Abstract
Prostate tumor cells, which characteristically metastasize to bone, initiate binding interactions with bone marrow endothelium under blood flow conditions through binding interactions with E-selectin. We hypothesized that E-selectin ligands on prostate tumor cells are directly associated with bone-metastatic potential. In this report, we elucidate the identity of E-selectin ligands on human metastatic prostate tumor cells and examine their association with prostate tumor progression and metastasis in vivo. To our surprise, we found that the E-selectin-binding form of P-selectin glycoprotein ligand-1 (PSGL-1) is expressed on the human bone-metastatic prostate tumor MDA PCa 2b cell line. Interestingly, we also found that human prostate tumor cells derived from bone, lymph node and brain metastases expressed another leukocyte E-selectin ligand, E-selectin ligand-1 (ESL-1). Immunohistochemical analysis of PSGL-1 and ESL-1 in normal prostate tissue and in localized and metastatic prostate tumors revealed that ESL-1 was principally localized to intracellular cell membrane and expressed on all normal and malignant prostate tissue, whereas PSGL-1 was notably detected on the surfaces of bone-metastatic prostate tumor cells. These findings implicate a functional role of PSGL-1 in the bone tropism of prostate tumor cells and establish a new perspective into the molecular mechanism of human prostate tumor metastasis.
Keywords: Metastasis, E-selectin Ligands, Prostate Cancer, Bone Marrow, Homing
Introduction
The tropism of prostate tumor cells to bone is a common pathologic process (1, 2) and, as evidenced by numerous reports, appears to be regulated by factors inherent to both prostate tumor cells and bone marrow (BM) microenvironment (3-6). The identity of bone-specific factors, which promote bone metastasis of prostate cancer, is currently undefined. It has been suggested that the fate of metastases formation in a particular organ is due to survival factors indigenous to that organ and that the initial seeding of tumor cells occurs by vascular lodgment (7). The presence of circulating prostate tumor cells and the conspicuous avidity of prostate tumor cells to BM endothelial cells (BMEC) suggest, to the contrary, that migration of prostate tumor cells to bone may be mediated through a bone-specific homing mechanism (8-11).
Human hematopoietic progenitor cells (HPC) require the coordinated functional expression ofthe chemokine receptor, CXCR4, and endothelial (E)-selectin ligands for migration to human BM(12-17). While CXCR4 binding to stromal cell-derived factor-1α (SDF-1α) mediates HPC migration across BMEC (13), E-selectin ligands expressed on HPC support rolling adhesive interactions on BMEC that initiate a cascade of molecular events and facilitate HPC entry into bone. HPC E-selectin ligands, PSGL-1 and CD44, are specialized membrane protein scaffolds bearing sialyl Lewis X epitopes (HECA-452 antigen) concomitant with E-selectin-binding activity (14). Recent in vivo homing experiments indicate that PSGL-1 is a major contributor of HPC E-selectin ligand activity (16, 17), highlighting the importance of BMEC E-selectin and HPC E-selectin ligand(s) in bone homing.
In the context of prostate tumor metastasis, SDF-1α mediates trans-BMEC migration of human prostate tumor cells, while CXCR4 expression is elevated on localized and bone-metastatic prostate tumors, implicating this mechanism in the bone tropism of prostate tumor cells (10, 18, 19). Recent data from our laboratory show that BMEC E-selectin helps initiate adhesion of bone-metastatic prostate tumor cells with BMEC and that HECA-452 antigen expression is associated with prostate tumor progression (20). We hypothesize, therefore, that circulating prostate tumor cells utilize a similar bone-homing mechanism as HPC and that acquisition of E-selectin ligand expression may correspond to a bone metastasis phenotype. Though CD44 is a viable E-selectin ligand candidate due to its noted expression on prostate tumor cells (21, 22), other leukocyte E-selectin ligands, such as PSGL-1, L-selectin and ESL-1, represent other potential E-selectin glycoprotein ligands by virtue of their potential expression of HECA-452 antigen (14, 23-25). The identity of E-selectin ligand(s) on human prostate tumor cells are currently unknown and is the focus of our work described herein.
In this study, we investigated the identity of E-selectin glycoprotein ligands on human prostate tumor cells derived from bone, lymph node (LN) or brain metastases. We found that PSGL-1 bearing HECA-452 antigen and E-selectin-binding determinants, otherwise known as cutaneous lymphocyte-associated antigen (CLA), was expressed on human bone-metastatic prostate tumor cells. In addition, we identified the E-selectin ligand, ESL-1, on all metastatic prostate tumor cells. Immunohistochemical analysis of ESL-1 on normal prostatic tissue and on low and high grade prostate tumors revealed that ESL-1 was highly expressed on all prostatic tissue and was principally localized to intracellular membranous structures. PSGL-1 expression, on the other hand, coincided with high E-selectin ligand activity on the bone-metastatic prostate tumor MDA PCa 2b cell line and was directly associated with bone metastases. Immunohistochemical analysis showed that PSGL-1 was conspicuously detected on the surface of prostate tumor cells in bone, whereas low to negligible levels of PSGL-1 were found on normal prostate epithelium, localized prostate cancer and prostate tumor metastases in non-bone tissue. These data are the first described to date and support the notion that PSGL-1/CLA may facilitate the bone tropism of prostate cancer.
Materials and Methods
Cell Lines. Human HPC KG1a cells and murine monocytic WEHI-3 cells (both from ATCC®, Manassas, VA) were maintained in RPMI-1640 with glutamine/10% FBS/1% penicillin/streptomycin (P/S) (all from Gibco™ Invitrogen Corp.; Grand Island, NY). Human prostate tumor MDA PCa 2b cells derived from bone metastases (26) were propagated in BRFF-HPCI (AthenaES™, Baltimore, MD)/20% FBS/1% P/S. Other human bone-metastatic prostate tumor cell lines, PC-3, PC-3M, PC-3M Pro-4 and PC-3M LN-4 (27,28), were maintained in RPMI-1640 with glutamine/10% FBS/1% P/S, while PC-R1 and PC-E1 cell lines (generously provided by Dr. Klaus Pantel; Hamburg, Germany) (29) were cultured in RPMI-1640 with glutamine/10% FBS, 1% P/S, 10μg/ml transferrin, 5μg/ml insulin, 10ng/ml recombinant human EGF and 10μg/ml recombinant human bFGF. Human LN-metastatic prostate tumor cell lines, LNCaP, LNCaP Pro-5, and LNCaP LN-3 (28), and human brain-metastatic prostate tumor DU-145 cells (ATCC®) were maintained in RPMI-1640 with glutamine/10% FBS/1% P/S. Human BMEC, HBMEC-60 (kindly provided by Dr. C. Ellen van der Schoot; Sanquin Research at CLB; Amsterdam, Netherlands (30)), were cultured in Medium199 with HEPES and glutamine/10% FBS/10% human serum/100μg/ml G418/5U/ml heparin/1ng/ml recombinant human bFGF/1% P/S.
Parallel-Plate Flow Analysis. For cell rolling assessments on E-selectin natively expressed on human BMEC, prostate tumor cells and (+) control KG1a cells were perfused over confluent cultures of HBMEC-60 grown in 35x10mm culture dishes (Corning Inc., Corning, NY) and stimulated for 4 hr with 10ng/ml IL-1β (Sigma Co., St. Louis, MO) prior to assay as previously described (20). To confirm E-selectin expression, cells were harvested with 0.5mM EDTA and stained with anti-human E-selectin moAb 68-5H11 (BD Biosciences, Inc., San Jose, CA) for flow cytometric analysis. IL-1β-stimulated HBMEC-60 cells treated with 10μg/ml neutralizing anti-human E-selectin monoclonal Ab 68-5H11 for 30 min. at RT was performed to confirm E-selectin-mediated adhesion. Prostate tumor cells released with 0.5mM EDTA and washed twice in PBS were suspended at 1x106cells/ml in HBSS/10mM HEPES/2mM CaCl2 assay medium and then infused into the chamber over HBMEC-60 cultures as previously described (20). Cell rolling was assessed at 0.6 dynes/cm2 from the mid-point of the chamber viewing field (4 fields of view, 3 different experiments) at 100X magnification as previously described (31). All experiments were observed in real time and videotaped for offline analysis.
Immunoprecipitations and Western Blot Analysis. Membrane preparations of prostate tumor, WEHI-3 and KG1a cells were prepared, and membrane protein was quantified by Bradford method as previously described (14, 20). E-selectin ligand, PSGL-1 and ESL-1 were immunoprecipitated from membrane protein solubilized in 2% NP-40 and precleared in Protein G-agarose with recombinant murine E-selectin-human Ig chimera (R & D Systems, Inc., Minneapolis, MN), mouse IgG anti-human PSGL-1 moAb KPL-1 (BD Biosciences, Inc., San Jose, CA) and rabbit polyclonal anti-sera against ESL-1, respectively. ESL-1 anti-sera was generously provided by Dr. Bruce Furie (Beth-Israel Deaconess Medical Center, Boston, MA) and was also prepared by immunizing rabbits with KLH-conjugated peptides from amino acids 157-176 of ESL-1 (Invitrogen Corp., Carlsbad, CA). Solubilized membrane protein precleared in protein G-agarose was mixed with antibody/chimera at a mass ratio of 100:4, incubated for 18hr on a rotator at 4°C, mixed with protein-G agarose for 2hr at 4°C, and then analyzed by Western blotting. Immunoprecipitations performed with respective isotype controls were conducted in parallel to control for non-specific protein binding. Where indicated, surfaces of WEHI-3 and prostate tumor cells were biotinylated with EZ-Link Sulfo-NHS-SS-Biotin (Pierce Biotechnology, Inc., Rockford, IL) according to manufacturer's protocol prior to membrane protein preparation. Biotinylated membrane protein was incubated with avidin-agarose (Vector Labs., Burlingame, CA) for 18hr on a rotator at 4°C and analyzed by Western blotting.
