Abstract
Human skin is a local source of corticotropin-releasing hormone (CRH) and expresses CRH and CRH receptors (CRH-R) at mRNA and protein levels. Epidermal melanocytes respond to CRH by induction of cAMP with up-regulation of pro-opiomelanocortin gene expression and subsequent production of adrenocorticotropin hormone. However, the role of CRH/CRH-R in melanocyte biology is complicated by the significant heterogeneity of cutaneous melanocyte subpopulations, from continuously active and UV-responsive melanocytes in epidermis to UV nonresponsive, hair growth cycle-coupled melanogenesis in hair follicles. In the present study we report that normal human scalp hair follicle melanocytes express CRH at the mRNA level. Furthermore, CRH, urocortin and CRH-R 1 and 2 were differentially expressed in follicular melanocytes, fibroblasts, and keratinocytes depending on anatomic location and differentiation status in situ and in vitro. Stimulation of follicular melanocytes with CRH and CRH peptides, modified for selectivity for CRH-R1 and/or CRH-R2, variably induced cell melanogenesis, dendricity, and proliferation. CRH-peptides also stimulated the expression and activity of Tyrosinase, and expression of Tyrosinase-related protein-1 and-2. However, a modified urocortin peptide highly selective for CRH-R2 down-regulated melanocyte differentiation phenotype. This study indicates that CRH peptides can differentially influence hair follicle melanocyte behavior not only via CRH-R1 signaling but also by complex cross-talk between CRH-R1 and CRH-R2.—Kauser, S., Slominski, A., Wei, E. T., Tobin, D. J. Modulation of the human hair follicle pigmentary unit by corticotropin-releasing hormone and urocortin peptides.
Keywords: hair follicle melanocytes, melanogenesis, corticotropin-releasing hormone, CRH receptors, hair growth
In the past decade much data have emerged to implicate the skin as a pivotal peripheral sensor (1–7), and it has become apparent that there is a cutaneous “stress response” system (reviewed in refs 1, 2, 5, 8). This is consistent with the skin’s strategic location at the interface of a noxious external environment (e.g., UV radiation, chemical and mechanical trauma, infections, etc.) and the internal metabolic space. Such an organ would need systems to both respond to and mediate the stress response (1, 2, 9). Recent evidence indicates that the skin as well as its principal appendage, the hair follicle, is indeed well equipped for this by having its own version of the body’s central and classical hypothalamic-pituitary-adrenal (HPA) axis (1, 2, 5–7, 10, 11). In this way, the skin appears to regulate local responses to stress independently of the central systemic system (2, 3, 12–14). Many of the elements of an HPA axis have been detected recently in the skin, including its most proximal element corticotropin-releasing hormone (CRH) (15) and pro-opiomelanocortin (POMC) with its cleaved peptides adrenocorticotropic hormone (ACTH), α-melanocyte-stimulating hormone (α-MSH), β-endorphin, etc. (16). CRH regulates the expression of POMC via its control of prohormone convertases, which cleave POMC into the above peptides. A final piece of the jigsaw, cortisol, was recently reported in the hair follicle itself (7), and so both the initiation and termination of the stress response (i.e., via the cortisol-associated attenuation of CRH/POMC production) now appear to be evident in skin.
Human skin, including the hair follicle, expresses the genes and proteins for CRH and POMC, POMC-derived melanocortin peptides (α-MSH, ACTH, β-endorphin), prohormone convertases (PC-1 and PC-2), and the receptors for CRH and melanocortins (e.g., MC-1R, μ-opiate receptors) (reviewed in refs 1, 2, 5). Human skin has been identified as a local source of CRH production (1, 17) and has been shown to express CRH and functional CRH-R1 at the mRNA and protein levels (1, 6, 18, 19). Incubation of normal epidermal melanocytes (EM) and dermal fibroblasts (DF) with CRH initiate a cascade of events that is hierarchically ordered in a manner similar to that in the HPA axis, whereby CRH activates CRH receptor 1, which induces cAMP accumulation and increases POMC gene expression with subsequent production of ACTH. In particular, melanocytes respond to CRH and ACTH with an enhanced production of cortisol/corticosterone (13, 14). Identification of a local CRH/CRH-R signaling system in human skin, together with the detection of POMC peptides as well as POMC processing machinery in melanocytes (20), supports the existence of a cutaneous equivalent of an HPA axis that controls local POMC synthesis and processing (1, 4, 6, 21).
The precise role of the CRH/CRH-R system in the differential regulation of cutaneous melanocytes is complicated by the significant melanocyte heterogeneity in this organ (22). Melanotic melanocytes are distributed in the basal layer of the epidermis, infundibulum of the hair follicle, basal layer of the sebaceous gland, and in the anagen hair bulb, while amelanotic melanocytes can be detected in the hair follicle outer root sheath as well as in the most peripheral and proximal hair bulb matrix. Follicular and EM diverge from a common origin in many important ways (5, 23, 24), the most striking difference being the tight coupling of hair pigmentation to the hair growth cycle (25). By contrast, melanogenesis in the epidermis appears to be continuous (26), though this is further up-regulated by UV radiation (UVR). UVB radiation does not reach the melanogenic cells of the anagen hair bulb located in the hypodermis, and so UVR is unlikely to directly influence the follicular-melanin unit.
We are only beginning to understand the mechanisms involved in regulating pigmentation in the human hair follicle (reviewed in refs 22, 23). However, some skin and hair pigmentation phenotypes are linked to polymorphisms in the melanocortin 1 receptor (MC-1R) gene (27). The MC-1R receptor is activated by α-melanocyte-stimulating hormone (α-MSH) and ACTH, and to some extent by proopiomelanocortin itself. Although, MC-1R-α-MSH/ACTH occupies the dominant position in our current concept of the regulation of mammalian pigmentation (5), there is accumulating evidence that non-MC-1R-dependent pathways can regulate pigmentation. For example, positive regulators of melanogenesis may include prostaglandin E, endothelin-1 and -3, and catecholamines (1, 5, 26, 28, 29). Moreover, we have found that β-endorphin, operating via the μ-opiate receptor, is also able to modulate epidermal and follicular melanocyte biology in vitro, and both ligand and high-affinity receptor are expressed in situ (30, 31). We have found that α-MSH, ACTH, and β-endorphin can all stimulate cell differentiation (dendricity and melanogenesis) and proliferation in epidermal and follicular melanocytes.
The present study was designed to examine whether the presence of CRH/CRH-R system in human scalp participates in regulating the hair follicle pigmentary unit. Expression analyses were carried out at both mRNA and protein levels in situ and in vitro using isolated primary cultures of hair follicle-derived cell types. Functional analysis included stimulation with CRH, urocortin, and CRH analogs designed to preferentially stimulate CRH receptor 1 and 2. We found that CRH and CRH-related peptides differentially influenced human scalp hair follicle melanocyte behavior via either CRH-R1- or CRH-R2 dependent mechanisms, although it is possible that CRH effects may be indirect via up-regulation of POMC peptides production in these cells (14).
MATERIALS AND METHODS
Isolation and culture of hair follicle cell subpopulations
Hair follicle melanocytes
Hair follicle melanocytes (HFM) were established from normal human haired scalp tissue, obtained from 7 females (43–59 years, mean 48 years, hair color light brown to black), after elective plastic surgery with informed consent and with local ethics committee approval. All cell culture reagents were obtained from Invitrogen Ltd. (Paisley, Scotland) unless otherwise stated. HFM cultures were established using described protocols (31, 32). Contaminating fibroblasts, when present in HFM cultures, were removed with 150 μg/ml geneticin sulfate (G418) in cycles of 48 h (33). Hair follicle keratinocytes (HFK) were separated from HFM cultures by differential trypsinization. The identity of isolated cells was confirmed by immunophenotyping at passage 1 with the melanocyte lineage-specific marker NKI/beteb against glycoprotein100 (gp100) (Monosan, Netherlands) (34).
Hair follicle keratinocytes
Hair follicle keratinocytes cultures were established by preparing single cell suspensions from isolated hair follicles as described previously (31). Selective trypsinization of HFM facilitated separation of both follicular melanocytes and keratinocytes, as described above. The separated follicular keratinocytes were subsequently transferred to K-SFM medium (Invitrogen).
Epidermal melanocytes
EM were established from normal human haired scalp tissue obtained from 7 females (43–59 years, mean 48 years, hair color light brown to black), after elective plastic surgery as described previously (30). Briefly, epidermal sheets were separated from the underlying dermis after an18 h incubation in 0.25% trypsin solution at 4°C, then used to prepare single cell suspensions using 0.05% trypsin and 0.53 mM EDTA solution. The isolated cells were transferred cell culture flasks (Corning Costar Corporation, Cambridge, MA, USA). Residual epidermal material was carefully removed and the medium was replenished after 48 h. Cells were incubated at 37°C in a 5% CO2 atmosphere and fed every third day.
Epidermal keratinocytes
EK were established by selectively trypsinizing EM from the coculture at the primary culture stage, using trypsin/EDTA solution. The detached EM were transferred into a separate culture dish, and the remaining EK were switched to keratinocyte serum-free medium.
Follicular papilla fibroblasts
Follicular papilla (FP) fibroblasts were isolated and cultured according to Magerl (35). Briefly, anagen hair follicles were isolated by microdissection from normal scalp from 5 females (age range 43–59 years, mean age 48 years). Isolated FP were transferred into Petri dishes and replenished every third day with culture medium after cell growth was evident.
Dermal fibroblasts
Dermal tissue derived from human haired scalp tissue (described above) was transferred into culture flasks, ensuring the tissue had direct contact with the surface of the culture dish. A small volume of RPMI 1640 medium supplemented with 10% bovine pituitary extract (FCS), 100 U/ml penicillin, 100 μg/ml streptomycin was added so that it just covered the surface of the tissue, and incubated at 37°C in a 5% CO2 atmosphere as before. Fresh medium was added every third day and the dermal pieces were removed once cell attachment and growth of DF were evident.
Isolation of RNA
Total RNA was isolated from follicular and EM, follicular and EK, DF, and FP fibroblasts by the guanidinium thiocyanate-phenol-chloroform based method, using Tri-Reagent™ (Sigma, Poole, Dorset, UK) according to the manufacturer’s instructions. To purify total RNA isolated from HFM, dyna-beads mRNA direct kit (Dynal AS, Oslo, Norway) was subsequently used according to the manufacturer’s instructions. Extracted total RNA samples were also treated with deoxyribonuclease I, amplification grade (Invitrogen Ltd.) to avoid possible contamination of genomic DNA.
