Abstract
Objective
To investigate potential associations between cognitive function and/or impairment and age-related macular degeneration (AMD) and visual impairment in the Age-Related Eye Disease Study (AREDS).
Methods
The AREDS is an 11-center natural history study of AMD and age-related cataract. The AREDS Cognitive Function Battery was administered to 2946 participants. The battery consists of 6 neuropsychological tests measuring performance in several cognitive domains. The Dunnett multiple comparison test was used to identify differences by AMD and visual acuity severity. The relationship with cognitive impairment was also assessed using logistic regression.
Results
Mean scores of instruments in the AREDS Cognitive Function Battery declined with increased macular abnormalities and reduced visual acuity. After adjustment for age, sex, race, education, smoking status, diabetes mellitus, hypertension, and depression, increased macular abnormalities (trend P value <.05) reduced mean cognitive function scores as measured by the Modified Mini-Mental State Examination and the Wechsler Logical Memory Scale. Reduced vision was found to be associated with reduced mean cognitive function scores as measured by the Modified Mini-Mental State Examination and letter and verbal fluency tasks. Persons with vision worse than 20/40 OU were more likely to be cognitively impaired (Modified Mini-Mental State Examination score <80) (odds ratio, 2.88 [95% confidence interval, 1.75–4.76]) compared with persons with visual acuity of 20/40 or better OU.
Conclusion
These data suggest a possible association of advanced AMD and visual acuity with cognitive impairment in older persons.
Cognitive impairment, OR an acquired deficit in memory function, problem solving, orientation, or abstraction, reduces an individual's ability to function independently and is a major component of age-related deterioration.1–4 Age-related macular degeneration (AMD) is a leading cause of irreversible vision loss in the United States among elderly individuals.5–8 It has been suggested that cognitive impairment and AMD may be associated because they are both neurodegenerative disorders related to aging.
There are limited data regarding a possible association between cognitive function and AMD. The Rotterdam Study found that late age-related maculopathy (ARM) was associated with 2-year incident Alzheimer disease (AD) in white persons 75 years or older.9 This association was attenuated after adjustment for smoking and atherosclerosis. The Atherosclerosis Risk in Communities Study found an association between cognitive function and severe cognitive impairment and early ARM, defined as falling in the lowest 10% of the population in 1 of 3 cognitive function tests, thus suggesting a weak association between cognitive function and early ARM in middle-aged persons.10
A few studies have been inconclusive regarding the relationship between cognitive and visual impairment.11–13 Visual impairment was found to be associated with both an increased risk and an increased clinical severity of AD in a case-control study of 87 patients 65 years and older with mild to moderate, clinically diagnosed AD and 87 controls without dementia matched to the cases by age, sex, and education.14 Rizzo et al15 found a correlation between impairment of visual function and overall severity of cognitive impairment in AD. A study of 156 elderly individuals from the Berlin Aging Study found an association between visual impairment and poor performance on intelligence tests covering 5 cognitive domains.16 The Australian Longitudinal Study of Aging found that a 2-year decline in vision was associated with memory decline in an elderly population.17 A large study of women 69 years and older participating in the Study of Osteoporotic Fractures found a 2-fold increase in the odds of cognitive and functional decline over time associated with vision impairment (best-corrected vision worse than 20/40).18 A cohort study of community-dwelling, older Mexican American individuals found that near-vision impairment was associated with low cognitive function along with cognitive decline during a 7-year period.19 This association remained significant after adjustment for possible confounders.
Although there are data to suggest a relationship between cognitive impairment and AMD and/or visual impairment, the studies have not been conducted in a large sample that includes a large number of patients with the advanced form of AMD and vision loss due to advanced AMD. The purpose of this article is to investigate a potential association between cognitive function and AMD as well as cognitive function and visual impairment among participants in the Age-Related Eye Disease Study (AREDS).
METHODS
STUDY POPULATION
The AREDS is a large multicenter research program designed to further our understanding of the predisposing factors, clinical course, and prognostic factors for AMD and cataract. Macular status of AREDS participants was assessed by trained and certified personnel at a reading center, using the AREDS system for classifying AMD20 based on stereoscopic color fundus photographs taken at regular intervals during study participation.
Macular status at the time of cognitive function assessment ranged from essentially no macular abnormality in either eye (AMD Category 1), to mild or borderline AMD features (AMD Category 2: many small or few intermediate drusen or pigment abnormalities), to at least 1 large druse, extensive intermediate drusen, or noncentral geographic atrophy (AMD Category 3), to advanced AMD in at least one eye (AMD Category 4).
Visual acuity was measured annually in each participant according to the ETDRS (Early Treatment of Diabetic Retinopathy Study) protocol (AREDS Manual of Operations, The EMMES Corporation, Rockville, Md) as the number of letters read with scores ranging from 0 to 100. The letter scores were transformed into approximate Snellen fractions.21 The AREDS participants were categorized into 3 groups based on visual acuity: (1) visual acuity 20/40 or better OU; (2) visual acuity worse than 20/40 in one eye; and (3) visual acuity worse than 20/40 OU.