For Western blotting, total membrane protein, biotinylated membrane protein or avidin/immunoprecipitates were subjected to reducing SDS-PAGE on 4-20% gradient gels, transferred to Immunoblot™ PVDF membrane (Bio-Rad, Inc., Hercules, CA), and blotted with respective antibody. To confirm that quantified membrane protein was equivalent, identically-loaded SDS-PAGE gels were prepared in parallel and stained with Coomassie blue R-250. Intensity analysis of Coomassie-blue stained protein showed that membrane protein loaded for blotting experiments was identical. Blots were first blocked in FBS and then incubated with E-selectin-Ig, anti-PSGL-1 moAb KPL-1, rat IgM anti-human CLA moAb HECA-452 (BD Biosciences) or ESL-1 anti-sera (all at 1 μg/ml) for 1 hr at RT. Isotype control immunoblots were performed in parallel to evaluate non-specific protein binding. Blots were then incubated with respective alkaline phosphatase (AP)-conjugated secondary Abs (all at 1:1000) (Zymed Labs. Inc., San Francisco, CA) for 1 hr at RT and developed with AP-substrate Western Blue® (Promega; Madison, WI) as previously described (20). These experiments were performed a minimum of 5-times.
Purification and Mass Spectrometry of E-selectin-Ig-reactive 150kDa Membrane Protein. To isolate the 150kDa protein(s), we separated MDA PCa 2b membrane protein on reducing 4-20% SDS-PAGE gradient gels and analyzed the E-selectin-Ig-reactive 150kDa protein as follows. To guide localization, excision and retention of the relevant protein, an E-selectin-Ig immunostained blot was prepared in parallel, and the stained blot was superimposed with the corresponding gel. Excised gel fragments from corresponding gels were loaded into a single well (three gel fragments/well) of a new 4-20% gradient gel and subjected to reducing SDS-PAGE. This semi-purification method was repeated 3-times, and the last excised gel fragment was digested with trypsin and analyzed by MALDI-TOF mass spectrometry (Molecular Biology Core Facility, Dana Farber Cancer Institute), and the NCBInr database was searched for possible peptide matches.
Flow Cytometry. Cells from suspension cultures or from adherent cultures harvested by 5mM EDTA were washed twice with cold PBS/2%FBS and suspended in PBS/1%FBS. MoAb HECA-452, anti-human PSGL-1 moAb PL-2 (BD Biosciences), anti-human CD44 moAb A3D8 (Sigma), ESL-1 rabbit anti-sera, anti-human E-selectin moAb 68-5H11 or appropriate isotype-matched control antibody (2μg/test) was incubated with cells for 30 min. on ice. Following two washes, cells were incubated with fluorochrome-conjugated secondary antibody for 30 min. on ice. After washing twice, flow cytometry was performed using a FACScan apparatus equipped with an argon laser tuned at 488 nm (Becton Dickinson). Cells stained with relevant isotype control antibody were subtracted from cells stained with test antibody to control for non-specific binding.
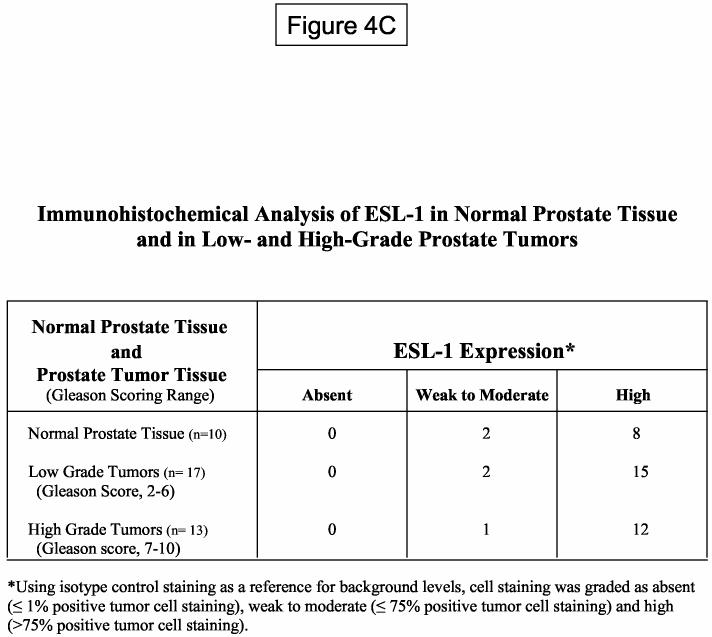
Immunohistochemical Analysis. For ESL-1 immunohistochemical analysis, we utilized tissue micro-arrays (TMA) containing 4μm sections of 2-mm cores from formalin-fixed, paraffin-embedded normal prostate tissue and prostate adenocarcinoma (Chemicon International, Inc.; Temecula, CA). Prostate tumors with a Gleason score of 2-6 were designated as low grade tumors, and tumors with a Gleason score ranging from 7-10 were designated as high grade tumors. TMA were deparaffinized and rehydrated according to manufacturer's protocol. For antigen retrieval, TMA were incubated in 10mM citrate buffer (pH 6.0) in a steam pressure cooker per manufacturer's instructions (Biocare Medical, Walnut Creek, CA). TMA were blocked in hydrogen peroxide and normal goat serum (1:20), and then incubated with rabbit anti-sera against ESL-1 (1:1000) for 1hr at RT. After washing, TMA were incubated with HRP linked-anti-rabbit IgG (Envision Plus Kit; DakoCytomation, Inc., Carpinteria, CA) for 30 min. at RT. Staining was performed using standardized development times and diaminobenzidine (Dako) as a substrate. All TMA were counterstained in hematoxylin. For semi-quantitative analysis of cell staining, brown-stained tumor cells were enumerated and divided by total tumor cell count per field of view at 200X magnification (0.785mm2) and multiplied by 100 to obtain a percent positive cell staining value. Two 2mm cores (a minimum of 4 fields of view) were examined per prostate tissue specimen. Using isotype control staining as a reference for background levels, cell staining was scored as absent (≤1% positive tumor cell staining), weak to moderate (≤75% positive tumor cell staining) and high (>75% positive tumor cell staining).
For PSGL-1 analysis, TMA were constructed and immunostained as follows:
TMA were generated from 30 rapid autopsies and represent prostate tumor specimens from the prostate gland and from all potential metastatic sites (32). The rapid autopsy program was approved by the Institutional Review Board of The University of Michigan. TMA cores were assembled using the manual tissue arrayer (Beecher Instruments, Silver Spring, MD) as previously described (33). Tissue cores from designated areas were targeted for transfer to array blocks. Tissue cores in triplicate (0.6mm in diameter) were sampled from each representative tissue block and spaced at 0.8mm from core-center to core-center. After construction, 4 μm sections were cut and stained with hematoxylin and eosin to verify histological diagnosis. All data are maintained on a relational database as previously described (34). TMA were deparaffinized and rehydrated and then subjected to antigen retrieval in 10mM citrate buffer (pH 6.0) in a pressure cooker for 2 minutes. For immunostaining, TMA were blocked in hydrogen peroxide and normal goat serum (1:20), and then incubated with mouse anti-human PSGL-1 antibody (clone PL-2) (1:200) for 1hr at RT. After washing, TMA were incubated with HRP-linked anti-murine IgG (Envision Plus Kit; Dako), developed and counterstained as described above.
Semi-Automated Quantitative Image Analysis. A semi-automated quantitative image analysis system, ACIS II (Chromavision, San Juan Capistrano, CA), was used to evaluate stained TMA. For immunohistochemical analysis, proprietary software for the ACIS II device was used to detect and quantify brown staining intensity and then compares this value to blue counterstain representing background. Theoretical intensity levels range from 0-255 chromogen intensity units (CIU). In pilot experiments for this study, reproducibility of the ACIS II system was tested and confirmed by scoring several TMA. The correlation coefficient for these experiments was r2=0.973. Due to tissue heterogeneity and to the potential of false-positive leukocyte staining with anti-PSGL-1, a pathologist electronically circled the areas of interest on each tissue core with the ACIS II software, ensuring that intensity measurements were consistent with selected stained areas. Benign prostate tissue cores were included on each TMA to help normalize each slide.
Statistical Analysis. Intensity values were obtained for each tissue core and then normalized within each TMA. Due to potential variation of immunohistochemical staining and intensity values for each TMA, data were normalized for each TMA and from multiple TMA. Staining intensity values for each tissue core from a given TMA were subtracted by the mean intensity for that same TMA and then divided by the standard deviation:
Where j = 1, …, ni ( ni is the total number of cores on TMAi ) . As a result, each normalized TMA had a mean score of 0 and standard deviation equal to 1. Data were then combined using this normalized scale and analyzed using the SPSS software (SPSS Systems, Chicago, IL).
Results
Analysis of Candidate E-selectin Ligands on Human Metastatic Prostate Tumor Cells.
To analyze candidate E-selectin ligands, we first confirmed the expression of E-selectin ligand activities on several bone-, LN- and brain-metastatic prostate tumor cell lines in the parallel-plate flow chamber (20). We assayed tumor cell rolling on E-selectin natively expressed on BMEC. In an E-selectin-dependent manner, we found that bone-metastatic MDA PCa 2b cells exhibited high rolling activity on IL-1β stimulated HBMEC-60 cultures, while PC-3M LN4 cells possessed low activity and all other cells lines did not support rolling adhesions (Figure 1A). Flow assays performed in the presence of neutralizing anti-human E-selectin Ab completely abrogated rolling activity (data not shown).
Figure 1.