Reverse transcriptase polymerase chain reaction (RT-PCR)
The synthesis of cDNA was performed using RevertAid™ M-MuLV Reverse Transcriptase (MBI Fermentas, Lithuania) according to the manufacturer’s instructions using 2 μg of total RNA or 0.2 μ g of mRNA and oligo (dT)18 and random hexamer primers (Sigma Genosys, Pampisford, Cambridgeshire, UK). All PCR reagents were obtained from MBI Fermentas (Vilnius, Lithuania) unless otherwise stated. One microliter of cDNA was used in the PCR amplification in a 50 μl reaction mixture containing the following components: 1× PCR buffer (75 mM Tris-HCl pH 8.8, 20 mM (NH4)2SO4, moles of CRH primers, 0.01% Tween 20), 2.0 mM MgCl2, 20 ρ (SIGMA Genosys, Cambridgeshire, UK) 0.2 mM dNTP mix, 1.0 U of TaqDNA polymerase, and finally nuclease-free water (Sigma, Dorset, UK) was added to make a final reaction vol of 50 μl. CRH was amplified using primer sets and PCR parameters as described previously (18). One cycle at 94°C for 3 min, 35 cycles at 96°C for 30 s, 55°C for 30 s, 72°C for 2 min, and a final extension of 72°C for 2 min. EM were used as a positive control cell line for CRH mRNA expression (6, 18). RNA samples that were not reverse transcribed and the omission of cDNA from the reaction mixture served as negative controls.
Amplifications were performed using the Hybaid PCR sprint temperature cycling system (Hybaid, Ashford, Middlesex, UK). 10 microliters of the reaction mixture was mixed with 4 μl of gel loading solution (MBI Fermentas, Lithuania) and loaded onto a 1.5% agarose gel (Sigma, Poole, Dorset, UK) containing 1 μg/ml of EtBr (Sigma, Poole, Dorset). A 100 base pair (bp) DNA ladder (New England Biolabs, Hitchin, Hertfordshire, UK) was also loaded. This was followed by electrophoresis at a constant voltage of 100 V using 0.5× tris-borate buffer. Gels were photodocumented using the UVitec gel documentation system (UVitec Limited, Cambridge, UK).
Immunohistochemistry
Normal human haired scalp tissue obtained after elective plastic surgery (5 females, age range 43–60 years, mean age 50 years) or occipital scalp tissue (2 males, age 23 years and 36 years) were cryosectioned (7 μm) and processed for double immunolabeling as described previously (30, 31, 36). Sections were incubated with primary antibodies against CRH (1:30), urocortin 1 (1:50), CRH-R1 (1:50), CRH-R2 (1:30) for 18 h at 4°C (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). Secondary antibody (Ab) incubations used a rhodamine-conjugated donkey anti-rabbit or anti-goat secondary antibodies (1:50) (Jackson Immunoresearch Laboratories, Inc., West Grove, PA, USA) for 60 min at room temperature (RT). To detect the melanocyte lineage-specific marker gp100, sections were further blocked with 10% normal donkey serum and incubated with the second primary Ab, NKI/beteb (1:30) (Monosan, Uden, Netherlands) for 2 h at RT, then incubated with a fluorescein-conjugated donkey antimouse secondary Ab (1:50) (Jackson Immunoresearch Laboratories, Inc., West Grove, USA) for 60 min at RT. Sections were washed in PBS and mounted in Vectashield mounting medium with 4′, 6-diamidino-2-phenylindole (DAPI) (Vector Laboratories Ltd., Peterborough, UK). Negative controls included incubating sections with peptide-blocked primary Ab (urocortin, CRH-R1, CRH-R2 according to the manufacturer’s instructions). Further negative controls included omission of primary antibodies and their replacement with preimmune serum from the host species of the secondary Ab. The staining was visualized with a Nikon eclipse 80i fluorescence microscope, (Nikon instruments Europe B.V., Badhoevedorp, The Netherlands) and photodocumented using a Nikon DS digital camera and the ACT-2U graphics program (Nikon). The images produced with the two different fluoro-chromes rhodamine (red) and fluorescein (green) were merged together using the Paint Shop Pro™ 7 graphics program (JascSoftware, Oxon, UK). Colocalization of CRH, urocortin 1 (urocortin), CRH-R1, and CRH-R2 with gp100-positive HFM was indicated by the production of a yellow color.
Immunocytochemistry
To establish whether the CRH/CRH-R system was retained under our in vitro culture conditions, the expression of this hormone receptor system was examined in cultured hair follicle cell subpopulations. Cultured HFM, HFK, and FPF (passage 2–5) were seeded into 8-well Lab-Tek® chamber slides (ICN Biomedicals, Inc., Aurora, OH, USA) at 5000 cells/well and cultured for 2–3 days. FCS and bovine pituitary extract (BPE) were omitted from the culture media 48 h prior to immunostaining to remove all exogenous sources of CRH peptide, with a half-life is ~30 min (37). Cells were rinsed in PBS for 5 min and fixed in ice-cold methanol for 10 min at −20°C. Cells were blocked in 10% normal goat serum or 10% normal horse serum rinsed briefly in PBS and incubated with CRH, urocortin, CRH-R1 and CRH-R2-specific antibodies and with positive control antibodies to gp100 (NKI/beteb; Monosan, Netherlands), Tyrosinase (Novacastra, Newcastle on Tyne, UK), Tyrosinase-related protein (TRP)-1, TRP-2 (Santa Cruz Biotechnology), hair follicle-specific keratins (AE13, a gift from T-T Sun NUY Medical Center, NY, USA) and vimentin (DAKO, Carpinteria, CA, USA), at 4°C for 18 h. Subsequent steps in immunostaining were performed either using the DAKO LSAB® 2 HRP kit and DAKO 3-amino-9-ethyl carbazole (AEC) substrate system (DAKO) according to the manufacturer’s instructions for CRH or by incubation with a biotinylated anti-goat IgG (1:300) (Vector Laboratories Ltd., Peterborough, UK) for urocortin, CRH-R1, and CRH-R2 followed by only the streptavidin peroxidase solution part of the LSAB® 2 HRP kit and subsequent AEC chromagen development as described above. Negative controls included peptide blocks, i.e., depletion of the primary Ab by incubation with the corresponding blocking peptides (according to the manufacturer’s instructions). The omission of both primary Ab, and replacement with preimmune serum from secondary Ab host and inclusion of secondary antibodies also served as negative controls.
Stimulation of melanocytes and assessment of melanin content, dendricity, and cell proliferation
Follicular melanocyte cultures (n = 7) were grown without FCS and BPE for 48 h prior to stimulation with native CRH (American Peptide Company, Sunnyvale, CA, USA) or its modified peptides [D-Glu20]-CRH, [D-Pro5]-CRH, and [D-Pro4]-r-urocortin at 10−7 M to 10−10 M concentrations (for details, see ref 38). HFM were stimulated for 72 h, and cells from the same donor and passage were maintained in parallel in the absence of hormone as the unstimulated control.
To assess melanocyte dendricity, cells were photographed 72 h after stimulation with native CRH or its modified peptides, [D-Glu20)]-CRH, [D-Pro5]-CRH, and [D-Pro4]-r-urocortin (10−7–10−10 M). Representative photographs were taken from up to 8 random and different fields (of 10 cells for each cell line), and the number of bipolar cells and the number of cells with more than three dendrites were counted and compared to controls. After trypsinization cells were counted using a Neubauer counting chamber. The cells were pelleted by centrifugation and solubilized in 1 M sodium hydroxide, boiled, and absorbancy was measured spectrophotometrically at 475 nm. A standard curve of synthetic melanin (Sigma, Poole, Dorset, UK) was the basis for relative melanin content determination. For each cell line examined, melanin content and cell counts were determined at least three times and average values were taken. Melanin content was determined as picogram melanin/cell and expressed as % increase in melanin content above control unstimulated cells. Increase in cell number was expressed as % increase in cell number above control, unstimulated levels.
Western immunoblot analysis
The effect of CRH peptide (10−7 M) and related analogs [D-Glu20]-CRH (10−8 M), [D-Pro5]-CRH (10−10 M), and [D-Pro4]-r-urocortin (10−10 M) on the expression of key melanogenic enzymes Tyrosinase, Tyrosinase-related protein-1 (TRP-1) and Tyrosinase-related protein-2 (TRP-2) was assessed by immunoblotting. N human HFM were stimulated for 72 h with the hormones at concentrations most effective at inducing melanogenesis. HFM (n=3) (passage 2–5) were scraped into basal melanocyte medium (without FCS or BPE), pelleted by centrifugation, and lysed on ice using Laemli’s buffer supplemented with 1:100 dilution of protease inhibitor cocktail (Sigma, Poole, Dorset, UK). Protein concentration was measured using the modified Bradford assay (Bio-Rad, Richmond, CA, USA), and identical amounts of extracted protein samples (25 μg) were separated by sodium dodecyl sulfate-8% PAGE under reducing conditions. After electrophoretic separation, proteins were electroblotted on to poly-vinylidene difluoride (PVDF) membranes (Immobilon, Millipore, Bedford, MA, USA), and blocked for 18 h at 4°C with 5% nonfat milk (Marvel Ltd., Merseyside, UK) in PBS. The membranes were immunoprobed for 18 h at 4°C with antibodies against Tyrosinase (1:200), TRP-1 (1:1000) and TRP-2 (1:200) (Santa Cruz Biotechnology). The membranes were then incubated for 2 h at RT with a horseradish peroxidase-conjugated donkey anti-sheep/goat IgG Ab (1:2000) (Serotec Ltd., Kidlington, Oxford, UK) and developed by the Enhanced Chemiluminescence plus Western blot detection system kit, according to the manufacturer’s instructions (Amersham Biosciences Ltd., Little Chalfont, Buckinghamshire, UK).
The concentration of expression of the target melanogenic proteins in the HFM protein extracts was assessed by densitometric analysis, with the density of the central darkest region of the band corresponding to “basal medium” set as 100 U. The increase and decrease in expression were calculated as a % increase or a % decrease compared to the control.
