TO THE EDITOR
Known steroidogenic functions of the skin that are represented by the synthesis and metabolism of androgens and estrogens (Chen et al., 2002; Zouboulis and Degitz, 2004) probably include corticosteroids (Slominski et al., 2000; Slominski, 2005). Thus, the skin expresses both the genes and proteins involved in the synthesis of adrenal corticosteroids (Slominski et al., 1996, 1999, 2004; Rogoff et al., 2001). Metabolism of progesterone to deoxycorticosterone and corticosterone has been documented in melanoma cells and in rat skin (Slominski et al., 1999, 2000); corticotropin-releasing hormone- and ACTH-regulated cortisol production was demonstrated in histocultured human hair follicles (Ito et al., 2005); and corticotropin-releasing hormone and ACTH enhancement of cortisol production was demonstrated in epidermal melanocytes (Slominski et al., 2005b). In contrast, in human dermal fibroblasts cortisol production was not enhanced by corticotropin-releasing hormone and ACTH (there was only selective stimulation of corticosterone production; Slominski et al., 2005a). However, since the cortisol-like immunoreactivity was detected by ELISA, we investigated the chemical nature of the antigen.
Normal human adult dermal fibroblasts (from lightly pigmented female: lot 1C0317 (Cascade Biologics Inc., Portland, OR)) were cultured in Epilife medium with EpiLife Defined Growth Supplement free of serum (Cascade Biologics). The cells were washed twice with phosphate-buffered saline before incubation in supplement-free EpiLife medium with progesterone (10−6 m), ACTH (10− m, but added again after 12 h), and/or IBMX (phosphodiesterase inhibitor) (10− m). After 24 hours, media were extracted for cortisol identification and quantification as described previously (Slominski et al., 2005b). Cortisol in supernatants was measured with ELISA (Bio-Quant Inc., San Diego, CA), whereas chemical nature of the compounds was assessed by liquid chromatography mass spectrometry analysis with auto-mass spectrometry/mass spectrometry as described previously (Slominski et al., 2005b). Data were analyzed using Prism 4.00 (GraphPad Software), and is presented as mean + SEM (n = 4).
Testing dermal fibroblasts for the release of cortisol into the media showed that addition of progesterone (10− m) stimulated significantly (4.3-fold) cortisol production; further enhancement (2.5 times) was observed after the addition of 10− m IBMX (phosphodiestearse inhibitor) but not ACTH (11-fold increase over the basal values obtained from unstimulated cells; Figure 1). The addition of corticotropin-releasing hormone did not affect the production of cortisol (not shown). Liquid chromatography mass spectrometry analyses of extracted steroids showed the presence of an [M + H]+ ion at m/z 363 (real mass 362) with retention time of 11 minutes identical to the cortisol standard (Figure 2). Final confirmation of the product identity as cortisol was obtained with mass spectrometry/mass spectrometry analysis that yielded the same fragment ions as those of the cortisol standard: m/z = 345 ([M + H]+ –H2O), m/z = 327 ([M + H]+ –2H2O), and m/z = 309 ([M + H]+ –3H2O) (Figure 2).
Figure 1. Effect of progesterone on cortisol synthesis.
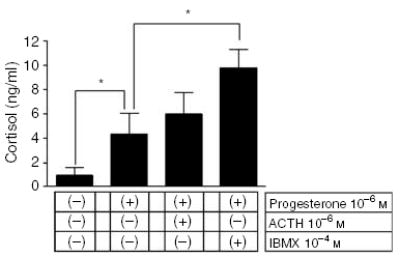
Fibroblasts were cultured in 24-well plates seeded at density of 105 cells/well. Differences between control and experimental treatments: *P<0.05.
Figure 2. Liquid chromatography mass spectrometry (LC/MS2) identifies cortisol in cultures of fibroblasts.
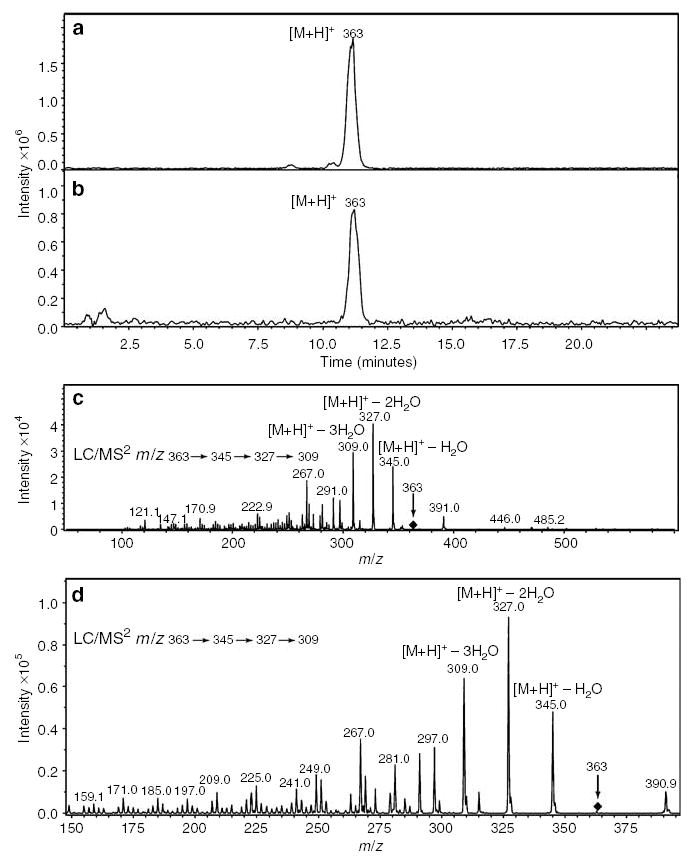
Fibroblasts were cultured in Petri dishes seeded at density of 2 × 106 cells/dish. (a, c) Cortisol standard and (b, d) extracts from conditioned media.
