Abstract
Two experiments investigated the derived transfer of functions through equivalence relations established using a stimulus pairing observation procedure. In Experiment 1, participants were trained on a simple discrimination (A1+/A2−) and then a stimulus pairing observation procedure was used to establish 4 stimulus pairings (A1–B1, A2–B2, B1–C1, B2–C2). Subsequently, a transfer of the simple discrimination functions through equivalence relations was observed (e.g., C1+/C2−). These procedures were modified in Experiment 2, which demonstrated that spider-fearful and non-spider-fearful participants show differing levels of a transfer of self-reported arousal functions for stimuli used in equivalence relations with video-based material depicting scenes with spiders. The results demonstrate that the stimulus pairing observation procedure provides a viable alternative to matching-to-sample, and also offer tentative support for a derived-relations model of the acquisition of anxiety responses in at least one sub-clinical population.
Keywords: simple discrimination, stimulus pairing observation procedure, derived transfer of functions, spider fearful, humans
A number of behavior-analytic researchers have proposed that derived stimulus relations, and the transfer of function through such relations, is one of the main components missing from a behavioral account of human language (e.g., S. Hayes, Barnes-Holmes, & Roche, 2001; Sidman, 1994). Although the specific theoretical interpretations differ across researchers (see Leslie & Blackman, 2000), overall, many do agree that the derived transfer of stimulus functions1 may provide a basic behavior-analytic model of symbolic control (e.g., Barnes & Holmes, 1991). Imagine, for example, that a young girl has learned to stop what she is doing whenever a caregiver has asked her to “stop”. If the novel words, “cease” and “desist” then come to participate in an equivalence relation with “stop” (e.g., she learns these synonyms at school), then she may respond appropriately to “cease” or “desist” without being trained explicitly to do so. In effect, the derived transfer of functions through equivalence relations may help to explain how words and other symbols acquire their psychological functions or meaning (Barnes, Browne, Smeets & Roche, 1995; Wulfert & Hayes, 1988).
The training procedures employed in the study of equivalence relations and derived transfer typically have involved some type of operant training before testing for equivalence responding. Generally, a matching-to-sample (MTS) procedure is used in which the participant is given positive feedback for choosing one comparison from one or more comparisons when presented with a specific sample stimulus, and is given negative feedback for choosing any of the other comparisons. MTS has long been considered one of the most robust and reliable research methods (Mackay, 1991). Recently, however, researchers have drawn on alternative procedures in the study of derived stimulus relations (Layng & Chase, 2001). Leader, Barnes, and Smeets (1996), for example, employed a stimulus pairing observation procedure (SPOP), which they called respondent-type training, to establish emergent equivalence relations. The SPOP involved the presentation of nine nonsense syllables in the form of six stimulus pairs (A1–B1, A2–B2, A3–B3, B1–C1, B2–C2, and B3–C3). The first stimulus of each pair appeared on a computer screen for 1 s, the screen then cleared for an intra-pair delay of 0.5 s before the second stimulus of the pair appeared for 1 s. There was a 3 s between-pair delay before the next stimulus pair was presented in the same manner. All six stimulus pairs were presented 10 times each in a quasi-random order over 60 trials. In contrast with the traditional MTS training procedure, explicit differential reinforcement was not provided. Participants were required only to observe the computer screen and not to demonstrate any overt response. Upon testing for symmetry and equivalence relations, 84% of the participants passed the standard MTS equivalence test.
As previously noted, the derived transfer-of-functions effect pervades the stimulus equivalence literature. As pointed out by Layng and Chase (2001), the MTS procedure is only one way in which stimuli are presented for learning that occurs in the real world (e.g., advertising often involves simply pairing a product with a positively valenced stimulus). Thus, if the derived transfer of functions is to provide a valid model of an important feature of human learning, then this effect also should occur when stimulus relations are created with procedures other than MTS. Leader et al. (1996) demonstrated that a SPOP can be used to establish equivalence relations without explicit reinforcement, and thus the SPOP provides another method for establishing stimulus relations. At this point in the study of the derived transfer of functions, therefore, it seems important to determine if the effect also will be observed when the SPOP is employed as a means of establishing the necessary equivalence relations. Thus, the primary purpose of Experiment 1 of the current study was to demonstrate the transfer of simple discriminative functions through equivalence relations established using a SPOP similar to that employed by Leader et al. (1996).
In the study of derived transfer effects, researchers have examined the transfer of emotional psychological functions. For example, Dougher, Auguston, Markham, Greenway, and Wulfert (1994) reported the derived transfer of aversive respondent elicitation functions through equivalence classes. The findings reported by Dougher et al. suggested a process by which individuals can come to fear stimuli to which they have never been exposed, or that have never been directly associated with an aversive event, and therefore may provide the basis for certain anxiety responses in clinical and sub-clinical populations (Dougher, et al., 1994, p. 349). Other researchers also have explored the extent to which the derived transfer-of-functions effect might shed light on the psychological processes involved in evaluating events without direct experience of those events (Barnes-Holmes, Keane, Barnes-Holmes & Smeets, 2000; Grey & Barnes, 1996).
These and other studies have demonstrated the ubiquity and reliability of the derived transfer effect across a wide range of psychological functions. However, to date no transfer research has attempted to determine if a transfer effect is differentially influenced by the psychological characteristics that participants bring with them to the laboratory. In fact, Dougher et al. (1994) explicitly acknowledged that research with clinical populations was necessary in this regard:
… the strength of and stability of the emotional reactions conditioned in the present study were not comparable with those in clinical populations, and the procedures by which participants' responses were acquired may not be directly analogous to those that lead to clinical disorders. Accordingly, generalizing from the present results is premature. More research is needed to determine the extent to which these processes play a role in the development of human emotional responding in general and clinical disorders in particular (Dougher et al., 1994, p. 349).
If derived transfer provides the basis for a valid model of at least some forms of anxiety disorders, as suggested by Dougher et al. (1994), then the transfer of anxiety-related functions should be predicted, in part, by the clinical or subclinical characteristics of the experimental participants. The current study attempted to address this issue in the context of a common fear that is found in the wider population, fear of spiders (see Marks, 1969; Mohlman & Zinbarg, 2000). More specifically, the primary purpose of Experiment 2 was to determine if individuals categorized as spider fearful and non-spider-fearful would show differing levels of a derived transfer of self-reported arousal functions for stimuli used in equivalence relations with visual material depicting scenes with spiders. Comparing the transfer performances of these two groups could provide support for a model of the acquisition of fear towards an object that was never paired with an aversive event. Experiment 2, therefore, aimed to establish the derived transfer of self-reported arousal with spider-fearful and non-spider-fearful individuals, screened initially using an established psychometric instrument and then finally selected using a behavioral approach task (BAT; see Mohlman & Zinbarg, 2000). Finally, the SPOP developed in Experiment 1 was employed in Experiment 2, thus providing an additional test of its ability to produce derived transfer effects.
Before continuing, it should be noted that a recent study reported a transfer of functions using a SPOP (Tonneau & Gonzalez, 2004). In each of the four experiments reported in this study, the second stimulus of each pair appeared on screen with the first, and thus the trained relations were not inherently unidirectional (i.e., participants could scan back and forth between the two stimuli while they were both on screen). It follows, therefore, that the observed transfer effects could occur through forward Pavlovian conditioning rather than via a bidirectional derived stimulus relation. In line with this reasoning, Tonneau and Gonzalez appealed to Pavlovian processes to explain the observed transfer. In contrast, the SPOP employed in the current study only presents one stimulus at a time, rendering the trained relations unidirectional, and thus the transfer effects are predicted based on derived stimulus relations rather than forward Pavlovian conditioning per se. The current study involved establishing a specific function for an A stimulus, and then presenting A–B and B–C stimulus pairs. A transfer of functions from the A to the C stimulus cannot occur through forward conditioning because the C stimulus follows, rather than precedes, the presentation of the B and A stimuli—forward Pavlovian conditioning requires that the conditioned stimulus predicts the subsequent presentation of the unconditioned stimulus.
Experiment 1
Method
Participants
Three individuals volunteered to serve as participants in Experiment 1. All participants were undergraduate students attending the National University of Ireland, Maynooth and all were experimentally naïve. Sessions were arranged so that participants would not meet each other in the vicinity of the laboratory, and they were instructed not to discuss their participation in the study with anyone.
Apparatus and Materials
In the experimental room, participants were seated at a table upon which there was a personal computer. The computer, programmed in Microsoft Visual Basic 6, was used to control stimulus presentations and record participants' responses throughout the experiment. Stimuli employed during Experiment 1 consisted of six nonsense syllables (CUG, ZID, DAX, BEH, PAF, JOM) that were arbitrarily divided into two classes for each participant. For convenience, the stimuli are alphanumerically designated (e.g., A1, B2, etc.); the participants did not see these designations.