The expression of CRH-R2 was also assessed in unstimulated extracts of hair follicle melanocytes (66 years female, passage 4) by probing for 18 h at 4°C using the same polyclonal antibody used in the immunocytochemistry assay above (Santa Cruz Biotechnology). The membrane was then incubated for 2 h at RT with a horseradish peroxidase-conjugated donkey anti-sheep/goat IgG Ab (Serotec Ltd., Kidlington, Oxford, UK) and developed by the Enhanced Chemiluminescence plus Western blot detection system kit (Amersham Biosciences Ltd., Little Chalfont, Buckinghamshire, UK).
DOPA-oxidase activity of Tyrosinase
Measurement of the dihydroxyphenylalanine (DOPA)-oxidase activity of Tyrosinase in HFM cultures after 72 h stimulation with CRH and its related analogs was conducted as follows. Stimulated cells were lysed as described above and 40 μg of extracted protein was separated by sodium dodecyl sulfate-8% PAGE under nonreducing conditions. After electrophoretic separation, proteins were electroblotted onto PVDF membranes and incubated 5 mM L-DOPA (Sigma, Poole, Dorset, UK) in 0.1 M sodium phosphate buffer (pH 6.8) at RT for 3 h. After several changes of DOPA solution the reaction was terminated by washing the membranes in distilled water, then photodocumented. The concentration of DOPA-oxidase activity expression in the HFM protein extracts was assessed by densitometric analysis as above.
Statistical analysis
Statistical significance was assessed by 1-way ANOVA and Dunnet’s post hoc test using Prism 4.00 (GraphPad Software, Chicago, IL, USA). Statistically significant differences are denoted with asterisks: *P < 0.05, **P < 0.01, ***P < 0.001.
RESULTS
Hair follicle cell subpopulations express CRH mRNA in vitro
RT-PCR with primers specific for CRH produced the expected 413 bp-sized amplification product in normal human follicular melanocytes, keratinocytes, and FP fibroblasts (Fig. 1). The bands for CRH were discrete and reproducible, and used the precise primer pairs confirmed in published protocols (18). Amplification of RNA samples that were not reverse transcribed and samples from which cDNA was omitted from the reaction mixture were also subjected to PCR. These negative controls did not reveal a specific product (Fig. 1). While the reaction product was similar for melanocytes derived either from the hair follicle and epidermis, the CRH mRNA product in FP fibroblasts was much greater than for dermal fibroblasts.
Figure 1.
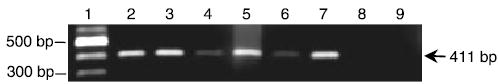
Detection of CRH-specific transcripts by RT-PCR in cultured follicular melanocytes, keratinocytes, and follicular papilla fibroblasts. Detection of a 413 bp product (arrow) representative of mRNA from the CRH coding region of exon 2. Lane 1: DNA ladder, lane 2: HFM, lane 3: EM (positive control), lane 4: HFK, lane 5: EK, lane 6: DF, lane7: FP fibroblasts, lane 8: negative control #1, omission of cDNA, lane 9: negative control #2, substitution of cDNA with total RNA.
Human scalp anagen hair follicle differentially expresses CRH, urocortin, CRH-R1, and CRH-R2
The expression of CRH and urocortin was prominent in the hair bulb and outer root sheath of anagen VI hair follicles (Fig. 2a, b). The cytoplasmic pattern, expression concentration, and anatomic distribution of CRH and urocortin were similar and largely restricted to differentiating keratinocytes of the precortex (future hair shaft keratinocytes) and inner root sheath, and also in the outer root sheath. Expression of both hormones was conspicuously down-regulated in the hair follicle melanogenic zone containing highly differentiated melanotic hair bulb melanocytes and relatively undifferentiated keratinocytes. By contrast, expression of both hormones was detectable in singly scattered melanocytes located in the outer root sheath and most peripheral and proximal hair bulb (Fig. 2a, arrowheads). In the latter, anatomic site expression of both hormones was detected in the most undifferentiated and proliferative hair bulb cells (Fig. 2a, b). Within the follicular mesenchyme only urocortin was readily detectable in the cytoplasm of papilla fibroblasts and within cells of the papilla stalk (Fig. 2b). By contrast, CRH was weakly expressed in the follicular papilla, restricted largely to presumptive endothelial cells of the capillary (Fig. 2a).
Figure 2.

Human hair follicles express CRH, urocortin, and CRH-R1 and CRH-R2. CRH and urocortin were detected in a minor subpopulation of melanocytes located in the proximal/peripheral matrix region and also in the ORS (arrowheads), but were down-regulated (solid arrowheads)/absent (arrows) in the melanogenic zone of anagen VI hair follicles. Considerable cytoplasmic expression of both CRH and urocortin was present in the peripheral layers of the hair bulb keratinocytes and also in the precortex, but weakly expressed in basal keratinocytes of the hair matrix next to the follicular dermal papilla (FP). Urocortin and, to a lesser degree, CRH peptide were also expressed in the cytoplasm of follicular fibroblasts, including the FP and follicular dermal sheath (DS) (a, b). CRH-R1 and CRH-R2 were down-regulated in most bulbar melanocytes including the differentiated melanocytes (solid arrowhead) in the melanogenic zone but expressed at higher levels in submaximally differentiated melanocytes (arrowheads) in the peripheral/proximal bulb. Expression of both CRH-R1 and CRH-R2 was observed to exhibit a marked nuclear pattern in the most peripheral layer of hair bulb keratinocytes but also, more moderately, throughout the entire hair bulb. Receptor expression was greater in the fibroblasts of the DS compared to the FP (c, d). CRH, urocortin, CRH-R1 and CRH-R2: red; gp100: green; colocalization: yellow. Scale bar: 50 μm.
Both CRH receptors were expressed in the pigmented anagen hair follicle, where they exhibited broadly overlapping expression profiles (Fig. 2c, d) in keratinocytes. While all follicular keratinocytes expressed CRH-R1, the expression pattern of CRH-R2 was greatest in undifferentiated and differentiating keratinocytes of the lower hair follicle (Fig. 2c, d). Both receptors were also expressed in follicular fibroblasts of the connective tissue sheath and sub-papillary cap or stalk. Unlike CRH-R2, weak CRH-R1 expression was seen in the nuclei of papillary fibroblasts. Moreover, differentiated melanocytes in the melanogenic zone of the anagen hair bulb only weakly expressed CRH receptors (Fig. 2c, d), while greater expression of both receptors was detected in scattered melanocytes of the proximal/peripheral hair bulb matrix and outer root sheath. These results demonstrate that ligands and receptors of the CRH/urocortin/CRH-R1/2 system are expressed in the pigmented anagen hair bulb according to anatomic/spatial location and differentiation status, though this appeared to be inversely correlated for keratinocyte and melanocytes. While CRH and urocortin were localized to cell cytoplasm, their receptors appeared to exhibit a broadly nuclear distribution.
Human hair follicle cell subpopulations differentially express CRH, urocortin, CRH-R1 and CRH-R2 in vitro
Hair follicle melanocytes
Melanocytes that expand in culture are derived from relatively undifferentiated melanocytes that originate in the hair follicle outer root sheath and hair bulb (31, 32, 36, 39). These cells in culture expressed CRH, urocortin, CRH-R1, and CRH-R2 (Fig. 3A). The expression of CRH and urocortin was restricted to the cell surface with some additional cytoplasmic staining (Fig. 3A). Unlike CRH, the expression of urocortin was also granular (Fig. 3A). Similarly, the expression of both CRH-R1 and CRH-R2 was greatest at the cell surface (Fig. 3A), though again some granular cytoplasmic staining was also seen. The expression of hormones receptors appears to be strongest in more differentiated (Fig. 3A, arrowheads), i.e., dendritic, melanocytes compared with the less differentiated flat cells (Fig. 3A, arrow). The expression of CRH-R2 in hair follicle melanocytes in vitro was confirmed by immunoblotting. Cell extracts were probed with an anti-CRH-R2 Ab and yielded a single band of the expected 45 kDa size (Fig. 3A). Immunostaining of HFM cultures with antibodies to the melanocyte-specific markers gp100, Tyrosinase, TRP-1 and TRP-2 confirmed the melanocyte identity of these cells (Fig. 3A).
Figure 3.
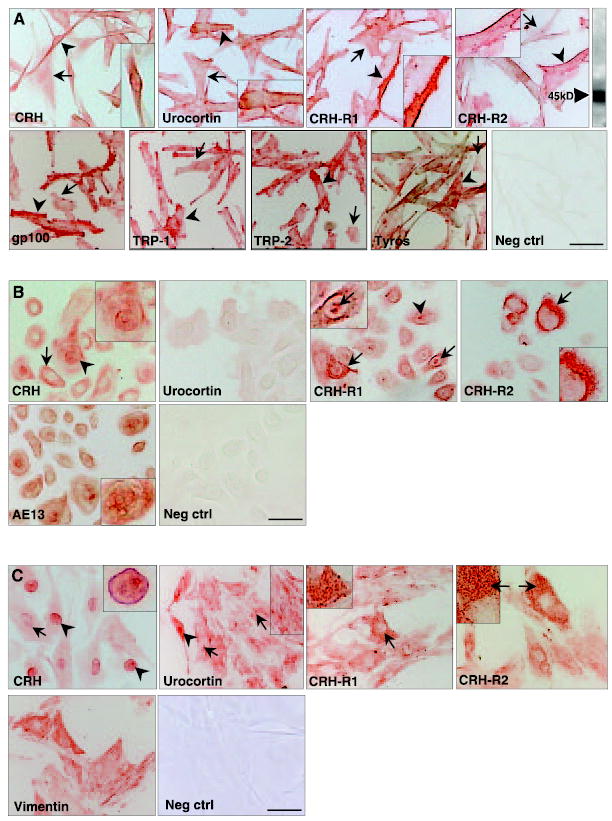
A) CRH, urocortin, CRH-R1, and CRH-R2 are expressed in hair follicle melanocytes in vitro. CRH, urocortin, CRH-R1, CRH-R2 was variably expressed in cultured hair follicle melanocyte (passage 3). CRH expression correlated positively with differentiation status (arrowhead), with fine cytoplasmic staining and nuclear/nuclear membrane expression also found (arrow). By contrast, urocortin expression exhibited strong cell membrane localization (arrowhead). CRH-R1 expression was located both cytoplasmically (arrowhead) and at the cell membrane (arrow), whereas CRH-R2 staining was intense at the cell membrane (arrowhead). These cells also exhibited CRH-R2 expression by immunoblotting (inset). Gp100, Tyrosinase, TRP-1, TRP-2 expression in all cells confirmed their melanocyte identity. Neg ctrl; negative control scale bar: 17 μm. B) CRH, urocortin, CRH-R1, and CRH-R2 are expressed hair follicle keratinocytes in vitro. CRH, urocortin, CRH-R1, and CRH-R2 were variably expressed in cultured hair follicle keratinocyte (passage 2). Expression of the CRH did not show any obvious correlation with cell differentiation, and was characterized by both cytoplasmic (arrow) and nuclear (arrowhead) staining. Some cells exhibited both nuclear and cytoplasmic expression, while other cells lacked nuclear expression. By contrast, urocortin expression was weak/negative in these matched follicular keratinocytes. CRH-R1 expression was detected in relatively high levels in all cells, where it exhibited a granular cytoplasmic distribution (arrow) and also in cell nuclei (arrowhead). CRH-R2 expression was weak in the vast majority of cells. Rare keratinocytes, with a broad flattened morphology, expressed high levels of this receptor in a cytoskeletal pattern (arrow). AE13 expression (hair follicle-specific keratins) confirmed their follicular keratinocyte identity. Neg crtl; negative control. Scale bar: 20 μm. C) CRH, urocortin, CRH-R1, and CRH-R2 are expressed follicular papilla fibroblasts in vitro. The pattern of expression of CRH, urocortin, CRH-R1, CRH-R2 was very heterogeneous in cultured of follicle papilla cells (passage 3). Expression of the CRH was strongly nuclear (arrowhead), though also located in the cell cytoplasm (arrow). Some cells exhibited very intense staining of the nuclear membrane and nucleoplasm (arrowhead), whereas other nuclei were relatively unstained. By contrast, urocortin expression was broadly cytoplasmic, where it exhibited a strong cytoskeletal pattern (arrow). Rare cells also exhibited nuclear expression (arrowhead). The expression profiles of both CRH-R1 and CRH-R2 were broadly similar and characterized by an intense granular cytoplasmic staining (arrow) in a subpopulation of cells. Vimentin (mesenchymal marker) expression in all cells supported a fibroblast identity. Neg crtl; negative control. Scale bar: 30 μm.