COGNITIVE FUNCTION ASSESSMENT
The reason for the addition of the Cognitive Function Battery in AREDS was to measure the potential beneficial or deleterious effect of the AREDS medication on cognitive function and to investigate potential associations between cognitive function and AMD. In June 2000, an ancillary study focusing on cognitive function was added to the AREDS follow-up visit protocol. The protocol for the cognitive function assessment was approved by an independent data and safety monitoring committee and the institutional review board for each clinical center. Written informed consent was obtained from AREDS participants to participate in this ancillary study. Between July 2000 and March 2004, trained and certified interviewers administered 6 neuropsychological tests in a standardized order. Of the 4360 AREDS participants alive at the time of the implementation of the ancillary study, 3070 (70%) gave consent and completed the AREDS Cognitive Function Battery. A total of 2946 (96%) of these participants had a complete battery as well as macular photographs of each study eye and a visual acuity measurement available from a study visit within 1 year of cognitive function testing.
A variety of instruments was used to measure the domains of cognitive function. Because some AREDS participants had vision impairment, the extent to which published cognitive tests were vision dependent was an important consideration in choosing the cognitive tests for this study. The AREDS Cognitive Function Battery included 6 validated and commonly used cognitive tests with 8 components.
(1) The Modified Mini-Mental State Examination (3MS) is a brief general cognitive battery with components for orientation, concentration, language, praxis, and immediate and delayed memory with a maximum (best) score of 100.22 Because the 3MS contains 4 questions that are vision dependent, we conducted an additional analysis that eliminated these questions for participants with vision worse than 20/200 in their better eye and prorated the score. The 4 questions involved writing, drawing, reading, or identifying displayed items.
Verbal fluency was measured using (2) animal category23 and (3) letter fluency24 tasks. For the animal category task, participants were asked to name as many animals as possible in 1 minute. The score is the total number of unique animals named. For the letter fluency task, the participants were asked to name as many words starting with the letter “F” as possible in 1 minute. The score is the total number of unique words named. This is repeated for letters “A” and “S.” The letter fluency task score was computed as the sum of the letter “F,” “A,” and “S” scores.
The Logical Memory test, a subtest of the Wechsler Memory Scale—Revised,25 was used to measure both immediate and delayed recall. Two brief stories are read aloud to the subject, and after each reading, (4) the subject is asked to recall as much as possible (Logical Memory Part I). (5) Delayed recall (Logical Memory Part II) is tested after 30 minutes, when the participant is again asked to recall each story from memory. Part I scores range from 0 to 75 and Part II scores range from 0 to 50.
The Buschke Selective Reminding Test26 also measures verbal memory. Participants are read a list of 12 words and asked to recall as many as possible after 1 trial. (6) Immediate recall is the percentage of words recalled on the first trial. (7) After 8 trials, a mean of correct words recalled per trial is calculated (mean word list).
(8) Digits Backward27 was used to measure working memory and attention. Twelve progressive sequences of 2 to 7 digits are presented for backward repetition; scores range from 0 to 12.
OTHER MEASUREMENTS
Demographic information, including age, sex, race, and education, along with history of smoking, use of cholesterol-lowering medications, and self-reported medical comorbidities, was obtained during the AREDS baseline clinical interview. Each participant also underwent a general physical examination at this baseline examination including standardized measurements of height, weight, and blood pressure. The AREDS clinical trial participants were randomly assigned to receive daily oral tablets containing either antioxidants (500 mg of vitamin C; 400 IU of vitamin E; and 15 mg of beta carotene) or no antioxidants. Participants at risk for advanced AMD (AREDS Categories 2, 3, and 4) were also randomly assigned to receive zinc (80 mg as zinc oxide) and copper (2 mg as cupric oxide) or placebo with or without antioxidants in a factorial design.
At the time of administration of the AREDS Cognitive Function Battery, depressive symptoms were assessed using the Center for Epiclemiological Studies Depression Scale (CES-D),28 which is a self-administered measure of depressive symptoms experienced during the previous week. The scores range from 0 to 60 with higher scores indicating more depressive symptoms. The CES-D was administered prior to the other instruments in the AREDS Cognitive Function Battery. Depression in this report is used as a covariate.29–33
STATISTICAL ANALYSIS
This report provides a cross-sectional association of AMD and visual acuity with cognitive impairment. The Dunnett multiple comparison test was used to compare the mean cognitive function scores by AMD category and visual acuity group. Unadjusted and covariate-adjusted means were computed using a generalized linear model. The covariates used included age (<70 years, 70–75 years, or >75 years), sex, education (≤high school or > high school), race (white or other), diabetes mellitus (yes or no), smoking status (never, former, or current), antioxidants (yes or no), use of cholesterol-lowering medication (yes or no), hypertension (yes or no), and depressive symptoms28 (yes, CES-D score >16 or no, CES-D score ≤16).
The potential relationship of cognitive impairment with AMD and vision was explored in unadjusted and adjusted logistic regression models. Cognitive impairment was predefined as a score of less than 80 on the 3MS.22,34 All analyses were carried out using SAS version 8.0 (SAS Institute Inc, Gary, NC).