Procedure
Experimental phases and blocks
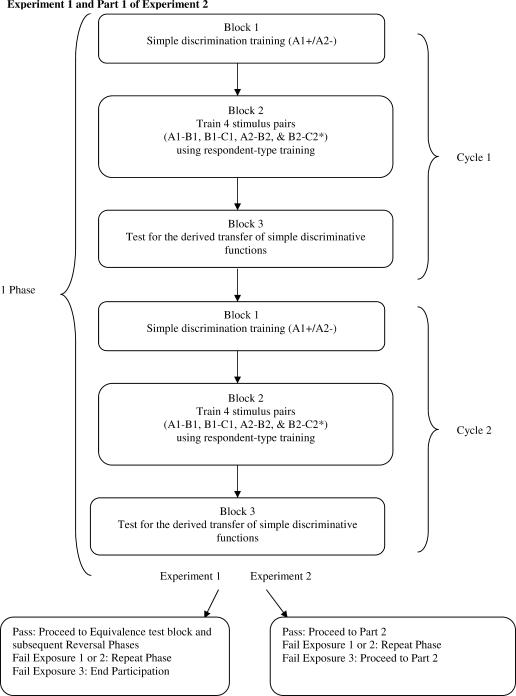
Each phase of Experiment 1 consisted of two cycles of three blocks of different trial types (see Figure 1). The first block involved simple discrimination training trials during which the nonsense syllable A1 served as an S+ and A2 as an S− (A1+/A2−). The second block involved training four stimulus pairings using the SPOP (A1–B1, A2–B2, B1–C1, B2–C2). The third block tested for the transfer of simple discriminative functions from the A to the B and C stimuli (B1+, C1+, B2−, C2−). No participant failed this block on the second cycle and thus all participants were immediately exposed to an equivalence test block. The equivalence test block consisted of twelve MTS trials that tested the baseline conditional discriminations (e.g., A1–B1), and symmetry (e.g., B1–A1), transitivity (e.g., A1–C1), and equivalence (e.g., C1–A1) relations.
Fig 1. A schematic overview of the procedure for Experiment 1 and Parts 1 and 2 of Experiment 2.
Simple discrimination training
The experiment commenced with simple discrimination training. On each simple discrimination training trial the A1 and A2 stimuli appeared to the left and right in the center of the computer screen. The left–right position of the stimuli was counterbalanced across all trials. The following instructions were also presented on the screen (for the first trial only) above the nonsense syllables: “Please click on one of the nonsense words. Try to get as many right as possible.” After clicking on one of the stimuli with the mouse, the screen cleared and a feedback message was presented immediately. Clicking on A1 produced the word correct and clicking on A2 produced the word wrong on the screen. The feedback remained on the screen for 1 s, and was followed by a 2 s intertrial interval (ITI), during which the screen remained blank, and then the next trial was presented. Training continued until the participant reached a performance criterion of six consecutively correct simple discrimination trials. Once this criterion was reached, the screen cleared for 2 s and then instructions for the SPOP training block appeared.
Stimulus pairing observation procedure
During this block of trials, participants were presented with four stimulus pairings; A1–B1, B1–C1, A2–B2, and B2–C2. The following instructions appeared on the screen at the start of the SPOP training phase: “Please just look at the nonsense words as they appear below. Do not click on the words with the mouse.” A button was positioned directly below these instructions with the following text “Click Here to Continue”. When the participant clicked on this button with the mouse, the screen cleared and 2 s later the first of the stimulus pairings was presented. On each SPOP training trial the first stimulus of a pair (e.g., A1) was presented for 1 s. The computer screen then cleared for a within-pair delay of 0.5 s. The second stimulus of the pair (e.g., B1) then was presented for 1 s and the screen cleared for a between-pair interval of 3 s before the first stimulus of the next stimulus pair was presented. All subsequent stimulus pairs were presented in the same manner. The four stimulus pairs were presented in a quasi-random order for eight trials, so that each stimulus pair was presented twice, once across every four trials. Following the completion of the SPOP training block, the screen cleared for 2 s before proceeding to the derived transfer-of-functions test.
Derived transfer-of-functions testing
Before exposure to the derived transfer testing block, the following instructions appeared on the screen: “Please click on one nonsense word. The computer will not tell you if you are correct or wrong. Please try to get as many correct as possible.” Stimulus presentations and the ITI were the same as for the simple discrimination training block; the stimuli appeared to the left and right in the center of the computer screen, and the left-right position of the stimuli was counterbalanced across all trials. However, unlike the training block, no feedback was provided for responding during the derived transfer test. The test consisted of three different trial types. One of the trial types consisted of the simple discrimination that had been explicitly trained (A1+/A2−) and the other two presented the B and C stimuli that were employed in the previous SPOP training block, the predicted derived functions of which would be: B1+/B2− and C1+/C2−. Each of the three trial types was presented twice in a quasi-random order. Although the number of test trials was small, participants were given up to three exposures to the training and test procedures (see below).
Following the final trial of the derived transfer test, the screen cleared and the statement “You may take a break now” appeared. In order to continue with the experiment the participant had to click a button labeled “Click Here to Continue” located immediately below the statement. The participant then was exposed to another cycle of blocks of simple discriminative function training, SPOP training, and transfer testing trials. Once this second cycle was completed, the screen cleared and the following appeared: “Thank you. That is the end of this part of the experiment. Please report to the experimenter.”
While the participant waited outside the room, the experimenter inspected the participant's performance on the second block of derived transfer test trials. If the participant had responded correctly to all six trials of the derived transfer test (A1+/A2−, B1+/B2−, and C1+/C2−) the experimenter initiated the equivalence-testing program. If the participant had responded incorrectly to any of the derived transfer testing trials, he or she was re-exposed to a second phase of two consecutive cycles of blocks of simple discriminative function training, SPOP training, and transfer testing trials. Following exposure to this second phase, performance on the second block of derived transfer test trials was inspected once again in order to determine if the participant should proceed at this point to the equivalence test or complete another phase of training and testing. It was decided that, should any participant consistently fail the derived functions testing block when exposed to a third phase, participation in the experiment would be terminated. However, all three participants responded correctly on all six trials of the derived transfer-of-functions test on the second cycle during the first phase.
Equivalence testing
During the equivalence testing block, participants were presented with a standard MTS equivalence test that examined the four trained relations A1–B1, B1–C1, A2–B2, B2–C2, the four symmetry relations B1–A1, B2–A2, C1–B1, C2–B2, the two transitivity relations A1–C1, A2–C2, and the two combined symmetry and transitivity relations C1–A1, C2–A2. On each task, the incorrect comparison was the alternatively numbered alphanumeric (e.g., when B1 was presented as the correct comparison, B2 was always the incorrect comparison, and vice versa). Thus, the equivalence test consisted of a block of twelve MTS tasks. During presentation of these tasks, the sample was always presented in the center top-half of the screen and the comparison stimuli, which only appeared after the participant had clicked on the sample, were located in the lower half of the screen, one to the right and one to the left. Participants selected a comparison by clicking on it with the mouse. Once again, instructions were presented on the computer screen: “Click on the nonsense word above and then click on the nonsense word below that goes with the one at the top. Try your best not to make any mistakes.” The twelve MTS trial types were each presented once in a random order. The screen then cleared and a message appeared thanking the participant for his or her co-operation with an instruction to report to the experimenter. Participants proceeded to the next phase irrespective of their performance on the equivalence test.
Reversal phase
In order to demonstrate control over the derived transfer of functions, either the B stimuli (for Participant 2) or the C stimuli (for Participants 1 and 3) were paired with opposite-class members during subsequent SPOP training. The participants were exposed to the entire experimental procedure of blocks of simple discriminative function training, SPOP training, derived transfer-of-function testing and equivalence testing trials, in exactly the same manner as before but with these new stimulus pairings. Thus, following the first block of simple discrimination training, the stimulus pairings presented during the SPOP were as follows: Participant 2, A1–B2, B2–C1 and A2–B1, B1–C2; Participants 1 and 3, A1–B1, B1–C2 and A2–B2, B2–C1 (those stimulus pairings that differed from the previous phase are italicized). Experiment 1 ended upon completion of the equivalence-testing block of the reversal phase.
Results and Discussion
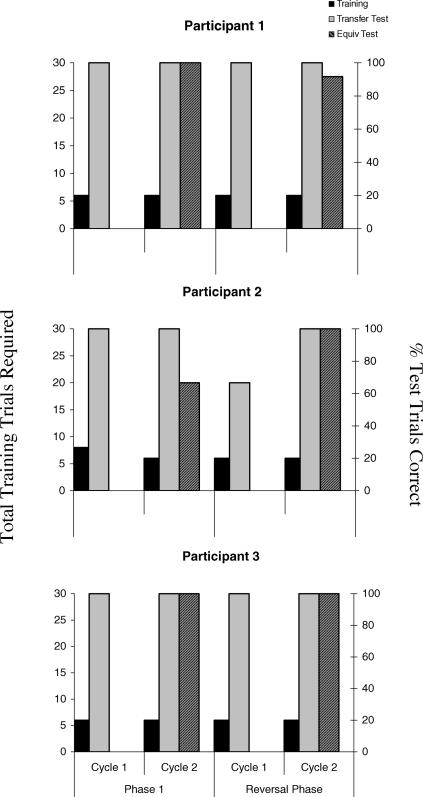
Two of the 3 participants (Figure 2) consistently reached the criterion of six consecutively correct simple discrimination training trials within the minimum required (i.e., six trials). Participant 2 required eight simple discrimination trials in Cycle 1 of Phase 1 but required only six trials in the subsequent block. One phase of training and test trials was the maximum required by all 3 participants in order to pass the derived transfer test in the second cycle. Participants 1 and 3 also responded correctly on all 12 MTS trials during the equivalence tests. Participant 2 scored 8 out of 12, responding incorrectly to a trained relation (B2–C1), two symmetry relations (B2–A1, C2–B1) and one transitivity relation (A2–C1), and thus was deemed to have failed the equivalence test.