Hair follicle keratinocytes
Follicular keratinocytes expressed CRH, CRH-R1, and CRH-R2 (Fig. 3B), though little or no urocortin was detectable under these culture conditions (Fig. 3B). Considerable heterogeneity in the expression of CRH was apparent in this follicular cell population. Some keratinocytes expressed CRH in both cell nuclei and cytoplasm, though all cells expressed CRH in the cell cytoplasm. However, these heterogeneous staining patterns did not correlate with morphological features of keratinocyte differentiation. Follicular keratinocytes expressed both CRH receptors with a broadly granular cytoplasmic pattern (Fig. 3B). However, some scattered keratinocytes expressed particularly high levels of CRH-R1 (including in cell nuclei), while some keratinocytes expressed intense staining for CRH-R2 with a cytoskeletal distribution (Fig. 3B). Expression of hair follicle-specific keratins confirmed the identity of these HFK cultures (Fig. 3B).
Follicular papilla fibroblasts
CRH, urocortin CRH-R1 and CRH-R2, were readily detected in FP fibroblasts (Fig. 3C). Similar to CRH expression in some keratinocytes, the expression of CRH in papilla fibroblasts was broadly nuclear, with lower expression detected in the cell cytoplasm (Fig. 3C). By contrast, the expression of urocortin was only rarely nuclear, but more commonly exhibited a uniquely granular/cytoskeletal pattern (Fig. 3C). Both CRH receptors were expressed in a highly granular pattern, with significant variability in expression levels between different cells. Expression of vimentin confirmed the mesenchymal identity of these cells (Fig. 3C).
CRH and modified CRH/urocortin peptides modulate hair follicle melanocyte phenotype in vitro
Cell proliferation
CRH, [D-Pro5]-CRH, [D-Glu20]-CRH, and [D-Pro4]-r-urocortin all significantly stimulated melanocyte proliferation at concentrations that varied from 10−7 to 10−10 M (Fig. 4). At a concentration of 10−8 M, the native CRH peptide was most active at (43%±5.1; P<0.001; n=7) compared to unstimulated controls, followed by both [D-Glu20]-CRH (30%±4.8; P<0.01, n=7). Although [D-Pro5]-CRH was not active at this concentration (−3%±2.3; P=0.05; n=7), this peptide was the most active of all the test peptides at 10−10 M (+30%±6.6, P<0.01, n=7). By contrast, [D-Pro4]-r-urocortin induced a small, though significant stimulation of proliferation at 10−7 M and 10−8 M (6.2%±0.9 and 10.3%±1.1 P<0.01 respectively, n=7), and a small but significant inhibition of proliferation at 10−9 M (−7.7%±0.99) and 10−10 M (−7.7%±1.0).
Figure 4.
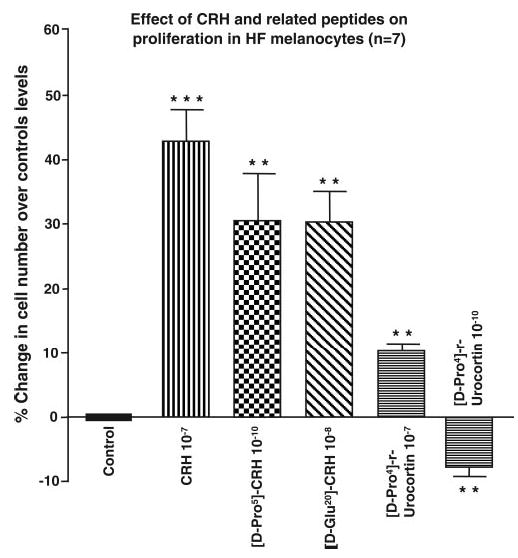
CRH and CRH-associated peptides stimulated proliferation in cultured hair follicle melanocytes. Cell proliferation was assessed by determining cell counts before and after stimulation cells with CRH, [D-Pro5]-CRH, [D-Glu20]-CRH, and [D-Pro4]-r-urocortin. Results are expressed as a % increase in cell number over control unstimulated levels and are a mean ± se of 7 cell lines. CRH (10−7 M) was the most potent inducer of melanocyte proliferation, while a marginal inhibition of proliferation was detected with urocortin at 10−10 M. Statistical significance was assessed by 1-way ANOVA ** P < 0.01; ***P < 0.001
Cell dendricity
CRH, [D-Pro5]-CRH, [D-Glu20]-CRH and [D-Pro4]-urocortin all stimulated melanocyte dendricity (Fig. 5). [D-Pro5]-CRH was the most active inducer of melanocyte dendricity, with a maximal dendritogenic effect observed at 10−10 M (Fig. 5a).
Figure 5.
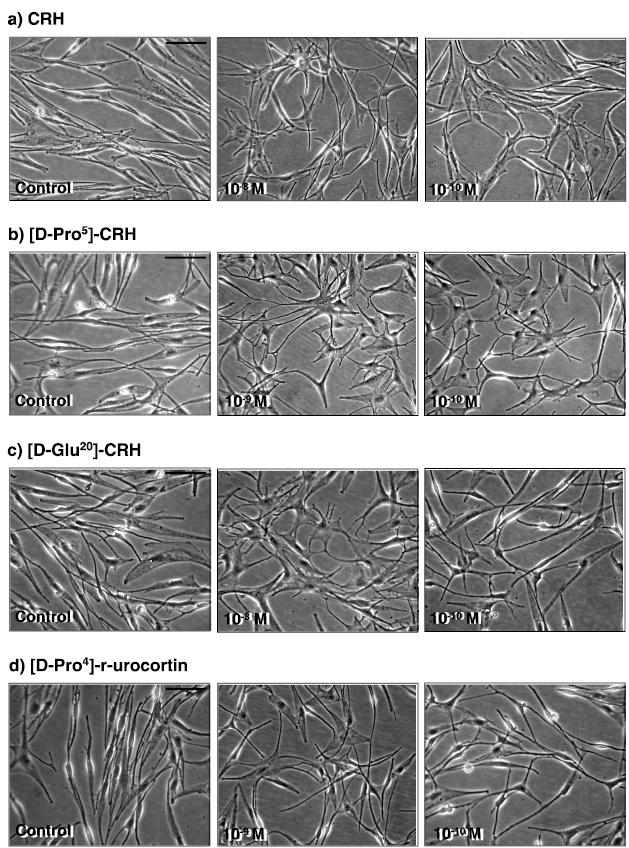
CRH and CRH-associated peptides stimulated dendricity in cultured hair follicle melanocytes. Cell dendricity was assessed by counting cells with 3 or more dendrites before and after stimulation cells with a) CRH; b) [D-Pro5]-CRH; c) [D-Glu20]-CRH, and d) [D-Pro4]-r-urocortin. HFM cultures (passage 2–5) were established in basic fibroblast growth factor (bFGF)/ET-1 (n=7) supplemented medium. FCS and BPE were omitted from the culture medium 48 h prior to stimulation. All peptides stimulated cell dendricity at 72 h, but most potently by [D-Pro5]-CRH at 10−10 M. Scale bar: 50 μm
Cell pigmentation
CRH, [D-Pro5]-CRH, [D-Glu20]-CRH, and [D-Pro4]-urocortin all significantly stimulated melanin production in HFM at concentrations that varied from 10−7 to 10−10 M, although there were significant differences between activities at different peptide concentrations (Fig. 6). For example, only the native CRH peptide significantly stimulated melanogenesis at a concentration of 10−7 M (27%±2.1; P<0.001, n=7) compared to unstimulated controls. However, at 10−8 M melanogenesis was also stimulated by CRH (20.2%±2.4; P<0.01, n=7), together with [D-Glu20]-CRH (9.2%±4.4; P<0.01, n=7) and [D-Pro4]-r-urocortin (3.6%±0.62, P<0.01, n=7). Only [D-Pro5]-CRH stimulated melanogenesis at 10−9 M (12.8%±3.1; P<0.01, n=7) (Fig. 6a).
Figure 6.
