36 | BASP | Q05175 | Brain acid soluble protein 1 (BASP1 protein) | 12.56 | 12.56 | 12.56 | G |
| 37 | BIN1 | O08839 | Myc box dependent interacting protein 1 | 2.34 | 2.34 | ||
| 38 | CAH2 | P27139 | Carbonic anhydrase II (EC 4.2.1.1) | 9.85 | |||
| 39 | CAP1 | Q08163 | Adenylyl cyclase-associated protein 1 (CAP 1) | 4.57 | 8.52 | 3.95 | A |
| 40 | CAP2 | P52481 | Adenylyl cyclase-associated protein 2 (CAP 2) | 3.09 | |||
| 41 | CATD | P24268 | Cathepsin D precursor (EC 3.4.23.5). | 4.35 | 4.35 | 4.35 | |
| 42 | CH10 | P26772 | 10 kDa heat shock protein, mitochondrial (Hsp10) | 13.59 | 25.24 | 13.59 | |
| 43 | CLCB | P08082 | Clathrin light chain B (Lcb) | 4.29 | 4.29 | ||
| 44 | CLH | P11442 | Clathrin heavy chain | 13.51 | 18.44 | 17.56 | A, G |
| 45 | CN37 | P13233 | 2′,3′-cyclic nucleotide 3′-phosphodiesterase | 13.38 | 3.41 | 9.00 | |
| 46 | COA1 | P11497 | Acetyl-CoA carboxylase 1 (EC 6.4.1.2) (ACC-alpha) | 0.67 | 0.67 | ||
| 47 | COF1 | P45592 | Cofilin, non-muscle isoform | 6.51 | 6.51 | A | |
| 48 | COX2 | P00406 | Cytochrome c oxidase polypeptide II (EC 1.9.3.1) | 4.33 | 4.33 | 4.33 | |
| 49 | COXA | P11240 | Cytochrome c oxidase polypeptide Va, mitochondrial | 30.20 | 10.07 | 20.13 | |
| 50 | CPV1 | P22443 | Cytochrome P450 19A1 (Aromatase) (EC 1.14.14.1) | 3.68 | |||
| 51 | CRP2 | P36201 | Cysteine-rich protein 2 (CRP2) (ESP1 protein) | 15.09 | 15.09 | ||
| 52 | CX41 | P10888 | Cytochrome c oxidase subunit IV isoform 1, mitochondrial | 6.98 | 6.40 | ||
| 53 | DCE2 | Q05683 | Glutamate decarboxylase, 65 kDa isoform | 3.70 | |||
| 54 | DDH1 | O08557 | NG,NG-dimethylarginine dimethylaminohydrolase 1 | 9.00 | A | ||
| 55 | DLG2 | Q63622 | Channel associated protein of synapse-110 | 1.61 | |||
| 56 | DLG4 | P31016 | Presynaptic density protein 95 (PSD-95) | 2.04 | |||
| 57 | DOPD | P80254 | D-dopachrome tautomerase | 10.08 | G | ||
| 58 | DPY1 | Q62950 | Dihydropyrimidinase related protein-1 (DRP-1) | 3.95 | 6.70 | 12.89 | |
| 59 | DPY2 | P47942 | Dihydropyrimidinase related protein-2 (DRP-2) | 15.98 | 34.36 | 37.11 | G |
| 60 | DPY4 | Q62951 | Dihydropyrimidinase related protein-4 (DRP-4) | 2.79 | 2.79 | ||
| 61 | DPY5 | Q9JHU0 | Dihydropyrimidinase related protein-5 (DRP-5) ULIP6 protein | 4.88 | |||
| 62 | DYN1 | P21575 | Dynamin-1 (EC 3.6.1.50) (D100) (Dynamin, brain) | 20.44 | 11.09 | 13.28 | G |
| 63 | DYN2 | P39052 | Dynamin 2 (EC 3.6.1.50) | 3.16 | 4.18 | 3.84 | |
| 64 | DYN3 | Q08877 | Dynamin 3 (EC 3.6.1.50) (Dynamin, testicular) | 3.82 | 1.97 | 2.78 | |
| 65 | EAA1 | P24942 | Sodium-dependent glutamate/aspartate transporter 2 | 6.33 | 3.62 | ||
| 66 | EAA2 | P31596 | Sodium-dependent glutamate/aspartate transporter 2 | 4.46 | 6.35 | 6.00 | A, G |
| 67 | ECHM | P14604 | Enoyl-CoA hydratase, mitochondrial precursor | 5.76 | 5.76 | ||
| 68 | ENOA | P04764 | Alpha enolase (EC 4.2.1.11) (2-phospho-D-glycerate) | 10.19 | 27.66 | 32.20 | |
| 69 | ENOB | P15429 | Beta enolase (EC 4.2.1.11) (2-phospho-D-glycerate) | 10.43 | G | ||
| 70 | ENOG | P07323 | Gamma enolase (EC 4.2.1.11) (2-phospho-D-glycerate) | 35.15 | 32.88 | 36.51 | |
| 71 | FKB1 | Q62658 | FK506-binding protein 1A (EC 5.2.1.8) | 24.77 | |||
| 72 | FRAP | P42346 | FKBP-rapamycin associated protein (FRAP) | 0.27 | |||
| 73 | FUMH | P14408 | Fumarate hydratase, mitochondrial precursor | 4.07 | |||
| 74 | G3P | P04797 | Glyceraldehyde 3-phosphate dehydrogenase | 36.98 | 30.77 | 40.53 | G |
| 75 | GABT | P50554 | 4-aminobutyrate aminotransferase, mitochondrial precursor | 5.70 | 7.86 | 2.95 | |
| 76 | GB01 | P59215 | Guanine nucleotide-binding protein G(O), alpha subunit | 27.86 | |||
| 77 | GB02 | P30033 | Guanine nucleotide-binding protein G(O), alpha subunit | 15.88 | 24.79 | ||
| 78 | GB12 | Q63210 | Guanine nucleotide-binding protein, alpha-12 subunit | 2.86 | 2.86 | ||
| 79 | GBAK | P08753 | Guanine nucleotide-binding protein G(k), alpha subunit | 3.06 | |||
| 80 | GBB1 | P54311 | Guanine nucleotide-binding protein G(I)/G(S)/G(T) beta subunit 1 | 10.69 | 8.96 | 10.69 | A |
| 81 | GDIA | P50398 | Rab GDP dissociation inhibitor alpha (Rab GDI alpha) | 40.66 | 28.35 | 29.45 | |
| 82 | GDIC | P50399 | Rab GDP dissociation inhibitor beta-2 (Rab GDI beta) | 4.19 | |||
| 83 | GLNA | P09606 | Glutamine synthetase (EC 6.3.1.2) | 4.21 | 11.58 | 11.58 | |
| 84 | GLSK | P13264 | Glutaminase, kidney isoform, mitochondrial precursor | 5.10 | 5.10 | 3.06 | |
| 85 | GR75 | P48721 | Stress-70 protein, mitochondrial precursor (GRP 75) | 1.74 | 4.49 | 1.59 | M |
| 86 | GR78 | P06761 | 78 kDa glucose-regulated protein precursor (GRP 78) | 2.41 | |||
| 87 | GTM2 | P08010 | Glutathione S-transferase Yb-2 (EC 2.5.1.18) | 7.69 | |||
| 88 | GTP | P04906 | Glutathione S-transferase P (EC 2.5.1.18) | 7.51 | 7.51 | ||
| 89 | GUAD | Q9WTT6 | Guanine deaminase (EC 3.5.4.3) (Guanase) | 3.03 | |||
| 90 | HCD2 | O70351 | 3-hydroxyacyl-CoA dehydrogenase type II | 9.81 | |||
| 91 | HEM0 | Q63147 | 5-aminolevulinic acid synthase, erythroid-specific | 1.84 | |||
| 92 | HES2 | P35429 | Transcription factor HES-2 | 6.25 | |||
| 93 | HS1A | P55063 | Heat shock protein 1A (Heat shock 70 kDa protein 3) | 4.45 | 4.45 | 1.99 | |
| 94 | HS72 | P14659 | Heat shock-related 70 kDa protein 2 | 6.52 | 6.52 | G | |
| 95 | HS9B | P34058 | Heat shock protein HSP 90-beta (HSP 84) | 4.76 | 3.53 | 3.53 | M |