Fig 2. Number of simple discrimination training trials required by each participant in Experiment 1 to reach criterion, the percentage of transfer trials correct across each cycle for each phase, and the equivalence test scores for each phase.
When the B or C stimuli “exchanged” classes for the SPOP during the reversal phase, all 3 participants successfully completed the derived transfer test across both cycles (P1 & P3) or during the second cycle (P2). Participants produced near perfect equivalence responding, and only Participant 1 responded incorrectly on one of the MTS tasks of the equivalence test.
This experiment demonstrated that it is possible to transfer simple discriminative functions through equivalence relations established using a SPOP. Experiment 2 was conducted to determine if such a procedure would facilitate the derived transfer of negative emotional stimulus functions.
Experiment 2
Method
Participants
Sixteen undergraduate students attending the National University of Ireland, Maynooth served as participants. These consisted of eight non-spider-fearful (P1–P8; one male and seven female), and eight spider-fearful individuals (P9–P16; all female) all of whom were purposively sampled using a self-report anxiety screen, the Spider Phobia Questionnaire (SPQ; Watts & Sharrock, 1984), followed by a behavioral approach test (BAT). All participants were experimentally naïve. Sessions were arranged so that participants would not meet each other in the vicinity of the laboratory, and all were instructed not to discuss their participation in the study with anyone.
Participant selection
The Spider Phobia Questionnaire (SPQ, Watts & Sharrock, 1984) was administered to approximately 150 volunteers. Watts and Sharrock reported a mean score of 21.97 for phobic and 5.83 for nonphobic participants. In the current experiment, volunteers who scored 4 or below, or 22 or above on the 43-item questionnaire were asked to meet with the experimenter individually at prearranged times to participate in a BAT. As part of the BAT, volunteers were informed that there was “an average-sized Irish house spider in an adjacent room in a glass terrarium” and they were asked if they would be willing to enter the room and approach and look at the spider for two minutes (cf. Mohlman & Zinbarg, 2000). All individuals were made fully aware that they could refuse the task or terminate it at any time. Twenty-five volunteers completed the BAT before the final 16 participants for the study had been identified. A single experimenter collected all data for the BAT.
The eight spider-fearful individuals who participated in the experiment were those who had scored highly on the SPQ and who had either refused to participate in the BAT or else had not successfully completed it. Unsuccessful completion of the BAT included terminating the task before the two minutes were up, or entering the room but not approaching the terrarium (i.e., remaining by the door; see Table 1). The eight non-spider-fearful participants were those who had a low score on the SPQ and who had then successfully completed the BAT by entering the room, approaching the terrarium, and looking at the spider for the full 2 min (see Table 1).
Table 1. SPQ scores and BAT performances for both spider fearful and non-spider fearful participants.
| Non-Spider Fearful |
Spider Fearful |
||||
| Participant | SPQ | BAT | Participant | SPQ | BAT |
| 1 | 4 | Approached & looked for 2 min | 9 | 28 | 10 s at door |
| 2 | 4 | Approached & looked for 2 min | 10 | 24 | 90 s at door |
| 3 | 4 | Approached & looked for 2 min | 11 | 24 | 10 s at door |
| 4 | 4 | Approached & looked for 2 min | 12 | 29 | 2 min at door |
| 5 | 2 | Approached & looked for 2 min | 13 | 29 | Refused to perform BAT |
| 6 | 4 | Approached & looked for 2 min | 14 | 27 | 30 s at door |
| 7 | 3 | Approached & looked for 2 min | 15 | 27 | Refused to perform BAT |
| 8 | 3 | Approached & looked for 2 min | 16 | 24 | Refused to perform BAT |
Apparatus and Materials
In the experimental room, participants were seated at a table upon which there was a personal computer. The computer, programmed in Microsoft Visual Basic 6, was used to control stimulus presentations and record participants' responses throughout the experiment. Stimuli employed during Experiment 2 consisted of six nonsense syllables (MAU, YIM, VEK, ROG, FID, ZOL) that were arbitrarily divided into two classes for each participant. For convenience, the stimuli are alphanumerically designated (e.g., A1, B2, etc.); the participants did not see these designations.
The video files used in the current experiment contained dramatized “spider- attack” scenes from a well-known Hollywood movie. The four files used were selected based on the ratings provided by three independent raters who viewed a total of 17 spider-attack scenes (available from the first author upon request). The raters were selected from among friends and acquaintances of the experimenter and did not classify themselves as spider fearful. The video files were rated on a scale ranging from 1 (not at all uncomfortable) to 100 (extremely uncomfortable). The mean rating for each of the 17 video files ranged from 30 to 90. Four of the videos were rated 75 or above and these were used in the study. The four video files were 85 s, 110 s, 55 s and 81 s in length, respectively.
The self-report measure administered during the initial screening to determine participant eligibility for Experiment 2 was the SPQ. This is a 43-item questionnaire, designed to measure various fearful thoughts about spiders. Thirty-three of the items load onto three subscales: vigilance, internal preoccupation, and avoidance coping. Watts and Sharrock (1984) did not report reliability data for the total SPQ scores, but they reported that the scales correlate .27 to .47 with one another suggesting moderate overlap, and that the scales have good internal consistency coefficients (.78, .81, and .77, respectively). Furthermore, nonfearful individuals were reported to differ significantly from fearful individuals on all three of the questionnaire scales. The current study did not aim to differentiate between the subtypes of spider-fearful individuals, and thus an overall score was calculated across all three subscales and a further 10 factual-knowledge and cognitive-behavioral items not included on any of the subscales. The questionnaire simply was used, therefore, as a prescreening instrument in order to select volunteers for the BAT.
Procedure
Experimental overview
Experiment 2 was divided into two parts. Part 1 aimed to determine if each of the participants would demonstrate a derived transfer of simple discriminative functions (as in Experiment 1) before being exposed to Part 2, which was designed to test for the derived transfer of self-reported arousal functions. This experimental sequence served two main purposes. First, it ensured that if participants failed to show a derived transfer of self-reported arousal functions in Part 2, this failure could not be attributed to unfamiliarity with the general experimental environment and the type of automated procedures that were employed. Second, providing two different procedures for training and testing for derived transfer performances (simple discriminative in Part 1 and self-reported arousal in Part 2) allows for a comparison of the effectiveness of these two procedures.
Part 1 of Experiment 2 was identical to Experiment 1, except that no reversal phase was included and participants did not receive an equivalence test after passing the test for the derived transfer of simple discriminative functions (see Figure 1). Furthermore, if a participant failed the derived transfer test after three exposures (i.e., six cycles of training and testing), then he or she continued directly to Part 2 of Experiment 2 (in Experiment 1, participation would have been terminated after three failures). Including participants who failed the transfer test in Part 1 was deemed to be a relatively conservative strategy in that Part 2 sought to demonstrate a derived transfer of self-reported arousal functions even with participants who had failed a prior transfer test. The reversal phase was omitted in Part 1 because successful reversal performances had already been demonstrated in the previous experiment and, moreover, Part 2 presented a second test for derived transfer with a novel set of stimuli, including baseline and postconditioning measures. The equivalence test was omitted in Part 1 so that any successful transfer of self-reported arousal functions would be demonstrated in the absence of a prior equivalence test. Some researchers have argued that the equivalence test, and the MTS format itself, may function as a powerful contextual cue for equivalence responding (e.g., Barnes & Holmes, 1991), and thus it was deemed important to avoid the presentation of such a test (or the MTS format itself) before the transfer-of-function test. In doing so, successful transfer performances could not be attributed to an experimental history of equivalence test exposures (see Barnes & Keenan, 1993; Barnes-Holmes et al., 2000).
Part 2 of Experiment 2 consisted of six blocks of different trial types, and a novel set of six nonsense syllables was employed (i.e., Part 2 required that two new stimulus classes be formed). For convenience, these syllables will be referred to using the same alphanumerics (A1, B1, etc.), but the actual stimuli were novel for the participants. The first block involved a baseline rating of the six stimuli, during which participants answered four questions for each stimulus by using sliding scales, which ranged from 0 to 100. The second block involved video pairing trials during which the A1 stimulus was paired with spider-attack video files, and the A2 stimulus was paired with a blank screen. The third block consisted of the SPOP (A1–B1, A2–B2, B1–C1, and B2–C2). The fourth block was a postconditioning measure of participants' ratings of the six stimuli, performed in the same manner as the baseline block. The fifth block involved assigning each of the six stimuli to either a spider video category or a blank video category. The sixth block consisted of twelve MTS trials that tested the baseline conditional discriminations (e.g., A1–B1), and symmetry (e.g., B1–A1), transitivity (e.g., A1–C1), and equivalence (e.g., C1–A1) relations.