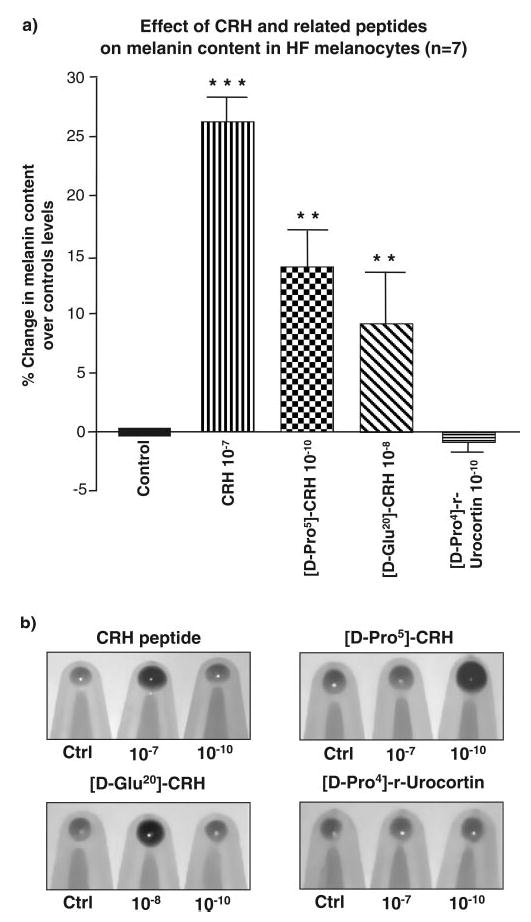
CRH and CRH-associated peptides stimulated melanogenesis in hair follicle melanocytes in culture. Melanin content was determined spectrophotometrically at 475 nm after sodium hydroxide solublization in cells stimulated with CRH; [D-Pro5]-CRH; [D-Glu20]-CRH and [D-Pro4)]-r-urocortin. a) Results are expressed as % increase in melanin content over control unstimulated levels and are a mean ± se of 7 cell lines. CRH was the most potent inducer of melanogenesis, while a marginal inhibition of melanogenesis was detected with [D-Pro4]-r-urocortin. b) Corresponding cells pellets revealing melanogenic effects of the test peptides. Statistical significance was assessed by 1-way ANOVA **P < 0.01; ***P < 0.001
CRH and modified CRH/urocortin peptides modulated the expression and activity of melanogenic enzymes in hair follicle melanocytes in vitro
For this assay, cell pellets taken from cultures that were maximally stimulated by test peptides for melanogenesis were analyzed for melanogenic enzyme expression and activity.
Tyrosinase expression and activity
CRH, [D-Pro5]-CRH, and [D-Glu20]-CRH all stimulated increased expression of Tyrosinase protein at their respective maximally active peptide concentrations. Densitometric analysis indicated that both CRH and [D-Pro5]-CRH were potent at increasing the concentration of Tyrosinase protein compared to unstimulated controls with 180% and 171% increases, respectively (Fig. 7a). [D-Glu20]-CRH also stimulated melanogenesis ~140% over control. However, no significant stimulation was observed in cultures incubated with [D-Pro4]-r-urocortin (Fig. 7a). A similar profile was observed for DOPA-oxidase activity of Tyrosinase. CRH was the most potent stimulator of Tyrosinase activity (138% above controls), followed by [D-Pro5]-CRH and [D-Glu20]-CRH (132% and 127%, respectively, above controls). By contrast, [D-Pro4]-r-urocortin did not exhibit any effect on Tyrosinase’s DOPA-oxidase activity compared to the unstimulated control (0.2% below control) (Fig. 7b).
Figure 7.

CRH and CRH-associated peptides modulated the expression and activity of melanogenic enzymes in hair follicle melanocytes in culture. a) Effect of CRH; [D-Pro5]-CRH; [D-Glu20]-CRH and [D-Pro4]-r-urocortin on Tyrosinase protein expression. CRH and [D-Pro5]-CRH markedly up-regulated Tyrosinase protein expression. No or slight inhibition of Tyrosinase expression was associated with [D-Pro4]-r-urocortin. Lane 1, MW markers; lane 2, CRH 10−7 M; lane 3, [D-Pro5]-CRH 10−10 M; lane 4, [D-Glu20]-CRH 10−8 M; lane 5, [D-Pro4]-r-urocortin 10−10 M; lane 6, control:BM; lane 7, negative control. b) Effect of CRH and CRH-associated peptides on Tyrosinase activity. CRH and [D-Pro5]-CRH up-regulated the activity of Tyrosinase. Little effect was detected in [D-Pro4]-r-urocortin-stimulated cells. Lane 1, CRH 10−7 M; lane 2, [D-Pro5]-CRH 10−10 M; lane 3, [D-Glu20]-CRH 10−8 M; lane 4, [D-Pro4]-r-urocortin 10−10 M; lane 5, control:BM; lane 6, negative control. c) Effect of CRH; [D-Pro5]-CRH; [D-Glu20]-CRH and [D-Pro4]-r-urocortin on TRP-1. CRH; [D-Pro5]-CRH markedly up-regulated the expression of this enzyme. Inhibition of TRP-1 expression was induced by [D-Pro4]-r-urocortin. Lane 1, MW markers; lane 2, CRH 10−7 M; lane 3, [D-Pro5]-CRH 10−10 M; lane 4, [D-Glu20]-CRH 10−8 M; lane 5, [D-Pro4]-r-urocortin 10−10 M; lane 6, control: BM; lane 7, negative control. d) Effect of CRH; [D-Pro5]-CRH; [D-Glu20]-CRH and [D-Pro4]-r-urocortin on TRP-2. CRH and [D-Pro5]-CRH markedly up-regulated the expression of this enzyme. urocortin strongly inhibited TRP-2 expression. Lane 1, MW markers; lane 2, CRH 10−7 M; lane 3, [D-Pro5]-CRH 10−10 M; lane 4, [D-Glu20]-CRH 10−8 M; lane 5, [D-Pro4]-r-urocortin 10−10 M; lane 6, control: BM; lane 7, negative control.
Tyrosinase-related protein-1
CRH, [D-Pro5]-CRH, and [D-Glu20]-CRH all stimulated an increase in the expression of Tyrosinase-related protein-1 (TRP-1). Densitometric analysis indicated that both CRH and [D-Pro5]-CRH were very potent at increasing the concentration of TRP-1 protein compared to unstimulated controls with 166% and 157% increases, respectively (Fig. 7c). [D-Glu20]-CRH also stimulated TRP-1 expression by 117% over control. However, an 18% reduction in the expression of TRP-1 was observed in cultures incubated with [D-Pro4]-r-urocortin (Fig. 7c).
Tyrosinase-related protein-2
CRH, [D-Pro5]-CRH, and [D-Glu20]-CRH all stimulated an increase in the expression of TRP-2/DCT. Densitometric analysis indicated that both CRH and [D-Pro5]-CRH were very potent at increasing the concentration of TRP-2 protein compared to unstimulated controls with 161% and 57% increases, respectively (Fig. 7d). [D-Glu20]-CRH also stimulated TRP-2 protein expression of ~117% above controls. However, an 18% reduction in the expression of TRP-2 was observed in cultures incubated with [D-Pro4]-r-urocortin (Fig. 7d). This inhibition was observed for both the 65 and 80 kDa band of the TRP-2 doublet, although a much greater reduction was observed in the expression of the 80 kDa band.
DISCUSSION
Recent evidence indicates that the human hair follicle expresses CRH, urocortin, and CRH receptors (1, 7, 10, 18, 19, 21, 40). However, it is not yet clear whether CRH can also influence the hair follicle pigmentary unit. In this study we present evidence that CRH is expressed at the mRNA level in human follicular melanocytes, follicular keratinocytes, and FP fibroblasts. These findings are in agreement with the detection of CRH mRNA in neonatal epidermal keratinocytes and melanocytes (18). Furthermore, we previously reported CRH-R1 and/or CRH-R2 mRNA expression in hair follicle-derived melanocytes, keratinocytes, and FP fibroblasts (10). Here we show that scalp hair follicle cells express CRH and urocortin and their cognate receptors CRH-R1 and -R2 at protein levels in the tissue environment (i.e., in situ) and in vitro. CRH and related peptides modified human follicular melanocyte phenotype (i.e., melanogenesis, dendricity and proliferation) in a receptor-specific manner and also up-regulated the expression and activity of Tyrosinase, and the expression of TRP-1 and TRP-2/DCT, providing underlying mechanisms for observed melanogenic effects. The least active of the test peptides was the modified urocortin peptide with an increased selectivity for CRH-R2, indicating that CRH and related peptides modulate follicular melanocyte behavior largely, though not solely, through CRH-R1.
In the present study, the melanogenic zone of the upper hair bulb matrix was conspicuous by its lack of CRH and urocortin expression. While this zone consists of relatively undifferentiated keratinocytes, it also houses highly differentiated and melanogenically active melanocytes (22, 23). By contrast, both CRH and urocortin were detected in a minor population of relatively undifferentiated melanocytes located in the ORS and in the most proximal and peripheral matrix. Thus, unlike keratinocytes, CRH expression in melanocytes was inversely correlated with cell differentiation status. This finding concurs with the observation that while CRH triggers the activation of CRH-R1 in EM in vitro (stimulating cAMP accumulation and increases POMC gene expression and ACTH production), no similar effect is seen in keratinocytes (14). Both CRH and urocortin were expressed in follicular melanocytes in vitro, an observation likely to be linked to the fact that these cells are much less differentiated than melanogenic zone cells in situ and are rather derived from poorly differentiated ORS and hair bulb melanocytes (31, 39) that express CRH/CRH-R1 in situ. Funasaka and colleagues (41) reported the presence of CRH in melanocytic cells, with greatest levels of expression in melanoma cells. The latter cells share similarities with proliferative and relatively undifferentiated follicular melanocytes in vitro (39). Low levels of CRH-R1/R2 expression were also restricted to undifferentiated melanocytes in situ. The low sensitivity of the immunofluorescence technique used is this study suggests that this concentration receptor expression is still likely to be physiologically relevant.
CRH, urocortin, and their receptors CRH-R1 and -R2 were found to be variably expressed in the human scalp anagen hair bulb, with greatest expression of the peptides found in the differentiating keratinocytes of the precortex and inner root sheath. These markers were strikingly down-regulated in the melanogenic zone of the hair bulb but were expressed at moderate levels in the most proximal and least differentiated hair bulb matrix. Moreover, the expression profiles of CRH-R1, -R2 did not fully overlap with their ligands, as has been observed in other tissues, including the brain (42). However, ligands colocalized with their receptors in differentiating keratinocytes of the keratogenous region of the growing hair follicle concur with recent findings in human immortalized (HaCaT) and normal EK where activation of CRH-R1 triggered both G0/1 arrest and early differentiation (43–46), and so represents a key component for the triggering of sequential cell differentiation (45).