RESULTS
Of the 4757 subjects enrolled in AREDS, 4360 were alive at the time of the implementation of the AREDS Cognitive Function Battery. Of the available AREDS subjects, 2946 (68%) are included in this report. Of the 1414 subjects not included, 270 (19%) were lost to follow-up, 102 (7%) did not have fundus photographs and/or a visual acuity measurement within 1 year, 22 (2%) had an incomplete battery, and the remaining 1020 participants (56%) refused participation in the ancillary study. Reasons for refusal included the following: time commitment (n = 178); illness (n= 139); diagnosis or suspected AD and/or dementia (n = 120); high anxiety and/or fear (n = 114); transportation problems (n = 66); relocation (n = 60); stopped in-clinic visits (n = 45); language barrier (n= 10); hearing loss (n = 6); and other or unknown (n = 282). Nonparticipants tended to have more macular abnormalities and worse visual acuity in the better eye, were older and less educated, had hypertension, and were nonwhite and current smokers at baseline (Table 1).
Table 1.
Baseline Characteristics of Subjects Included vs Those Not Included in the Analysis*
| Baseline Characteristics | Participants (n = 2496) | Nonparticipants (n = 1414) | PValue† |
|---|---|---|---|
| AMD category | <.01 | ||
| Category 1 | 774 (26) | 276 (19) | |
| Category 2 | 708 (24) | 283 (20) | |
| Category 3 | 987 (34) | 504 (36) | |
| Category 4 | 477 (16) | 351 (25) | |
| Visual acuity in better eye, mean (SD) | 85 (5) | 84 (5) | <.01 |
| Male | 1281 (43) | 593 (42) | .33 |
| White | 2833 (96) | 1338 (95) | .02 |
| Age at randomization, y, mean (SD) | 68 (5) | 70 (5) | <.01 |
| >High school education‡ | 2036 (69) | 792 (56) | <.01 |
| Use of cholesterol-lowering medications | 259 (9) | 122 (9) | .86 |
| Hypertension | 1056(36) | 611 (43) | <01 |
| History of diabetes mellitus | 148 (5) | 88 (6) | .10 |
| Antioxidants | 1467 (50) | 694 (49) | .66 |
| Current smoker | 192 (7) | 124 (9) | <.01 |
Abbreviation: AMD, age-related macular degeneration.
Values are expressed as number (%) of subjects unless otherwise indicated.
Pvalue derived from t test for means and χ2 test for percentages.
Three participants refused to answer.
Results on the association between cognitive function and the AREDS clinical trial treatment have been reported.35 That report found that supplementation with high doses of antioxidants or zinc did not have an effect on cognitive function. Because of this finding, the AREDS treatment was not considered a covariate in the analyses to follow.
The 2946 participants included in this report had a mean age of 75 years (range, 61–88 years) at the time of AREDS Cognitive Function Battery administration. Forty-four percent of the participants had AMD status assessed on the same date of Cognitive Function Battery administration. The mean (SD) length of time between AMD photographs and Cognitive Function Battery administration was 77 (90) days. Fifty-five percent of the participants had visual acuity status assessed on the same date of Cognitive Function Battery administration. The mean (SD) length of time between visual acuity assessment and Cognitive Function Battery administration was 58 (81) days. At the time of the Cognitive Function Battery administration, 23% of the participants were classified as AMD Category 1, 29% were AMD Category 2, 26% were AMD Category 3, and 22% were classified as AMD Category 4. Seventy-two percent had visual acuity of 20/40 or better, 18% had visual acuity of worse than 20/40 in one eye, and 10% had visual acuity of worse than 20/40 OU at the time of Cognitive Function Battery administration.
Characteristics by AMD category and visual acuity group are given in Table 2. Persons with AMD or reduced vision were older, more likely to be of white race, less educated, and current smokers. In addition, persons with reduced vision were more likely to have hypertension at baseline.
Table 2.
Participant Characteristics by AMD and Visual Acuity Category*
|
AMD Category |
Visual Acuity |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | 1 | 2 | 3 | 4 | PValue† | 20/40 or Better OU | worse Than 20/40 in One Eye | Worse Than 20/40 OU | PValue† |
| Sample size (%) | 670 (23) | 852 (29) | 773 (26) | 651 (22) | 2121 (72) | 525 (18) | 300 (10) | ||
| Age, y, mean (SD) | 74 (4) | 74(5) | 75 (5) | 77 (5) | <.01 | 74(5) | 77 (5) | 78 (5) | <.01 |
| Male | 44 | 43 | 41 | 45 | .51 | 43 | 46 | 40 | .18 |
| White | 95 | 94 | 98 | 99 | <.01 | 95 | 99 | 99 | <.01 |
| >High school education‡ | 74 | 71 | 69 | 62 | <.01 | 73 | 62 | 57 | <.01 |
| Current smoker | 6 | 5 | 5 | 12 | <.01 | 5 | 7 | 14 | <.01 |
| History of diabetes mellitus | 6 | 4 | 4 | 6 | .35 | 5 | 6 | 7 | .08 |
| Hypertension | 35 | 33 | 36 | 40 | .10 | 34 | 38 | 42 | .02 |
| Use of cholesterol-lowering medications | 9 | 9 | 8 | 10 | .55 | 8 | 10 | 11 | .11 |
| Antioxidants | 45 | 51 | 53 | 50 | .02 | 49 | 53 | 51 | .32 |
Abbreviation: AMD, age-related macular degeneration.