Baseline measure
A baseline measure of emotional reaction to each of the six stimuli was taken before conditioning commenced. This was accomplished by posing four questions that were designed to provide self-reported measures of arousal. These questions were devised based upon evidence that extent of fear, disgust, emotional control and intensity tap into important components of fear-related arousal (e.g., see Forsyth & Eifert, 1998; Forsyth, Eifert, & Thompson, 1996). The purpose of this block was to provide a baseline comparison to determine if the subsequent video pairing and SPOP training influenced postconditioning ratings of the same stimuli.
At the beginning of the block of baseline measures, the following instructions appeared at the top of the computer screen: “Please look at the nonsense word below, think about it, and then use the sliding scales to answer each of the following questions.” The first nonsense syllable (e.g., A1) was presented below these instructions. The rest of the screen was divided into four boxes, each of which contained one of the four questions. A sliding scale, ranging from −1 to 100 was located below each question (the sliding scale control is available with all versions of Microsoft Visual Basic 6). In order to answer a question the participant operated the sliding scale by clicking on the slider with the mouse and dragging the cursor to the desired number.
The first question asked participants to “Rate the extent of fear you experienced when seeing and thinking about the nonsense word above” (0 = extreme calm and 100 = extreme fear). The second question asked participants to “Rate the extent of disgust (feeling grossed out) you experienced when seeing and thinking about the nonsense word above” (0 = extremely pleasant, 100 = extreme disgust). The third question asked participants to “Rate the extent to which you could control your emotional reactions when seeing and thinking about the nonsense word above” (0 = complete control, 100 = extreme lack of control). Finally, the fourth question asked participants to “Rate the intensity of your emotional reaction you experienced when seeing and thinking about the nonsense word above” (0 = extremely mild, 100 = extremely intense) At the bottom of the screen was the instruction “Click here when you have answered all of the questions.”
If all of the questions had been answered the following appeared upon clicking the button: “Thank you – Please click the continue button to present the next nonsense word and then answer the questions again.” If the participant had not answered all of the questions by moving each of the sliding scales, from its default position of −1 to 0 or above, the following instruction appeared: “Please answer ALL of the questions.” The screen only cleared once all four questions had been answered for that stimulus and the participant had clicked the continue button. The next nonsense syllable appeared immediately at the top of the screen and the participant was required to answer the same four questions for this stimulus in the same manner as before. Once the participant had answered all four questions for each of the six stimuli (each of which were presented once in a quasi-random order) the screen cleared and the video-pairing block began immediately.
Video pairing and stimulus observation pairing procedure
In the video-pairing block, participants were not required to emit any overt response but only to observe while the A stimuli were paired with either a spider-attack video file or a blank screen. A1 was paired with the spider-attack scenes and A2 was paired with a blank screen. In any one video-pairing block, one of the blank screen presentations was 72 s and the other was 92 s. The mean duration of these two presentations was roughly equal to the mean duration of the four video files.
At the start of the video-pairing block the following instructions appeared at the top of the screen: “Please look at the nonsense word and video clip when they appear below. Sometimes a video clip will NOT appear – This is part of the experiment. Click on the button to continue.” Once the participant had clicked on the button with the mouse, the screen cleared for 500 ms and the first nonsense syllable appeared in a white box on a gray background, and remained in the center of the screen for 2 s. Immediately thereafter a video box (15 cm wide × 13 cm high, approximately) appeared in the center of the screen and, depending on the class to which the preceding nonsense syllable belonged, either a digital video file began to play or the video box remained blank (i.e., black). The previously presented stimulus flashed on and off once per sec in the top left-hand corner of the video box for the length of time that the video-file or blank screen was presented.
Once the presentation ended, the screen cleared and the same instructions (as presented above) appeared immediately. When the participant clicked on the button the next nonsense syllable appeared and was paired with a video file or blank screen in the same manner as before. Participants were exposed to four video pairing trials, during which the A1 (spider-attack scenes) and A2 (blank screen) stimuli were each presented twice in a quasi-random order. Different video files were employed for each of the two A1 pairings. The SPOP training block began immediately after exposure to the video-pairing block.
The SPOP of Experiment 2 was identical to Experiment 1, except that the new stimulus set was used. Once all four stimulus pairs had been presented in a quasi-random order for eight trials, the screen cleared and the instructions for the video-pairing block were, once again, presented immediately. The participant was exposed to another block of four video-pairing trials, during which the A1 and A2 stimuli were each presented twice in a quasi-random order. The video files depicted two different spider-attack scenes than those employed in the earlier block of video-pairing trials. Following this second block of four video-pairing training trials, participants were exposed once more to eight SPOP training trials, presented in the same manner as before.
Post-conditioning measure
A post-conditioning measure of emotional reaction to each of the six stimuli was now taken. This block was identical to the baseline measure block. The same four questions were employed and once again participants were asked to answer them for all six stimuli using 100-point scales. Once the participant had answered all four questions for each of the six stimuli, the screen cleared and the video categorization block began immediately.
Video categorization
Participants were presented with six video categorization tasks, which required them to match each of the nonsense syllables with either the phrase ‘spider video’ or ‘blank video’. The following instructions were presented on the screen at the start of the video categorization block: “Please look at each nonsense word below and then click on the blue box that you think goes with the nonsense word. When you have finished making your choices, click the finish button at the bottom of the screen.” The computer screen was divided into six boxes, each of which contained one of the six categorization tasks. Once the participant had completed all six of the tasks by clicking upon the chosen category for each of the stimuli, he or she clicked on a button at the bottom of the screen labeled “Click Here To Finish”. The screen then cleared and the following instructions appeared immediately: “Thank you. That is the end of this part of the experiment. Please report to the experimenter.”
Equivalence testing
The experimenter then set the computer to commence the equivalence-testing block. The procedure for this block, and the tasks employed, were exactly the same as for Experiment 1, except that the new stimulus set was used. Experiment 2 ended upon completion of the equivalence-testing block.
Results and Discussion
Part 1
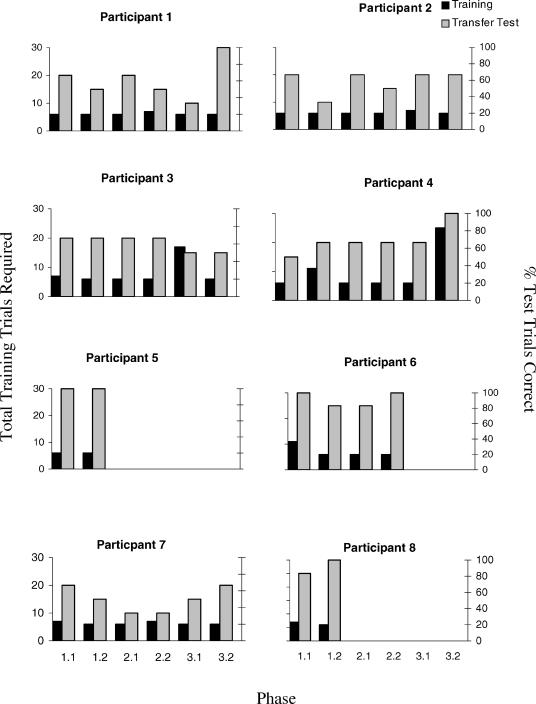
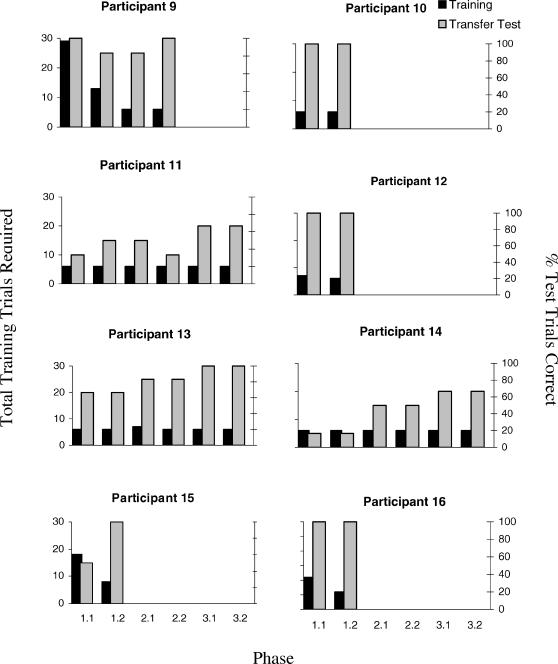
Four of the 16 participants (P5 of the nonfearful group and P10, P11, and P14 of the fearful group) reached the criterion of six consecutively correct simple discrimination training trials within the minimum required (i.e., six trials) across all blocks of simple discrimination training. All other participants required more than six trials in at least one of these training blocks.
As can be seen in Figures 3 (Nonfearful) and 4 (Fearful), 11 of the 16 participants passed the derived transfer test (A1+/A2−, B1+/B2−, and C1+/C2−) by responding in accordance with all of the stimulus pairings that were presented during the SPOP (A1–B1–C1, and A2–B2–C2). Six of these 11 participants (P5 and P8 of the nonfearful group and P10, P12, P15, and P16 of the fearful group) required just one phase of training and test trials in order to pass the second block of derived-transfer test trials. As indicated in the procedure, the maximum number of exposures permitted to the training and testing phases was three, and thus the five participants who showed a consistently incorrect performance after three phases were not retrained and retested, but proceeded with the other 11 participants to the next part of the experiment.