| 96 | HXK1 | P05708 | Hexokinase, type I (EC 2.7.1.1) (HK I) (Brain form) | 8.89 | 6.96 | 4.60 | A, G |
| 97 | HXK2 | P27881 | Hexokinase type II (EC 2.7.1.1) (HK II) | 1.18 | |||
| 98 | IDHG | P41565 | Isocitrate dehydrogenase [NAD] subunit gamma | 5.00 | 5.00 | ||
| 99 | JAG2 | P97607 | Jagged 2 (Jagged2) (Fragment) | 1.06 | |||
| 100 | K6PF | P47858 | 6-phosphofructokinase, muscle type (EC 2.7.1.11) | 1.39 | |||
| 101 | K6PL | P30835 | 6-phosphofructokinase, liver type (EC 2.7.1.11) | 2.40 | |||
| 102 | K6PP | P47860 | 6-phosphofructokinase, type C (EC 2.7.1.11) | 3.00 | 6.00 | 6.00 | |
| 103 | KAD1 | P39069 | Adenylate kinase isoenzyme 1 (EC 2.7.4.3) | 7.07 | 7.07 | ||
| 104 | KCCA | P11275 | Calcium/calmodulin-dependent protein kinase type II | 19.55 | 22.84 | 22.84 | G |
| 105 | KCCB | P08413 | Calcium/calmodulin-dependent protein kinase type II | 18.84 | 16.67 | 21.20 | M, O |
| 106 | KCCD | P15791 | Calcium/calmodulin-dependent protein kinase type II | 10.15 | 7.75 | 7.75 | |
| 107 | KCCG | P11730 | Calcium/calmodulin-dependent protein kinase type II | 12.87 | 8.21 | 12.87 | |
| 108 | KCRB | P07335 | Creatine kinase, B chain (EC 2.7.3.2) (B-CK) | 30.93 | 38.92 | 34.79 | G, M |
| 109 | KCRS | P09605 | Creatine kinase, sarcomeric mitochondrial precursor | 2.11 | 3.76 | ||
| 110 | KCRU | P25809 | Creatine kinase, ubiquitous mitochondrial precursor | 23.06 | 11.06 | 13.18 | G |
| 111 | KILO | Q9Z0J8 | Kilon protein precursor (Kindred of IgLON) | 3.67 | |||
| 112 | KPRB | O08618 | Phosphoribosyl pyrophosphate synthetase-associated protein 2 | 6.12 | |||
| 113 | KPYM | P11980 | Pyruvate kinase, M1/M2 isozyme (EC 2.7.1.40) | 44.90 | 35.62 | 31.91 | |
| 114 | KPYR | P12928 | Pyruvate kinase, isozymes R/L (EC 2.7.1.40) (L-PK) | 1.88 | 1.88 | ||
| 115 | LDHA | P04642 | L-lactate dehydrogenase A chain (EC 1.1.1.27) (LDH) | 12.72 | 14.20 | 8.28 | G |
| 116 | LDHB | P42123 | L-lactate dehydrogenase B chain (EC 1.1.1.27) (LDH) | 13.86 | 12.98 | 4.72 | |
| 117 | MA32 | O35796 | Complement component 1, Q subcomponent binding protein | 15.55 | 10.60 | G | |
| 118 | MAPB | P15205 | Microtubule-associated protein 1B (MAP 1B) | 0.72 | |||
| 119 | MBP | P02688 | Myelin basic protein S (MBP S) | 17.53 | 8.25 | 11.34 | G |
| 120 | MDHM | P04636 | Malate dehydrogenase, mitochondrial precursor | 32.56 | 38.08 | 34.59 | G |
| 121 | MDR1 | P43245 | Multidrug resistance protein 1 (P-glycoprotein 1) | 1.00 | |||
| 122 | MDR2 | Q08201 | Multidrug resistance protein 2 (P-glycoprotein 2) | 1.00 | |||
| 123 | MIF | P30904 | Macrophage migration inhibitory factor (MIF) | 25.86 | 18.10 | ||
| 124 | MPCP | P16036 | Phosphate carrier protein, mitochondrial precursor | 3.31 | |||
| 125 | MYHA | Q9JLT0 | Myosin heavy chain, nonmuscle type B | 1.34 | |||
| 126 | MYOG | P20428 | Myogenin | 3.08 | 3.08 | ||
| 127 | NCA1 | P13596 | Neural cell adhesion molecule 1, 140 kDa isoform | 3.44 | 2.06 | ||
| 128 | NCP1 | P55161 | Nck-associated protein 1 (NAP 1) (p125Nap1) | 1.22 | 1.74 | ||
| 129 | NDKB | P19804 | Nucleoside diphosphate kinase B (EC 2.7.4.6) | 5.81 | |||
| 130 | NEUM | P07936 | Neuromodulin (Axonal membrane protein GAP-43) | 10.00 | 10.00 | 10.00 | |
| 131 | NP25 | P37805 | Neuronal protein NP25 | 15.25 | 8.97 | 8.97 | |
| 132 | NPX1 | P47971 | Neuronal pentraxin I precursor (NP-I) (NP1) | 4.09 | |||
| 133 | NTRI | Q62718 | Neurotrimin precursor (GP65) | 6.29 | |||
| 134 | NUHM | P19234 | NADH-ubiquinone oxidoreductase 24 kDa subunit | 5.28 | 4.47 | 9.76 | |
| 135 | ODO2 | Q01205 | Dihydrolipoamide succinyltransferase component of 2-oxoglutarate dehydrogenase complex | 2.67 | 4.67 | ||
| 136 | ODP2 | P08461 | Dihydrolipoamide acetyltransferase component of pyruvate dehydrogenase complex | 8.14 | 12.74 | 9.56 | G |
| 137 | ODPA | P26284 | Pyruvate dehydrogenase E1 component alpha subunit | 14.61 | 17.13 | 8.56 | P |
| 138 | ODPB | P49432 | Pyruvate dehydrogenase E1 component beta subunit | 7.40 | 20.27 | 11.78 | G |
| 139 | OPCM | P32736 | Opioid binding protein/cell adhesion molecule precursor | 11.11 | 5.41 | ||
| 140 | OPLA | P97608 | 5-oxoprolinase (EC 3.5.2.9) (5-oxo-L-prolinase) | 0.46 | |||
| 141 | PAC1 | Q9Z0W5 | Protein kinase C and casein kinase substrate | 9.58 | 12.69 | 15.37 | G |
| 142 | PDX5 | Q9R063 | Peroxiredoxin 5, mitochondrial precursor (Prx-V) | 24.42 | 16.59 | 5.99 | |
| 143 | PDX6 | O35244 | Peroxiredoxin 6 (EC 1.11.1.-) | 8.81 | 10.13 | 7.49 | |
| 144 | PEBP | P31044 | Phosphatidylethanolamine-binding protein (PEBP) | 36.32 | 48.42 | 36.84 | A, G |
| 145 | PGK2 | P16617 | Phosphoglycerate kinase, testis specific (EC 2.7.2) | 18.68 | 18.44 | 24.59 | g |
| 146 | PHS3 | P53534 | Glycogen phosphorylase, brain form (EC 2.4.1.1) | 1.41 | |||
| 147 | PIMT | P22062 | Protein-L-isoaspartate(D-aspartate) O-methyltransferase | 8.26 | |||
| 148 | PMG1 | P25113 | Phosphoglycerate mutase 1 (EC 5.4.2.1) | 15.12 | 8.14 | ||
| 149 | POR1 | Q9Z2L0 | Voltage-dependent anion-selective channel protein | 18.69 | 17.38 | 25.25 | |
| 150 | POR2 | P81155 | Voltage-dependent anion-selective channel protein | 10.00 | 10.00 | 9.67 | |
| 151 | POR3 | Q9R1Z0 | Voltage-dependent anion-selective channel protein | 14.93 | 10.42 | ||
| 152 | PPIA | P10111 | Peptidyl-prolyl cis-trans isomerase A (EC 5.2.1.8) | 45.78 | 42.17 | 34.34 | |