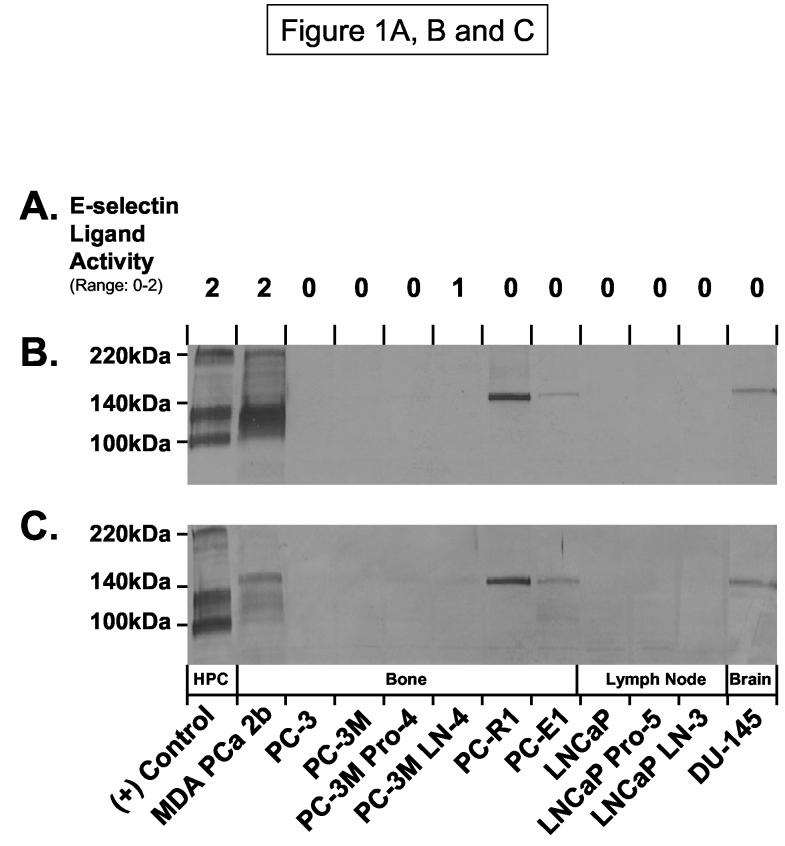
Candidate E-selectin Ligand(s) on Human Metastatic Prostate Tumor Cells. E-selectin ligand activities of human prostate tumor cell lines derived from bone, LN or brain were analyzed at 0.6 dynes/cm2 on IL-1β stimulated HBMEC-60 in the parallel-plate flow chamber (Panel A). Results are expressed as a scoring range from 0 to 2 (0 = no, 1 = low and 2 = high rolling activity) relative to (+) control E-selectin ligand activity of KG1a cells. Positive control KG1a membrane protein (10μg/lane) and tumor membrane protein (40μg/lane) were subjected to SDS-PAGE on 4-20% gradient gels, transferred to PVDF membrane and immunoblotted with moAb HECA-452 (Panel B) or with E-selectin-Ig chimera (Panel C). As indicated in Panels A, B and C, MDA PCa 2b cells possessed high E-selectin ligand activity and exhibited HECA-452-reactive membrane protein from 110-130kDa and at 220kDa, while E-selectin-Ig-reactive protein was detected at ∼110-130 kDa and 150kDa. All other cell lines possessed low to no activity, though PC-R1, PC-E1 and DU-145 expressed a HECA-452 and E-selectin-Ig reactive protein at 150kDa. Binding data and immunoblots are representative of a minimum of 5 experiments, and blots stained with AP-secondary Ab alone showed no detectable proteins.
We subsequently wanted to determine the repertoire of potential E-selectin glycoprotein ligands on human metastatic prostate tumor cells. Prior studies have indicated that the reactivity of moAb HECA-452 to human HPC membrane proteins on Western blots conferred E-selectin-binding function (14, 23-25). Accordingly, we performed Western blot analysis of HECA-452 antigen on membrane protein prepared from prostate tumor cell lines. As shown in Figure 1B, HECA-452-reactive membrane protein from HPC KG1a cells was represented by CD44 at 100kDa and PSGL-1 at 130 (monomer) and 220kDa (dimer). Interestingly, prominent HECA-452-stained membrane protein from MDA PCa 2b cells was evident from 110-130kDa and at 220kDa. Of note, a single HECA-452-reactive band at 150kDa was detectable on cell lines that did not express E-selectin ligand activity (Figure 1B). To help confirm that HECA-452-reactive proteins corresponded to potential E-selectin glycoprotein ligand(s), we performed Western blotting experiments on membrane protein using E-selectin-Immunoglobulin (Ig) chimera as a probe. Similar to HECA-452 immunostaining analysis, we found that KG1a membrane protein stained at 100, 130 and 220kDa (representing CD44 and PSGL-1) and MDA PCa 2b membrane protein(s) was stained at 110-130kDa with E-selectin-Ig. A distinct protein at 150kDa from MDA PCa 2b cells as well as from E-selectin ligand (−) cell lines PC-R1, PC-E1 and DU-145 was also stained with E-selectin-Ig (Figure 1C).
Since HECA-452-reactive PSGL-1 migrates at 120-130kDa and 220kDa (14), we examined the expression of HECA-452 antigen on anti-human PSGL-1 immunoprecipitate from MDA PCa 2b membrane protein. HECA-452 immunostaining of anti-PSGL-1 immunoprecipitate from KG1a membrane protein clearly demonstrated the expression and gel mobility pattern of PSGL-1 (Figure 2A). To our surprise, we found that anti-human PSGL-1 immunoprecipitate from MDA PCa 2b membrane protein was also reactive to moAb HECA-452 at 130 and 220kDa (Figure 2A). By definition, HECA-452-reactivity of PSGL-1 confers E-selectin-binding activity and designation as CLA (14, 23, 31). CLA is a major E-selectin ligand on human HPC and on human skin-homing T-cells (14, 23, 31). Anti-human PSGL-1 immunoprecipitate from KG1a membrane protein was similarly immunostained with Eselectin-Ig at 130kDa and 220kDa, while anti-PSGL-1 immunoprecipitate from MDA PCa 2b cells was stained with E-selectin-Ig at 120 and 130kDa (Figure 2B). Absence of staining at 220kDa in anti-PSGL-1 immunoprecipitate from MDA PCa 2b cells was also noted, suggesting that E-selectin-binding species were only detectable on Western blots in the monomer form. Staining of isotype control immunoprecipitates at 100kDa and not at 120 and 130kDa with E-selectin-Ig indicated that staining at 100kDa was non-specific and that staining of anti-PSGL-1 immunoprecipitate at 120 and 130kDa was PSGL-1 (Figure 2B). Since all other prostate tumor cell lines did not express CLA, we investigated by FACS analysis whether PSGL-1 polypeptide was inherently expressed on metastatic prostate tumor cells. We found that PSGL-1 polypeptide was variably expressed on all metastatic prostate tumor cell lines (from 5-75% positive) (Table 1). As previously reported, prostate tumor cells express CD44 (21, 22) and thus, we considered the possibility that CD44 could represent HECA-452-reactive membrane protein at 110kDa on MDA PCa 2b cells. FACS analysis of CD44 showed that CD44 expression was high on most metastatic prostate tumor cell lines; however, CD44 was notably absent on MDA PCa 2b cells (Table 1). Collectively, these results showed that CLA expression and not CD44 expression was associated with robust E-selectin ligand activity on prostate tumor cells and that CLA was expressed on human bone-metastatic prostate MDA PCa 2b tumor cells. The fact that CLA expression was noted only on MDA PCa 2b cells among several other bone-metastatic prostate tumor cell lines should not weaken the mechanistic implications of these findings. For example, studies on the identification of E-selectin ligands on hematopoietic stem cells, CD44 (HCELL) and PSGL-1, were performed utilizing a single human HPC line (KG1a), which led to subsequent analyses and confirmation that HCELL and E-selectin-binding PSGL-1 were also expressed on freshly-isolated human HPC (14).
Figure 2.
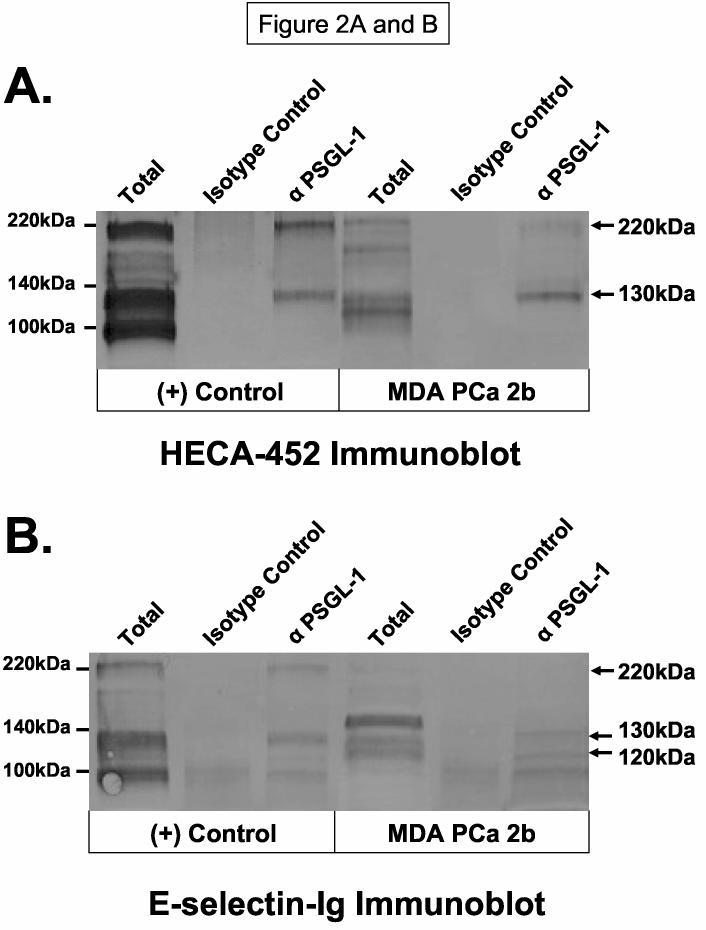
HECA-452-Reactive and E-selectin-binding PSGL-1 is Expressed on Human Bone-Metastatic Prostate Tumor Cells. Anti-PSGL-1 immunoprecipitates from MDA PCa 2b membrane protein were subjected to SDS-PAGE on 4-20% gradient gels, transferred to PVDF membrane and immunoblotted with moAb HECA-452 (Panel A) or E-selectin Ig (Panel B). Compared with immunostaining of anti-PSGL-1 immunoprecipitate from KG1a membrane protein and isotype control immunoprecipitates, MDA PCa 2b cells expressed HECA-452-reactive protein at ∼130 and 220kDa and E-selectin-Ig-reactive protein at 120 and 130kDa. Stainable protein at 100kDa was non-specific as isotype control immunoprecipitates also contained 100kDa stained protein. Immunoblots are representative of at least 5 experiments and isotype control blots (AP-secondary Ab alone) showed no staining.