These data document the capability of human dermal fibroblasts to produce cortisol molecules identified by rigorous analysis with liquid chromatography mass spectrometry2. Moreover, and similar to normal (Slominski et al., 2005b) and malignant melanocytes (Slominski et al., 1999), fibroblasts also displayed a precursor substrate relationship in the accumulation of cortisol, for example, the addition of progesterone-stimulated production of the final corticosteroid cortisol, which was further enhanced by a phosphodiesterase inhibitor (IBMX). Nevertheless, in contrast to melanocytes ACTH and corticotropin-releasing hormone did not stimulate cortisol production in fibroblasts, related, perhaps, to defective coupling of MC-2R (if expressed) to intracellular signaling of the glucosteroidogenic pathway in cultured fibroblasts. Thus, these studies extend the ex vivo finding of cortisol production by hair follicles (Ito et al., 2005), adding an important component of heterogeneity to the pleiotropic expression of hypothalamic-pituitary-adrenal mediators in the skin (Slominski and Wortsman, 2000; Slominski, 2005). The role of these factors in the cutaneous response to stress remains to be fully characterized.
Acknowledgments
This work was supported by NIH Grant AR047079.
References
- Chen W, Thiboutot D, Zouboulis CC. Cutaneous androgen metabolism: basic research and clinical perspectives. J Invest Dermatol. 2002;119:992–1007. doi: 10.1046/j.1523-1747.2002.00613.x. [DOI] [PubMed] [Google Scholar]
- Ito N, Ito T, Kromminga A, Bettermann A, Tokigawa M, Kees F et al (2005) Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal (HPA) axis and synthesize cortisol. FASEB J 10.1096/fj04-1968fje: Published online June 9, 2005. [DOI] [PubMed]
- Rogoff D, Gomez-Sanchez CE, Foecking MF, Wortsman J, Slominski A. Steroidogenesis in the human skin: 21-hydroxylation in cultured keratinocytes. J Steroid Biochem Mol Biol. 2001;78:77–81. doi: 10.1016/s0960-0760(01)00076-0. [DOI] [PubMed] [Google Scholar]
- Slominski A, Ermak G, Mihm M. ACTH receptor, CYP11A1, CYP17 and CYP21A2 genes are expressed in skin. J Clin Endocrinol Metab. 1996;81:2746–9. doi: 10.1210/jcem.81.7.8675607. [DOI] [PubMed] [Google Scholar]
- Slominski A, Gomez-Sanchez C, Foecking MF, Wortsman J. Metabolism of progesterone to DOC, corticosterone and 18OHDOC in cultured human melanoma cells. FEBS Lett. 1999;445:364–6. doi: 10.1016/s0014-5793(99)00889-3. [DOI] [PubMed] [Google Scholar]
- Slominski A, Gomez-Sanchez C, Foecking MF, Wortsman J. Active steroidogenesis in the normal rat skin. Biochim Biophys Acta. 2000;1474:1–4. doi: 10.1016/s0304-4165(99)00215-9. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21:457–87. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- Slominski A, Zjawiony J, Wortsman J, Semak I, Stewart J, Pisorchik A, et al. A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. Eur J Biochem. 2004;271:4178–88. doi: 10.1111/j.1432-1033.2004.04356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A. Neuroendocrine system of the skin. Dermatology. 2005;211:199–208. doi: 10.1159/000087012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Zbytek B, Semak I, Sweatman T, Wortsman J. CRH stimulates POMC activity and corticosterone production in dermal fibroblasts. J Neuroimmunol. 2005a;162:97–102. doi: 10.1016/j.jneuroim.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Slominski A, Zbytek B, Szczesniewski A, Semak I, Kaminski J, Sweatman T, et al. CRH stimulation of corticosteroids production in melanocytes is mediated by ACTH. Am J Physiol Endocrinol Metab. 2005b;288:E701–6. doi: 10.1152/ajpendo.00519.2004. [DOI] [PubMed] [Google Scholar]
- Zouboulis CC, Degitz K. Androgen action on human skin – from basic research to clinical significance. Exp Dermatol. 2004;13(Suppl 4):5–10. doi: 10.1111/j.1600-0625.2004.00255.x. [DOI] [PubMed] [Google Scholar]