Fig 3. Number of simple discrimination training trials required by each non-fearful participant to reach criterion, the percentage of transfer trials correct across each cycle for each phase, and the equivalence test scores for each phase.
Fig 4. Number of simple discrimination training trials required by each fearful participant to reach criterion, the percentage of transfer trials correct across each cycle for each phase, and the equivalence test scores for each phase.
Part 2
Baseline and postconditioning measures
The raw data for both the baseline and postconditioning blocks consisted of the ratings for all six stimuli given by each participant in answer to the four questions—fear, disgust, control, and intensity. For both sets of data, responses to each of the four questions for each of the six stimuli were summed yielding overall fear, disgust, control, and intensity scores for each participant. Statistical analyses were conducted in order to determine if the self-reported arousal ratings of both groups differed between each question type. The data for the baseline sliding scale consisted of a large number of zero ratings and were not normally distributed, and thus a series of four Friedman tests was conducted in order to analyze this data set. Each of these tests proved to be nonsignificant. Unlike the baseline block, the postconditioning sliding-scale ratings contained very few zero scores. Furthermore, standard deviations and errors across the spider-fearful and non-spider-fearful groups for each of the four questions for each stimulus were broadly similar, indicating homogeneity across question type for each stimulus. Four one-way repeated measures Analyses of Variance (ANOVAs) were therefore employed to determine if ratings differed significantly across the four question types, and once again, these tests were nonsignificant. Because no significant differences were found between question type across all participants for either the baseline or the postconditioning block, the results of these analyses are not presented here. Accordingly, for both blocks, the four scores for each participant to each stimulus were collapsed into a single mean self-reported arousal score for each of the six stimuli. These individual mean self-reported arousal scores may be seen in Figure 5 (non-fearful participants) and Figure 6 (fearful participants).
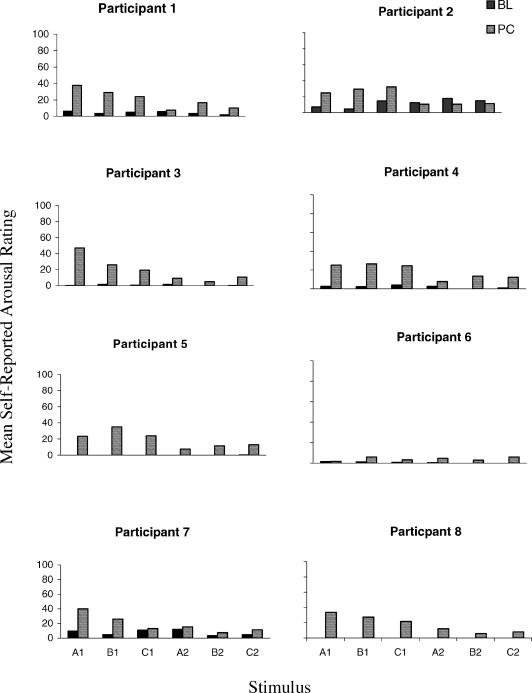
Fig 5. Mean self-reported ratings for each non-fearful participant to each stimulus, recorded during the baseline (BL) and postconditioning (PC) blocks.
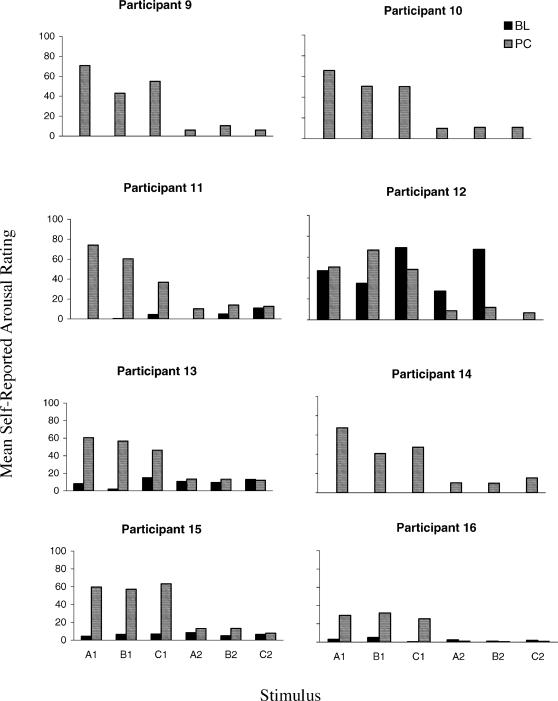
Fig 6. Mean self-reported ratings for each fearful participant to each stimulus, recorded during the baseline (BL) and postconditioning (PC) blocks.
Visual inspection of Figures 5 and 6 indicates that a number of patterns of responding emerged between phases and groups. With the exception of Participant 12 (Fearful), self-reported arousal scores in the baseline block (dark bars) were relatively low across all participants and stimuli. Overall, therefore, the nonsense syllables prior to conditioning appeared to have no specific self-reported arousal functions for 15 of the 16 participants. Subsequently, all participants, except Participant 12, demonstrated higher self-reported arousal levels to all Class 1 stimuli in the postconditioning compared to the baseline block (striped bars versus dark bars for A1, B1, and C1). Furthermore, even Participant 12 demonstrated this pattern for two of the three Class 1 stimuli (A1 and B1). Twelve of the participants (7 non-fearful and 5 fearful) also showed increased self-reported arousal for each of the Class 2 stimuli in the postconditioning relative to the baseline block (striped bars versus dark bars for A2, B2, and C2). Although increased self-reported arousal to the Class 2 stimuli may seem surprising it is important to note that the video clips consisted of highly dramatized spider attack scenes. It is possible therefore that general levels of arousal may have increased for some participants as a result of watching these film clips. In comparing self-reported arousal scores between all Class 1 and Class 2 stimuli for the postconditioning block (striped bars), 15 participants produced higher self-reported arousal scores for each of the former, relative to each of the latter stimuli. Participant 7 demonstrated this effect for A1 and B1. Comparing across Figures 5 and 6, seven of the fearful participants gave higher ratings to each of the Class 1 stimuli than did any of the non-fearful participants (i.e., comparing A1 with A1, B1 with B1, and C1 with C1 for each participant). The remaining nonfearful participant (16) demonstrated higher Class 1 ratings than three of the nonfearful participants (2, 4, and 6).
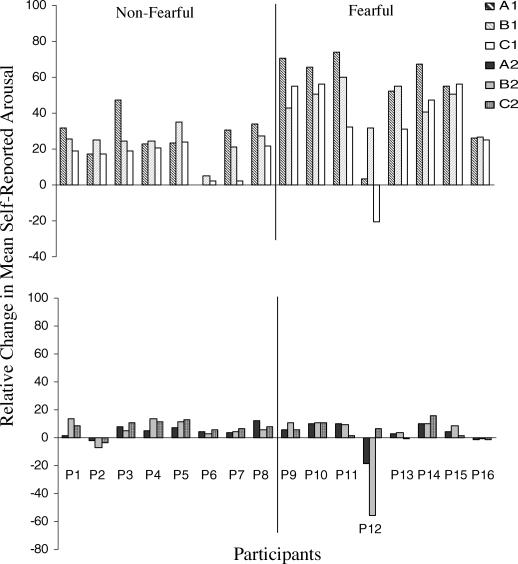
To illustrate the between-group differences, the relative change in self-reported arousal from baseline to postconditioning for all six stimuli is presented in Figure 7. As can be seen, with the exception of Participant 12, all participants demonstrated an increase in self-reported arousal ratings (of the class 1 stimuli) in the postconditioning relative to the baseline phase. Participant 12's performance could be explained in terms of spurious stimulus control in the baseline phase, during which she gave all but one of the nonsense syllables inflated ratings (see Figure 6). Nevertheless, in the postconditioning block, Participant 12 rated the Class 1 and Class 2 stimuli differently (see Figure 5), thereby demonstrating the derived transfer of self-reported arousal functions. Overall, however, fearful participants produced greater relative change, compared to the nonfearful participants, in ratings for the Class 1 stimuli (P3 nonfearful and P16 fearful were exceptions). Conversely, there was little difference between fearful and nonfearful participants for the Class 2 stimuli, in that both groups produced very little difference in baseline and postconditioning ratings for these stimuli.
Fig 7. Relative change for each participant in Class 1 and Class 2 mean self-reported arousal ratings from the baseline to the postconditioning block.
In order to explore further the differences in self-reported arousal ratings between the two groups for the Class 1 stimuli, three independent t tests were conducted. Each test investigated the impact of participant type on self-reported arousal ratings for one of the three Class 1 stimuli. Significant differences were found between the ratings of fearful and nonfearful participants for the A1 [t (14) = −4.316, p = 0.001], B1 [t (14) = −4.598, p = 0.0005], and C1 [t (14) = −5.188, p = 0.0005] stimuli. Therefore, fearful participants reported a significantly greater arousal response than nonfearful participants, to stimuli that were directly and indirectly related to spider video files.
