Abstract
Study objectives
The aim of this study was to characterize the elemental composition of exhaled breath condensate (EBC) in order to identify new biomarkers of exposure and susceptibility in COPD patients. Serum pneumoproteins were used as lung-specific biomarkers of effect.
Design
EBC was obtained from 50 healthy subjects, 30 healthy smokers, 30 asthmatics, and 50 patients with stable COPD, and was collected by cooling exhaled air. Trace elements and toxic metals in the samples were measured by means of inductively coupled plasma-mass spectrometry and electrothermal atomic absorption spectroscopy. The serum pneumoproteins were immunoassayed.
Results
The EBC of COPD subjects had higher levels of such toxic elements as lead, cadmium, and aluminum, and lower levels of iron and copper, than that of the nonsmoking control subjects. There were no between-group differences in surfactant protein (SP)-A and SP-B levels. Clara-cell protein and SP-D levels were negatively and positively influenced, respectively, by tobacco smoke.
Conclusions
Our results show that toxic metals and transition elements are detectable in the EBC of studied subjects. We propose new biomarkers of exposure as a means of assessing the target tissue dose of carcinogenic and pneumotoxic substances from tobacco smoke or polluted workplaces, and the use of the transition elements involved in redox systems of oxidative stress as disease biomarkers associated with effect or susceptibility. Together with biomarkers of effect, such as serum pneumoproteins, the elemental composition of EBC may be clinically useful in distinguishing similar diseases.
Keywords: COPD, exhaled breath condensate, metals, pneumoproteins, trace elements
Abbreviations: ANOVA = analysis of variance, CC16 = Clara-cell secretary protein, EBC = exhaled breath condensate, ETAAS = electrothermal atomic absorption spectroscopy, ICP-MS = inductively coupled plasma-mass spectrometry, LOD = limit of detection, PM = particulate matter, SOD = superoxide dismutase, SP = surfactant protein
COPD is one of the leading causes of morbidity and mortality worldwide, and represents a substantial economic burden on global health.1 It is characterized by small airways disease, mucus hyper-secretion, and chronic bronchitis, which lead to an obstructed airflow that impairs ventilatory capacity and gas exchange, and causes shortness of breath.1
Various instruments have been developed for diagnosing, monitoring, and evaluating COPD. Lung function tests offer insights into changes in airway caliber, exhaled air flow, lung volumes, and gas exchange,2 and imaging techniques (especially high-resolution CT) offer insight into the loss of lung tissue3; both provide indirect measurements of the adverse effects occurring in a biological system as a consequence of exposure to toxic or noxious agents. More direct evaluation of biological lung events can be obtained using more or less invasive measurements (bronchoscopy, induced sputum). These have improved our understanding of the biological processes occurring in lung diseases and still represent the “gold standard” for evaluating the diseases themselves,4 but their applicability is limited mainly because the invasiveness of the sampling procedures makes them unsuitable for routine clinical use.
Modern research relies on biomarkers, which are defined as any substance, structure, or process that can be measured in the body or its products, and which influence or predict the incidence of outcome or disease.5 Biomarkers of lung diseases can be developed on the basis of exhaled air and blood analyses.
The analysis of exhaled air is feasible and noninvasive.6 Exhaled breath condensate (EBC) obtained by cooling exhaled air under conditions of spontaneous breathing is a promising biological fluid that could provide a real-time assessment of pulmonary pathobiology. It can be easily and noninvasively collected from patients of any age using portable devices in an outpatient setting or even at home. EBC is particularly suitable for the sequential and longitudinal sampling of the lower respiratory tract, and published data on inflammation mediators suggest that it reflects the abnormalities noted in bronchoscopic specimens.7 Despite the enthusiasm of a few research groups, there is skepticism concerning the diagnostic and monitoring validity of EBC because of the analytical problems associated with measuring trace amounts of unstable and nonspecific mediators, which mainly relies on immunochemistry techniques that lack reference methods and materials, and are affected by their poor sensitivity, specificity, and selectivity.8
As EBC mainly consists of water that is practically free of potentially interfering solutes, it is an ideal biological fluid for elemental determinations based on relatively common techniques, such as electrothermal atomic absorption spectroscopy (ETAAS), or less frequently available reference instruments such as inductively coupled plasma-mass spectrometry (ICP-MS).
The aim of this study was to investigate the elemental composition of EBC in order to identify new biomarkers of exposure, or susceptibility, in COPD patients. The working hypothesis was that long-term exposure to tobacco smoke (which may cause the development of COPD) leads to an increased lung uptake of toxic metals that, because of their stability, can also be used as tracers of environmental pollution. Likewise, hard metals in occupationally exposed workers9 and toxic metals (such as lead, cadmium, chromium, nickel, and aluminum) should provide a quantitative estimate of target tissue burden.
EBC was also used to quantify essential elements as biomarkers of susceptibility, which have been defined as “indicators of an inherited or acquired limitation of an organism’s ability to respond to the challenge of exposure to a xenobiotic substance.”5 Individual detoxifying capacity modulates the lung response to inhaled pneumotoxic substances, and there is considerable variability in individual responses to toxic substances. A growing body of evidence indicates that many transition elements play important roles in biological processes by activating or inhibiting enzymatic reactions, by competing with other elements and metalloproteins for binding sites, or by affecting the permeability of cell membranes.