Values are expressed as percentage of participants unless otherwise indicated.
P value derived from generalized linear model for means and χ2 test for percentages.
One participant refused to answer.
Cognitive impairment was associated with increased age, less education, hypertension, diabetes mellitus, and current smoking status. Nonwhite participants tended to be more cognitively impaired compared with white participants (data not shown).
Mean cognitive function instrument scores significantly decreased with increased macular abnormality (Table 3). After adjustment for age, sex, race, education, smoking status, diabetes mellitus, use of cholesterol-lowering medications, antioxidants, and hypertension, significant associations for participants with advanced AMD in at least one eye compared with participants with little or no small drusen were attenuated for all instruments except the 3MS and Logical Memory Part I. A significant trend remained for the Logical Memory Part I and 3MS instruments (trend P value <.05). In an analysis restricted to 2121 subjects with vision 20/40 or better OU, a significant association remained for Logical Memory Part I and increased macular abnormality (Table 4). In addition, a significant trend was found for the Logical Memory Part II instrument and increased macular abnormality.
Table 3.
Mean (SEM) of the AREDS Cognitive Function Battery Instruments by AMD Category*
|
Unadjusted Model |
Adjusted Model† |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
AMD Category |
AMD Category |
|||||||||
| Instrument | 1 | 2 | 3 | 4 | pValue‡ | 1 | 2 | 3 | 4 | pValue‡ |
| Logical Memory Part I26 | 37.7 (0.42) | 37.6 (0.37) | 35.9 (0.39)§ | 35.6 (0.43)§ | <.01 | 37.2 (0.40) | 37.3 (0.35) | 35.9 (0.37)|| | 36.5 (0.41) | .048 |
| Logical Memory Part II26 | 22.0 (0.33) | 21.9 (0.29) | 20.8 (0.31)|| | 20.6 (0.33)§ | <.01 | 21.6 (0.32) | 21.6 (0.28) | 20.8 (0.29) | 21.2 (0.32) | .13 |
| 3MS22 | 93.8 (0.24) | 93.6 (0.21) | 92.8 (0.22) § | 91.3 (0.24)§ | <.01 | 93.5 (0.22) | 93.3 (0.20) | 92.8 (0.21) | 91.9 (0.23)§ | <.01 |
| Letter fluency24 | 39.2 (0.52) | 39.6 (0.46) | 38.9 (0.49) | 37.1 (0.53)|| | <.01 | 38.8 (0.51) | 39.3 (0.45) | 38.9 (0.47) | 37.9 (0.52) | .20 |
| Buschke Immediate Recall26 | 27.6 (0.51) | 28.0 (0.45) | 27.1 (0.47) | 25.8 (0.51)|| | <.01 | 27.1 (0.49) | 27.5 (0.44) | 27.1 (0.46) | 26.9 (0.51) | .68 |
| Buschke Overall Word List Mean26 | 6.0 (0.08) | 5.9 (0.07) | 5.9 (0.08) | 5.8 (0.08) | .07 | 5.9 (0.08) | 5.9 (0.07) | 5.9 (0.07) | 6.0 (0.08) | .47 |
| Animal category23 | 17.6 (0.19) | 17.6 (0.17) | 17.3 (0.18) | 16.6 (0.1 9)§ | <.01 | 17.4 (0.19) | 17.4 (0.16) | 17.3 (0.17) | 17.0 (0.19) | .10 |
| Digits Backward27 | 6.4 (0.07) | 6.4 (0.07) | 6.3 (0.07) | 6.1 (0.08)|| | <.01 | 6.3 (0.07) | 6.4 (0.07) | 6.3 (0.07) | 6.2 (0.08) | .17 |
Abbreviations: 3MS, Modified Mini-Mental State Examination; AMD, age-related macular degeneration; AREDS, Age-Related Eye Disease Study.
Values are expressed as mean (SEM) unless otherwise indicated. Reference category, AMD Category 1.
Adjusted for age, sex, race, education, smoking status, diabetes mellitus, use of cholesterol-lowering medications, antioxidants, hypertension, and depression.
Trend P value.
P<.01.
P<.05.
Table 4.