Stimulus categorization and equivalence test measures
The results of the stimulus categorization task and the equivalence test were highly consistent across all participants and therefore are not presented here. All participants in both the spider- fearful and non-spider-fearful conditions responded correctly to all six tasks in the stimulus categorization test. Similarly, all participants were deemed to have passed the equivalence test; 15 of the 16 participants responded correctly to all 12 MTS test trials. Participant 8 of the fearful group scored 11 out of 12, but stated during debriefing, “I made a mistake on one of the tasks.”
The data from Experiment 2 demonstrate that the SPOP, when combined with the video-pairing procedure, produces the derived transfer of self-reported arousal functions. Furthermore, the spider-fearful group showed significantly higher levels of self-reported arousal than the non-spider-fearful group, for stimuli directly paired with spider material. Finally, these different levels of self-reported arousal transferred through equivalence relations, thus demonstrating that the derived transfer of emotional functions was sensitive to the subclinical characteristics of the experimental participants.
General Discussion
The current study demonstrates that it is possible to produce the derived transfer of both simple-discriminative (Experiments 1 and 2) and self-reported arousal (Experiment 2) functions through equivalence relations established using a SPOP. The results of Experiment 2 support and extend the findings of Experiment 1 and other studies of derived transfer in a number of ways. First, the experiment provides the clearest evidence currently available for the derived transfer of functions using a SPOP. Second, the differences observed in the derived transfer of functions across the spider-fearful and non-spider-fearful participants provides the type of evidence that Dougher et al. (1994) argued was needed to begin to support a derived-relations model of the acquisition of anxiety responses. Third, the findings are also important with respect to the literature on stimulus-class formation more generally (e.g., Zentall & Smeets, 1996, for an extensive review). Specifically, the results indicate that the derived transfer of self-reported arousal functions, based on the SPOP, is also indicative of equivalence class formation, as measured by standard MTS procedures and other categorization tests (see Pilgrim & Galizio, 1996). Fourth, the findings of Experiment 2 are important because the experimental procedures were entirely automated and thus there was no interaction between the experimenter and participant between exposure to the baseline measure and the final categorization test. This level of automated control reduces the possibility of inadvertent experimenter cueing, which is particularly important when self-report measures are employed. Finally, Experiment 2 extends previous research that successfully employed video-based material as the basis for derived transfer (Grey & Barnes, 1996) and likert-type scales as measures of that transfer (Barnes-Holmes et al., 2000).
The reversal phase of Experiment 1 demonstrated control over the derived transfer of simple discrimination functions. Furthermore, the patterns of MTS responding shown on the equivalence tests indicated that the participants' performances were sensitive to the reversed stimulus pairings. These data contrast with a number of other studies (Pilgrim & Galizio, 1990, 1995, 1996; Roche, Barnes, & Smeets, 1997; Saunders, Drake & Spradlin, 1999; Saunders, Saunders, Kirby, & Spradlin, 1988). Some researchers have reported that only symmetry relations, and not transitivity relations, were sensitive to reversed reinforcement contingencies during MTS training. The fact that the current study demonstrated the successful reversal of both derived-transfer effects and equivalence-class formation, suggests that perhaps the SPOP is more effective in reversing previously derived performances than is MTS training. However, caution is required here, because other researchers using MTS have reported successful reversal of both symmetrical and transitive relations (e.g., Garotti, de Souza, De Rose, Molina, & Gil, 2000; Smeets, Barnes-Holmes, Akpinar, & Barnes-Holmes, 2003). Further research clearly is needed, therefore, on the effects of reversing baseline contingencies on subsequent test performances, and the current data suggest that the SPOP may be of some use in these investigations.
Because the current study employed a stimulus-pairing procedure that did not require a programmed operant response, it may be tempting to interpret the derived transfer-of-function effects, and the formation of equivalence relations, as the result of a Pavlovian conditioning process. As suggested in the introduction, however, the current transfer effects could not occur through forward respondent conditioning, and thus it would be necessary to appeal to backward associative conditioning. In fact, given that the transfer occurred from A to C via a mediating stimulus, a combination of backward and higher-order or sensory preconditioning would be required. Although associative learning theorists have reported so-called backward sensory preconditioning (Ward-Robinson & Hall, 1996), the effects they document are explained in terms of a type of mediated forward conditioning (Hall, 1996; Urcuioli, 1996; cf. L. Hayes, 1992). Specifically, they argue that training an A–B relation causes A to generate an internal representation of B. If an A–C relation is then trained, a forward associative chain is created in which A activates the internal representation of B, which is then paired with C (i.e., more informally, given A the participant thinks of B and then chooses C). Given this internally-mediated associative chain, acquiring a new B–C relation should be facilitated, and indeed, this is what researchers have reported (Nakagawa, 2005; see Hall, 1996; Urcuioli, 1996, for reviews).
The linear design of the SPOP used in the current study, however, does not permit the type of mediated forward conditioning outlined above. Indeed, Hall (1996), specifically argued that the current design should fail to produce the observed transfer effect:
Associative links formed in the first stage of training… might allow A1 and A2, when presented as comparison stimuli in the test, to evoke representations of B1 and B2. But the new sample stimulus (C1) would be able to evoke the representation of the trained sample (A1 for the choice between B1 and B2) only by way of a chain of backward associations (i.e., C–B–A). Such backward associations are not readily formed… In different terminology, these training procedures do not establish the symmetry relation, and, hence, the equivalence test—which depends on the effectiveness of this relation—will be failed (p. 248).
As an aside, Hall does recognize the evidence for backward associative conditioning but points out that such conditioning occurs “only in a rather restricted set of conditions… and these conditions are not especially well met” (p. 238) in the transfer studies that he reviews.
Leaving aside Hall's (1996) prediction of failure for the linear design used in the current study, it also is important to acknowledge the possibly important role played by the current participants' pre-experimental behavioral and verbal histories. Indeed, leading researchers in associative conditioning have argued that human verbal behavior, or what they describe as a “propositional system associated with consciousness”, often mediates the effect of respondent contingencies on verbally able humans (Lovibond & Shanks, 2002). In fact, these authors conclude that this type of verbal mediation may be necessary in order to observe the types of Pavlovian conditioning effects that have been reported with human participants (see also Lovibond, 2003). A broadly similar argument has been made from a behavior-analytic perspective in terms of Relational Frame Theory (RFT; e.g., Barnes-Holmes & Hayes, 2003; Leader et al., 1996; but see Sidman, 1994 who suggested that respondent conditioning, as a two-term contingency, may establish an equivalence relation). For RFT, however, the propositional system in human Pavlovian conditioning is defined in terms of generalized verbal operant classes (Leader et al., 1996 pp. 703–704) rather than as a mediational cognitive mechanism. Irrespective of the theoretical issues surrounding human Pavlovian conditioning effects, the systematic analysis of nonoperant stimulus-pairing procedures on derived transfer seems to be important on the grounds of ecological validity because the typically employed MTS preparation is far from ubiquitous in the natural environment (Smeets & Barnes-Holmes, 2003). The current study is important in this regard because it is the first to demonstrate a clear derived transfer of functions using a SPOP rather than some form of programmed operant training, such as MTS.
Previous research has demonstrated that differential stimulus equivalence performances may be observed between heterogeneous groups that differ on some social-psychological dimension (e.g., Watt, Keenan, Barnes, & Cairns, 1991). Subsequent studies have demonstrated similar group differences in equivalence responding based on levels of anxiety (Leslie, Tierney, Robinson, Keenan, & Barnes, 1993), gender (Moxon, Keenan, & Hine, 1993), and learning disability (Barnes, Lawlor, Smeets, & Roche, 1996). The current study, however, is the first to demonstrate a differential transfer of derived functions based on group differences that were measured systematically using a psychometric instrument and a well-established behavioral test. The current findings therefore support and extend previous research that has demonstrated the differential sensitivity of equivalence responding to individual differences. Moreover, the data support the idea that equivalence and derived transfer are functionally related behavioral repertoires, in that both seem to be differentially sensitive to a subclinical characteristic of the participant groups involved.
The current procedures and data may be of interest to researchers working in the area of laboratory-induced fears and phobias (e.g., Cook & Mineka, 1989; Schell, Dawson & Marinkovic, 1991). For example, there has been widespread disenchantment with traditional conditioning theories of anxiety reactions, with leading researchers pointing to the fact that only a minority of investigators have reported reliable acquisition effects (McNally, 1987). Based on this and other criticisms, many researchers have argued for explanations of human anxieties that rely on cognitive processes, such as beliefs and expectancies (e.g., Mineka & Tomarken, 1989; Reiss, 1980). From a behavior-analytic perspective, however, such explanations are incomplete because they leave the expectancies and beliefs, which are also behaviors, unexplained (Barnes, 1989; S. Hayes & Brownstein, 1986). If derived stimulus relations provide an adequate behavioral account of human language and cognition (e.g., S. Hayes, et al., 2001; Sidman, 1994), research on the derived transfer of aversive functions in clinically relevant populations could elucidate the role played by language and thought in the acquisition and manipulation of human emotional reactions. Indeed, the success of the current study, and others that also have demonstrated a derived transfer of emotional arousal (e.g., Barnes-Holmes et al., 2000; Dougher, et al., 1994; Markham, Dougher, & Augustson, 2002; Roche & Barnes, 1997; Roche, Barnes-Holmes, Smeets, Barnes-Holmes, & McGeady, 2000), suggests that the concept of derived transfer could be an extremely useful tool in the study of laboratory-induced fears.