The possible biomarkers of susceptibility were investigated bearing in mind that some transition elements play a fundamental role in the respiratory chain (iron), or are components of mitochondrial (manganese) or cytoplasmic (copper, zinc) superoxide dismutases (SODs), or glutathione peroxidase (selenium). Given their key roles in the generation and detoxification of reactive oxygen species, and the scavenging of free radicals, transition elements modulate the response to toxic substances,10 thus possibly accounting for the limited proportion (15 to 20%) of smokers acquiring COPD. Biomarkers of susceptibility may be useful in identifying and counseling people at increased risk of disease when exposed to tobacco smoke or environmental pollutants.
Finally, we analyzed serum pneumoproteins (Clara-cell secretory protein [CC16]), and three surfactant proteins (SPs), SP-A, SP-B, and SP-D, which were used as a complementary approach to develop biomarkers of effect suitable for the long-term monitoring of COPD patients. As these proteins are mainly (if not exclusively) secreted within the respiratory tract, their occurrence in the vascular compartment can only be explained by assuming their leakage from the lung into the bloodstream. Excluding changes in renal function, their serum concentrations can be expected to reflect their rate of synthesis and the permeability of the lung epithelium.11
Materials and Methods
Subject Characteristics
The characteristics of the study subjects are shown in Table 1. The sample size required to obtain a sufficient power was calculated taking into account the mean values and SDs of EBC elements in control subjects, and assuming that a 100% increase or 50% decrease in comparison with the central tendency of the controls might reflect biologically relevant processes. All of the COPD patients met the Global Initiative for Chronic Obstructive Lung Disease1 diagnostic criteria: history of cough and sputum production for > 2 consecutive years and for most days in a consecutive 3-month period, as well as fixed airflow obstruction, which was defined as a postbronchodilator FEV1/FVC ratio < 70%, and a postbronchodilator reversibility of FEV1 of < 12% measured at baseline and after the inhalation of a β2-agonist (salbutamol, 400 μg, via a metered-dose inhaler).
Table 1.
Characteristics of Study Subjects*
| Characteristics | COPD | Asthma | Smokers | Control |
|---|---|---|---|---|
| Subjects | 50 | 30 | 30 | 50 |
| Female gender | 14 | 19 | 10 | 12 |
| Age, yr | 65.6 (2.1) | 44.6 (2.7) | 43.6 (1.8) | 54.9 (2.1) |
| Smokers/ex-smokers/nonsmokers | 16/28/6 | 0/4/26 | 30/0/0 | 0/6/44 |
| Pack/yr for smokers | 28.9 (4.3) | 0 | 17.7 (2.3) | 0 |
| FVC, L | 4.5 (1.6) | 3.8 (0.2) | 4.5 (0.1) | 4.6 (0.1) |
| FVC, % of predicted | 83.3 (4.4) | 104.3 (3.1) | 109.5 (2.2) | 111.8 (2.2) |
| FEV1, L | 1.7 (0.1) | 2.9 (0.2) | 3.5 (0.1) | 3.7 (0.1) |
| FEV1, % of predicted | 60.3 (3.8) | 94.1 (3.5) | 103.6 (2) | 106.1 (0.9) |
| FEV1/FVC | 0.5 (0.2) | 0.75 (0.01) | 0.8 (0.08) | 0.77 (0.8) |
Data are presented as mean (SEM) or No.
Asthma was diagnosed according to the National Institutes of Health guidelines12 as a ≥ 12% increase in FEV1 in response to a bronchodilator. None of the COPD or asthma patients were receiving oral corticosteroids, and none reported any worsening in their symptoms (exacerbation) during at least the 4 weeks preceding EBC collection.
The other study subjects were control smokers and nonsmokers with normal spirometry results and no significant history of respiratory diseases. Tobacco smoke exposure was evaluated in terms of self-reported current smoking status, the number of cigarettes smoked per day, the number of years of tobacco smoking, and (in ex-smokers) the number of years since stopping. Ex-smokers were defined as those who had stopped smoking at least 1 year before the test (mean, 12.7 years; SEM, 2.3 years). In order to avoid possible confounding factors, subjects using drugs such as salicylates, trace element-containing supplements, and patients with concomitant renal, hepatic, cardiac, immunologic, or inflammatory diseases or cancer were excluded.
The study was conducted in conformity with the Declaration of Helsinki and was approved by the Ethics Committee of the University of Parma. All of the patients gave informed written consent.
EBC Collection and Analysis
EBC was collected using a portable condenser (TURBO-DECCS; ItalChill; Parma, Italy) specifically designed to collect EBC in clinical and working settings (TURBO-DECCS is the acronym of “transportable unit for research on biomarkers obtained from disposable exhaled condensate collection systems”), and whose validity has recently been shown.13 The condenser has a refrigerating system that thermostatically controls the working temperature, and a disposable respiratory system consisting of a mouthpiece connected to a one-way aspiration valve and (through a tube with a special stopper) an EBC collection test tube at the end. On the basis of preliminary data, we chose a condensation temperature of − 5°C.13 The concentrations of all residual elements on the condenser walls were below the limit of detection (LOD).
The subjects were asked to breathe tidally through the mouthpiece for 15 min while sitting comfortably in the laboratory. They were instructed to form a complete seal around the mouthpiece with their mouths, which had to be kept dry by periodically swallowing excess saliva; they were also asked to rinse their mouths thoroughly before the maneuver and every 5 min during the test. In preliminary experiments, we observed that the mouth-rinsing maneuver did not influence the elemental composition of EBC.