CRH/urocortin and their receptors were also expressed variably within follicular fibroblast subpopulations, critical for hair follicle growth and cycling (reviewed in ref 47). The urocortin/CRH-R1 pairing was more highly expressed than CRH/CRH-R2 in fibroblasts of the follicular papilla. CRH and urocortin signal via both CRH receptors, with urocortin equipotent for both receptors, with CRH showing higher potency for CRH-R1 (38, 48). Thus, it would appear that signaling through CRH-R1 system predominates in this cell population. Human DF (but not EK) respond to CRH with stimulation of cAMP, induction of POMC gene and protein expression, and ACTH production and release (13). Moreover, ex vivo stimulation of hair follicles with ACTH can up-regulate cortisol in FP cells (11). The greater expression of CRH message and protein in the fibroblasts of the follicular papilla—the growth regulator of the hair follicle—than in interfollicular dermal fibroblasts is of particular interest. Given the close proximity of FP fibroblasts to hair bulb melanocytes, and the observation that eumelanin is still produced in POMC knock-out C57BL6 mice (2), it is possible that CRH from the FP could stimulate melanogenesis in bulbar melanocytes.
Curiously, in situ expression of CRH peptides was broadly cytoplasmic whereas CRH receptors exhibited a nuclear pattern. The implications of this distribution of ligand and receptor localization are unclear, although it may reflect significant relocalization of receptors and ligands intracellularly, with associated physiological functions (intracrine mechanism of action). This pattern may provide an explanation for our previous finding on urocortin stimulated increase of Ca+2 predominantly in the nuclei of immortalized keratinocytes (49). However, receptor/ligand expression patterns in situ often did not correlate with those in vitro. It should be noted, however, that the activation and differentiation status of follicular cells is likely to be somewhat different in vitro, with associated variability in the intracellular processing of receptors and hormones. This is particularly relevant for FP fibroblasts, which divide rarely in situ (50). CRH expression was also not associated with differentiation status in cultured hair follicle keratinocytes, although the concentration of receptor expression (CRH-R2) was greater in the more differentiated cells. The additional expression of CRH in nuclei of some keratinocytes is similar to that observed in CHO-K1 ovary fibroblasts transfected with preproCRH and also in human T cells (51–53). Nuclear localization of CRH has, however, not been reported before in skin cells and could suggest a physiological role for CRH at this intracellular site.
A major focus of the present study was the assessment of CRH/CRH-Rs in regulating human HFM biology. We provide evidence for the existence of a functionally active CRH/CRH-Rs system in human follicular melanocytes, where stimulation of HFM cultures with CRH and urocortin peptides modulated melanogenesis, dendricity, and proliferation in a CRH receptor-specific manner. All CRH peptides up-regulated pigmentation and dendricity in relatively undifferentiated cultured HFM, indicating an important role for these peptides in HFM differentiation and concurs with the in situ expression of CRH peptides only in submaximally differentiated HFM. Moreover, the magnitude of these phenotypic effects was as great or often greater than those observed for ACTH, α-MSH, and β-END (31, 36), suggesting that these effects must include a direct CRH-R1 activity in addition to possible indirect CRH influences on POMC peptide levels (2). Support for a direct CRH effect also derives from POMC knockout C57/Bl6 mice, which express normal hair pigmentation with eumelanin production despite lack of ACTH, α-MSH, and β-END ligands (54). While CRH and urocortin have similar binding affinities to CRH-R1, urocortin binds CRH-R2 with a much higher affinity than CRH (55). Therefore, the modest effect of the modified urocortin peptide (that has less affinity for CRH-R1) (38) seen in the current study indicates that melanocyte effects of CRH and related peptides are mediated principally via CRH-R1.
The current study demonstrated that CRH itself is effective at up-regulating melanocyte proliferation, as the D-Pro5-CRH and D-Glu20-CRH modifications to the CRH peptide resulted in some reduction in mitogenic activity compared to the parent peptide at the same concentration. By contrast, D-Pro4-r-urocortin exhibited only a very modest, though significant, proliferative effect at 10−7 M and 10−8 M but a mildly inhibitory effect at 10−9 M and 10−10 M. The reduction in the mitogenic potential of these modified peptides compared to native CRH is likely due to changes in their affinity for CRH receptors. For example, the D-Glu20 CRH modification increases its selectivity for CRH-R1 by a factor of 25 as D-Glu20 is required for signaling through CRH-R2 (38) without any observed increase in activity over CRH at any tested concentration. By contrast, the D-Pro5 modification, which results in a relative 5-fold increase in its selectivity for CRH-R2 compared with CRH (38), dramatically increased its proliferative activity at low concentrations suggests an important contribution via signaling through CRH-R2 as well as at CRH-R1 at 10−9 M and 10−10 M in regulating mitogenic activity.
Alternatively, some of the observed effects of CRH peptides on HFM biology may be indirect, via activation of POMC. We previously reported that ACTH, α-MSH, and β-endorphin are all active in stimulating proliferation of these cells in culture (5, 23, 31, 36) and that, in EM, CRH activation of CRH-R1 results in increased POMC gene expression and production of ACTH (14). The inhibition of proliferation by CRH in some melanoma cell lines (56) may result from a failure of CRH to stimulate POMC in these cells (2). In addition, CRH stimulation of human scalp hair follicle organ cultures is associated with an increase in POMC transcription and in the expression of both ACTH and α-MSH (7). It could be argued that part of the observed CRH effect on melanocyte numbers is due to improved hair follicle melanocyte survival rather than proliferation per se, as reported previously in neural cell systems (57). A similar prosurvival action of CRH was observed in normal EM; the latter proliferation, however, was inhibited by CRH when cells cultured in the presence of growth factors (58). Note that a CRH-associated up-regulation of POMC peptides may lead to α-MSH and ACTH-associated protection from apoptosis (59, 60).
All CRH and urocortin peptides stimulated follicular melanocyte dendricity, suggesting that both CRH-R1 and R2 are involved in this important pigmentary response. Peptide blocking experiments indicated that both CRH-R1 and CRH-R2 are expressed by HFM. CRH peptide (10−7 M) induced a significant increase in melanogenesis at levels comparable to POMC peptides (31, 36). However, the D-Pro5-CRH peptide, which exhibits a 5-fold relative increase in selectivity for CRH-R2, was more potent at stimulating both melanogenesis and dendricity at 10−10 M than was CRH. This is consistent with findings showing that dendricity and melanogenesis (markers of melanocyte differentiation) are regulated by separate yet overlapping pathways (61). If CRH-R2 is indeed also involved in the differentiation response, this observation infers the existence of cross-talk between the two CRH receptors. This is supported by the observation that D-Glu20-CRH (25-fold more selective for CRH-R1 than CRH itself) was equipotent to CRH at inducing melanogenesis at the same concentration. It is well accepted that CRH-R1 is more effective than CRH-R2 at transducing the CRH signal (55), leading to a greater intracellular accumulation of cAMP; there is some suggestion that similar ligands may be bound to both CRH receptors at the same time. Under these circumstances two receptors may even exert opposite effects, as seen in the brain, where CRH-R1 can exert anxiogenic actions while CRH-R2 can exert anxiolytic properties (62).
It is becoming increasingly clear that at least two interdependent signaling pathways (i.e., Gsα/cAMP and Gq/11/PLC, IP3, and PKC) are involved in mediating the effects of CRH and urocortin (55). CRH signaling in the skin involves cAMP, IP3, and Ca2+ signaling systems (1, 6, 45, 49, 63). In addition, observations from other cell systems (e.g., endothelium) suggest that urocortin can signal, via CRH-R2β, to increase both cAMP and NO (64). Both of these second messengers have been associated with melanogenesis in EM (65). Our observation that D-Pro4-r-urocortin failed to induce a melanogenic response despite its selectivity for CRH-R2 suggests that HFM may not express the relevant isoforms of CRH-R2. It has been shown that CRH-related peptides exhibit a 10-fold greater potency in stimulating CRH-R2β (in terms of activation of adenylate cyclase) compared with either CRH-R2α or γ (48). Although the Ab used in this study was raised against the amino terminus of CRH-R2, it is not clear which of the known or yet unreported isoforms it preferentially recognizes. The latter would explain our inability to detect by PCR of common CRH-R2 gene fragment in cultured HFM (10). Alternatively, there is interindividual heterogeneity in the ability to express CRH-R2 by HFM.
To gain insight into the possible mechanisms involved in the CRH-stimulated melanogenic response, we examined the influence of these peptides on the expression of melanogenic enzymes (Tyrosinase, TRP-1, and TRP-2). Immunoblot analysis of enzyme expressions fully correlated with the observed melanogenic effects in vitro. Thus, using extracts of maximally stimulated cells (i.e., at optimal concentrations of peptides), the greatest induction of Tyrosinase expression was observed with the CRH parent peptide, followed by D-Pro5-CRH. Both the CRH R1-selective (D-Glu20-CRH and the CRH R2-selective D-Pro4-r-urocortin) agonists induced lower amounts of this enzyme, again suggesting the involvement of both receptors and potential cross-talk between them. Similarly, CRH and D-Pro5-CRH induced similar inductions in the DOPA-oxidase activity of Tyrosinase compared to unstimulated controls. However, the induction of DOPA-oxidase activity by D-Glu20-CRH peptide (25-fold selectivity for CRH-R2) above control levels, albeit less potently that CRH or D-Pro5-CRH, suggests the existence of cross-talk between both CRH receptors. This is supported by the observation that D-Pro4-r-urocortin (ineffective at CRH-R1) failed to affect DOPA-oxidase activity at its most promelanogenic concentration (data not shown), consistent with reduced signaling through CRH-R2 in cultured HFM, despite the immunodetection of CRH-R2 protein. An identical picture was observed regarding the effects of CRH peptides on the expression of TRP-1 and TRP-2, where CRH and D-Pro5-CRH induced a significant increase in protein expression. By contrast, expression of both enzymes was reduced by the modified urocortin peptide. Thus, it appears that signaling through CRH-R1 is necessary to result in the maximal stimulation of the CRH melanogenic effect.