Table 1.
FACS Analysis of Candidate E-selectin Ligands on Human Metastatic Prostate Tumor Cells.
| Percent Positive Cell Staining (MCF)* | ||
|---|---|---|
| Cell Lines | CD44 | PSGL-1 |
| Positive Control (KG1a Cells) | 100 (470) | 100 (36) |
| Bone-Metastatic Lines | ||
| MDA PCa 2b | 1 (20) | 75 (31) |
| PC-3 | 100 (79) | 6 (21) |
| PC-3M | 100 (161) | 35 (20) |
| PC-3M Pro-4 | 72 (41) | 9 (18) |
| PC-3M LN-4 | 100 (51) | 37 (50) |
| PC-R1 | 100 (193) | 13 (17) |
| PC-E1 | 98 (365) | 27 (74) |
| LN-Metastatic Lines | ||
| LNCaP | 13 (50) | 22 (196) |
| LNCaP Pro-5 | 21 (96) | 37 (166) |
| LNCaP LN-3 | 4 (13) | 34 (22) |
| Brain-Metastatic Lines | ||
| DU-145 | 99 (137) | 5 (31) |
Flow cytometric analysis of CD44 and PSGL-1 on human metastatic prostate tumor cell lines with anti-CD44 moAb A3D8 (1μg/test) and anti-PSGL-1 moAb PL-2 (1μg/test). Percent positive cell staining and mean channel fluorescence (MCF) indicates the number of cells staining greater than negative isotype control cell staining.
The distinct E-selectin-Ig-reactive 150kDa membrane protein on MDA PCa 2b cells prompted subsequent biochemical analyses to identify this protein. We performed sequential SDS-PAGE purification of the gel fragments corresponding to the E-selectin-Ig-stained 150kDa protein and analyzed resolved protein(s) in the resultant gel fragment by MALDI-TOF mass spectrometry. Estimated molecular weights of trypsin-digested peptides in this gel fragment strongly corresponded to the molecular identity of a sialylated membrane glycoprotein known as E-selectin glycoprotein ligand (ESL-1) (35, 36). ESL-1 is a major E-selectin ligand on murine myelocytes and has high homology to the type 1 sialo-membrane protein of the medial cisternae of the Golgi apparatus, MG-160 (35-37). To confirm the localization of ESL-1 at 150kDa, we immunoblotted MDA PCa 2b membrane protein and membrane protein from ESL-1 positive WEHI-3 cells with rabbit IgG anti-sera raised against ESL-1. We found that ESL-1 expression was evident and co-localized with the E-selectin-Ig-reactive 150kDa protein from MDA PCa 2b cells (Figure 3A). To verify that the 150kDa protein on MDA PCa 2b cells was ESL-1, we blotted E-selectin-Ig-purified protein with ESL-1 anti-sera, and alternatively, blotted anti-ESL-1 immunoprecipitate with E-selectin-Ig. As shown in Figure 3B, ESL-1 was detectable in E-selectin-Ig affinity-purified eluate and not in eluate prepared in the presence of EDTA, which confirmed the requirement of Ca++ for ligand binding activity. Likewise, anti-ESL-1 immunoprecipitate from WEHI-3 and MDA PCa 2b membrane protein was stained with E-selectin-Ig, whereas isotype control immunoprecipitates did not stain with E-selectin-Ig (Figure 3C).
Figure 3.
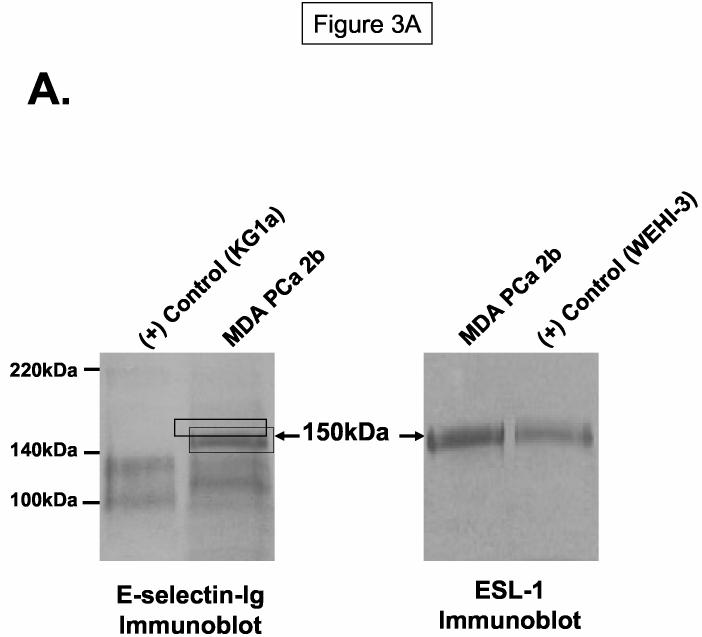
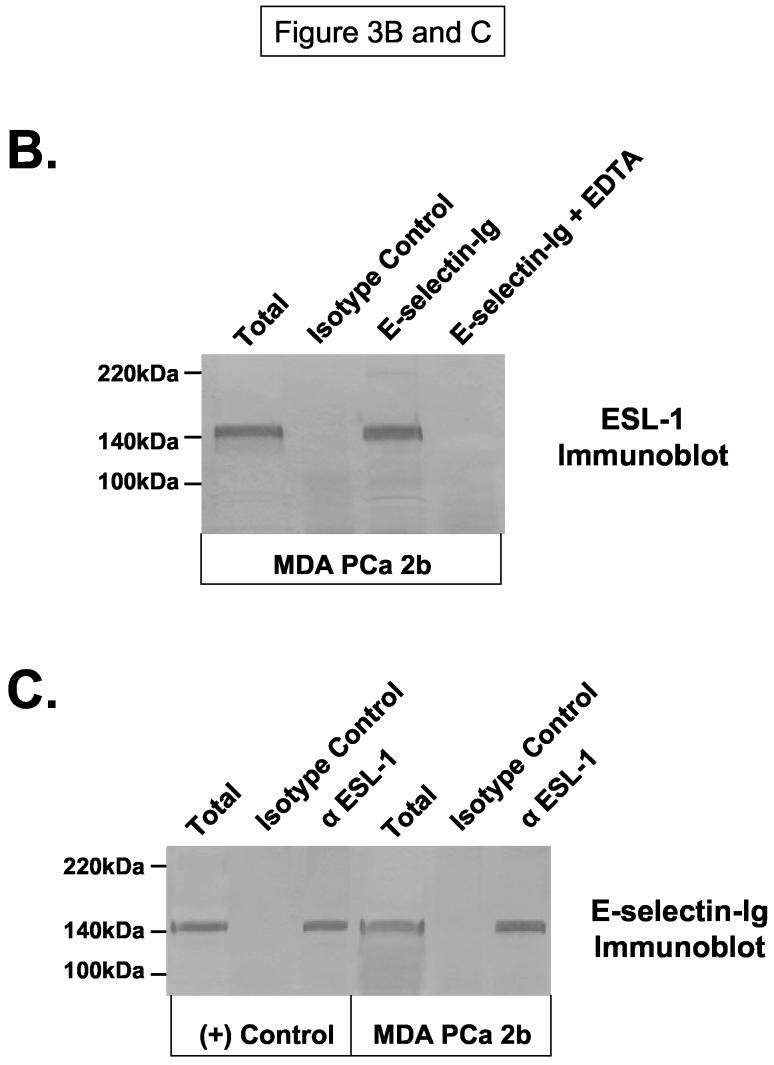
ESL-1 is a Candidate E-selectin Ligand on Human Bone-Metastatic Prostate Tumor Cells. Membrane protein and immunoprecipitates were resolved on 4-20% SDS-PAGE gels, transferred to PVDF membrane and blotted as indicated. In Panel A, ESL-1 was identified from the gel fragment (box) corresponding to the E-selectin-Ig-reactive membrane protein at 150kDa from MDA PCa 2b cells by mass spectrometry. Immunoblot analysis of (+) control WEHI-3 and of MDA PCa 2b membrane protein (40μg/Lane) with ESL-1 anti-sera showed a stainable protein at 150kDa. To determine whether ESL-1 was the E-selectin glycoprotein ligand at 150kDa, E-selectin-Ig affinity-purified eluate from MDA PCa 2b membrane protein (300μg) was blotted with ESL-1 anti-sera (Panel B). Also, anti-ESL-1 immunoprecipitates of MDA PCa 2b membrane protein (300μg) were immunoblotted with E-selectin-Ig (Panel C). In Panel B, the 150kDa protein was detected in E-selectin-Ig eluate with ESL-1 anti-sera and not in the E-selectin-Ig eluate containing EDTA. In Panel C, the 150kDa protein was blotted with E-selectin-Ig in membrane protein (40μg/Lane) and anti-ESL-1 immunoprecipitates from WEHI-3 and MDA PCa 2b cells. Immunoblots performed with AP-secondary Ab alone did not result in background staining. Immunoblots are representative of a minimum of 5 experiments.