Salivary contamination was excluded by means of the colorimetric detection of α-amylase (Infinity Amylase Reagent; Sigma; Milan, Italy), whose LOD is approximately 1/5,000 to 1/10,000 of the activity measured in saliva. However, as stated in the recent European Respiratory Society/American Thoracic Society task force report,14 it is not very useful to measure EBC amylase without also measuring the salivary concentration of the studied biomarkers. In order to address this issue further, we measured the concentrations of the selected metals in the saliva and EBC of 15 healthy subjects, and calculated their saliva/EBC ratio, whose maximum was as follows: nickel: 7 times; lead, 12 times; cadmium, 15 times; copper, 45 times; manganese, 140 times; aluminum, 285 times; and iron, 700 times. A dilution factor of 5,000 (the worst case in terms of sensitivity of the amylase kit) would imply a maximum of approximately 14% of salivary iron in EBC and a proportion even lower for the other elements, thus not enough to justify the differences we observed between groups and intersubject variability. Therefore, the amylase kit was adequate to rule out salivary contamination, at least for the selected biomarkers we measured. Furthermore, we observed that the elemental pattern in saliva and in EBC is significantly different from each other (data not shown), thus making the salivary contamination of EBC negligible. However, according to American Thoracic Society/European Respiratory Society guidelines,14 further studies are in progress to develop an alternative and more sensitive test than amylase one. Contamination due to ambient air was also excluded; in fact, when outpatients’ room ambient air was passed through cooled condensers for 120 min, the concentration of elements was below the LOD.
The EBC samples were transported in dry ice to the laboratory and stored at − 80°C until analysis. Their elemental composition was determined by means of ETAAS or ICP-MS, as appropriate in terms of analytical reliability. Both the ICP-MS (ELAN 5000; Perkin Elmer; Wellesley, MA) and Zeeman ETAAS (SpectrAA 220Z; Varian; Palo Alto, CA) analyses were made using the external calibration method15,16 and reference material 1643 from the National Institute of Standards and Technology (Gaithersburg, MD) for the assessment of analytical accuracy.
The data concerning the validity of ETAAS and ICP-MS methods in measuring some toxic elements (cobalt and tungsten) in EBC samples have been previously published.9 We further controlled the analytical validity of other elemental determinations in EBC in order to obtain accurate and reliable results: briefly, as observed for cobalt and tungsten, an agreement between data obtained with two techniques relying on different principles (optical and mass-spectrometric techniques) was observed, thus strongly supporting the validity of analytical results.
Of the 33 elements determined, only those with measurable levels in > 50% of the samples from COPD or control subjects are reported. The LOD for selenium and aluminum by ICP-MS was 0.01 μg/L (calculated as 3 SDs of the blank), the limit of quantification being approximately 0.03 μg/L; the sensitivity for the other elements was even higher (LOD and limit of quantification values of 0.005 μg/L and 0.015 μg/L, respectively). The coefficients of variation for the measurements of standard samples (5 μg/L and 0.5 μg/L) in water and in a pool of EBC were < 10% (higher concentration) and 20% (lower concentration) for all intraday and interday determinations.
Serum Analysis
CC16 concentration was determined by means of a latex immunoassay using rabbit anti-Clara cell antibody (Dakopatts; Glostrup, Denmark) and CC16 purified in our laboratory as standards,17 with all of the samples being run in duplicate at two different dilutions. This assay has validated against a monoclonal antibody-based enzyme-linked immunosorbent assay,18 and the between-run and within-run coefficients of variation range from 5 to 10%. The serum concentrations of SP-A and SP-B were determined by enzyme-linked immunosorbent assay,19 and that of SP-D was measured by SP-D enzyme immunoassay.20
Statistics
The statistical analysis was performed using statistical software (Prism 4; GraphPad; San Diego, CA; and SPSS 13.0; SPSS; Chicago, IL). The Gaussian nature of the data distribution was assessed using Kolmogorov-Smirnov test. The between-group comparisons were made by means of one-way analysis of variance (ANOVA) followed by Tukey test in the case of a normal or log-normal distribution, or by means of the Kruskal-Wallis test followed by Dunn test in the case of nonnormally distributed variables. Depending on the data distribution, the correlations between the variables were assessed using Pearson or Spearman tests. The significance level for all of the tests was p = 0.05 (two sided). Finally, a parametric multivariate ANOVA of the log-transformed data, reinforced by a nonparametric ANOVA (http://www.folk.uio.no/ohammer/past/index.html), was performed to test the overall significance of the differences between groups and the role of age as a possible covariate.
Results
Biomarkers of Exposure
The toxic metals (lead, cadmium, nickel, aluminum) found in the EBC samples are shown in Figure 1. The COPD patients had higher levels of lead, cadmium, and aluminum than the nonsmoking control subjects. Interestingly, only nickel levels were higher in the asthmatics than in the smokers. The control smokers had higher lead and cadmium levels than the control nonsmokers. When the COPD patients were subdivided into smokers vs ex-smokers and nonsmokers, the former had higher lead, cadmium, and aluminum levels, whereas there was no difference in nickel levels (Fig 2).
Figure 1.