| 153 | RB10 | P35281 | Ras-related protein Rab-10 | 5.39 | |||
| 154 | RB1A | Q6NYB7 | Ras-related protein Rab-1A | 5.26 | 5.26 | ||
| 155 | RB2A | P05712 | Ras-related protein Rab-2A | 7.41 | 7.41 | ||
| 156 | RB3A | P63012 | Ras-related protein Rab-3A | 40.45 | 40.45 | 36.82 | |
| 157 | RB3C | P62824 | Ras-related protein Rab-3C | 16.74 | 16.74 | ||
| 158 | RIM1 | Q9JIR4 | Regulating synaptic membrane exocytosis protein 1 | 0.85 | 0.85 | G | |
| 159 | RPA1 | O54889 | DNA-directed RNA polymerase I largest subunit | 0.52 | |||
| 160 | RTN1 | Q64548 | Reticulon 1 (Neuroendocrine-specific protein) | 3.67 | |||
| 161 | RUN1 | Q63046 | Runt-related transcription factor 1 | 2.62 | |||
| 162 | S109 | P50116 | Calgranulin B | 10.53 | |||
| 163 | S6A1 | P23978 | Sodium- and chloride-dependent GABAb transporter | 1.97 | |||
| 164 | SAP | P10960 | Sulfated glycoprotein 1 precursor (SGP-1) | 2.66 | 4.61 | 2.66 | G |
| 165 | SEP7 | Q9WVC0 | Septin 7 (CDC10 protein homolog) | 5.18 | 5.86 | 3.83 | |
| 166 | SFX1 | Q63965 | Sideroflexin 1 (Tricarboxylate carrier protein) | 4.57 | |||
| 167 | SH31 | O35964 | SH3-containing GRB2-like protein 1 | 3.20 | 3.20 | ||
| 168 | SH32 | O35179 | SH3-containing GRB2-like protein 2 | 11.07 | 6.32 | 6.32 | G |
| 169 | SNAA | P54921 | Alpha-soluble NSF attachment protein (SNAP-alpha) | 3.67 | 3.67 | ||
| 170 | SNGP | Q9QUH6 | Ras GTPase-activating protein SynGAP | 1.14 | 1.37 | 1.37 | |
| 171 | SODC | P07632 | Superoxide dismutase [Cu-Zn] (EC 1.15.1.1) | 7.05 | 8.33 | 8.33 | G |
| 172 | SODM | P07895 | Superoxide dismutase [Mn], mitochondrial precursor | 12.39 | 12.39 | 12.39 | |
| 173 | SPCN | P16086 | Spectrin alpha chain, brain | 2.74 | 5.53 | 5.45 | G |
| 174 | SSDH | P51650 | Succinate semialdehyde dehydrogenase (EC 1.2.1.24) | 3.62 | |||
| 175 | ST1A | P32851 | Syntaxin 1A(Synaptotagmin associated 35 kDa protein) | 9.22 | 9.22 | 13.65 | |
| 176 | SUCA | P13086 | Succinyl-CoA ligase [GDP-forming] alpha-chain | 4.72 | G | ||
| 177 | SX10 | 0O55170 | Transcription factor SOX-10 | 1.48 | |||
| 178 | SYJ1 | Q62910 | Synaptojanin 1 (EC 3.1.3.36) | 1.25 | G | ||
| 179 | SYN1 | P09951 | Synapsin I | 25.98 | 24.02 | 27.65 | G |
| 180 | SYN2 | Q63537 | Synapsin II | 22.48 | 22.15 | 19.97 | |
| 181 | SYPH | P07825 | Synaptophysin (Major synaptic vesicle protein p38) | 3.19 | 7.35 | 3.19 | A |
| 182 | SYT1 | P21707 | Synaptotagmin I (SytI) (p65) | 20.51 | 19.11 | 15.15 | G |
| 183 | SYT2 | P29101 | Synaptotagmin II (SytII) | 5.12 | 5.12 | 5.12 | |
| 184 | SYT5 | P47861 | Synaptotagmin V (SytV) | 3.05 | 3.05 | ||
| 185 | SYUA | P37377 | Alpha-synuclein | 25.17 | 26.57 | 26.57 | g |
| 186 | SYUB | Q63754 | Beta-synuclein (Phosphoneuroprotein 14) (PNP 14) | 28.57 | 28.57 | 28.57 | g, M |
| 187 | TAU | P19332 | Microtubule-associated protein tau | 6.28 | 11.52 | 1.57 | |
| 188 | TBB1 | P04691 | Tubulin beta chain (T beta-15) | 50.55 | 56.95 | 57.40 | G, O |
| 189 | TERA | P46462 | Transitional endoplasmic reticulum ATPase (TER ATPase) | 4.76 | |||
| 190 | THIL | P17764 | Acetyl-CoA acetyltransferase, mitochondrial precursor | 3.94 | 8.33 | ||
| 191 | THIO | P11232 | Thioredoxin | 12.26 | 12.26 | 12.26 | |
| 192 | THY1 | P01830 | Thy-1 membrane glycoprotein precursor | 10.37 | 8.54 | 8.54 | G |
| 193 | TKT | P50137 | Transketolase (EC 2.2.1.1) (TK) | 3.00 | 3.00 | ||
| 194 | TMO2 | P70566 | Neuronal tropomodulin (N-Tmod) (Tropomodulin 2) | 10.08 | 10.08 | ||
| 195 | TPIS | P48500 | Triosephosphate isomerase (EC 5.3.1.1) (TIM) | 40.71 | 42.29 | 42.29 | |
| 196 | TPM2 | P58775 | Tropomyosin beta chain (Tropomyosin 2) | 3.46 | |||
| 197 | UBL1 | Q00981 | Ubiquitin carboxyl-terminal hydrolase isozyme L1 | 6.61 | 7.93 | 7.93 | |
| 198 | UCR2 | P32551 | Ubiquinol-cytochrome C reductase complex core protein | 17.17 | 16.09 | 13.70 | |
| 199 | UCRI | P20788 | Ubiquinol-cytochrome C reductase iron-sulfur subunit | 3.07 | 13.03 | ||
| 200 | VP3B | Q63616 | Vacuolar protein sorting 33B (r-vps33b) | 4.30 | |||
| 201 | VPP1 | P25286 | Vacuolar proton translocating ATPase 116 kDa subunit | 4.93 | 5.05 | 3.76 | G |
Absence of sequence coverage indicates identification was based on the observation of peptides in the chromatogram without MS/MS data A: acetylation; G, g: glycosylation and G indicates the presence of N-glycosylation motif; M: methylation; O: oxidation; P: phos-phorylation.
Regarding PTM of the 201 proteins identified by LC-MS/MS, 47 proteins were found to be glycosylated, five proteins were methylated, 11 proteins were acetylated, two were oxidized, and one was phosphorylated (Table 2). An additional 71 proteins that were not identified during the initial database search were found to be post-translationally modified in some way (glycosylated, methylated, acetylated, oxidized, or phosphorylated, Table 3). The majority of glycosylated proteins, which were determined by LC/MSMS analysis of lectin trapped proteins, possessed the N-glycan motif, suggesting several proteins involved in synaptic vesicle trafficking/docking underwent PTM (e.g., Synapsin I, Synaptotagmin, Synaptophysin, Syntaxin 1B, syntaxin binding protein 1 (Unc-18A), CaM, actin, protein kinase C, and casein kinase substrate (PACSIN1 or syndapin1)) as did several with synaptic membrane function (e.g., excitatory amino acid transporter, clathrin, Septin 7, Dynamin).
Table 3.