Mean (SEM) of the AREDS Cognitive Function Battery Instruments by AMD Category for Participants With Vision 20/40 or Better OU*
|
Unadjusted Model |
Adjusted Model† |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
AMD Category |
AMD Category |
|||||||||
| Instrument | 1 | 2 | 3 | 4 | pValue‡ | 1 | 2 | 3 | 4 | pValue‡ |
| Logical Memory Part I26 | 38.0 (0.43) | 37.8 (0.38) | 36.4 (0.44)§ | 35.3(1.31) | .02 | 37.8 (0.41) | 37.9 (0.36) | 36.5 (0.41) | 35.2 (1.23) | .02 |
| Logical Memory Part II26 | 22.1 (0.34) | 22.0 (0.30) | 21.3 (0.34) | 20.1 (1.02) | .04 | 21.9 (0.32) | 22.1 (0.28) | 21.4 (0.32) | 19.9 (0.96) | .03 |
| 3MS22 | 94.0 (0.23) | 93.6 (0.20) | 93.0 (0.23) | 93.2 (0.70) | .25 | 93.9 (0.21) | 93.7 (0.19) | 93.4 (0.22) | 93.1 (0.64) | .22 |
| Letter fluency24 | 39.4 (0.54) | 39.6 (0.48) | 39.6 (0.55) | 40.4 (1.64) | .57 | 39.2 (0.52) | 39.7 (0.45) | 39.7 (0.52) | 40.2 (1.57) | .58 |
| Buschke Immediate Recall26 | 27.8 (0.51) | 28.3 (0.45) | 27.6 (0.52) | 27.9 (1.55) | .97 | 27.6 (0.49) | 28.2 (0.44) | 27.8 (0.50) | 27.9 (1.50) | .94 |
| Buschke Overall Word List Mean26 | 6.0 (0.08) | 6.09 (0.07) | 6.1 (0.09) | 6.0 (0.26) | .95 | 6.0 (0.08) | 6.0 (0.07) | 6.1 (0.08) | 5.9 (0.24) | .98 |
| Animal category23 | 17.7 (0.20) | 17.6 (0.17) | 17.4 (0.20) | 17.6 (0.60) | .72 | 17.6 (0.19) | 17.7 (0.17) | 17.5 (0.19) | 17.5 (0.57) | .73 |
| Digits Backward27 | 6.4 (0.08) | 6.4 (0.07) | 6.3 (0.08) | 6.1 (0.24) | .23 | 6.4 (0.08) | 6.5 (0.07) | 6.4 (0.08) | 6.1 (0.23) | .20 |
Abbreviations: see Table 3.
Values are expressed as mean (SEM) unless otherwise indicated. Reference category, AMD Category 1.
Adjusted for age, sex, race, education, smoking status, diabetes mellitus, use of cholesterol-lowering medications, antioxidants, hypertension, and depression.
Trend P value.
P<.05.
A covariate-adjusted logistic regression model was used to evaluate associations between cognitive impairment and AMD status. Participants in AMD Categories 3 and 4 had an increased yet nonsignificant risk of depression (Figure 1). The risk of cognitive impairment (3MS score <80) for participants in AMD Categories 3 and 4 was increased yet not significantly compared with Category 1 with odds ratios of 1.09 and 1.63, respectively.
Figure 1.
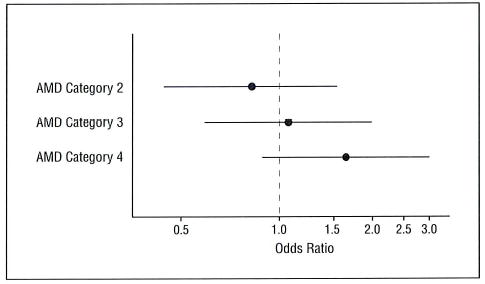
The likelihood (odds ratio and 95% confidence interval) of cognitive impairment (Modified Mini-Mental State Examination score <80) by age-related macular degeneration (AMD) category. Reference group is AMD Category 1.
Mean cognitive function instrument scores significantly decreased with increased visual impairment (Table 5). After adjustment for age, sex, race, education, smoking status, diabetes mellitus, use of cholesterol-lowering medications, antioxidants, and hypertension, significant associations remained for the 3MS and letter fluency and animal category tasks.
Table 5.
Mean (SEM) of the AREDS Cognitive Function Battery Instruments by Visual Acuity Category*
|
Unadjusted Model |
Adjusted Model† |
|||||||
|---|---|---|---|---|---|---|---|---|
|
Visual Acuity |
Visual Acuity |
|||||||
| Instrument | Vision 20/40 or Better OU | Vision Worse Than 20/40 One Eye | Vision Worse Than 20/40 OU | PValue‡ | Vision 20/40 or Better OU | Vision Worse Than 20/40 One Eye | Vision Worse Than 20/40 OU | PValue‡ |
| Logical Memory Part I26 | 37.3 (0.24) | 35.1 (0.48)§ | 35.3 (0.63)§ | <.01 | 36.9 (0.23) | 36.0 (0.46) | 36.6 (0.61) | .63 |
| Logical Memory Part II26 | 21.8 (0.19) | 20.1 (0.37)§ | 20.2 (0.49)§ | <.01 | 21.5 (0.18) | 20.7 (0.36) | 21.2 (0.48) | .55 |
| 3MS22 | 93.6 (0.13) | 92.2 (0.27)§ | 89.3 (0.35)§ | <.01 | 93.4 (0.13) | 92.7 (0.25)|| | 90.2 (0.34)§ | <.01 |
| Letter fluency24 | 39.6 (0.29) | 37.4 (0.59)§ | 35.7 (0.78)§ | <.01 | 39.2 (0.29) | 38.2 (0.57) | 37.0 (0.77)|| | <.01 |
| Buschke Immediate Recall26 | 27.9 (0.28) | 25.1 (0.57)§ | 25.7 (0.75)|| | <.01 | 27.4 (0.28) | 26.2 (0.56) | 27.1 (0.75) | .71 |
| Buschke Overall Word List Mean26 | 6.0 (0.05) | 5.6 (0.09)§ | 5.8 (0.12) | .07 | 5.9 (0.04) | 5.8 (0.09) | 6.0 (0.12) | .47 |
| Animal category23 | 17.6 (0.11) | 16.8 (0.22)§ | 16.1 (0.28)§ | <.01 | 17.4 (0.10) | 17.1 (0.21) | 16.7 (0.28)|| | .02 |
| Digits Backward27 | 6.4 (0.04) | 6.0 (0.08)§ | 6.1 (0.11)|| | .02 | 6.4 (0.04) | 6.1 (0.08)|| | 6.3 (0.11) | .53 |
Abbreviations: 3MS, Modified Mini-Mental State Examination; AREDS, Age-Related Eye Disease Study.