One criticism of Experiment 2 could be that it lacked a psycho-physiological measure of arousal. Research suggests that fear-related behavior is multicomponential, involving three systems—verbal-evaluative, physiological, and overt motor actions (e.g., escape/avoidance; see Barlow, 2002; Lang, 1971). In human respondent conditioning work, including studies evaluating fearful and anxious responding to fear-evoking challenges, researchers typically assess the verbal-evaluative and physiological domains (e.g., extent of fear, like vs. dislike, etc; e.g., see Forsyth & Eifert, 1998; Forsyth et al., 1996). Rarely do researchers also assess the overt motor component, though this is typical of studies using tasks requiring approach toward a fear-evoking stimulus (e.g., Behavioral Approach Task). On balance, although physiological responses often are employed, they are not alone regarded as being synonymous with fearful or anxious responding—self-report, particularly when assessed concurrently with fear-evoking procedures, can be a reliable index of an important component of fearful responding in humans (e.g., Cavanagh & Davey, 2000).
Of course, one might argue that self-report measures are more susceptible than physiological measures to demand characteristics. In the current study, however, the fearful group reported significantly higher rates of arousal than the nonfearful group. If the only variable at work here was the participants' desire to please the experimenter, then no differences should have emerged across the two groups, unless each of the participants had access to the data from the members of the other group (which of course they did not). Nonetheless, other researchers have employed physiological measures of derived transfer (e.g., Dougher et al., 1994), and thus subsequent research with spider-fearful and non-spider-fearful participants might employ similar measures.
An interesting issue arising from Experiment 2 is that all 16 participants demonstrated the derived transfer of self-reported arousal during Part 2, but 5 of these individuals previously had failed to demonstrate the derived transfer of simple discriminative functions during Part 1. Why did this difference in transfer performances occur? Although Parts 1 and 2 both employed a SPOP, they were otherwise quite dissimilar and thus any one of a number of variables (e.g., greater salience of the video stimuli and longer trial duration in Part 2) may have been responsible for the different success rates observed across the two parts of Experiment 2. One possible explanation for the difference in performance is that the two functions were trained using very different conditioning procedures. The simple discrimination of Part 1 was trained using an operant task, which involved the provision of feedback when the participant chose one of two stimuli (by clicking with the mouse). In contrast, during the video-pairing exercise of Part 2 the participants were required simply to observe the pairing of stimuli with either spider material or a blank screen in the absence of feedback. In effect, the video-pairing procedure was formally, and perhaps functionally, similar to the SPOP in that they both involved stimulus pairing in the absence of an overt response requirement and feedback. Perhaps this formal, and possible functional, similarity facilitated the greater number of transfer performances observed in Part 2 relative to Part 1.
Of course, the improvement in transfer performances from Part 1 to Part 2 could have been due to a simple order effect. However, it is remarkable that 5 participants failed to demonstrate a transfer of simple discriminative functions across six cycles of training and testing, but then immediately demonstrated transfer when exposed to the video-pairing procedure. It seems likely, therefore, that an order effect is not the only variable at work here, and that future studies of derived transfer should explore systematically the extent to which differences, such as the presence or absence of feedback and/or an overt response requirement, impacts upon the derived transfer of functions (cf. Dymond & Barnes, 1998).
A related issue concerns the possible criticism that the high level of transfer performances observed in Part 2 was, to some degree, a function of the participants' prior exposure to Part 1 (whether or not they passed). Perhaps fewer participants would have demonstrated the predicted transfer effects if they had been exposed immediately to Part 2 alone. Although this may have been the case, the current procedures involved a number of novel features that have not been employed in previous transfer studies, including computer presented video clips and a SPOP designed to generate derived transfer. Consequently, it was deemed important to ensure that any failure to show derived transfer in a subclinical population was not due to extraneous variables, such as unfamiliarity with the highly automated procedures. However, it is important to note that the set of nonsense syllables in Part 2 was completely novel relative to Part 1. Thus, the derived transfer performances observed in Part 2 were genuinely novel in that they did not simply reflect the transfer of additional functions through already existing derived relations (cf. Healy, Barnes-Holmes, & Smeets, 2000).
A final issue arising from Experiment 2 that may warrant further investigation concerns the almost perfect equivalence test performances of all 16 participants. At the present time it remains unclear to what extent the prior successful exposure to the video categorization task facilitated equivalence responding. Indeed, the simultaneous presentation of all six stimuli during the former task may have been important in this regard. However, other studies have reported a dissociation between performance on equivalence and other types of categorization tasks in which all stimuli are presented simultaneously (e.g., Smeets & Barnes-Homes, in press). Clearly therefore, further research will be needed to explore the correlation, or lack thereof, between performances on different types of equivalence tests.
In summary, the current results suggest that the use of new methods of training and testing may benefit the advance of derived stimulus relations research. The findings also indicate that a SPOP is a powerful means for establishing derived relational responding. Furthermore, by demonstrating that the subclinical characteristics of participants may differentially affect their performance in a derived transfer-of-functions test, weight is given to Dougher et al.'s (1994) claim that stimulus equivalence, and the derived transfer of functions, may provide an important part of the behavior-analytic explanation for the acquisition of fear and anxiety responses.
Acknowledgments
The preparation of this manuscript was funded in part by a scholarship from the Irish Research Council for Humanities and Social Sciences.
Footnotes
Some researchers have argued that the term “transformation of stimulus function”, rather than “transfer”, provides a more generic concept that can be applied when changes in stimulus functions involve relations other than equivalence relations (Barnes-Holmes, Hayes, Dymond & O'Hora, 2001). In certain cases, however, the two terms may be used interchangeably and we will adopt that practice in this article.
References
- Barlow D.H. Anxiety and its disorders: The nature and treatment of anxiety and panic (2nd Ed.) New York: Guilford; 2002. [Google Scholar]
- Barnes D. Behavior-behavior analysis, human schedule performance, and radical behaviorism. The Psychological Record. 1989;39:339–350. [Google Scholar]
- Barnes D, Browne M, Smeets P.M, Roche B. A transfer of functions and a conditional transfer of functions through equivalence relations in three to six year old children. The Psychological Record. 1995;45:405–430. [Google Scholar]
- Barnes D, Holmes Y. Radical behaviorism, stimulus equivalence and human cognition. The Psychological Record. 1991;41:19–31. [Google Scholar]
- Barnes D, Keenan M. A transfer of functions through derived arbitrary and nonarbitrary stimulus relations. Journal of the Experimental Analysis of Behavior. 1993;59:61–81. doi: 10.1901/jeab.1993.59-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D, Lawlor H, Smeets P.M, Roche B. Stimulus equivalence and academic concept among mildly handicapped and nonhandicapped children. The Psychological Record. 1996;46:87–107. [Google Scholar]
- Barnes-Holmes D, Hayes S.C. A reply to Galizio's “The abstracted operant: A review of Relational Frame Theory: A post-Skinnerian account of human language and cognition”. The Behavior Analyst. 2003;26:305–310. doi: 10.1007/BF03392084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes-Holmes D, Hayes S.C, Dymond S, O'Hora D. Multiple stimulus relations and the transformation of stimulus functions. In: Hayes S.C, Barnes-Holmes D, Roche B, editors. Relational Frame Theory: A post-Skinnerian account of human language and cognition. New York: Kluwer Academic/Plenum Publishers; 2001. pp. 51–72. [Google Scholar]
- Barnes-Holmes D, Keane J, Barnes-Holmes Y, Smeets P.M. A derived transfer of emotive functions as a means of establishing differential preferences for soft drinks. The Psychological Record. 2000;50:493–511. [Google Scholar]
- Cavanagh K, Davey G.C.L. UCS expectancy bias in spider phobics: Underestimation of aversive consequences following fear irrelevant stimuli. Behavior Research and Therapy. 2000;38:641–651. doi: 10.1016/s0005-7967(99)00077-7. [DOI] [PubMed] [Google Scholar]
- Cook M, Mineka S. Observational conditioning of fear to fear-relevant versus fear-irrelevant stimuli in rhesus monkeys. Journal of Abnormal Psychology. 1989;98:448–459. doi: 10.1037//0021-843x.98.4.448. [DOI] [PubMed] [Google Scholar]