Synaptosomal protein PTM of synaptosomal proteins determined by LC-MS/MS
| No. | Swiss-Prot Entry | NCBI Accession | Protein | Acetylationa) | Glycosylationb) | Methylation Methylester (DE)/Methylester (C-term) | Oxidation (HW) | Phosphorylation (ST) |
|---|---|---|---|---|---|---|---|---|
| 1 | 143E HUMAN | P62258 | 14-3-3 protein epsilon | *MDDREDLVYQAK | ||||
| 2 | 143E HUMAN | P42655 | 14-3-3 protein epsilon (Mitochondrial import stimulation factor L subunit) | G | ||||
| 3 | 143T MOUSE | P35216 | 14-3-3 protein tau (14-3-3 protein theta) | G | ||||
| 4 | 143Z MOUSE | P35215 | 14-3-3 protein zeta/delta (Protein kinase C inhibitor protein-1) (KCIP-1) | *MDKNELVQK | G | |||
| 5 | A2B1 HUMAN | P21851 | Adapter-related protein complex 2 beta 1 subunit (Beta-adaptin) | G | ||||
| 6 | AATM RAT | P00507 | Aspartate aminotransferase, mitochondrial precursor (EC 2.6.1.1) (Transaminase A) | G | ||||
| 7 | ACTB HUMAN | P60709 | Actin, cytoplasmic 1 (Beta-actin) | YPIE*HGIVTNWDDMEK | HQGVM*VGMGQK | |||
| 8 | ACTG HUMAN | P02571 | Actin, cytoplasmic 2 (Gamma-actin) actin 1) | G | HQGVM*VGMGQK | |||
| 9 | ACTS HUMAN | P02568 | Actin, alpha skeletal muscle (Alpha- | G | ||||
| 10 | AKA1 RAT | P51635 | Alcohol dehydrogenase [NADP+] | *TASSVLLHTGQK | ||||
| 11 | ALBU RAT | P02770 | Serum albumin precursor [Contains: Neurotensin-related peptide (NRP)] | q | ||||
| 12 | BCKD RAT | Q00972 | 3-methyl-2-oxobutanoate dehydrogenase [lipoamide]] kinase, mitochondrial precursor | G | ||||
| 13 | CALB HUMAN | P06705 | Calcineurin B subunit isoform 1 (Protein phosphatase 2B regulatory subunit 1) | q | ||||
| 14 | CALM HUMAN | P02593 | Calmodulin | *ADQLTEEQIAEFKG | G | |||
| 15 | CCAH RAT | Q9EQ60 | Voltage-dependent T-type calcium channel alpha-1H subunit (Cav3.2) | G | ||||
| 16 | CH60 MOUSE | P19226 | 60 kDa heat shock protein, mitochondrial precursor (Hsp60) (60 kDa chaperonin) (CPN60) | G | ||||
| 17 | CYC MOUSE | P00009 | Cytochrome c, somatic | q | ||||
| 18 | DHPR RAT | P11348 | Dihydropteridine reductase | SM*PEADFSSWTPLEFLVETFHDWITGNK | ||||
| 19 | DJC5 MOUSE | P60904 | DnaJ homolog subfamily C member 5 SLS*TSGESLYHVLGLDK | |||||
| 20 | DYHC RAT | P38650 | Dynein heavy chain, cytosolic (DYHC) (Cytoplasmic dynein heavy chain) (MAP 1C) | G | ||||
| 21 | F262 RAT | Q9JJH5 | 6-phosphofructo-2-kinase/fructose- 2,6-biphosphatase 2 | G | ||||
| FRIH RAT | P19132 | Ferritin heavy chain (Ferritin H sub-unit) | G | |||||
| 23 | GIT1 RAT | Q9Z272 | ARF GTPase-activating protein GIT1 (G protein-coupled receptor kinase-interactor 1) | G | ||||
| 24 | GSN2 RAT | Q64232 | Synaptic glycoprotein SC2 | G | ||||
| 25 | GTM1 RAT | P04905 | Glutathione S-transferase Yb-1 (EC 2.5.1.18) (Chain 3) (GSTYb1) (GST M1-1) (GST class-mu) | q | ||||
| 26 | HBB1 or 2 RAT | P02091 | Hemoglobin beta chain, major-form | q | ||||
| HS7C MOUSE | P08109 | Heat shock cognate 71 kDa protein | G | |||||
| 28 | I13A HUMAN | P62206 | Mitochondrial import inner membrane translocase subunit TIM13 A | *MDSGFGSDFGGTGGGK | ||||
| 29 | IL18 RAT | P97636 | Interleukin-18 precursor (IL-18) (Interferon-gamma inducing factor) | G | ||||
| 30 | IP3S RAT | P29995 | Inositol 1,4,5-trisphosphate receptor type 2 | G | ||||
| 31 | K1CS RAT | Q63279 | Keratin, type I cytoskeletal 19 (Cytokeratin 19) (K19) (CK 19) (Fragment) | G | ||||
| 32 | KAP3 RAT | P12369 | cAMP-dependent protein kinase type II-beta regulatory chain | *SIEIPAGLTEL-LQGFTVEVLR | ||||
| 33 | KC1A RAT | P97633 | Casein kinase I, alpha isoform (EC 2.7.1.-) (CKI-alpha) (CK1) | G | ||||
| 34 | KC21 RAT | P19139 | Casein kinase II, alpha chain (CK II) (EC 2.7.1.37) | G | ||||
| 35 | KPY1 or 2 RAT | P11981 | Pyruvate kinase, M2 isozyme (EC 2.7.1.40) | G | ||||
| 36 | KPY1 RAT | P11981 | Pyruvate kinase, M2 isozyme (EC 2.7.1.40) | FGVE*QD*VDMVFASFIR | ||||
| MK01 MOUSE | P27703 | Mitogen-activated protein kinase 1 | *AAAAAAGPEMVR | |||||
| 38 | MRP2 RAT | Q63120 | Canalicular multispecific organic anion transporter 1; Multidrug resistance-associated protein 2 | IMNE*ILSGIKILK | ||||
| 39 | NAC1 RAT | Q01728 | Sodium/calcium exchanger 1 precursor (Na(+)/Ca(2+)-exchange protein 1) | G | ||||
| 40 | NAC2 RAT | P48768 | Sodium/calcium exchanger 2 precursor (Na(+)/Ca(2+)-exchange protein 2) | G | ||||
| 41 | P2BA MOUSE | P20652 | Serine/threonine protein phosphatase 2B catalytic subunit, alpha isoform (EC 3.1.3.16) | G | ||||
| 42 | PPAL RAT | P20611 | Lysosomal acid phosphatase precursor (EC 3.1.3.2) (LAP) | G | ||||
| 43 | PPIA RAT | P10111 | Peptidyl-prolyl cis-trans isomerase A (EC 5.2.1.8) (PPIase) (Rotamase) (Cyclophilin A) | q | ||||
| 44 | PRRA RAT | P09320 | Placental prolactin-like protein A precursor (PLP-A) | G | ||||
| 45 | PTB RAT | Q00438 | Polypyrimidine tract-binding protein 1 | ID*FSKLTSLNVK | ||||
| 46 | PXR RAT | Q9R1A7 | Orphan nuclear receptor PXR (Pregnane X receptor) | G | ||||
| 47 | RB3A MOUSE | P05713 | Ras-related protein Rab-3A | G | ||||
| 48 | RS3A RAT | P49242 | 40S ribosomal protein S3a | FK*LITEDVQGK | ||||
| 49 | RSG1 RAT | P50904 | Ras GTPase-activating protein 1 (GTPase-activating protein) (GAP) | WPTNNTM*R | ||||
| 50 | RSG1 RAT | P50904 | Ras GTPase-activating protein 1 (GTPase-activating protein) (GAP) | G | ||||
| RT26 RAT | Q9EPJ3 | 28S ribosomal protein S26, mitochondrial precursor (MRP-S26) (5′OT-EST protein) | G | |||||
| 52 | S10A RAT | P35467 | S-100 protein, alpha chain | *GSELETA-METLINVFHAHSGK | ||||
| 53 | S10B RAT | P04631 | S-100 protein, beta chain | *SELEKAMVA-LIDVFHQYSGRG | G | |||
| 54 | SFX3 RAT | Q9JHY2 | Sideroflexin 3 | G | ||||
| 55 | SLA1 RAT | P59622 | SRC-like-adapter (Src-like-adapter protein 1) | G | ||||
| 56 | ST1B MOUSE | P32853 | Syntaxin 1B (P35B) SAKDS*DDEEEVVHVDR | G | ||||
| 57 | STB1 HUMAN | Q64320 | Syntaxin binding protein 1 (Unc-18 homolog) (Unc-18A) (Unc-18-1) (N-Sec1) (rbSec1) (p67) | G | ||||
| 58 | STX7 RAT | O70257 | Syntaxin 7 | *SYTPGIGGDPAQLAQR | ||||
| 59 | TBA1 MOUSE | P02551 | Tubulin alpha-1 chain | G | AVFVDLEPTVIDE*VR | |||
| 60 | TBB5 HUMAN | P05218 | Tubulin beta-5 chain | G | GFWE-VISDEHGIDPTG-TYHGDSDLQLD*R | EVDEQM*LNVQNK | ||
| 61 | TPIS RAT | P48500 | Triosephosphate isomerase (EC 5.3.1.1) (TIM) | G | ||||