Values are expressed as mean (SEM) unless otherwise indicated. Reference category, vision 20/40 or better OU.
Adjusted for age, sex, race, education, smoking status, diabetes mellitus, use of cholesterol-lowering medications, antioxidants, hypertension, and depression.
Trend P value.
P<.01.
P<.05.
In a covariate-adjusted logistic regression model, persons with visual acuity worse than 20/40 OU were more likely to be cognitively impaired compared with persons with visual acuity of 20/40 or better OU (Figure 2). Persons with visual acuity worse than 20/40 in one eye had an increased yet nonsignificant risk of cognitive impairment.
Figure 2.
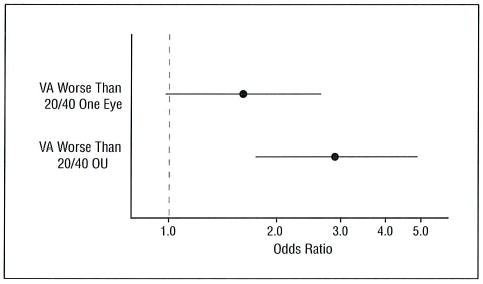
The likelihood (odds ratio and 95% confidence interval) of cognitive impairment (Modified Mini-Mental State Examination score <80) by visual acuity (VA) group. Reference group is vision 20/40 or better OU.
COMMENT
In this study, we document the potential association between cognitive impairment, as determined from the AREDS Cognitive Function Battery, and AMD, as determined from detailed standardized retinal photographic grading and visual acuity. We found a potential association of cognitive impairment with reduced vision and AMD. Persons with cognitive impairment, as determined by the 3MS, were more likely to have reduced visual acuity and/or AMD compared with those not cognitively impaired.
Age-related macular degeneration and cognitive impairment are both chronic neurodegenerative disorders affecting an increasing number of persons as they age. Two prospective studies have hypothesized that AMD and cognitive impairment may be associated because they share a common pathogenesis.9,10 For example, the main common characteristic of these diseases is the loss in cells of the nervous system. In both AMD and AD, early signs include drusen and basal laminar deposits and senile plaques, which are associated with macular and neuronal malfunction and cell loss on accumulation of these deposits.9,36
Two studies have evaluated the relationship between ARM and cognitive function.9,10 These studies included small numbers of subjects with advanced AMD. The Rotterdam Study found a weak association with late ARM and incident AD. This association was attenuated after adjustment for various risk factors. The Atherosclerosis Risk in Communities Study found an association between late ARM and reduced scores for a delayed recall instrument. Significant associations were not found for 2 additional instruments: digit symbol subtest and word fluency. The AREDS found a significant association after covariate adjustment for the 3MS and Logical Memory Part I instrument with advanced AMD. We also found a significant association for the 3MS and letter and verbal fluency tasks with worsening visual acuity. Because of the clinical reliability of the 3MS in diagnosing suspected dementia, there is a suggestion of an association between AMD and cognitive impairment.
The relationship between visual impairment and cognitive function may be due to degeneration of the optic nerve and the retina. Electrophysiological studies have shown that the visual pathways in patients with AD are impaired compared with those in persons without AD.37 Also, optimal cognitive function depends on processing and retrieval of information acquired through the visual sensory system, thus possibly affecting performance on mental assessment tests.19
In addition, it has been hypothesized that the relationship between visual and cognitive impairment is based on the influence of visual impairment on the level and quality of interactive experiences of older adults, thus reducing their capacity to develop and maintain relationships and to participate in activities that may improve their physical, mental, and psychosocial well-being.38 It has been postulated that vision impairment affects cognitive performance by reducing the level of participation in these types of stimulating activities and thus leads to a decrease in brain reserve.39,40 The lack of activity may exacerbate cognitive impairment indirectly if it predisposes a person to depression and social isolation.41
A major strength of this report is the large number of participants with the advanced stage of AMD or vision loss due to advanced AMD, along with the use of standardized photographs assessed by trained and certified personnel at a reading center. Cognitive function was not the primary outcome of our study, and this has led to a number of study limitations. The cognitive testing occurred at the end of the trial, and we only can assess the cross-sectional comparison of AMD and visual acuity and cannot determine whether increased macular abnormalities influence the rate of cognitive decline. This report presents a cross-sectional look at the relationship between cognitive impairment and AMD and visual loss and makes it impossible to ascertain whether cognitive impairment occurred soon after the progression to advanced AMD or visual loss or whether patients were already cognitively impaired for other reasons. Another limitation is that not all subjects in the trial had cognitive testing. The fact that the nonparticipants tended to be older and less educated and have worse vision and more severe AMD suggests that if they would have participated, there may have been a greater proportion of subjects who met criteria for cognitive impairment. Because some AREDS participants had vision impairment, the extent to which published cognitive tests were vision dependent was an important consideration in choosing the cognitive tests for this study. The 3MS contains 4 questions that are vision dependent. However, scoring of the 3MS allows for the prorating of scores for subjects unable to perform visual-dependent tasks. The selection of the participants in AREDS may have, in part, led to the association reported. Participants in AREDS Categories 3 and 4 at baseline were recruited by their retinal doctors in the clinic whereas persons in AREDS Categories 1 and 2 were more likely to be volunteers recruited outside of the clinic. These volunteers may have been less cognitively impaired than persons in AMD Categories 3 and 4. The fact that our findings remained consistent after controlling for various baseline health status factors may have reduced the likelihood of this bias.