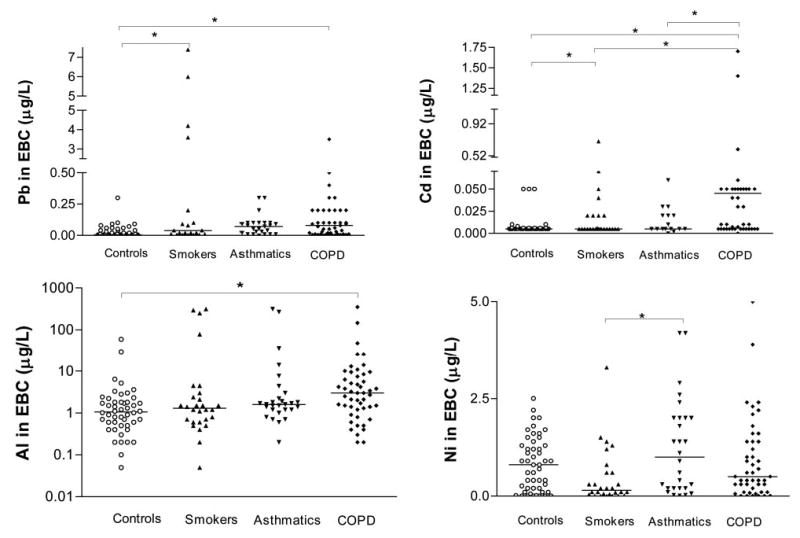
Lead, cadmium, aluminum (expressed in log10 scale), and nickel levels in the EBC of the studied groups. Between-group differences were sought using the Kruskal-Wallis test (p < 0.0001), followed by Dunn multiple comparison test (*p < 0.05). The horizontal lines represent median values.
Figure 2.
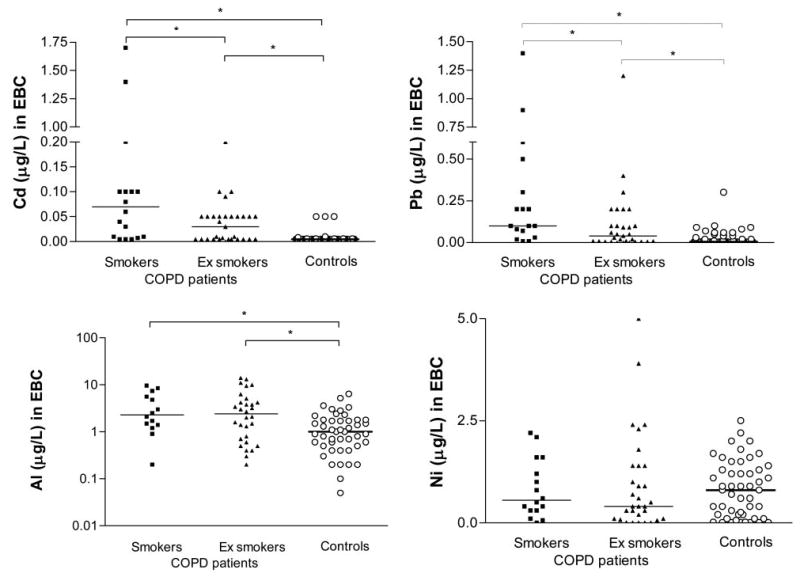
Lead, cadmium, aluminum (expressed in log10 scale), and nickel levels in the EBC of COPD patients (smokers vs ex-smokers and nonsmokers) and control subjects (*p < 0.05, Mann-Whitney U test). The horizontal lines represent median values.
The cadmium levels in the COPD patients positively correlated with smoking history (pack-years) [r = 0.5; p = 0.001; data not shown]. No correlations were observed between spirometric values and EBC toxic metal concentrations.
Biomarkers of Susceptibility
Figure 3 shows the iron, selenium, copper, and manganese levels in the EBC samples. The COPD patients had lower iron and copper levels than the control nonsmokers. There were no between-group differences in manganese and selenium levels. No differences were observed in the levels of transition elements when the COPD patients were subclassified into smokers vs ex-smokers and nonsmokers (data not shown). The copper levels in the COPD patients positively correlated with their FEV1 values (Fig 4).
Figure 3.
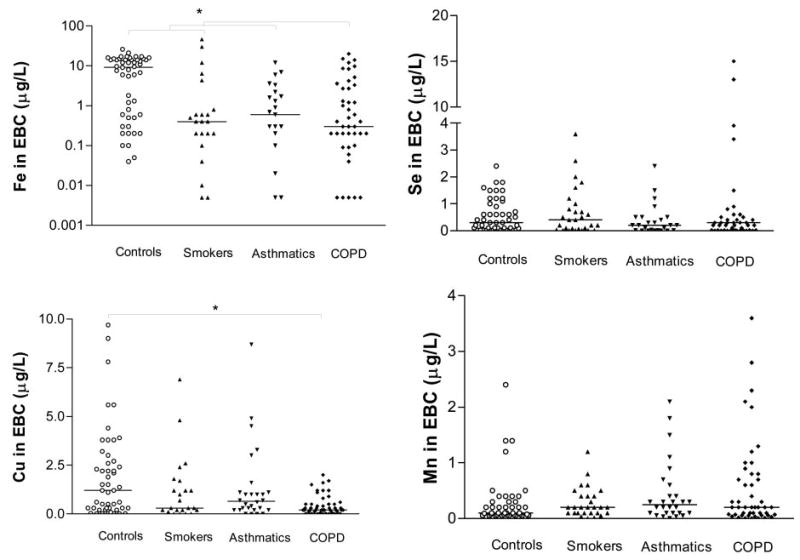
Iron (expressed in log10 scale), selenium, copper, and manganese levels in the EBC of the studied groups. Between-group differences in iron and copper were sought using the Kruskal-Wallis test (p < 0.0001), followed by Dunn multiple comparison test (*p < 0.05); one-way analysis of variance was used for selenium and manganese. The horizontal lines represent the median values of iron and copper, and the mean values of selenium and manganese.
Figure 4.
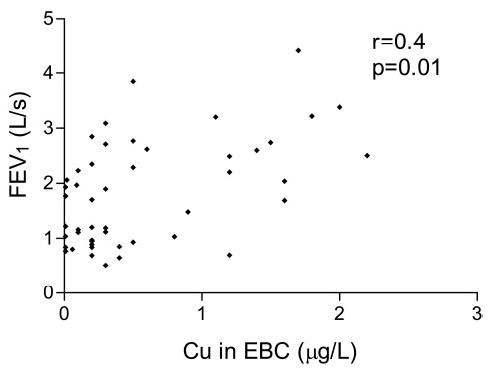
Spearman correlations between EBC copper levels and FEV1 in COPD patients.