- Dougher M.J, Auguston E, Markham M.R, Greenway D.E, Wulfert E. A transfer of self-discrimination response functions through equivalence relations. Journal of the Experimental Analysis of Behavior. 1994;62:251–267. doi: 10.1901/jeab.1994.62-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dymond S, Barnes D. The effects of prior equivalence testing and verbal instructions on derived self-discrimination transfer: A follow-up study. The Psychological Record. 1998;48:147–170. [Google Scholar]
- Forsyth J.P, Eifert G.H. Response intensity in content-specific fear conditioning comparing 20% versus 13% CO2-enriched air as unconditioned stimuli. Journal of Abnormal Psychology. 1998;107:291–304. doi: 10.1037//0021-843x.107.2.291. [DOI] [PubMed] [Google Scholar]
- Forsyth J.P, Eifert G.H, Thompson R.N. Systematic alarms in fear conditioning II: An experimental methodology using 20% carbon dioxide inhalations as an unconditioned stimulus. Behavior Therapy. 1996;27:391–415. [Google Scholar]
- Garotti M, de Souza D.G, de Rose J.C, Molina R.C, Gil M.A. Reorganizaion of equivalence classes after reversal of baseline relations. The Psychological Record. 2000;50:35–48. [Google Scholar]
- Grey I.M, Barnes D. Stimulus equivalence and attitudes. The Psychological Record. 1996;46:243–270. [Google Scholar]
- Hall G. Learning about associatively activated stimulus representations: Implications for acquired equivalence and perceptual learning. Animal Learning and Behavior. 1996;24:233–255. [Google Scholar]
- Hayes L.J. Equivalence as process. In: Hayes S.C, Hayes L.J, editors. Understanding verbal relations. Reno, NV: Context Press; 1992. pp. 97–108. [Google Scholar]
- Hayes S.C, Barnes-Holmes D, Roche B. Relational Frame Theory: A post-Skinnerian account of human language and cognition. New York: Kluwer Academic/Plenum Publishers; 2001. [DOI] [PubMed] [Google Scholar]
- Hayes S.C, Brownstein A.J. Mentalism, behavior-behavior relations, and a behavior analytic view of the purposes of science. The Behavior Analyst. 1986;9:175–190. doi: 10.1007/BF03391944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy O, Barnes-Holmes D, Smeets P. Derived relational responding as generalized operant behavior. Journal of the Experimental Analysis of Behavior. 2000;74:207–227. doi: 10.1901/jeab.2000.74-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P.J. The application of psychophysiological methods to the study of psychotherapy and behavior modification. In: Bergin A.E, Garfield S.L, editors. Handbook of psychotherapy and behavior change. New York: John Wiley & Sons; 1971. [Google Scholar]
- Layng M.P, Chase P.N. Stimulus-stimulus pairing, matching-to-sample testing and emergent relations. The Psychological Record. 2001;51:605–628. [Google Scholar]
- Leader G, Barnes D, Smeets P.M. Establishing equivalence relations using a respondent-type training procedure. The Psychological Record. 1996;46:685–706. [Google Scholar]
- Leslie J.C, Blackman D. Experimental and applied analysis of human behavior. Reno, NV: Context Press; 2000. [Google Scholar]
- Leslie J.C, Tierney K.J, Robinson C.P, Keenan M, Barnes D. Differences between clinically anxious and non-anxious subjects in a stimulus equivalence training task involving threat words. The Psychological Record. 1993;43:153–161. [Google Scholar]
- Lovibond P.F. Causal beliefs and conditioned responses: Retrospective revaluation induced by experience and by instruction. Journal of Experimental Psychology: Learning, Memory, & Cognition. 2003;29:97–106. [PubMed] [Google Scholar]
- Lovibond P.F, Shanks D.R. The role of awareness in Pavlovian Conditioning: Empirical evidence and theoretical implications. Journal of Experimental Psychology. 2002;28:3–26. [PubMed] [Google Scholar]
- Mackay H.A. Conditional stimulus control. In: Huston J.P, Iverson I.H, Lattal K.A, editors. Techniques in the behavioral and neural sciences: Vol 6. Experimental analysis of behavior, Part 1. Amsterdam: Elsevier; 1991. pp. 300–350. (Series Ed.) (Vol. Eds.), [Google Scholar]
- Markham M.R, Dougher M.J, Augustson E.M. Transfer of operant discrimination and respondent elicitation via emergent relations of compound stimuli. The Psychological Record. 2002;52:325–350. [Google Scholar]
- Marks I.M. Fears and phobias. New York: Academic Press; 1969. [Google Scholar]
- McNally R.J. Preparedness and phobias: A review. Psychological Bulletin. 1987;101:283–303. [PubMed] [Google Scholar]
- Mineka S, Tomarken A.J. The role of cognitive biases in the origins and maintenance of fear and anxiety disorders. In: Archer T, Nilsson L, editors. Aversion, avoidance and anxiety. Hillsdale, NJ: Erlbaum; 1989. pp. 195–221. [Google Scholar]
- Mohlman J, Zinbarg R.E. What kind of attention is necessary for fear reduction? An empirical test of the emotional processing model. Behavior Therapy. 2000;31:113–133. [Google Scholar]
- Moxon P.D, Keenan M, Hine L. Gender-role stereotyping and stimulus equivalence. The Psychological Record. 1993;43:381–393. [Google Scholar]
- Nakagawa E. Emergent, untrained stimulus relations in many-to-one matching-to-sample discrimination in rats. Journal of the Experimental Analysis of Behavior. 2005;83:185–195. doi: 10.1901/jeab.2005.41-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilgrim C, Galizio M. Relations between baseline contingencies and equivalence probe performances. Journal of the Experimental Analysis of Behavior. 1990;54:213–224. doi: 10.1901/jeab.1990.54-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilgrim C, Galizio M. Reversal of baseline relations and stimulus equivalence: I. Adults. Journal of the Experimental Analysis of Behavior. 1995;63:225–238. doi: 10.1901/jeab.1995.63-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilgrim C, Galizio M. Stimulus equivalence: A class of correlations, or a correlation of classes? In: Zentall T.R, Smeets P.M, editors. Stimulus class formation in humans and animals. New York: Elsevier Science; 1996. pp. 173–195. [Google Scholar]
- Reiss S. Pavlovian conditioning and human fear: An expectancy model. Behavior Therapy. 1980;11:380–396. [Google Scholar]
- Roche B, Barnes D. A transformation of respondently conditioned stimulus function in accordance with arbitrarily applicable relations. Journal of the Experimental Analysis of Behavior. 1997;67:275–301. doi: 10.1901/jeab.1997.67-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche B, Barnes D, Smeets P.M. Incongruous stimulus pairing contingencies and conditional discrimination training: Effects on relational responding. Journal of the Experimental Analysis of Behavior. 1997;68:143–160. doi: 10.1901/jeab.1997.68-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche B, Barnes-Holmes D, Smeets P.M, Barnes-Holmes Y, McGeady S. Contextual control over the derived transformation of discriminative and sexual arousal functions. The Psychological Record. 2000;50:267–291. [Google Scholar]
- Saunders R.R, Drake K.M, Spradlin J.E. Equivalence class establishment, expansion, and modification in preschool children. Journal of the Experimental Analysis of Behavior. 1999;7:195–214. doi: 10.1901/jeab.1999.71-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders R.R, Saunders K.J, Kirby K.C, Spradlin J.E. The merger and development of equivalence classes by unreinforced conditional selection of comparison stimuli. Journal of the Experimental Analysis of Behavior. 1988;50:145–162. doi: 10.1901/jeab.1988.50-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell A.M, Dawson M.E, Marinkovic K. Effects of potentially phobic conditioned stimuli on retention, reconditioning, and extinction of the conditioned skin conductance response. Psychophysiology. 1991;28:140–153. doi: 10.1111/j.1469-8986.1991.tb00403.x. [DOI] [PubMed] [Google Scholar]
- Sidman M. Equivalence relations and behavior: A research story. Boston, MA: Authors Cooperative; 1994. [Google Scholar]
- Smeets P.M, Barnes-Holmes D. Children's emergent preferences for soft drinks: Stimulus-equivalence and transfer. Journal of Economic Psychology. 2003;24:603–618. [Google Scholar]
- Smeets P.M, Barnes-Holmes D. Auditory-visual and visual-visual equivalence relations in children. The Psychological Record. in press. [Google Scholar]
- Smeets P.M, Barnes-Holmes Y, Akpinar D, Barnes-Holmes D. Reversal of equivalence relations. The Psychological Record. 2003;53:91–119. [Google Scholar]
- Tonneau F, Gonzalez C. Function transfer in human operant experiments: The role of stimulus pairings. Journal of the Experimental Analysis of Behavior. 2004;81:239–255. doi: 10.1901/jeab.2004.81-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcuioli P.J. Acquired equivalences and mediated generalization in pigeon's matching-to-sample. In: Zentall T.R, Smeets P.M, editors. Stimulus class formation in humans and animals. Holland: Elsevier Science; 1996. pp. 55–70. [Google Scholar]
- Ward-Robinson J, Hall G. Backward sensory preconditioning. Journal of Experimental Psychology: Animal Behavior Processes. 1996;22:395–404. [Google Scholar]
- Watt A, Keenan M, Barnes D, Cairns E. Social categorization and stimulus equivalence. The Psychological Record. 1991;41:33–50. [Google Scholar]
- Watts F.N, Sharrock R. Questionnaire dimensions of spider phobia. Behavior Research and Therapy. 1984;22:575–580. doi: 10.1016/0005-7967(84)90061-5. [DOI] [PubMed] [Google Scholar]
- Wulfert E, Hayes S.C. The transfer of conditional sequencing through conditional equivalence classes. Journal of the Experimental Analysis of Behavior. 1988;50:125–144. doi: 10.1901/jeab.1988.50-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentall T.R, Smeets P.M, editors. Stimulus class formation in humans and animals. New York: Elsevier Science; 1996. [Google Scholar]