| 62 | TRY2 RAT | P00763 | Trypsin II, anionic precursor (EC 3.4.21.4) (Pretrypsinogen II) | q | ||||
| 63 | TRY3 RAT | P08426 | Trypsin III, cationic precursor (EC 3.4.21.4) (Pretrypsinogen III) | q | ||||
| 64 | TSHR RAT | P21463 | Thyrotropin receptor precursor (TSH-R) (Thyroid stimulating hormone receptor) | G | ||||
| 65 | TYB0_ HUMAN | P13472 | Thymosin beta-10 | *ADKPDMGEIASFDK | ||||
| 66 | TYB4 HUMAN | P62328 | Thymosin beta-4 | *SDKPDMAEIEK | ||||
| 67 | UBIQ HUMAN | P02248 | Ubiquitin | g | ||||
| 68 | UCP2 RAT | P56500 | Mitochondrial uncoupling protein 2 | SLY*NGLVAGLQR | ||||
| 69 | UCR2 RAT | P32551 | Ubiquinol-cytochrome C reductase complex core protein 2, mitochondrial precursor | G | ||||
| 70 | VAB2 MOUSE | P50517 | Vacuolar ATP synthase subunit B, brain isoform (EC 3.6.3.14) (V-ATPase B2 subunit) | G | ||||
| 71 | VAM2 MOUSE | Q64357 | Vesicle-associated membrane protein 2 (VAMP-2) (Synaptobrevin 2) | G |
Only N-terminal acetylat ion was observed
G indicates the presence of N-glycosylation motif, while g indicates the absence of such a motif
modified amino acid
When the two sets of identified proteins are compared, as expected, there is some overlap in the proteins identified by either 2-DE/MS or LC-MS/MS. Of the 91 unique proteins identified by the former and the 201 unique proteins identified by the latter, 46 were found in both sets. Accounting for this intersection, the total number of unique proteins identified by the combined methods was 246. Of these 246 proteins, 61 were identified by PubMed literature search as having synapse-specific function (Fig. 2a and b). Nineteen identified proteins are involved in synaptic vesicle trafficking or docking, nine serve receptor or transporter functions, nine are involved in intra-cellular signaling cascades that affect synaptic transmission, and 24 have other synapse-specific functions.
Figure 2.
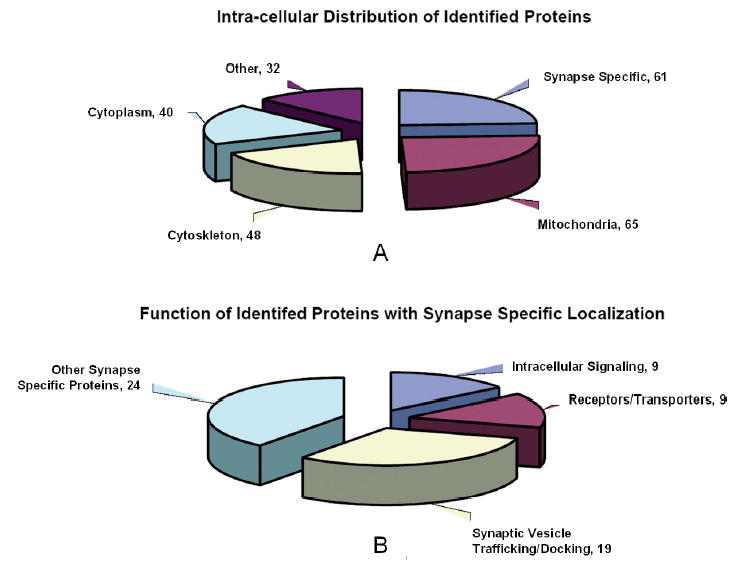
A: Intracellular distribution of all 254 unique synaptosomal proteins identified by either 2-DE/MS or LC-MS/MS. The synapse-specific fraction was determined using a manual search of PubMed™. Automated categorization of the remaining 193 proteins was performed using the GO via the webtool Pandora. Of these 193 proteins, 24 were successfully categorized. Estimated fractions were then extrapolated from the distribution of proteins in this subset. B: Distribution by synaptic function of those 61 proteins identified by manual PubMed search as having a synapse-specific function.
The remaining 185 proteins were categorized in a semiautomated manner. Swiss-Prot accession numbers of these proteins were uploaded to Pandora and were categorized by sub-cellular compartment according to the GO. Twenty-four proteins were categorized in this fashion. Assuming that this subset of 24 proteins is representative of the larger set of 185, extrapolation leads to an estimate that 65 of the 185 non-synapse-specific proteins are mitochondrial, 48 are cytoskeletal, and 40 are cytoplasmic.
4 Discussion
In the present study, multiple protein separation and identification approaches were used in conjunction to analyze the synaptosomes isolated from rat cerebral cortex, providing both confirmatory and complementary proteomic information. The identified proteins confirm that the primary objective of the study was accomplished – perhaps the single most important functional portion of the CNS, the synapse, has been isolated for proteomic analysis, providing for significant enrichment of synaptic proteins when compared to prior techniques.
Application of 2-DE to rat cerebral cortex synaptosome fractions resulted in the separation and detection of >900 protein spots, among which 163 of those with the highest abundance were identified by either MALDI-TOF or LC/MS-MS. These 163 spots represent various forms of 91 distinct proteins. Among these, a number of synaptic vesicle proteins were detected including vesicle-associated membrane protein (VAMP, synaptobrevin, no. 147 in Table 1, also listed as VAMP-3). VAMP is a synaptic vesicle docking protein (v-SNARE) that plays a fundamental role in synaptic vesicle exocytotic fusion, initiated by the binding of v-SNARES and t-SNARES. Another integral vesicle membrane protein, synaptotagmin, was detected as spot 25 (synaptotagmin I). Synaptotagmin serves as a calcium sensor for exocytosis, yet may also be considered a v-SNARE due to its interaction with t-SNARE syntaxin.
Synapsin II, a vesicle-associated protein was shown as spots 26, 29, and 32. Synapsins are anchor proteins, which tether the synaptic vesicles to the actin filaments of the nerve terminal in a Ca2+/phosphorylation-dependent manner, regulating the distribution of the vesicles between the reserved pool and the active zone for exocytotic release [26]. Vacuolar ATP synthase (V-ATPase) F subunit shown as spot 155, is a part of vacuolar proton pump present on all acidic cellular organelles, such as clathrin-coated vesicles, endosomes, lysosomes, and Golgi membranes. The acidification of the synaptic vesicle’s lumen is critical to the packaging and processing of the contents of synaptic vesicles [27]. Since synaptosomes are pinched-off nerve endings, containing both pre and post-synaptic structures, it was not surprising to detect proteins associated with the post-synaptic membranes. For example, spots 93, 94, 95, and 111 were identified as the β-subunit of G protein (GBB1 or GBB2), a membrane-associated protein that mediates the effects of numerous G protein-coupled receptors (GPCRs). In the brain, neurotransmitter receptors can be classified as two distinct super-families: ligand-gated channels (LGCs) and GPCRs. The receptors belonging to the GPCR family include muscarinic ACh receptors, DA receptors, adrenergic receptors, most 5-HT receptors, metabotropic glutamate receptors, GABAB receptors, histamine receptors, cannabinoid receptors, and neuropeptide receptors. While most of these receptors can be either a post-synaptic component or a pre-synaptic autoreceptor depending on the receptor sub-type, some of them, such as GABAB, can be found both pre- and post-synaptically [28, 29]. GPCRs can also be pre-synaptic, as has been reported in the regulation of voltage-dependent Ca++ channels during neurotransmitter release [30].