Biomarkers of Effect
The data concerning serum pneumoprotein levels are shown in Figure 5. CC16 levels were lower in the current smokers than in the healthy nonsmoking control subjects and COPD patients. There were no between-group differences in SP-A and SP-B levels, but SP-D levels were higher in the control smokers than in the control nonsmokers and asthmatics. The COPD patients had higher SP-D levels than the asthmatics and control smokers. When the COPD patients were subclassified on the basis of their smoking habits, CC16 levels were lower in the smokers than in the ex-smokers or nonsmokers (p = 0.005); there were no differences in SP-D levels. The control smokers showed a negative correlation between serum CC16 levels and the number of cigarettes per day, whereas in the COPD ex-smokers, they positively correlated with the number of years since stopping smoking. Serum SP-D levels in the COPD patients positively correlated with their smoking history (cigarette smoking, r = 0.4, p = 0.003; pack-years, r = 0.4, p = 0.04). Serum CC16 levels negatively correlated with the EBC concentrations of lead (r = − 0.2, p = 0.02) and cadmium (r = − 0.2, p = 0.04).
Figure 5.
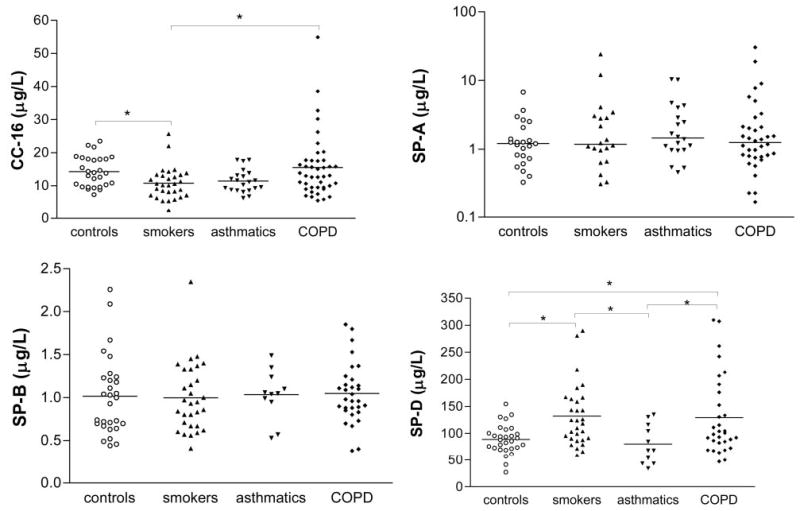
Serum CC-16, SP-A (expressed in log10 scale), SP-B, and SP-D levels in the studied groups. Between-group differences in SP-A were sought using the Kruskal-Wallis test; one-way analysis of variance followed by Tukey multiple comparison test were used for CC-16, SP-D (*p < 0.05), and SP-B. The horizontal lines represent the median values of SP-A, and the mean values of the other pneumoproteins.
Discussion
The results of this study show that toxic metals and transition elements are detectable in the EBC of healthy and nonsmoking control subjects, and COPD patients. Together with biomarkers of effect, such as pneumoproteins, the metal composition of EBC could be clinical useful in distinguishing potentially overlapping diseases. Elemental analysis could also provide mechanistic insights that may be useful in setting up preventive and possibly curative interventions.
To the best of our knowledge, this is the first report concerning the metal composition of the EBC of COPD patients, and so our results cannot be compared with previous findings. Although a comparison between EBC and BAL fluid element levels would be very interesting, it may be difficult because some authors21 have measured BAL element levels per number of cells. Romeo et al22 reported BAL metal concentrations in micrograms per liter, and it is interesting to note that these were of the same order of magnitude of those measured by us (apart from iron, whose intracellular concentrations may affect the data).
It is also difficult to compare published serum elemental composition23 with EBC data because the first is representative of the entire body rather than lung burden. EBC elemental analysis is a novel approach that reflects specific pulmonary levels of pneumotoxic or essential transition elements, and can be considered a significant advance in lung physiology in comparison with the analysis of alternative media (BAL fluid, blood, serum, urine, hair), which are not as reliable because of the presence of interfering substances in the complex matrix.
The source of the elements found in EBC is the first obvious question we tried to answer. Any possible release from plastics or contamination during EBC collection was excluded in repeated experiments (not shown) involving the extensive washing of each component of the cooling circuit. Furthermore, any relevant contribution to the exhaled metals from the upper airways was ruled out by the fact that the metal levels in EBC collected from the mouth are of the same order of magnitude as those obtained in EBC below the glottides of intubated subjects (data not shown).
In relation to the biomarkers of exposure, cadmium, lead, and aluminum levels were higher in the EBC of our COPD patients than in the control subjects. Given that these toxic metals are all contained in tobacco smoke,24,25 EBC metal levels could be biomarkers of current tobacco smoking (like exhaled carbon monoxide, urinary cotinine), but there were still differences between the COPD patients and control subjects when the former were classified into smokers and ex-smokers or nonsmokers. This means that exhaled metal levels may also provide a measure of cumulative long-term exposure to tobacco smoke and environmental exposure to toxic metals.
When the COPD patients were classified on the basis of disease severity (Global Initiative for Chronic Obstructive Lung Disease guidelines), we did not find any differences among subgroups (multivariate analysis) in EBC levels of toxic metals; however, the present study was not powered enough to address this issue. Further studies are therefore warranted to evaluate whether the levels of toxic elements in EBC change depending on the severity of the underlying chest disease.