In conclusion, these data suggest a possible association of advanced AMD and visual acuity with cognitive impairment in older persons.
Footnotes
Financial Disclosure: None.
Group Information: A complete list of the members of the AREDS Research Group appears in Arch Ophthalmol.
Funding/Support: This study was supported by contracts from the National Eye Institute, the National Institute on Aging, and the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Services, Bethesda, Md.
References
- 1.Jama JW, Launer LJ, Witteman JCM, et al. Dietary antioxidants and cognitive function in a population-based sample of older persons: the Rotterdam Study. Am J Epidemiol. 1996;144:275–280. doi: 10.1093/oxfordjournals.aje.a008922. [DOI] [PubMed] [Google Scholar]
- 2.Johnson LE. Vitamin disorders in the elderly. In: Morley JE, Glick Z, Rubenstein LZ, eds. Geriatric Nutrition. New York, NY: Raven Press; 1990:117–147.
- 3.Ortega RM, Requejo AM, Andres P, et al. Dietary intake and cognitive function in a group of elderly people. Am J Clin Nutr. 1997;66:803–809. doi: 10.1093/ajcn/66.4.803. [DOI] [PubMed] [Google Scholar]
- 4.Nandy K, Sherwin I, eds. The Aging Brain and Senile Dementia. New York, NY: Plenum Press; 1997.
- 5.National Advisory Eye Council. Vision Research: A National Plan, 1983–1987. Bethesda, Md: US Dept of Health and Human Services; 1984. Publication NIH 83–2471.
- 6.Klein R, Klein BEK, Linton KLP. Prevalence of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1992;99:933–943. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- 7.Bressler NM, Bressler SB. Preventative ophthalmology: age-related macular degeneration. Ophthalmology. 1995;102:1206–1211. doi: 10.1016/s0161-6420(95)30889-5. [DOI] [PubMed] [Google Scholar]
- 8.Seddon J. Epidemiology of age-related macular degeneration. In: Ryan SJ, ed. Retina. 3rd ed. Vol 2. St Louis, Mo: CV Mosby; 2001:1039–1050.
- 9.Klaver CC, Ott A, Hofman A, Assink JJ, Breteler MM, de Jong PT. Is age-related maculopathy associated with Alzheimer’s Disease? the Rotterdam Study. Am J Epidemiol. 1999;150:963–968. doi: 10.1093/oxfordjournals.aje.a010105. [DOI] [PubMed] [Google Scholar]
- 10.Wong TY, Klein R, Nieto FJ, et al. Is early age-related maculopathy related to cognitive function? the Atherosclerosis Risk in Communities Study. Am J Ophthalmol. 2002;134:828–835. doi: 10.1016/s0002-9394(02)01672-0. [DOI] [PubMed] [Google Scholar]
- 11.Salthouse TA, Hancock HE, Meinz EJ, Hambrick DZ. Interrelations of age, visual acuity and cognitive functioning. J Gerontol B Psychol Sci Soc Sci. 1996;51:P317–P330. doi: 10.1093/geronb/51b.6.p317. [DOI] [PubMed] [Google Scholar]
- 12.Fagerstrom R. Correlations of memory and learning with vision in aged patients before and after cataract operation. Psychol Rep. 1992;71:675–686. doi: 10.2466/pr0.1992.71.3.675. [DOI] [PubMed] [Google Scholar]
- 13.Mangione CM, Seddon JM, Cook EF, et al. Correlates of cognitive function scores in elderly outpatients. J Am Geriatr Soc. 1993;41:491–497. doi: 10.1111/j.1532-5415.1993.tb01883.x. [DOI] [PubMed] [Google Scholar]
- 14.Uhlmann RF, Larson EB, Koepsell TD, Rees TS, Duckert LG. Visual impairment and cognitive dysfunction in Alzheimer’s disease. J Gen Intern Med. 1991;6:126–132. doi: 10.1007/BF02598307. [DOI] [PubMed] [Google Scholar]
- 15.Rizzo M, Anderson SW, Dawson J, Nawrot M. Vision and cognition in Alzheimer’s disease. Neuropsychologia. 2000;38:1157–1169. doi: 10.1016/s0028-3932(00)00023-3. [DOI] [PubMed] [Google Scholar]
- 16.Lindenberger U, Baltes PB. Sensory functioning and intelligence in old age: a strong connection. Psychol Aging. 1994;9:339–355. doi: 10.1037//0882-7974.9.3.339. [DOI] [PubMed] [Google Scholar]
- 17.Anstey KJ, Luszcz MA, Sanchez L. Two-year decline in vision but not hearing is associated with memory decline in very old adults in a population-based sample. Gerontology. 2001;47:289–293. doi: 10.1159/000052814. [DOI] [PubMed] [Google Scholar]
- 18.Lin MY, Gutierrez PR, Stone KL, et al. Study of Osteoporotic Research Group. Vision impairment and combined vision and hearing impairment predict cognitive and functional decline in older women. J Am Geriatr Soc. 2004;52:1996–2002. doi: 10.1111/j.1532-5415.2004.52554.x. [DOI] [PubMed] [Google Scholar]