2-DE also successfully displayed numerous non-membrane bound and cytosolic proteins, some of which play an important role in synaptic and neuronal function. For instance, protein kinase C and casein kinase substrate in neurons (PACSIN1, spots 40 and 41), also named Syndapin I, is a cytoplasmic protein. Its interaction with dynamin (a GTPase implicated in clathrin-mediated endocytosis of synaptic vesicle membranes) and neural Wiskott-Aldrich syndrome protein (an actin-depolymerizing protein), suggests its role in cytoskeletal dynamics and synaptic vesicle formation, and transport and recycling at the pre-synaptic nerve terminal [31]. Glutamine synthetase (GS, GLNA, no. 60) is a key enzyme in the brain’s glutamate-glutamine cycle. It also plays an important role in protecting neurons against excitotoxicity by converting excess ammonia and glutamate into glutamine [32]. Though commonly found in astrocytes, the detection of GS in cortical synaptosomes should not be surprising. This is because of the extreme anatomical and communicational proximity of astrocytes and neuron, and the enrichment of glutamatergic neurons in the cortex.
Neuron-specific enolase (NSE) is an enzyme of the glycolytic pathway, which is found in numerous isomeric forms. Alpha (ENOA) and gamma enolases (ENOG), enzymes of the glycolytic pathway, are present specifically in neuronal cell cytoplasm and dendrites [33] and constitute the so-called NSE. ENOG has been shown to be located in cells of neuroectodermal origin and constitutes approximately 1.5% of the total soluble protein in the brain. Both ENOA and ENOG are also found in the synaptic membrane as homo- and hetero-dimers [34]. On the 2-D map, spots 51–53 were identified as ENOG and spots 47–50 as ENOA. Beyond being a neuronal marker, NSE can be released from distressed neurons into the cerebrospinal fluid and peripheral blood, serving as a biomarker of parenchymal brain injury [35]. Neuronal protein NP25 (no. 133) is also a neuron-specific protein present in highly differentiated neural cells [36].
In addition to the proteins that serve specific neuronal or synaptic structure and function, some of the proteins resolved by 2-DE are present universally in various cell types, but still play a crucial role in neurotransmission. For instance, actin (ACTB, nos. 54 and 55) is a cytoplasmic cytoskeleton protein that can be found in all cell types. At the synapse, actin filaments harbor some of the synaptic vesicles, forming a reserve pool. As mentioned above, synapsins serve as anchors for the vesicles. CaM (nos. 136, 139, 142, and 143) is a universal acidic calcium-binding protein, in virtually all eukaryotic cells, which regulates the activity of target molecules such as protein kinases, adenylyl cyclase, and nitric oxide (NO) synthase. Binding of CaM to various cytoskeletal proteins, such as the tubulins (nos. 11–15, nos. 18–24), microtubule-associated protein-2 (MAP-2), tau, and fodrin, appears to affect the cell shape, motility, secretion, and transport [37]. The activity of CaM is regulated by a variety of covalent modifications, such as methylation, phosphorylation, ubiquitinylation [38, 39] and glycoslylation (see Table 2), and these likely account for its heterogeneous appearance on the 2-D gel pattern. While methylation and phosphorylation only cause slight mass alterations, ubiquitylation can increase the mass of CaM by 50% or more [40]. In Fig. 1, CaM appears as a group of unique spots with similar pI, but different molecular weights. Whether the heterogeneities in CaM migration (mass and charge) observed here are the result of the above modifications or proteolysis, as suggested by the ID of spot 143 as a CaM fragment, remains to be determined.
Intact synaptosomal preparations are expected to contain mitochondria that reside near the synapse, and several mitochondrial proteins are found in Table 1. For example, ATP synthase is a mitochondrial protein that catalyzes ATP production in the presence of proton gradient [41]. Several subunits of the ATP synthase complex were resolved on the 2-D map, including the α-chain (ATPA, nos. 33–39), β-chain (ATPB, nos. 43–46), D-chain (ATPQ, no. 132), and E-chain (ATPJ, no. 160). The presence of these ATP synthase components is essential to synaptic function because they are involved in the synthesis of ATP. The packaging of neuro-transmitters into the synaptic vesicles through vesicle transporters [42] and the transportation of Ca++ from the cytoplasm into the ER or extracellular fluid via the Ca++ pump are fueled by the hydrolysis of ATP [43]. Another mitochondrial protein detected by 2-DE and shotgun proteomics is VDAC. VDAC1 (POR1) was resolved as a complex charge train (~pI 8.4) (nos. 101, 103–108), suggesting possible PTMs or heterogeneous isoforms. VDAC2 (POR2) also appears on the 2-D map (nos. 99 and 102) with both VDACs resolved at or near their predicted pI. VDACs are outer mitochondrial membrane proteins with weak anion selectivity in the open state, producing anion fluxes, including ATP, which regulate mitochondrial function. Several reports have confirmed their multi-topological localization, particularly in post-synaptic membrane structures [44, 45]. Interestingly, it has been shown that certain isoforms of these channel proteins can be up- or down-regulated in a certain cortical area in pathological conditions.
In comparison to 2-DE which identified two t-SNARE proteins, VAMP-3, and the Ca++ sensor synaptotagmin I, LC-MS/MS identified VAMP-2 in its glycosylated form (Table 3) and three isoforms of synaptotagmin (I, II, V, Table 2). The detection of two of the three forms of the VAMPs is supported by their tissue-specific expression, because VAMP-1 is more abundant in the spinal cord, while VAMP-2 is highly expressed in the brain, and VAMP-3 has ubiquitous tissue distribution [46, 47]. Although all three forms of synaptotagmin are abundantly expressed in the brain, synaptotagmin I is preferentially expressed in rostral, phylogenetically younger brain regions; synaptotagmin II is predominant in caudal, phylogenetically older brain regions, and synaptotagmin V has a wider peripheral tissue distribution [48, 49]. In addition to synapsin II, also identified by 2-DE, LC-MS/MS detected synapsin I. Three forms of free syntaxins, 1A, 1B, and seven were also identified, as was a syntaxin binding protein 1 (n-Sec1/Unc-18–1). As either cytoplasmic or membrane-associated, n-Sec1/Unc-18–1 binds to syntaxin, thereby regulating synaptic transmission.
Two subunits of clathrin were identified by LC-MS/MS, light chain B and heavy chain. The latter was also found to be modified by acetylation and glycosylation (Table 2). Clathrin is the major protein of polyhedral coat of coated pits and vesicles, playing an important role in the endocytotic retrieval and transport (recycling process) of vesicle membrane components from the pre-synaptic membrane [50]. Two additional proteins related to clathrin that were identified include clathrin coat assembly protein (AP180) and subunits of clathrin-associated adaptor protein complexes.
While most proteomic platforms have an inherent and variable bias in identifying certain types of proteins (e.g., hydrophilic, ionizable peptides, etc.), the combination of several proteomic techniques in the present study has offered complementary approaches. Figure 3 summarizes the major pre-synaptic proteins identified by 2-DE/MS and/or shotgun proteomics. Interestingly, most of the neuro-transmission regulating proteins were identified either by one or both the technique(s). The application of the PTM-detection option in the sequence database search greatly increased the likelihood of protein identification and suggests that PTM is common in synaptosomal proteins. Several proteins, such as neurexins, vesicular neurotransmitter reuptake transporters, and some components of SNAPs were left unidentified, as were neurotransmitter receptor proteins. Their absence is likely due to their unique biochemical properties (hydrophobicity, low abundance, etc.), which are unfavorable for identification in this proteomic approach.
Figure 3.