Exhaled metals are not just a means of assessing exposure to tobacco smoke, but they are also markers of the dose because of their inherent lung toxicity. For example, it is known that cadmium exposure leads to the development of emphysema,26 and in vitro evidence suggests that cadmium may play an important role in the pathogenesis of the emphysema associated with the long-term inhalation of cadmium fumes by inhibiting the production of connective tissue proteins.27 It is interesting to note that, in a representative US population, higher urinary cadmium levels were found to be significant predictors of lower FVC, FEV1, and FEV1/FVC values in current and former smokers, but not in neversmokers.28
Although there is no clear evidence of a relationship between lead exposure and the development of COPD, lead may play a pathogenetic role because of its high affinity for sulfhydryl groups, which may cause glutathione depletion and thus reduce its scavenging potential. It is worth noting that the study of transition metals in exhaled air may also help to assess the toxic lung effects of particulate air pollution (ie, particulate matter [PM] up to 10 μm in diameter, and PM up to 2.5 μm in diameter) because transition elements may modulate the toxic effects of PM up to 10 μm in diameter by means of oxidative stress,29 and there is now considerable epidemiologic evidence supporting a relationship between increased levels of particulate air pollution and increased morbidity and mortality due to respiratory diseases.30
Blood or urine levels of toxic metals may reflect their absorption, but exhaled toxic metal analysis may make it possible to assess metal dose at target tissue level. This may give rise to new therapeutic strategies (procedures and/or interventions aimed at altering the levels of trace element and toxic metals, such as the use of detoxifying and metal chelating agents or scavengers) in a disease for which no curative therapies are currently available.1,31
Although the changes in EBC manganese and selenium levels were not statistically significant, our COPD patients had reduced EBC copper and iron levels. This is at least partially in line with a study32 showing a decrease in the levels of various metals (calcium, manganese, iron, copper, zinc, selenium) in cancerous lung tissue, and thus supports the view that chronic oxidative stress may be associated with the metal depletion involved in the antioxidant responsive element.
EBC copper levels in COPD patients may be of particular interest because of their positive correlation with lung function parameters. Although there are published studies of the expression of SODs in the lung tissue of patients with COPD, and we did not evaluate lung SOD expression, we speculate that low copper levels may be associated with a reduction in SOD expression and activity, which could be due to the direct toxic effects of cigarette smoke or neutrophil-derived free radicals. Numerous studies have investigated whether injected or inhaled SOD enzyme proteins can protect lung tissue against oxidant injury, and a number of classes of synthetic SOD mimetics have been developed and shown to possess significant antioxidant capacity.33 However, we are aware that the information obtained by means of elemental analysis may be ambiguous, not least because certain transition elements can be essential or toxic depending on their valence, physical state, and total burden; further work is therefore needed to support our hypothesis and relate the transition elements of biological relevance with the pulmonary proteins that have them as cofactors.
Nonvolatile substances such as elements are mainly expired in small droplets and further diluted with exhaled water vapors.34 It is thought that droplet formation is due to random convective processes and may not be directly related to water vapor production, something that has raised the issue of variable droplet dilution in EBC and given rise to some concerns regarding the interpretation of EBC biomarkers on the basis of their absolute concentration. However, Effros et al34 found that the mean values of three different putative dilution factors (urea, conductivity, and total cation levels) were not significantly different between normal and COPD subjects, and so the differences in mediator concentrations between our study groups may be attributable to differences in respiratory fluid concentrations rather than the number of droplets.
All of the EBC produced for this study was dedicated to elemental analysis, and so no data are available concerning the exhaled biomarkers of effect and we were unable to detect pneumoproteins in EBC. However, we have further ongoing studies aimed at evaluating whether there is a correlation between elemental levels and biomarkers of effect in COPD.
The few extreme data points that seem to distinguish different groups are unlikely to affect (influence) statistical differences, which have been calculated relying either on nonparametric tests (whose significance depends on ranks rather than on absolute values) or on parametric tests after log-transformation (necessary to obtain a normal distribution of data). In either cases, extreme values do not influence the statistical significance of observed differences.
Our COPD patients were older than the subjects belonging to the other groups. However, age had a significant effect only on EBC iron levels (F = 19.83, p < 0.001). Differences between groups remained highly significant considering age as a covariate (F = 20.44, p < 0.001).
Our CC16 data (the negative correlations between serum CC16 levels and smoking history, as well as EBC cadmium and lead levels) confirm that cigarette smoke reduces serum CC16 concentrations.32 The levels of this antiinflammatory protein returned to normal in the COPD patients who had stopped smoking (note the positive correlation between serum CC16 levels and the number of years as an ex-smoker).
There is sufficient published evidence35,36 to attribute the decrease in serum CC16 levels in smokers and COPD patients to chronic tobacco-induced damage to Clara cells. When renal function is normal or just slightly impaired (as in the volunteers we selected), serum CC-16 levels are only influenced by the intrapulmonary pool (eg, decreased in smokers) and the rate at which CC16 leaks into serum.