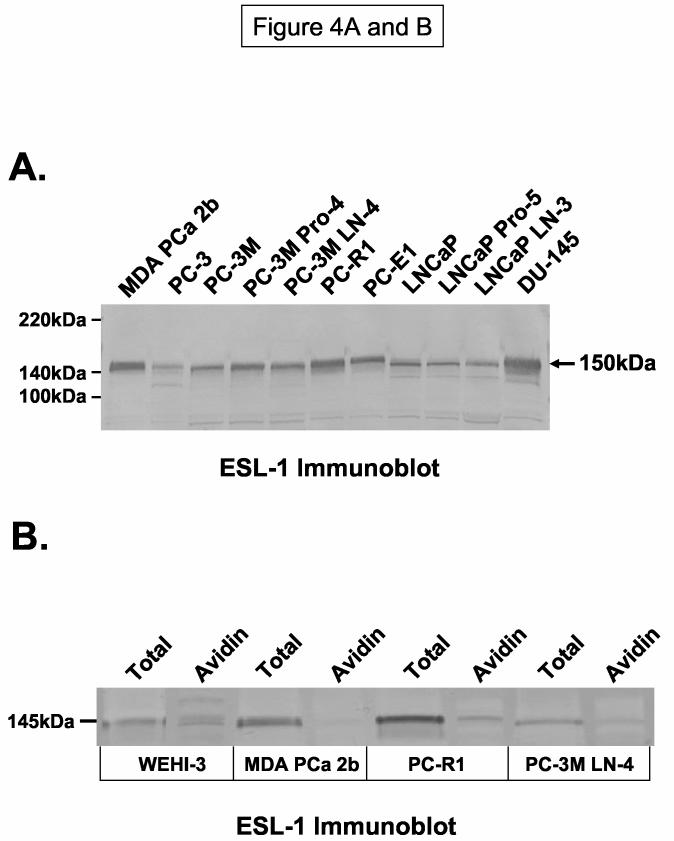
To examine the presence of ESL-1 polypeptide on all metastatic cell lines, we blotted tumor cell membrane protein with ESL-1 anti-sera. We found that ESL-1 polypeptide, representing both functional and non-functional forms, was expressed all cell lines (Figure 4A). However, FACS analysis of ESL-1 was relatively low to absent on most cell lines (0-13% positive), with the exception of WEHI-3 cells (91% positive) and LNCaP LN-3 cells (63% positive) (Table 2). These data suggested that ESL-1 expression as detected by Western blotting experiments may be localized to intracellular membranous structures. To help validate this notion, we performed Western blot analysis of ESL-1 in avidin-agarose purified eluate of surface biotinylated membrane protein. Compared with the presence of ESL-1 in avidin-purified eluate from WEHI-3 cells, ESL-1 was relatively absent in avidin-purified eluate from prostate tumor cell lines (Figure 4B). Results from these experiments indicated that ESL-1 in either E-selectin-binding or non-binding forms was expressed on metastatic prostate tumor cells, and that ESL-1/MG-160 appeared to be an intracellular membrane protein as previously reported (35-37).
Figure 4.
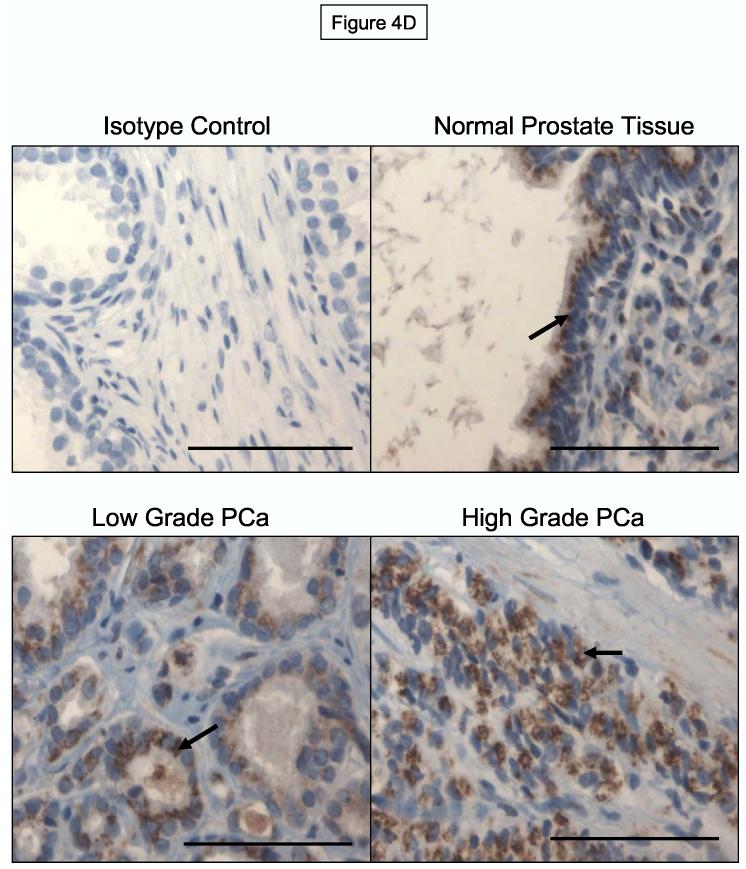
ESL-1 is Constitutively Expressed and Localized to Intracellular Membranes of Normal and Malignant Prostate Epithelium. Panel A: Membrane protein (40μg) from human prostate tumor cells were resolved on 4-20% SDS-PAGE gradient gels, transferred to PVDF membrane and blotted with ESL-1 anti-sera. Panel B: Biotinylated membrane protein from the surface of tumor cells was affinity purified with avidin-agarose and blotted with ESL-1 anti-sera. Compared with staining of ESL-1 from total protein and from avidin-purified (+) control WEHI-3 membrane protein, ESL-1 was relatively absent in avidin-purified protein from prostate tumor cells. Immunoblots in Panel A and B were performed a minimum of 5-times. In Panel C, representative photomicrographs of immunohistochemical analysis of ESL-1 on normal prostate tissue and on localized, low and high grade prostate cancer showed that ESL-1 staining (in brown) was found on intracellular Golgi structures (Arrows indicate peri-nuclear membranous structures). Bar=100μM.
Table 2.
FACS Analysis of ESL-1 on Human Metastatic Prostate Tumor Cells.
| Percent Positive Cell Staining (MCF)* | |
|---|---|
| Cell Lines | ESL-1 |
| Positive Control (WEHI-3 Cells) | 91 (46) |
| Bone-Metastatic Lines | |
| MDA PCa 2b | 1 (11) |
| PC-3 | 14 (11) |
| PC-3M | 13 (21) |
| PC-3M Pro-4 | 3 (1) |
| PC-3M LN-4 | 2 (1) |
| PC-R1 | 10 (9) |
| PC-E1 | 7 (8) |
| LN-Metastatic Lines | |
| LNCaP | 13 (10) |
| LNCaP Pro-5 | 9 (12) |
| LNCaP LN-3 | 62 (30) |
| Brain-Metastatic Lines | |
| DU-145 | 0 (10) |
Flow cytometric analysis of ESL-1 on human metastatic prostate tumor cell lines with rabbit IgG anti-sera against ESL-1 (1μg/test). Percent positive cell staining indicates the number of cells staining greater than negative isotype control cell staining.
In vivo Expression of ESL-1 on Normal and Malignant Prostate Tissue.
To investigate the role of ESL-1 as a marker of prostate tumor progression, we performed immunohistochemical analysis of ESL-1 on TMA of normal prostatic tissue and of low (score of 2-6) and high (score of 7-10) grade prostate tumor tissue. Control staining experiments were performed using paraffin-embedded MDA PCa 2b cell pellets to determine appropriate ESL-1 anti-sera concentrations for antigen-specific detection (data not shown). As summarized in Figure 4C, ESL-1 expression was relatively high on all normal prostate epithelial cells and prostate tumor cells from localized cancer, as 75% of cells stained positive with ESL-1 anti-sera. Furthermore, we observed intense peri-nuclear staining, which was localized to intracellular structures of the Golgi apparatus (Figure 4D). These results in collaboration with Western blot and FACS analysis of ESL-1 on metastatic prostate tumor cell lines suggested that ESL-1 was expressed as an intracellular membrane protein on all normal and malignant prostate tissue and was not associated with prostate tumor progression and metastasis.
In vivo Expression of PSGL-1 in Prostate Cancer.