- 19.Reyes-Ortiz CA, Kuo Y-F, DiNuzzo AR, Ray LA, Raji MA, Markides KS. Near vision impairment predicts cognitive decline: data from the Hispanic established populations for epidemiologic studies of the elderly. J Am Geriatr Soc. 2005;53:681–686. doi: 10.1111/j.1532-5415.2005.53219.x. [DOI] [PubMed] [Google Scholar]
- 20.The Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study (AREDS) system for classifying age-related macular degeneration from stereoscopic color fundus photographs: AREDS Report No. 6. Am J Ophthalmol. 2001;132:668–681. doi: 10.1016/s0002-9394(01)01218-1. [DOI] [PubMed] [Google Scholar]
- 21.Ferris FL, Kassoff A, Bresnick GH, et al. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94:91–96. [PubMed] [Google Scholar]
- 22.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 23.Morris JC, Heyman A, Mohs RC, et al. The consortium to establish a registry for Alzheimer’s disease (CERAD), I: clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 24.Benton AL. Differential behavioral effects of frontal lobe disease. Neuropsychologia. 1968;6:53–60. [Google Scholar]
- 25.Wechsler DA. Wechsler Memory Scale—Revised. San Antonio, Tex: Psychological Corp; 1987.
- 26.Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24:1019–1025. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- 27.Wechsler D. A standardized memory scale for clinical use. J Psychol. 1945;19:87–95. [Google Scholar]
- 28.Radloff LS. The CES-D scale: a self report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 29.Yaffe K, Blackwell T, Gore R, et al. Depressive symptoms and cognitive decline in nondemented elderly women: a prospective study. Arch Gen Psychiatry. 1999;56:425–430. doi: 10.1001/archpsyc.56.5.425. [DOI] [PubMed] [Google Scholar]
- 30.Geerlings MI, Schoevers RA, Beekman ATF, et al. Depression and risk of cognitive decline and Alzheimer’s disease. Br J Psychiatry. 2000;176:568–575. doi: 10.1192/bjp.176.6.568. [DOI] [PubMed] [Google Scholar]
- 31.Wilson RS, Barnes LL, Mendes de Leon CF, et al. Depressive symptoms, cognitive decline, and risk of AD in older persons. Neurology. 2002;59:364–370. doi: 10.1212/wnl.59.3.364. [DOI] [PubMed] [Google Scholar]
- 32.Gatz JL, Tyas SL, St John P, Montgomery P. Do depressive symptoms predict Alzheimer’s disease and dementia? J Gerontol A Biol Sci Med Sci. 2005;60:744–747. doi: 10.1093/gerona/60.6.744. [DOI] [PubMed] [Google Scholar]
- 33.Biringer E, Mykletun A, Dahl AA, et al. The association between depression, anxiety, and cognitive function in the elderly general population: the Hordaland Health Study. Int J Geriatr Psychiatry. 2005;20:989–997. doi: 10.1002/gps.1390. [DOI] [PubMed] [Google Scholar]
- 34.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 35.The Age-Related Eye Disease Study Research Group. The impact of antioxidants and zinc on cognition in the elderly—a randomized, controlled trial: AREDS Report No. 12. Neurology. 2004;63:1705–1707. doi: 10.1212/01.wnl.0000142969.19465.8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deschamps V, Barberger-Gateau P, Peuchant E, Orgogozo JM. Nutritional factors in cerebral aging and dementia: epidemiological arguments for a role of oxidative stress. Neuroepidemiology. 2001;20:7–15. doi: 10.1159/000054752. [DOI] [PubMed] [Google Scholar]
- 37.Jackson GR, Owsley C. Visual dysfunction, neurodegenerative diseases, and aging. Neurol Clin. 2003;21:709–728. doi: 10.1016/s0733-8619(02)00107-x. [DOI] [PubMed] [Google Scholar]
- 38.Resnick HE, Fries BE, Verbrugge LM. Windows to their world: the effect of sensory impairments on social engagement and activity time in nursing home residents. J Gerontol B Psychol Sci Soc Sci. 1997;52:S135–S144. doi: 10.1093/geronb/52b.3.s135. [DOI] [PubMed] [Google Scholar]
- 39.Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348:2508–2516. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- 40.Wilson RS, Mendes de Leon CF, Barnes LL, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA. 2002;287:742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen HT, Black SA, Ray LA, Espino DV, Markides KS. Predictors of decline in MMSE scores among older Mexican Americans. J Gerontol A Biol Sci Med Sci. 2002;57:M181–M185. doi: 10.1093/gerona/57.3.m181. [DOI] [PubMed] [Google Scholar]