No between-group differences were observed in SP-A and SP-B levels, but the SP-D results were interesting. It is particularly interesting to note the different SP-D levels in asthmatics and COPD patients because these may be clinically useful in distinguishing potentially overlapping diseases. Unlike serum CC16 levels, serum SP-D concentrations were higher in both smokers and COPD patients than in normal control subjects. This may be explained on the basis of the role of SP-D in local host defense, and our data are in line with the increased serum SP-D levels reported in patients with various lung diseases.35 However, other plausible hypotheses concerning increased SP-D levels include increased lung epithelium permeability and increased surfactant degradation; although it has been demonstrated that SP-D is a “collectin” that is also found outside of the lungs and is therefore not an exclusively pulmonary marker.37
We conclude that EBC elemental analysis is a novel noninvasive approach to investigating lung pathobiology that is suitable for identifying the following: (1) the target tissue dose of carcinogenic and pneumotoxic substances from tobacco smoke and polluted workplaces or environments (cadmium, chromium, nickel, lead); and (2) the transition elements (manganese, copper, selenium, iron) involved in oxidative stress as part of redox systems. Together with biomarkers of effect such as serum pneumoproteins, the elemental composition of EBC may be clinically useful in distinguishing overlapping diseases.
Footnotes
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal.org/misc/reprints.shtml).
This study was supported by National Heart, Blood, and Lung Institute grant R01 HL72323. The contents of this article are solely the responsibility of the authors, and do not necessarily represent the official views of the National Heart, Blood, and Lung Institute or the National Institutes of Health.
From the Laboratory of Industrial Toxicology (Dr. Mutti), Department of Clinical Medicine, Nephrology and Health Sciences, University of Parma, Parma, Italy; National Institute of Occupational Safety and Prevention (Drs. Corradi and Vettori, and Mr. Goldoni), Research Centre at the University of Parma, Parma, Italy; Unit of Toxicology (Dr. Bernard), Catholic University of Louvain, Brussels, Belgium; and Laboratory of Industrial Hygiene (Dr. Apostoli), Department of Experimental and Applied Medicine, University of Brescia, Brescia, Italy.
References
- 1.Pauwels RA, Buist AS, Calverly PMA, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Am J Respir Crit Care. 2001;163:1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 2.Pellegrino R, Brusasco V. On the causes of lung hyperinflation during bronchoconstriction. Eur Respir J. 1997;10:468–475. doi: 10.1183/09031936.97.10020468. [DOI] [PubMed] [Google Scholar]
- 3.Baldi S, Miniati M, Bellina CR, et al. Relationship between extent of pulmonary emphysema by high resolution computed tomography and lung elastic recoil in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:585–589. doi: 10.1164/ajrccm.164.4.2010066. [DOI] [PubMed] [Google Scholar]
- 4.Saetta M, Turato G, Maestrelli P, et al. Cellular and structural bases of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:1304–1309. doi: 10.1164/ajrccm.163.6.2009116. [DOI] [PubMed] [Google Scholar]
- 5.NRC (National Research Council). Biological markers in environmental health research. Environ Health Perspect. 1987;74:1–19. doi: 10.1289/ehp.74-1474499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kharitonov SA, Barnes PJ. Exhaled markers of pulmonary disease. Am J Respir Crit Care Med. 2001;163:1693–1722. doi: 10.1164/ajrccm.163.7.2009041. [DOI] [PubMed] [Google Scholar]
- 7.Mutlu GM, Garey KW, Robbins RA, et al. Collection and analysis of exhaled breath condensate in humans. Am J Respir Crit Care Med. 2001;164:731–737. doi: 10.1164/ajrccm.164.5.2101032. [DOI] [PubMed] [Google Scholar]
- 8.Rahman I, Biswas SK. Non-invasive biomarkers of oxidative stress: reproducibility and methodological issues. Redox Rep. 2004;9:125–143. doi: 10.1179/135100004225005219. [DOI] [PubMed] [Google Scholar]
- 9.Goldoni M, Catalani S, De Palma G, et al. Exhaled breath condensate as a suitable matrix to assess lung dose and effects in workers exposed to cobalt and tungsten. Environ Health Perspect. 2004;112:1293–1298. doi: 10.1289/ehp.7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kondo H, Nakagaki I, Sasaki S, et al. Mechanism of oxidative stress in skeletal muscle atrophied by immobilization. Am J Physiol. 1993;265:E839–E844. doi: 10.1152/ajpendo.1993.265.6.E839. [DOI] [PubMed] [Google Scholar]
- 11.Hermans C, Bernard A. Lung epithelium-specific proteins: characteristics and potential applications as markers. Am J Respir Crit Care Med. 1999;159:646–678. doi: 10.1164/ajrccm.159.2.9806064. [DOI] [PubMed] [Google Scholar]
- 12.Revised GINA guidelines 2002: Global initiative for asthma. Bethesda, MD: National Institutes of Health, National Heart, Lung and Blood Institute. NIH publication No. 02–3659 2002
- 13.Goldoni M, Caglieri A, Andreoli R, et al. Influence of condensation temperature on selected exhaled breath parameters. BMC Pulm Med. 2005;5:10. doi: 10.1186/1471-2466-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horvath I, Hunt J, Barnes PJ. Exhaled breath condensate: methodological recommendations and unresolved questions. Eur Respir J. 2005;26:523–548. doi: 10.1183/09031936.05.00029705. [DOI] [PubMed] [Google Scholar]