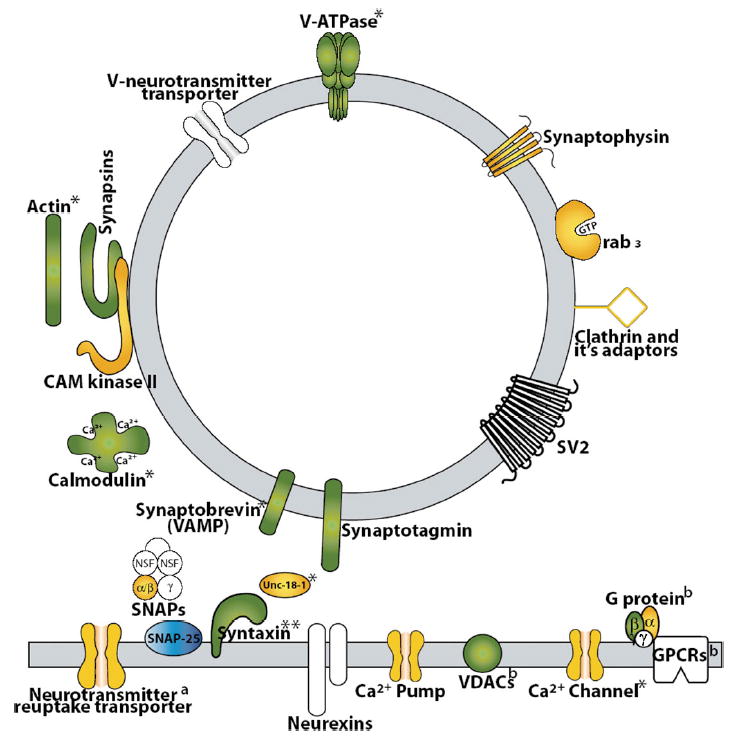
Diagrammatic illustration of the major pre- and post-synaptic proteins identified by 2-DE/MS and/or shotgun proteomics, and normally expected as constituent in synaptosomal preparations. Blue: The proteins that were identified by 2-DE/MS; yellow, the proteins that were identified by shotgun proteomics; green, the proteins that were identified by both 2-DE/MS and shotgun proteomics; and blank (white), those major constituents expected but not identified. *Proteins were identified by shotgun proteomics only after the PTM analysis; **Proteins were identified as a complex with other proteins by 2-DE/MS; (a) EAA1, EAA2, and GABA transporter. b: Post-synaptic proteins.
Several adjacent spots on the 2-D map were assigned identical protein IDs, but by different MS analysis. In such cases, the LC-MS/MS results provide confirmatory evidence for the accuracy of the PMF ID. More importantly these “charge trains” on a 2-D map typically represent a single protein resolved at varying pI, due to PTM. For example, spot nos. 20–24 were all identified as tubulin β-chain (TBB1), with spot 20 identified by LC-MS and the rest by PMF. It has been shown that brain tubulins exhibit a significant charge heterogeneity, with up to 21 charge variants (for both α- and β-subunits) observed in different studies [51, 52]. Phospho-rylation [53] and polyglycosylation [54] have been reported for tubulin β. As indicated in Tables 2 and 3, tubulin β was detected by LC-MS/MS with methylation, M and H oxidation, and glycosylation of various peptides. It has been shown that reductive methylation of the tubulin dimer with formaldehyde and sodium cyanoborohydride greatly inhibits the microtubule assembly [55], with the β-subunit being more susceptible to methylation than the α-subunit [56], although we observed methylation in both. The ability to observe and determine modifications in this way will be of great importance in future studies using this synaptosomal preparation in assessing the effects of alcohol ingestion, neurotoxins, etc.
Other modified proteins identified by LC-MS/MS include glyceraldehyde-3-phosphate dehydrogenase (GAPDH, G3P, nos. 82, 84, 86–90, 92, and 97), creatine kinase B chain (KCRB, nos. 56 and 57), triosephosphate isomerase (nos. 117, 120, 122–124), ubiquitin carboxy-terminal hydrolase isozyme L1 (nos. 112 and 113), ATP synthase β-subunit (ATPB, nos. 43–46), actin (nos. 54 and 55), and protein kinase C and casein kinase substrate in neurons protein 1 (PAC1, nos. 40 and 41). Though the chemical nature and the physiological relevance of these PTMs is beyond the scope of this manuscript, these results demonstrate the unique power of 2-DE in resolving the differentially modified protein charge forms and quantifying the extent of modification [57] established by mass spectrometric techniques.
Overall, the results of the current study indicate that a sample preparation incorporating pre-fractionation and enrichment of specific cell components can improve the capability of proteomics techniques to detect important synaptic proteins from brain tissues. In the present study, the proteome profile of cerebral cortical synaptosomes indicates that major protein components involved in synaptic vesicle trafficking and docking, post-synaptic densities, transporters and receptors, mitochondrial function and the glycolytic pathway can be detected and their relative expression studied. Because these are the proteins normally present in intact nerve endings, an approach that uses sub-cellular fractionation to produce synaptosomes may prove to be useful in proteomic studies of brain function where neural, not glial proteins, are of interest.
Acknowledgments
The authors wish to acknowledge the following individuals whose assistance was instrumental in translating proteomic accession numbers to genomic accession numbers, allowing us to more readily access Gene Oncology classifications: Tzulip Phang, Daniel J. McGoldrick, and Susan C. Trapp of the Center for Computational Pharmacology, Department of Pharmacology, University of Colorado Health Sciences Center, Aurora, CO, USA. This work was supported in part by AA07611 (WJM), grants from the National Institute of Alcohol Abuse and Alcoholism, NIH (AA U01 5–72387-INIA Project) (FAW), and grant no. (GM24349) from the National Institute of General Medical Sciences, U.S. Department of Health and Human Services, and the Indiana Genomics Initiative (INGEN) (MVN).
References
- 1.Fountoulakis M, Schuller E, Hardmeier R, Berndt P, et al. Electrophoresis. 1999;20:3572–3579. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3572::AID-ELPS3572>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 2.Gauss C, Kalkum M, Lowe M, Lehrach H, et al. Electrophoresis. 1999;20:575–600. doi: 10.1002/(SICI)1522-2683(19990301)20:3<575::AID-ELPS575>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 3.Beranova-Giorgianni S, Pabst MJ, Russell TM, Giorgianni F, et al. Brain Res Mol Brain Res. 2002;98:135–140. doi: 10.1016/s0169-328x(01)00333-3. [DOI] [PubMed] [Google Scholar]
- 4.Langen H, Berndt P, Roder D, Cairns N, et al. Electrophoresis. 1999;20:907–916. doi: 10.1002/(SICI)1522-2683(19990101)20:4/5<907::AID-ELPS907>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 5.Edgar PF, Douglas JE, Knight C, Cooper GJ, et al. Hippocampus. 1999;9:644–650. doi: 10.1002/(SICI)1098-1063(1999)9:6<644::AID-HIPO5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 6.Witzmann FA, Li J, Strother WN, McBride WJ, et al. Proteomics. 2003;3:1335–1344. doi: 10.1002/pmic.200300453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams RW, Herrup K. Annu Rev Neurosci. 1988;11:423–453. doi: 10.1146/annurev.ne.11.030188.002231. [DOI] [PubMed] [Google Scholar]
- 8.Hansson E, Ronnback L. FASEB J. 2003;17:341–348. doi: 10.1096/fj.02-0429rev. [DOI] [PubMed] [Google Scholar]
- 9.Stadler H, Tashiro T. Eur J Biochem. 1979;101:171–178. doi: 10.1111/j.1432-1033.1979.tb04229.x. [DOI] [PubMed] [Google Scholar]
- 10.Sherman AD. Neurochem Res. 1989;14:97–101. doi: 10.1007/BF00969765. [DOI] [PubMed] [Google Scholar]
- 11.Krapfenbauer K, Fountoulakis M, Lubec G. Electrophoresis. 2003;24:1847–1870. doi: 10.1002/elps.200305401. [DOI] [PubMed] [Google Scholar]