In conclusion, we have shown that the human scalp hair follicle contains several melanocyte subpopulations that appear to be variably modulated by the CRH/urocortin-CRH-Rs systems. The CRH/CRH-R1 system appears to be expressed mainly during the early stages of melanocyte differentiation and becomes down-regulated in mature and fully melanogenic melanocytes. Consistent with the interpretation is the observed down-regulation of CRH-R1 during melanization of melanoma cells when compared with their amelanotic phenotype (17). Thus, these findings suggest a role for CRH peptides in regulating human hair follicle melanocyte differentiation. There appears to be a requirement for significant cross-talk between the CRH-R1 and CRH-R2 signaling systems and that neither alone can provide optimal induction of melanocyte proliferation, dendricity, or melanogenesis. Thus, it may be possible to down-regulate melanocyte phenotype by alternately antagonizing signaling via either receptor type.
Acknowledgments
This research was supported by NIH Grant 1R01-AR047079 to A.S. and D.T.
References
- 1.Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21:457–487. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- 2.Slominski, A. (2005) Neuroendocrine system of the skin. Dermatology In press [DOI] [PMC free article] [PubMed]
- 3.Slominski A, Wortsman J, Kohn L, Ain KB, Venkataraman GM, Pisarchik A, Chung JH, Giuliani C, Thornton M, Slugocki G, Tobin DJ. Expression of hypothalamic-pituitary-thyroid axis related genes in the human skin. J Invest Dermatol. 2002;119:1449–1455. doi: 10.1046/j.1523-1747.2002.19617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80:979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- 5.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 6.Slominski A, Wortsman J, Pisarchik A, Zbytek B, Linton EA, Mazurkiewicz JE, Wei ET. Cutaneous expression of corticotropin-releasing hormone (CRH), urocortin, and CRH receptors. FASEB J. 2001;15:1678–1693. doi: 10.1096/fj.00-0850rev. [DOI] [PubMed] [Google Scholar]
- 7.Ito N, Ito T, Kromminga A, Bettermann A, Takigawa M, Kees F, Straub RH, Paus R. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB J. 2005;19:1332–1334. doi: 10.1096/fj.04-1968fje. [DOI] [PubMed] [Google Scholar]
- 8.Botchkarev VA. Stress and the hair follicle: exploring the connections. Am J Pathol. 2003;162:709–712. doi: 10.1016/S0002-9440(10)63866-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slominski A, Mihm MC. Potential mechanism of skin response to stress. Int J Dermatol. 1996;35:849–851. doi: 10.1111/j.1365-4362.1996.tb05049.x. [DOI] [PubMed] [Google Scholar]
- 10.Slominski A, Pisarchik A, Tobin DJ, Mazurkiewicz JE, Wortsman J. Differential expression of a cutaneous corticotropin-releasing hormone system. Endocrinology. 2004;145:941–950. doi: 10.1210/en.2003-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito N, Ito T, Betterman A, Paus R. The human hair bulb is a source and target of CRH. J Invest Dermatol. 2004;122:235–237. doi: 10.1046/j.1523-1747.2003.22145.x. [DOI] [PubMed] [Google Scholar]
- 12.Slominski A, Wortsman J, Tobin DJ. The cutaneous serotoninergic/melatoninergic system: securing a place under the sun. FASEB J. 2005;19:176–194. doi: 10.1096/fj.04-2079rev. [DOI] [PubMed] [Google Scholar]
- 13.Slominski A, Zbytek B, Semak I, Sweatman T, Wortsman J. CRH stimulates POMC activity and corticosterone production in dermal fibroblasts. J Neuroimmunol. 2005;162:97–102. doi: 10.1016/j.jneuroim.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 14.Slominski A, Zbytek B, Szczesniewski A, Semak I, Kaminski J, Sweatman T, Wortsman J. CRH stimulation of corticosteroids production in melanocytes is mediated by ACTH. Am J Physiol. 2005;288:E701–E706. doi: 10.1152/ajpendo.00519.2004. [DOI] [PubMed] [Google Scholar]
- 15.Aguilera G, Rabadan-Diehl C, Nikodemova M. Regulation of pituitary corticotropin releasing hormone receptors. Peptides. 2001;22:769–774. doi: 10.1016/s0196-9781(01)00390-4. [DOI] [PubMed] [Google Scholar]
- 16.Orth DN. Corticotropin-releasing hormone in humans. Endocr Rev. 1992;13:164–191. doi: 10.1210/edrv-13-2-164. [DOI] [PubMed] [Google Scholar]
- 17.Slominski A, Ermak G, Mazurkiewicz JE, Baker J, Wortsman J. Characterization of corticotropin-releasing hormone (CRH) in human skin. J Clin Endocrinol Metab. 1998;83:1020–1024. doi: 10.1210/jcem.83.3.4650. [DOI] [PubMed] [Google Scholar]
- 18.Slominski A, Ermak G, Hwang J, Chakraborty A, Mazurkiewicz JE, Mihm M. Proopiomelanocortin, corticotropin releasing hormone and corticotropin releasing hormone receptor genes are expressed in human skin. FEBS Lett. 1995;374:113–116. doi: 10.1016/0014-5793(95)01090-2. [DOI] [PubMed] [Google Scholar]
- 19.Slominski AT, Botchkarev V, Choudhry M, Fazal N, Fechner K, Furkert J, Krause E, Roloff B, Sayeed M, Wei E, Zbytek B, Zipper J, Wortsman J, Paus R. Cutaneous expression of CRH and CRH-R. Is there a “skin stress response system?” Ann. NY Acad Sci. 1999;885:287–311. doi: 10.1111/j.1749-6632.1999.tb08686.x. [DOI] [PubMed] [Google Scholar]
- 20.Peters EM, Tobin DJ, Seidah NG, Schallreuter KU. Proopiomelanocortin-related peptides, prohormone convertases 1 and 2 and the regulatory peptide 7B2 are present in melanosomes of human melanocytes. J Invest Dermatol. 2000;114:430–437. doi: 10.1046/j.1523-1747.2000.00913.x. [DOI] [PubMed] [Google Scholar]
- 21.Zouboulis CC, Seltmann H, Hiroi N, Chen W, Young M, Oeff M, Scherbaum WA, Orfanos CE, McCann SM, Bornstein SR. Corticotropin-releasing hormone: an autocrine hormone that promotes lipogenesis in human sebocytes. Proc Natl Acad Sci USA. 2002;99:7148–7153. doi: 10.1073/pnas.102180999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tobin DJ, Paus R. Graying: gerontobiology of the hair follicle pigmentary unit. Exp Gerontol. 2001;36:29–54. doi: 10.1016/s0531-5565(00)00210-2. [DOI] [PubMed] [Google Scholar]
- 23.Slominski A, Wortsman J, Plonka PM, Schallreuter KU, Paus R, Tobin DJ. Hair follicle pigmentation. J Invest Dermatol. 2005;124:13–21. doi: 10.1111/j.0022-202X.2004.23528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tobin, D. J. (2004) Biology of hair pigmentation. In Hair, Nail and Skin (Forslind, B., and Lindberg, M., eds) pp. 319–364, Marcel Dekker, New York
- 25.Slominski A, Paus R. Melanogenesis is coupled to murine anagen: toward new concepts for the role of melanocytes and the regulation of melanogenesis in hair growth. J Invest Dermatol. 1993;101:90S–97S. doi: 10.1111/1523-1747.ep12362991. [DOI] [PubMed] [Google Scholar]
- 26.Nordlund, J. J., and Ortonne, J. P. (1998) The normal color of human skin. In The Pigmentary System: Physiology and Pathophysiology (Nordlund, J. J., Boissy, R. E., Hearing, V. J., King, R. A., and Ortonne, J. P., eds) pp. 475–487, Oxford University Press, Oxford
- 27.Rees JL. Genetics of hair and skin color. Annu Rev Genet. 2003;37:67–90. doi: 10.1146/annurev.genet.37.110801.143233. [DOI] [PubMed] [Google Scholar]
- 28.Hearing VJ. Biochemical control of melanogenesis and melanosomal organization. J Invest Dermatol Symp Proc. 1999;4:24–28. doi: 10.1038/sj.jidsp.5640176. [DOI] [PubMed] [Google Scholar]
- 29.Gillbro JM, Marles LK, Hibberts NA, Schallreuter KU. Autocrine catecholamine biosynthesis and the beta-adrenoceptor signal promote pigmentation in human epidermal melanocytes. J Invest Dermatol. 2004;123:346–353. doi: 10.1111/j.0022-202X.2004.23210.x. [DOI] [PubMed] [Google Scholar]
- 30.Kauser S, Schallreuter KU, Thody AJ, Gummer C, Tobin DJ. Regulation of human epidermal melanocyte biology by beta-endorphin. J Invest Dermatol. 2003;120:1073–1080. doi: 10.1046/j.1523-1747.2003.12242.x. [DOI] [PubMed] [Google Scholar]
- 31.Kauser S, Thody AJ, Schallreuter KU, Gummer CL, Tobin DJ. beta-Endorphin as a regulator of human hair follicle melanocyte biology. J Invest Dermatol. 2004;123:184 –195. doi: 10.1111/j.0022-202X.2004.22724.x. [DOI] [PubMed] [Google Scholar]
- 32.Tobin DJ, Colen SR, Bystryn JC. Isolation and long-term culture of human hair-follicle melanocytes. J Invest Dermatol. 1995;104:86–89. doi: 10.1111/1523-1747.ep12613573. [DOI] [PubMed] [Google Scholar]
- 33.Halaban R, Alfano FD. Selective elimination of fibroblasts from cultures of normal human melanocytes. In Vitro. 1984;20:447–450. doi: 10.1007/BF02619590. [DOI] [PubMed] [Google Scholar]
- 34.Vennegoor C, Hageman P, Van Nouhuijs H, Ruiter DJ, Calafat J, Ringens PJ, Rumke P. A monoclonal antibody specific for cells of the melanocyte lineage. Am J Pathol. 1988;130:179–192. [PMC free article] [PubMed] [Google Scholar]
- 35.Magerl M, Kauser S, Paus R, Tobin DJ. Simple and rapid method to isolate and culture follicular papillae from human scalp hair follicles. Exp Dermatol. 2002;11:381–385. doi: 10.1034/j.1600-0625.2002.110414.x. [DOI] [PubMed] [Google Scholar]