- 15.Apostoli P, Morandi C, Menditto A. Multiple determination of elements in human seminal plasma. J Trace Elem Med Biol. 1998;11:182–184. doi: 10.1016/S0946-672X(97)80052-1. [DOI] [PubMed] [Google Scholar]
- 16.Apostoli P. Elements in environmental and occupational medicine. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;778:63–97. doi: 10.1016/s0378-4347(01)00442-x. [DOI] [PubMed] [Google Scholar]
- 17.Bernard A, Roels H, Buchet JP, et al. Decrease of serum Clara cell protein in smokers [letter] Lancet. 1992;339:1620. doi: 10.1016/0140-6736(92)91891-b. [DOI] [PubMed] [Google Scholar]
- 18.Hermans C, Aly O, Nyberg BI, et al. Determinants of Clara cell protein (CC16) concentration in serum: a reassessment with two different immunoassays. Clin Chim Acta. 1998;272:101–110. doi: 10.1016/s0009-8981(98)00006-0. [DOI] [PubMed] [Google Scholar]
- 19.Doyle IR, Hermans C, Bernard A, et al. Clearance of Clara cell secretory protein 16 (CC16) and surfactant proteins A and B from blood in acute respiratory failure. Am J Respir Crit Care Med. 1998;158:1528–1535. doi: 10.1164/ajrccm.158.5.9712097. [DOI] [PubMed] [Google Scholar]
- 20.Nagae H, Takahashi H, Kuroki Y, et al. Enzyme-linked immunosorbent assay using F(ab′) 2 fragment for the detection of human pulmonary surfactant protein D in sera. Clin Chim Acta. 1997;266:157–171. doi: 10.1016/s0009-8981(97)00124-1. [DOI] [PubMed] [Google Scholar]
- 21.Maier EA, Rastegar F, Heimburger R, et al. Simultaneous determination of trace elements in lavage fluids from human bronchial alveoli by energy dispersive x-ray fluorescence: 1. Technique and determination of the normal reference interval. Clin Chem. 1985;31:551–555. [PubMed] [Google Scholar]
- 22.Romeo L, Maranelli G, Malesani F, et al. Tentative reference values for some elements in broncho-alveolar lavage fluid. Sci Total Environ. 1992;120:103–110. doi: 10.1016/0048-9697(92)90221-d. [DOI] [PubMed] [Google Scholar]
- 23.Karadag F, Cildag O, Altinisik M, et al. Trace elements as a component of oxidative stress in COPD. Respirology. 2004;9:33–37. doi: 10.1111/j.1440-1843.2003.00534.x. [DOI] [PubMed] [Google Scholar]
- 24.Chiba M, Masironi R. Toxic and trace elements in tobacco and tobacco smoke. Bull World Health Org. 1992;70:269–275. [PMC free article] [PubMed] [Google Scholar]
- 25.Rustemeier K, Stabbert R, Haussmann HJ, et al. Evaluation of the potential effects of ingredients added to cigarettes: Part 2. Chemical composition of mainstream smoke. Food Chem Toxicol. 2002;40:93–104. doi: 10.1016/s0278-6915(01)00085-0. [DOI] [PubMed] [Google Scholar]
- 26.Hendrick DJ. Smoking, cadmium, and emphysema. Thorax. 2004;59:184–185. doi: 10.1136/thx.2003.018432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tatrai E, Kovacikova Z, Hudak A, et al. Comparative in vitro toxicity of cadmium and lead on redox cycling in type II pneumocytes. J Appl Toxicol. 2001;21:479–483. doi: 10.1002/jat.784. [DOI] [PubMed] [Google Scholar]
- 28.Mannino DM, Holguin F, Greves HM, et al. Urinary cadmium levels predict lower lung function in current and former smokers: data from the Third National Health and Nutrition Examination Survey. Thorax. 2004;59:194–198. doi: 10.1136/thorax.2003.012054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacNee W, Donaldson K. Mechanism of lung injury caused by PM10 and ultrafine particles with special reference to COPD. Eur Respir J. 2003;21:47–51. doi: 10.1183/09031936.03.00403203. [DOI] [PubMed] [Google Scholar]
- 30.Pope CA, Dockery DW. Epidemiology of particle effects. In: Holgate ST, Samet JM, Koren HS, et al, eds. Air pollution and health. San Diego, CA: Academic Press, 1999; 673–705
- 31.Bachelet M, Pinot F, Polla RI, et al. Toxicity of cadmium in tobacco smoke: protection by antioxidants and chelating resins. Free Radic Res. 2002;36:99–106. doi: 10.1080/10715760210159. [DOI] [PubMed] [Google Scholar]
- 32.Kubala-Kukus A, Braziewicz J, Banas D, et al. Trace element load in cancer and normal lung tissue. Nucl Instrum Methods Phys Res Sect B. 1999;150:193–199. [Google Scholar]
- 33.Jiang F, Guo Y, Salvemini D, et al. Superoxide dismutase mimetic M40403 improves endothelial function in apoli-poprotein(E)-deficient mice. Br J Pharmacol. 2003;139:1127–1134. doi: 10.1038/sj.bjp.0705354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Effros RM, Peterson B, Casaburi R, et al. Epithelial lining fluid solute concentrations in chronic obstructive lung disease patients and normal subjects. J Appl Physiol. 2005;99:1286–1292. doi: 10.1152/japplphysiol.00362.2005. [DOI] [PubMed] [Google Scholar]
- 35.Hermans C, Bernard A. Lung epithelium-specific proteins: characteristics and potential applications as markers. Am J Respir Crit Care Med. 199;159:646–678. doi: 10.1164/ajrccm.159.2.9806064. [DOI] [PubMed] [Google Scholar]
- 36.Hermans C, Dong P, Robin M, et al. Determinants of serum levels of surfactant proteins A and B and Clara cell protein CC16. Biomarkers. 2003;8:461–471. doi: 10.1080/13547500310001647021. [DOI] [PubMed] [Google Scholar]
- 37.Leth-Larsen R, Floridon C, Nielsen O, et al. Surfactant protein D in the female genital tract. Mol Hum Reprod. 2004;10:149–154. doi: 10.1093/molehr/gah022. [DOI] [PubMed] [Google Scholar]