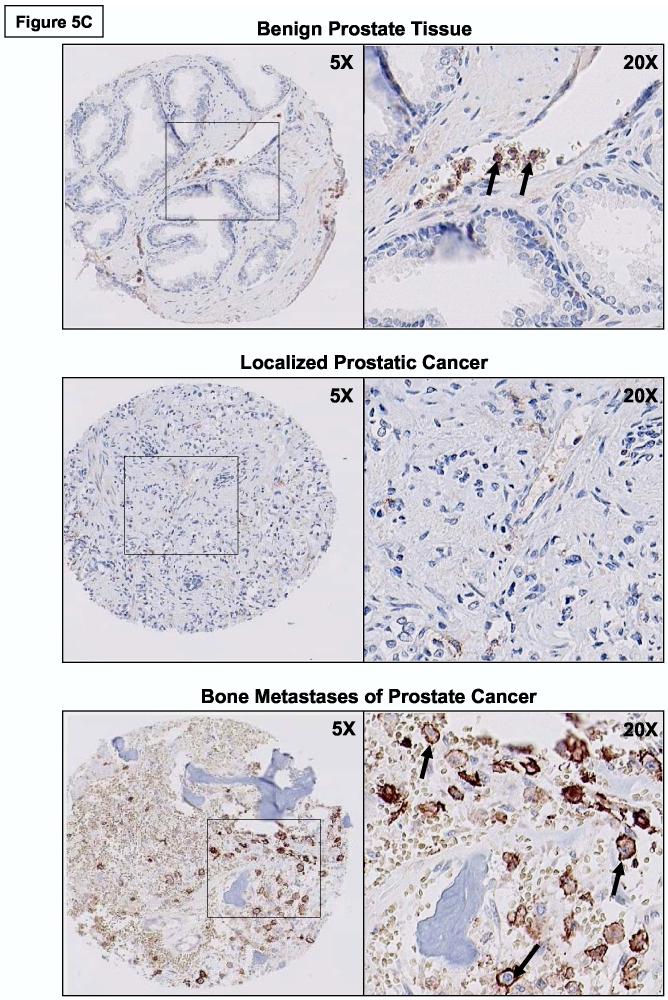
Prior immunohistochemical analysis of PSGL-1 has indicated that PSGL-1 antigen is primarily found on hematopoietic cells: PSGL-1 is broadly expressed on immature HPC and on mature leukocytes, including monocytes, granulocytes, dendritic cells and lymphocytes (38). To investigate the expression of PSGL-1 in prostate cancer, we performed immunohistochemical analysis of PSGL-1 on TMA constructed from benign prostate tissue, localized prostate cancer and metastatic deposits from a number of tissues. Preliminary immunohistochemical experiments were first performed on paraffin-embedded MDA PCa 2b and (+) control KG1a cell pellets with anti-human PSGL-1 moAb PL-2 to ascertain relevant dilutions of moAb PL-2 and of secondary Abs and development reagents for PSGL-1-specific reactivity (data not shown). Following immunohistochemistry with moAb PL-2 on TMA, benign prostate epithelium and malignant prostate tumor cells within the cores were identified by histologic examination, and staining intensities pertaining to selected prostatic tissue were normalized and analyzed using ACIS II image analysis. Of note, intravascular granulocytes were routinely detected with moAb PL-2, serving as an internal positive control for moAb PL-2 reactivity. Normalized PL-2 staining intensity data from these experiments showed that PSGL-1 expression was significantly elevated on prostate tumor cells from bone metastases compared with staining levels on benign prostate epithelial cells and on prostate tumor cells from localized cancer (Independent t-test; p<0.009) (Figure 5A). There was also evidence that PSGL-1 was expressed on prostate tumor cells in lung, LN, liver, bladder and soft tissue metastases (Figure 5B). As illustrated in Figure 5C, PSGL-1 expression on prostate tumor cells within bone-metastatic tissue was robust, whereas PSGL-1 levels on benign prostate epithelium and localized prostate cancer were largely negative. Granulocytes in blood vessels were distinctly positive for PSGL-1 (Figure 5C) and PSGL-1 (+) HPC were routinely detected in BM tissue.
Figure 5.
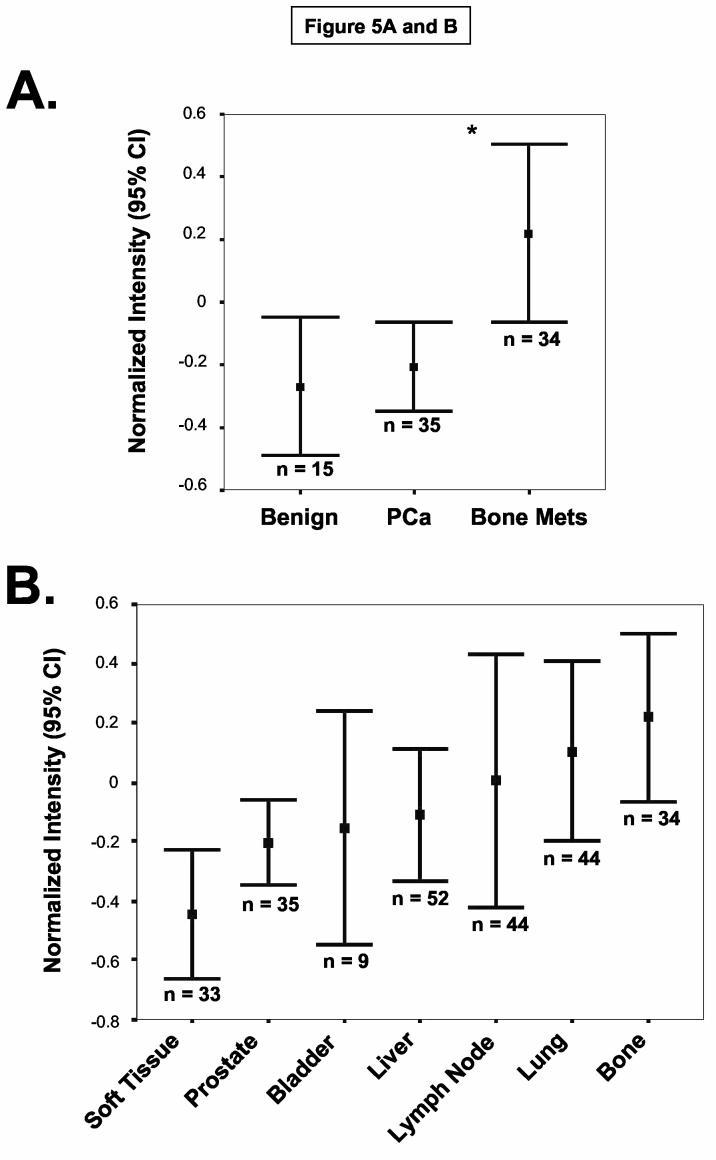
PSGL-1 Expression is Up-regulated on Prostate Tumor Cells in Bone Metastasis. TMA containing benign prostatic tissue and localized and metastatic prostate cancer were immunostained with anti-human PSGL-1 moAb PL-2. Confidence intervals (95%) of normalized mean staining intensity indicate that bone metastases possessed a higher level of PSGL-1 expression than on benign prostate tissue and on localized prostate cancer (Panel A) (*p<0.009, Independent t-test). Also, prostate tumor cells in bone possessed the highest level of PSGL-1 expression compared with expression on prostate tumor cells from other metastatic sites (Panel B) (n = number of core specimens). Panel C: Representative photomicrographs of PSGL-1 staining showed that PSGL-1 (in brown) was detectable on the surface of prostate tumor cells in bone metastases (arrows) and largely absent on benign prostatic epithelium and on localized prostate tumor cells. Of note, as a positive control, PSGL-1 expression on neutrophils was routinely detected in blood vessels as illustrated with arrows in the 20X magnification image of benign prostate tissue.
Discussion
We have previously shown that expression of the sialyl Lewis X-like epitope, HECA-452 antigen, is associated with prostate tumor progression (20). HECA-452 antigen expression on prostate tumor cell membrane glycoprotein(s) and not HECA-452 expression itself correlates with the capacity of human bone-metastatic prostate tumor cells to initiate adhesive contact with BMEC through E-selectin-mediated rolling adhesions (20). Since prostate cancer characteristically metastasizes to bone, we have speculated that binding interactions between E-selectin glycoprotein ligand(s) on circulating prostate tumor cells and E-selectin constitutively expressed on BMEC (39) are critical to formation of prostate tumor metastasis. The importance of E-selectin ligand - BMEC E-selectin adhesive interactions in bone tropism is not unfounded, as human HPC homing to bone in part requires the functional expression of E-selectin ligands, namely HCELL and PSGL-1/CLA (14-17). The coordinated expression of E-selectin ligands and chemokine receptor(s), such CCR4 on skin-homing T-cells and CXCR4 on bone-homing HPC, more notably, is critical for tissue-specific migration (40-42). The fact that human prostate tumor cells express functional CXCR4 and CXCR4 expression is related to prostate tumor metastasis to bone strongly suggests that E-selectin ligands may help promote bone tropism of prostate tumor cells (10, 18, 19).
In this report, we investigated the identity of E-selectin glycoprotein ligands on human metastatic prostate tumor cells. Using the parallel-plate flow chamber, we initially ascertained the prevalence of E-selectin ligand activities on prostate tumor cell lines derived from bone, LN or brain metastases. We observed that E-selectin ligand activity was notably robust on the bone-metastatic MDA PCa 2b cell line, whereas all other cell lines exhibited low to nil activity (20). Since we have previously speculated that HECA-452-reactive glycoproteins represent candidate E-selectin ligand(s) (14, 20), we first performed Western blot analysis of HECA-452 antigen on membrane proteins from both E-selectin (+) and (−) cell lines. Western blotting membrane protein with E-selectin-Ig chimera was also conducted to help show whether prospective HECA-452-reactive glycoprotein(s) expressed E-selectin ligand activity. We found that distinct HECA-452- and E-selectin-Ig-reactive membrane proteins from MDA PCa 2b cells were resolved at ∼120 and 220kDa as well as at 150kDa protein, which was also detected on cells that did not possess ligand activity. To identify E-selectin glycoprotein ligand(s) at ∼120 and 220kDa, we exercised a candidate immunoprobe approach due to the known molecular weights of leukocyte E-selectin ligand PSGL-1 (14). We performed Western blot analysis of HECA-452 antigen and E-selectin ligand expression on anti-PSGL-1 immunoprecipitates from MDA PCa 2b cells and found that the E-selectin-binding glycoform of PSGL-1 was present. Subsequent FACS analysis of PSGL-1 revealed that PSGL-1 polypeptide was variably expressed at low levels on most metastatic prostate tumor cell lines. To identify the E-selectin-Ig-reactive membrane protein at 150kDa, we performed mass spectrometry on a trypsin-digested gel fragment corresponding to E-selectin-Ig-staining region at 150kDa. We found that the molecular weights of digested peptides closely correlated with a known sialo-membrane glycoprotein, ESL-1 (also known as MG-160) (35-37). Western blot analysis of affinity-purified E-selectin ligand(s) with ESL-1 anti-sera and of anti-ESL-1 immunoprecipitates with E-selectin-Ig chimera was subsequently executed to confirm ESL-1 expression and E-selectin-binding activity. Further analysis of ESL-1 expression revealed that all metastatic prostate tumor cells expressed ESL-1 in membrane preparations, whereas ESL-1 expression was negative as determined by flow cytometry. Indeed, ESL-1 immunoblotting experiments using avid-purified membrane protein from biotinylated whole cells strongly suggested that ESL-1 was not be expressed on the cell surface. Collectively, results from these experiments showed that both leukocyte E-selectin ligands, PSGL-1 and ESL-1, were expressed on human metastatic prostate tumor cells. However, only PSGL-1 bearing HECA-452 antigen (known as CLA) (at 120 and 220kDa) expressed at high levels on MDA PCa 2b cells was coincident with robust cellular E-selectin ligand activity. ESL-1, on the other hand, was expressed, either in its E-selectin-binding or non-E-selectin-binding form, on all cells independent of cellular E-selectin ligand activity. It should be noted that the MDA PCa 2b cell line, opposed to all other bone-metastatic prostate tumor cell lines, is unique in its capacity to recapitulate the molecular and growth characteristics of bone metastases from patients with late stage disease (26). Since the synthetic pathway of E-selectin ligands is governed by a coordinated set of glycosyltransferases and a distinct molecular trafficking mechanism that helps target the glycosylation of specific membrane scaffolds, we believe that MDA PCa 2b cells provided a biological tool to help dissect the potential molecular mediators of E-selectin binding to BMEC. The peculiarity of PSGL-1 and ESL-1 expression on cell lines was subsequently analyzed on localized and metastatic prostate tumor cells in vivo.