- 36.Kauser S, Thody AJ, Schallreuter KU, Gummer CL, Tobin DJ. A fully functional proopiomelanocortin/melanocortin-1 receptor system regulates the differentiation of human scalp hair follicle melanocytes. Endocrinology. 2005;146:532–543. doi: 10.1210/en.2004-1145. [DOI] [PubMed] [Google Scholar]
- 37.Saphier PW, Faria M, Grossman A, Coy DH, Besser GM, Hodson B, Parkes M, Linton EA, Lowry PJ. A comparison of the clearance of ovine and human corticotrophin-releasing hormone (CRH) in man and sheep: a possible role for CRH-binding protein. J Endocrinol. 1992;133:487–495. doi: 10.1677/joe.0.1330487. [DOI] [PubMed] [Google Scholar]
- 38.Wei ET, Thomas HA, Christian HC, Buckingham JC, Kishimoto T. D-amino acid-substituted analogs of corticotropin-releasing hormone (CRH) and urocortin with selective agonist activity at CRH1 and CRH2beta receptors. Peptides. 1998;19:1183–1190. doi: 10.1016/s0196-9781(98)00085-0. [DOI] [PubMed] [Google Scholar]
- 39.Tobin DJ, Bystryn JC. Different populations of melanocytes are present in hair follicles and epidermis. Pigment Cell Res. 1996;9:304–310. doi: 10.1111/j.1600-0749.1996.tb00122.x. [DOI] [PubMed] [Google Scholar]
- 40.Slominski A, Roloff B, Curry J, Dahiya M, Szczesniewski A, Wortsman J. The skin produces urocortin. J Clin Endocrinol Metab. 2000;85:815–823. doi: 10.1210/jcem.85.2.6381. [DOI] [PubMed] [Google Scholar]
- 41.Funasaka Y, Sato H, Chakraborty AK, Ohashi A, Chrousos GP, Ichihashi M. Expression of proopiomelanocortin, corticotropin-releasing hormone (CRH), and CRH receptor in melanoma cells, nevus cells, and normal human melanocytes. J Invest Dermatol Symp Proc. 1999;4:105–109. doi: 10.1038/sj.jidsp.5640192. [DOI] [PubMed] [Google Scholar]
- 42.Bittencourt JC, Vaughan J, Arias C, Rissman RA, Vale WW, Sawchenko PE. Urocortin expression in rat brain: evidence against a pervasive relationship of urocortin-containing projections with targets bearing type 2 CRF receptors. J Comp Neurol. 1999;415:285–312. [PubMed] [Google Scholar]
- 43.Slominski AT, Roloff B, Zbytek B, Wei ET, Fechner K, Curry J, Wortsman J. Corticotropin releasing hormone and related peptides can act as bioregulatory factors in human keratinocytes. In Vitro Cell Dev Biol Anim. 2000;36:211–216. doi: 10.1290/1071-2690(2000)036<0211:CRHARP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 44.Zbytek B, Pikula M, Slominski RM, Mysliwski A, Wei E, Wortsman J, Slominski AT. Corticotropin-releasing hormone triggers differentiation in HaCaT keratinocytes. Br J Dermatol. 2005;152:474 – 480. doi: 10.1111/J.1365-2133.2005.06217.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zbytek B, Slominski AT. Corticotropin-releasing hormone induces keratinocyte differentiation in the adult human epidermis. J Cell Physiol. 2005;203:118–126. doi: 10.1002/jcp.20209. [DOI] [PubMed] [Google Scholar]
- 46.Quevedo ME, Slominski A, Pinto W, Wei E, Wortsman J. Pleiotropic effects of corticotropin releasing hormone on normal human skin keratinocytes. In Vitro Cell Dev Biol Anim. 2001;37:50–54. doi: 10.1290/1071-2690(2001)037<0050:peocrh>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 47.Tobin DJ, Gunin A, Magerl M, Paus R. Plasticity and cytokinetic dynamics of the hair follicle mesenchyme during the hair growth cycle: implications for growth control and hair follicle transformations. J Invest Dermatol Symp Proc. 2003;8:80–86. doi: 10.1046/j.1523-1747.2003.12177.x. [DOI] [PubMed] [Google Scholar]
- 48.Wille S, Sydow S, Palchaudhuri MR, Spiess J, Dautzenberg FM. Identification of amino acids in the N-terminal domain of corticotropin-releasing factor receptor 1 that are important determinants of high-affinity ligand binding. J Neurochem. 1999;72:388–395. doi: 10.1046/j.1471-4159.1999.0720388.x. [DOI] [PubMed] [Google Scholar]
- 49.Wiesner B, Roloff B, Fechner K, Slominski A. Intracellular calcium measurements of single human skin cells after stimulation with corticotropin-releasing factor and urocortin using confocal laser scanning microscopy. J Cell Sci. 2003;116:1261–1268. doi: 10.1242/jcs.00301. [DOI] [PubMed] [Google Scholar]
- 50.Tobin DJ, Gunin A, Magerl M, Handijski B, Paus R. Plasticity and cytokinetic dynamics of the hair follicle mesenchyme: implications for hair growth control. J Invest Dermatol. 2003;120:895–904. doi: 10.1046/j.1523-1747.2003.12237.x. [DOI] [PubMed] [Google Scholar]
- 51.Morrison E, Hodge P, Tomasec P, Lowenstein PR, Linton EA, Lowry PJ, Castro MG. Nuclear localisation of corticotrophin releasing hormone (CRH) in transfected CHO-K1 cells. Biochem Soc Trans. 1993;21:318S. doi: 10.1042/bst021318s. [DOI] [PubMed] [Google Scholar]
- 52.Castrol MG, Tomasec P, Morrison E, Murray CA, Hodge P, Blanning P, Linton E, Lowry PJ, Lowenstein PR. Mitogenic effects and nuclear localisation of procorticotrophin-releasing hormone expressed within stably transfected fibroblast cells (CHO-K1) Mol Cell Endocrinol. 1995;107:17–27. doi: 10.1016/0303-7207(94)03416-q. [DOI] [PubMed] [Google Scholar]
- 53.Ekman R, Servenius B, Castro MG, Lowry PJ, Cederlund AS, Bergman O, Sjogren HO. Biosynthesis of corticotropin-releasing hormone in human T-lymphocytes. J Neuroimmunol. 1993;44:7–13. doi: 10.1016/0165-5728(93)90262-w. [DOI] [PubMed] [Google Scholar]
- 54.Slominski A, Plonka PM, Pisarchik A, Smart JL, Tolle V, Wortsman J, Low MJ. Preservation of eumelanin hair pigmentation in proopiomelanocortin-deficient mice on a nonagouti (a/a) genetic background. Endocrinology. 2005;146:1245–1253. doi: 10.1210/en.2004-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grammatopoulos DK, Chrousos GP. Functional characteristics of CRH receptors and potential clinical applications of CRH-receptor antagonists. Trends Endocrinol Metab. 2002;13:436–444. doi: 10.1016/s1043-2760(02)00670-7. [DOI] [PubMed] [Google Scholar]
- 56.Carlson KW, Nawy SS, Wei ET, Sadee W, Filov VA, Rezsova VV, Slominski A, Quillan JM. Inhibition of mouse melanoma cell proliferation by corticotropin-releasing hormone and its analogs. Anticancer Res. 2001;21:1173–1179. [PubMed] [Google Scholar]
- 57.Pedersen WA, Wan R, Zhang P, Mattson MP. Urocortin, but not urocortin II, protects cultured hippocampal neurons from oxidative and excitotoxic cell death via corticotropin-releasing hormone receptor type I. J Neurosci. 2002;22:404–412. doi: 10.1523/JNEUROSCI.22-02-00404.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Slominski A, Zbytek B, Pisarchik A, Slominski RM, Wortsman J. CRH functions as a growth factor/cytokine in the skin. J Cell Physiol. 2005;206:780–791. doi: 10.1002/jcp.20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bohm M, Wolff I, Scholzen TE, Robinson SJ, Healy E, Luger TA, Schwarz T, Schwarz A. alpha-Melanocyte-stimulating hormone protects from ultraviolet radiation-induced apoptosis and DNA damage. J Biol Chem. 2005;280:5795–5802. doi: 10.1074/jbc.M406334200. [DOI] [PubMed] [Google Scholar]
- 60.Kadekaro AL, Kavanagh R, Kanto H, Terzieva S, Hauser J, Kobayashi N, Schwemberger S, Cornelius J, Babcock G, Shertzer HG, Scott G, Abdel-Malek ZA. alpha-Melanocortin and endothelin-1 activate antiapoptotic pathways and reduce DNA damage in human melanocytes. Cancer Res. 2005;65:4292–4299. doi: 10.1158/0008-5472.CAN-04-4535. [DOI] [PubMed] [Google Scholar]
- 61.Slominski A, Zjawiony J, Wortsman J, Semak I, Stewart J, Pisarchik A, Sweatman T, Marcos J, Dunbar C, R CT. A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. Eur J Biochem. 2004;271:4178–4188. doi: 10.1111/j.1432-1033.2004.04356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kishimoto T, Radulovic J, Radulovic M, Lin CR, Schrick C, Hooshmand F, Hermanson O, Rosenfeld MG, Spiess J. Deletion of crhr2 reveals an anxiolytic role for corticotropin-releasing hormone receptor-2. Nat Genet. 2000;24:415–419. doi: 10.1038/74271. [DOI] [PubMed] [Google Scholar]
- 63.Fazal N, Slominski A, Choudhry MA, Wei ET, Sayeed MM. Effect of CRF and related peptides on calcium signaling in human and rodent melanoma cells. FEBS Lett. 1998;435:187–190. doi: 10.1016/s0014-5793(98)01067-9. [DOI] [PubMed] [Google Scholar]
- 64.Miki I, Seya K, Motomura S, Furukawa K. Role of corticotropin-releasing factor receptor type 2 beta in urocortin-induced vasodilation of rat aortas. J Pharmacol Sci. 2004;96:170–176. doi: 10.1254/jphs.fp0040364. [DOI] [PubMed] [Google Scholar]
- 65.Romero-Graillet C, Aberdam E, Biagoli N, Massabni W, Ortonne JP, Ballotti R. Ultraviolet B radiation acts through the nitric oxide and cGMP signal transduction pathway to stimulate melanogenesis in human melanocytes. J Biol Chem. 1996;271:28052–28056. doi: 10.1074/jbc.271.45.28052. [DOI] [PubMed] [Google Scholar]