- 12.Walikonis RS, Jensen ON, Mann M, Provance DW, Jr, et al. J Neurosci. 2000;20:4069–4080. doi: 10.1523/JNEUROSCI.20-11-04069.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friso G, Wikstrom L. Electrophoresis. 1999;20:917–927. doi: 10.1002/(SICI)1522-2683(19990101)20:4/5<917::AID-ELPS917>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 14.Jimenez CR, Eyman M, Lavina ZS, Gioio A, et al. J Neurochem. 2002;81:735–744. doi: 10.1046/j.1471-4159.2002.00873.x. [DOI] [PubMed] [Google Scholar]
- 15.Stevens SM, Jr, Zharikova AD, Prokai L. Brain Res Mol Brain Res. 2003;117:116–128. doi: 10.1016/s0169-328x(03)00282-1. [DOI] [PubMed] [Google Scholar]
- 16.Ong SE, Pandey A. Biomol Eng. 2001;18:195–205. doi: 10.1016/s1389-0344(01)00095-8. [DOI] [PubMed] [Google Scholar]
- 17.Barnouin K. Methods Mol Biol. 2004;261:479–498. doi: 10.1385/1-59259-762-9:479. [DOI] [PubMed] [Google Scholar]
- 18.Freeman WM, Hemby SE. Neurochem Res. 2004;29:1065–1081. doi: 10.1023/b:nere.0000023594.21352.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin PT. Glycobiology. 2002;12:1R–7R. doi: 10.1093/glycob/12.1.1r. [DOI] [PubMed] [Google Scholar]
- 20.Simon JR, Martin DL. Arch Biochem Biophys. 1973;157:348–355. doi: 10.1016/0003-9861(73)90649-8. [DOI] [PubMed] [Google Scholar]
- 21.Gray EG, Whittaker VP. J Anat. 1962;96:79–88. [PMC free article] [PubMed] [Google Scholar]
- 22.Neuhoff V, Arold N, Taube D, Ehrhardt W. Electrophoresis. 1988;9:255–262. doi: 10.1002/elps.1150090603. [DOI] [PubMed] [Google Scholar]
- 23.Hale JE, Butler JP, Gelfanova V, You JS, et al. Anal Biochem. 2004;333:174–181. doi: 10.1016/j.ab.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan N, Vaaknin A, Linial M. Nucleic Acids Res. 2003;31:5617–5626. doi: 10.1093/nar/gkg769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris MA, Clark J, Ireland A, Lomax J, et al. Nucleic Acids Res. 2004;32(database issue):D258–D261. doi: 10.1093/nar/gkh036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferreira A, Rapoport M. Cell Mol Life Sci. 2002;59:589–595. doi: 10.1007/s00018-002-8451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng SB, Crider BP, Tsai SJ, Xie XS, et al. J Biol Chem. 1996;271:3324–3327. doi: 10.1074/jbc.271.6.3324. [DOI] [PubMed] [Google Scholar]
- 28.Chu DC, Albin RL, Young AB, Penney JB. Neuroscience. 1990;34:341–357. doi: 10.1016/0306-4522(90)90144-s. [DOI] [PubMed] [Google Scholar]
- 29.Gehlert DR, Yamamura HI, Wamsley JK. Neurosci Lett. 1985;56:183–188. doi: 10.1016/0304-3940(85)90126-0. [DOI] [PubMed] [Google Scholar]
- 30.Hille B. Trends Neurosci. 1994;17:531–536. doi: 10.1016/0166-2236(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 31.Qualmann B, Roos J, DiGregorio PJ, Kelly RB. Mol Biol Cell. 1999;10:501–513. doi: 10.1091/mbc.10.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suarez I, Bodega G, Fernandez B. Neurochem Int. 2002;41:123–142. doi: 10.1016/s0197-0186(02)00033-5. [DOI] [PubMed] [Google Scholar]
- 33.Forss-Petter S, Danielson P, Sutcliffe JG. J Neurosci Res. 1986;16:141–156. doi: 10.1002/jnr.490160114. [DOI] [PubMed] [Google Scholar]
- 34.Ueta H, Nagasawa H, Oyabu-Manabe Y, Toida K, et al. Neurosci Res. 2004;48:379–386. doi: 10.1016/j.neures.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Kulig E. Postepy Biochem. 1991;37:112–120. [PubMed] [Google Scholar]
- 36.Ren WZ, Ng GY, Wang RX, Wu PH, et al. Brain Res Mol Brain Res. 1994;22:173–185. doi: 10.1016/0169-328x(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 37.Gnegy ME. Annu Rev Pharmacol Toxicol. 1993;33:45–70. doi: 10.1146/annurev.pa.33.040193.000401. [DOI] [PubMed] [Google Scholar]
- 38.Ishida A, Kameshita I, Okuno S, Kitani T, et al. Arch Biochem Biophys. 2002;407:72–82. doi: 10.1016/s0003-9861(02)00514-3. [DOI] [PubMed] [Google Scholar]
- 39.Laub M, Steppuhn JA, Bluggel M, Immler D, et al. Eur J Biochem. 1998;255:422–431. doi: 10.1046/j.1432-1327.1998.2550422.x. [DOI] [PubMed] [Google Scholar]
- 40.Jennissen HP. J Mol Recognit. 1995;8:116–124. doi: 10.1002/jmr.300080121. [DOI] [PubMed] [Google Scholar]
- 41.Boulet D, Poirier J, Cote C. Biochem Biophys Res Commun. 1989;159:1184–1190. doi: 10.1016/0006-291x(89)92235-3. [DOI] [PubMed] [Google Scholar]
- 42.Nicholls DG. Neurochem Res. 2003;28:1433–1441. doi: 10.1023/a:1025653805029. [DOI] [PubMed] [Google Scholar]
- 43.Chaudhary J, Walia M, Matharu J, Escher E, et al. Am J Physiol Cell Physiol. 2001;280:C1027–C1030. doi: 10.1152/ajpcell.2001.280.4.C1027. [DOI] [PubMed] [Google Scholar]
- 44.Shafir I, Feng W, Shoshan-Barmataz V. J Bioenerg Biomembr. 1998;30:499–510. doi: 10.1023/a:1020598315287. [DOI] [PubMed] [Google Scholar]
- 45.Buettner R, Papoutsoglou G, Scemes E, Spray DC, et al. Proc Natl Acad Sci USA. 2000;97:3201–3206. doi: 10.1073/pnas.060242297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elferink LA, Trimble WS, Scheller RH. J Biol Chem. 1989;264:11061–11064. [PubMed] [Google Scholar]
- 47.McMahon HT, Ushkaryov YA, Edelmann L, Link E, et al. Nature. 1993;364:346–349. doi: 10.1038/364346a0. [DOI] [PubMed] [Google Scholar]
- 48.Geppert M, Archer BT, III, Sudhof TC. J Biol Chem. 1991;266:13548–13552. [PubMed] [Google Scholar]
- 49.Hudson AW, Birnbaum MJ. Proc Natl Acad Sci USA. 1995;92:5895–5899. doi: 10.1073/pnas.92.13.5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heuser JE, Reese TS. J Cell Biol. 1973;57:315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee JC, Field DJ, George HJ, Head J. Ann NY Acad Sci. 1986;466:111–128. doi: 10.1111/j.1749-6632.1986.tb38388.x. [DOI] [PubMed] [Google Scholar]
- 52.Wolff A, Denoulet P, Jeantet C. Neurosci Lett. 1982;31:323–328. doi: 10.1016/0304-3940(82)90041-6. [DOI] [PubMed] [Google Scholar]
- 53.Alexander JE, Hunt DF, Lee MK, Shabanowitz J, et al. Proc Natl Acad Sci USA. 1991;88:4685–4689. doi: 10.1073/pnas.88.11.4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xia L, Hai B, Gao Y, Burnette D, et al. J Cell Biol. 2000;149:1097–1106. doi: 10.1083/jcb.149.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Szasz J, Burns R, Sternlicht H. J Biol Chem. 1982;257:3697–3704. [PubMed] [Google Scholar]
- 56.Sherman G, Rosenberry TL, Sternlicht H. J Biol Chem. 1983;258:2148–2156. [PubMed] [Google Scholar]
- 57.Witzmann FA, Fultz CD, Mangipudy RS, Mehendale HM. Fundam Appl Toxicol. 1996;31:124–132. doi: 10.1006/faat.1996.0083. [DOI] [PubMed] [Google Scholar]