Immunohistochemical analysis of ESL-1 on normal and malignant prostate tissue showed that ESL-1 was notably expressed at high levels on all prostatic tissue and restricted to intracellular membranous structures. These results paralleled data from other studies showing ESL-1 or MG-160 expression on the Golgi apparatus of human cells (36, 37, 43). MG-160, first identified as a 160kDa sialoglycoprotein of the medial cisternae of the Golgi apparatus in rat brain (44), has been detected as a Golgi membrane protein in several human fetal and adult tissues, including normal and malignant human brain tissue (43, 45). Our prior findings showing that HECA-452-reactive membrane protein contributes 40% of cellular E-selectin activity (20) coupled with evidence of intracellular localization of ESL-1 and of PSGL-1 expression on metastatic prostate tumor cells indicate that ESL-1 may not be a functional E-selectin ligand and that PSGL-1 could be a key contributor to E-selectin ligand activity on human bone-metastatic prostate tumor cells.
To investigate the relationship between PSGL-1 expression and prostate tumor progression and metastasis, we immunostained normal, benign and malignant prostate tissue, as well as a number of metastatic lesions from various tissues. Other groups investigating PSGL-1 expression by immunohistochemical analysis have surveyed both hematopoietic and non-hematopoietic tissues (38) and have shown that PSGL-1 staining is expressed almost exclusively on immature and mature leukocytes from peripheral blood and from within hematopoietic and non-hematopoietic tissues (38). In our analysis, with the exception of granulocytes and lymphocytes in vessels and hematopoietic tissues, we found that normal, benign and malignant prostatic tissues were largely negative for PSGL-1 expression. However, confidence intervals (95%) from normalized PSGL-1 staining intensities of prostate tumor cells in bone metastases compared with staining intensities of epithelia in benign prostate tissue and localized prostate cancer showed that PSGL-1 expression was statistically higher on prostate tumor cells in bone metastases (Independent t-test; p<0.009). In addition, there was PSGL-1 staining on prostate tumor cells from other potential metastatic tissues, which corroborated with detection of PSGL-1 on other non-bone metastatic prostate tumor cell lines. Because metastatic tumor tissues were not traceable to the primary prostate tumors, we could not directly ascertain the potential causal relationship between PSGL-1 expression and tumor metastasis. Whether acquisition of PSGL-1 expression occurs on specialized tumor cells within localized prostate cancer, on circulating prostate tumor cells or on prostate tumor cells within the BM parenchyma is unknown and currently under investigation.
In summary, our studies elucidate potential E-selectin glycoprotein ligands on human metastatic tumor cells and show that PSGL-1/CLA is a major candidate E-selectin glycoprotein ligand on bone-metastatic tumor cells and PSGL-1 expression may be associated with bone metastasis. Coupled with the role of CXCR4 in prostate tumor metastasis, we speculate that bone metastasis of prostate cancer is a patho-biological outcome, which mirrors the process of HPC homing to bone. Interestingly, we found that E-selectin-binding form of CD44 (HCELL) was not expressed on metastatic prostate tumor cells as previously described on HPC (14). CD44, in the context of prostate tumor metastasis, most likely functions as a hyaluronic acid receptor mediating firm adhesion with BMEC (21, 22). The hyaluronan-binding feature of CD44 has previously been found to play a role in the trafficking of human CD34+ stem cells to BM (46), which further argues the mechanistic similarities between bone metastasis of prostate cancer and HPC homing to bone. Other adhesion molecules mediating prostate tumor cell binding interactions with BMEC and with BM hematopoietic, osteogenic and stromal elements are undoubtedly also required for seeding, retention and growth of prostate tumor cells in bone (22, 47-50). We believe that broadening our understanding of the “HPC mimicry” or bone-homing behavior of prostate tumor cells will help expand our current view of the pathogenesis of bone metastasis. In vivo mouse experiments tailored to examine the homing efficiency of E-selectin ligand (+) or (−) human metastatic prostate tumor cell into human bone will need to be performed to fully appreciate the functional role of E-selectin ligand(s). Information from these analyses will instigate the development of new therapeutic strategies for inhibiting the bone tropism of prostate tumor cells.
Acknowledgements
We would like extend to special thanks to Dr. Glenn Bubley (Department of Medicine, Beth-Israel Deaconess Medical Center, Boston, MA) for his helpful criticism of pilot experiments, and Dr. Mirna Lechpammer (Children's Hospital, Boston, MA) for her technical assistance in preliminary studies. This work was supported by NIH grants, NCI 1R21 CA102913-02 (C.J. Dimitroff), NCI 1R21 CA104828-01 (C.J. Dimitroff), NIAMS Harvard Skin Disease Research Center Core Grant 5P30 AR042689 and the Prostate Pathology Core of the Dana Farber/Harvard Cancer Center Grant (CA06516-37). The rapid autopsy program was supported by Specialized Program of Research Excellence in Prostate Cancer (NCI Grant CA69568) and The Prostate Cancer Foundation.
References
- 1.Rubin MA, Putzi M, Mucci N, et al. Rapid (“warm”) autopsy study for procurement of metastatic prostate cancer. Clin Cancer Res. 2000;6(3):1038–45. [PubMed] [Google Scholar]
- 2.Bubendorf L, Schopfer A, Wagner U, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Human Pathol. 2000;31(5):578–83. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 3.Lange PH, Vessella RL. Mechanisms, hypotheses and questions regarding prostate cancer micrometastases to bone. Cancer Met Rev. 1999;17:331–6. doi: 10.1023/a:1006106209527. [DOI] [PubMed] [Google Scholar]
- 4.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2003;2(8):584–93. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 5.Reddi AH, Roodman D, Freeman C, Mohla S. Mechanisms of tumor metastasis to the bone: challenges and opportunities. J. Bone Miner Res. 2003;18(2):190–4. doi: 10.1359/jbmr.2003.18.2.190. [DOI] [PubMed] [Google Scholar]
- 6.Tantivejkul K, Kalikin LM, Pienta KJ. Dynamic process of prostate cancer metastasis to bone. J Cell Biochem. 2003;91(4):706–17. doi: 10.1002/jcb.10664. [DOI] [PubMed] [Google Scholar]
- 7.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2(8):563–72. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 8.Ellis WJ, Pfitzenmaier J, Colli J, Arfman E, Lange PH, Vessella RL. Detection and isolation of prostate cancer cells from peripheral blood and bone marrow. Urology. 2003;61(2):277–81. doi: 10.1016/s0090-4295(02)02291-4. [DOI] [PubMed] [Google Scholar]
- 9.Loberg RD, Fridman Y, Pienta BA, et al. Detection and isolation of circulating tumor cells in urologic cancers: a review. Neoplasia. 2004;6(4):302–9. doi: 10.1593/neo.03484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002;62(6):1832–7. [PubMed] [Google Scholar]
- 11.Lehr JE, Pienta KJ. Preferential adhesion of prostate cancer cells to a human bone marrow endothelial cell line. J Natl Can Inst. 1998;90(2):118–23. doi: 10.1093/jnci/90.2.118. [DOI] [PubMed] [Google Scholar]
- 12.Peled A, Petit I, Kollet O, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283(5403):845–8. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 13.Kollet O, Spiegel A, Peled A, et al. Rapid and efficient homing of human CD34 (+)CD38 (−/low)CXCR4(+) stem and progenitor cells to the bone marrow and spleen of NOD/SCID and NOD/SCID/B2m(null) mice. Blood. 2001;97(10):3283–91. doi: 10.1182/blood.v97.10.3283. [DOI] [PubMed] [Google Scholar]
- 14.Dimitroff CJ, Lee JY, Rafii S, Fuhlbrigge RC, Sackstein R. CD44 is a major E-selectin ligand on human hematopoietic progenitor cells. J Cell Biol. 2001;153:1277–86. doi: 10.1083/jcb.153.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia L, McDaniel JM, Yago T, Doeden A, McEver RP. Surface fucosylation of human cord blood cells augments binding to P-selectin and E-selectin and enhances engraftment in bone marrow. Blood. 2004;104(10):3091–6. doi: 10.1182/blood-2004-02-0650. [DOI] [PubMed] [Google Scholar]
- 16.Hidalgo A, Weiss LA, Frenette PS. Functional selectin ligands mediating human CD34(+) cell interactions with bone marrow endothelium are enhanced postnatally. J Clin Invest. 2002;110(4):559–69. doi: 10.1172/JCI14047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katayama Y, Hidalgo A, Furie BC, Vestweber D, Furie B, Frenette PS. PSGL-1 participates in E-selectin-mediated progenitor homing to bone marrow: evidence for cooperation between E-selectin ligands and alpha4 integrin. Blood. 2003;102(6):2060–7. doi: 10.1182/blood-2003-04-1212. [DOI] [PubMed] [Google Scholar]
- 18.Sun YX, Wang J, Shelburne CE, et al. Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. J Cell Biochem. 2003;89(3):462–73. doi: 10.1002/jcb.10522. [DOI] [PubMed] [Google Scholar]
- 19.Darash-Yahana M, Pikarsky E, Abramovitch R, et al. Role of high expression levels of CXCR4 in tumor growth, vascularization, and metastasis. FASEB J. 2004;18(11):1240–2. doi: 10.1096/fj.03-0935fje. [DOI] [PubMed] [Google Scholar]
- 20.Dimitroff CJ, Lechpammer M, Long-Woodward D, Kutok JL. Rolling of Human Bone-Metastatic Prostate Tumor Cells on Human Bone Marrow Endothelial Cells under Shear Flow is Mediated by Eselectin. Cancer Res. 2004;64(15):5261–9. doi: 10.1158/0008-5472.CAN-04-0691. [DOI] [PubMed] [Google Scholar]
- 21.Paradis V, Eschwege P, Loric S, et al. De novo expression of CD44 in prostate carcinoma is correlated with systemic dissemination of prostate cancer. J Clin Pathol. 1998;51(11):798–802. doi: 10.1136/jcp.51.11.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Draffin JE, McFarlane S, Hill A, Johnston PG, Waugh DJ. CD44 potentiates the adherence of metastatic prostate and breast cancer cells to bone marrow endothelial cells. Cancer Res. 2004;64(16):5702–11. doi: 10.1158/0008-5472.CAN-04-0389. [DOI] [PubMed] [Google Scholar]
- 23.Fuhlbrigge RC, Kieffer D, Armerding D, Kupper TS. Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T-cells. Nature. 1997;389:978–81. doi: 10.1038/40166. [DOI] [PubMed] [Google Scholar]
- 24.Picker LJ, Warnock RA, Burns AR, Doerschuk CM, Berg EL, Butcher EC. The neutrophil selectin LECAM-1 presents carbohydrate ligands to the vascular selectins ELAM-1 and GMP-140. Cell. 1991;66(5):921–33. doi: 10.1016/0092-8674(91)90438-5. [DOI] [PubMed] [Google Scholar]
- 25.Zollner O, Vestweber D. The E-selectin ligand-1 is selectively activated in Chinese hamster ovary cells by the alpha(1,3)-fucosyltransferases IV and VII. J Biol Chem. 1996;271(51):33002–8. doi: 10.1074/jbc.271.51.33002. [DOI] [PubMed] [Google Scholar]
- 26.Navone NM, Olive M, Ozen M, et al. Establishment of two human prostate cancer cell lines derived from a single bone metastasis. Clin Cancer Res. 1997;3(12 Pt 1):2493–500. [PubMed] [Google Scholar]
- 27.Kozlowski JM, Fidler IJ, Campbell D, Xu ZL, Kaighn ME, Hart IR. Metastatic behavior of human tumor cell lines grown in the nude mouse. Cancer Res. 1984;44(8):3522–9. [PubMed] [Google Scholar]
- 28.Pettaway CA, Pathak S, Greene G, et al. Selection of highly metastatic variants of different human prostatic carcinomas using orthotopic implantation in nude mice. Clin Cancer Res. 1996;2(9):1627–36. [PubMed] [Google Scholar]
- 29.Putz E, Witter K, Offner S, et al. Phenotypic characteristics of cell lines derived from disseminated cancer cells in bone marrow of patients with solid epithelial tumors: establishment of working models for human micrometastases. Cancer Res. 1999;59(1):241–8. [PubMed] [Google Scholar]
- 30.Rood PM, Calafat J, von dem Borne AE, Gerritsen WR, van der Schoot CE. Immortalisation of human bone marrow endothelial cells: characterisation of new cell lines. Eur J Clin Invest. 2000;30(7):618–29. doi: 10.1046/j.1365-2362.2000.00672.x. [DOI] [PubMed] [Google Scholar]
- 31.Fuhlbrigge RC, King S, Dimitroff CJ, Kupper TS, Sackstein R. Direct real-time observation of Eand P-selectin-mediated rolling on cutaneous lymphocyte-associated antigen immobilized on western blots. J Immunol. 2002;168:5645–51. doi: 10.4049/jimmunol.168.11.5645. [DOI] [PubMed] [Google Scholar]
- 32.Shah RB, Mehra R, Chinnaiyan AM, et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer Res. 2004;64(24):9209–16. doi: 10.1158/0008-5472.CAN-04-2442. [DOI] [PubMed] [Google Scholar]
- 33.Rubin MA, Zhou M, Dhanasekaran SM, et al. Alpha-Methylacyl coenzyme A racemase as a tissue biomarker for prostate cancer. JAMA. 2002;287(13):1662–70. doi: 10.1001/jama.287.13.1662. [DOI] [PubMed] [Google Scholar]
- 34.Manley S, Mucci NR, De Marzo AM, Rubin MA. Relational database structure to manage high-density tissue microarray data and images for pathology studies focusing on clinical outcome: the prostate specialized program of research excellence model. Amer J Pathol. 2001;159(3):837–43. doi: 10.1016/S0002-9440(10)61759-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levinovitz A, Muhlhoff J, Isenmann S, Vestweber D. Identification of a glycoprotein ligand for E-selectin on mouse myeloid cells. J Cell Biol. 1993;121(2):449–59. doi: 10.1083/jcb.121.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steegmaier M, Borges E, Berger J, Schwarz H, Vestweber D. The E-selectin-ligand ESL-1 is located in the Golgi as well as on microvilli on the cell surface. J Cell Sci. 1997;110(Pt 6):687–94. doi: 10.1242/jcs.110.6.687. [DOI] [PubMed] [Google Scholar]
- 37.Stieber A, Mourelatos Z, Chen YJ, Le Douarin N, Gonatas NK. MG160, a membrane protein of the Golgi apparatus which is homologous to a fibroblast growth factor receptor and to a ligand for E-selectin, is found only in the Golgi apparatus and appears early in chicken embryo development. Exp Cell Res. 1995;219(2):562–70. doi: 10.1006/excr.1995.1265. [DOI] [PubMed] [Google Scholar]
- 38.Laszik Z, Jansen PJ, Cummings RD, Tedder TF, McEver RP, Moore KL. P-selectin glycoprotein ligand-1 is broadly expressed in cells of myeloid, lymphoid, and dendritic lineage and in some nonhematopoietic cells. Blood. 1996;88(8):3010–21. [PubMed] [Google Scholar]
- 39.Schweitzer KM, Drager AM, van der Valk P, et al. Constitutive expression of E-selectin and vascular cell adhesion molecule-1 on endothelial cells of hematopoietic tissues. Amer J Path. 1996;148:165–75. [PMC free article] [PubMed] [Google Scholar]
- 40.Dimitroff CJ, Bernacki RJ, Sackstein R. Glycosylation-dependent inhibition of cutaneous lymphocyte-associated antigen: Implications in modulating lymphocyte migration to skin. Blood. 2003;101(2):602–10. doi: 10.1182/blood-2002-06-1736. [DOI] [PubMed] [Google Scholar]
- 41.Dimitroff CJ, Kupper TS, Sackstein R. Prevention of leukocytic migration to inflamed skin with a novel fluorosugar modifier of cutaneous lymphocyte-associated antigen. J Clin Invest. 2003;112:1008–18. doi: 10.1172/JCI19220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343(14):1020–34. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 43.Mourelatos Z, Gonatas JO, Cinato E, Gonatas NK. Cloning and sequence analysis of the human MG160, a fibroblast growth factor and E-selectin binding membrane sialoglycoprotein of the Golgi apparatus. DNA Cell Biol. 1996;15(12):1121–8. doi: 10.1089/dna.1996.15.1121. [DOI] [PubMed] [Google Scholar]
- 44.Gonatas JO, Mezitis SG, Stieber A, Fleischer B, Gonatas NK. MG-160. A novel sialoglycoprotein of the medial cisternae of the Golgi apparatus. J Biol Chem. 1989;264(1):646–53. [PubMed] [Google Scholar]
- 45.Yamaguchi F, Morrison RS, Gonatas NK, Takahashi H, Sugisaki Y, Teramoto A. Identification of MG-160, a FGF binding medial Golgi sialoglycoprotein, in brain tumors: an index of malignancy in astrocytomas. Int J Oncol. 2003;22(5):1045–9. [PubMed] [Google Scholar]
- 46.Avigdor A, Goichberg P, Shivtiel S, et al. CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34+ stem/progenitor cells to bone marrow. Blood. 2004;103(8):2981–9. doi: 10.1182/blood-2003-10-3611. [DOI] [PubMed] [Google Scholar]
- 47.Romanov VI, Whyard T, Adler HL, Waltzer WC, Zucker S. Prostate cancer cell adhesion to bone marrow endothelium: the role of prostate-specific antigen. Cancer Res. 2004;64(6):2083–9. doi: 10.1158/0008-5472.can-03-3487. [DOI] [PubMed] [Google Scholar]
- 48.Angelucci A, Festuccia C, Gravina GL, et al. Osteopontin enhances the cell proliferation induced by the epidermal growth factor in human prostate cancer cells. Prostate. 2004;59(2):157–66. doi: 10.1002/pros.20008. [DOI] [PubMed] [Google Scholar]
- 49.Knerr K, Ackermann K, Neidhart T, Pyerin W. Bone metastasis: Osteoblasts affect growth and adhesion regulons in prostate tumor cells and provoke osteomimicry. Int J Cancer. 2004;111(1):152–9. doi: 10.1002/ijc.20223. [DOI] [PubMed] [Google Scholar]
- 50.Cooper CR, Chay CH, Gendernalik JD, et al. Stromal factors involved in prostate carcinoma metastasis to bone. Cancer. 2003;97(3 Suppl):739–47. doi: 10.1002/cncr.11181. [DOI] [PubMed] [Google Scholar]