Abstract
An understanding of posttranslational events in nuclear receptor signaling is crucial for drug design and clinical therapeutic strategies. Phosphorylation is a well-characterized posttranslational modification that regulates subcellular localization and function of nuclear receptors and coregulators. Although the role of single phosphorylation sites in nuclear receptor function has been described, the contribution of combinations of multiple phosphorylation sites to receptor function remains unclear. The development of phosphoantibodies to each phosphorylation site in a nuclear receptor is a powerful tool to address the role of phosphorylation in multiply phosphorylated receptors. However, phosphoantibodies must be rigorously validated prior to use. This review describes the general methodology for design, characterization and validation of phosphoantibodies using the example of eight phosphoantibodies raised against phosphorylation sites in estrogen receptor α (ERα).
Introduction
Phosphorylation is the well-established posttranslational event that regulates subcellular localization, dimerization, DNA binding, coregulator interaction and transcriptional activity of nuclear receptors [Orti et al., 1992]. Understanding the role of phosphorylation in nuclear receptor function is limited by receptor expression levels, intracellular phosphatase activity, and low stoichiometry and/or rapid turnover of some phosphorylation sites.
Several approaches may be used to study previously identified phosphorylation sites in nuclear receptors [Rowan et al., 2003]. Cells may be labeled in vivo with 32P orthophosphate followed by immunopurification and phosphopeptide mapping. Similarly, purified receptor may be phosphorylated in vitro with different kinases and individual sites assessed by phosphopeptide mapping [Rowan et al., 2003]. However these approaches are labor intensive, time consuming, and require radioactive material and large amounts of purified receptor. Mass spectrometry is another approach to study protein phosphorylation [Garcia et al., 2005]. This non-quantitative approach requires large amounts of purified receptor and is limited primarily by costly instrumentation and the need for a highly skilled operator.
The phosphoantibody approach to study receptor phosphorylation overcomes several limitations inherent in other approaches and provides the additional benefit of allowing rapid assessment of combinations of receptor phosphorylation sites by Western blotting [Rowan et al., 2003]. The advantages of the phosphoantibody approach include: 1) a highly sensitive assay that can detect phosphorylation in crude extracts containing low receptor levels and/or low stoichiometry of phosphorylation without the need for purified receptor; 2) the ability to profile total receptor phosphorylation and to quantify relative levels of each phosphorylation site; 3) immunoprecipitation of receptors phosphorylated at specific sites to investigate the recruitment of phosphoproteins to promoters (chromatin immunoprecipitation (ChIP)) and to identify phosphorylation sites involved in specific protein-protein interaction (co-immunoprecipitation); 4) identification of phosphorylation sites associated with real time dynamics of receptor subcellular localization and recycling and other cellular processes (immunofluorescence; flow cytometry sorting); 5) the ability to monitor changes in the total receptor phosphorylation profile during disease processes such as the progression from benign to malignant cancer (immunohistochemistry).
The phosphoantibody approach has been widely applied to characterize nuclear receptor activity and subcellular localization [Wang et al., 2002]. Glucocorticoid receptor (GR) GR-P-S203 and GR-P-S211 phosphoantibodies have been used by immunoblotting to study GR activation by different ligands and by indirect immunofluorescence to investigate the subcellular localization of the active GR. Progesterone receptor (PR) phosphospecific antibodies to sites S400 [Pierson-Mullany and Lange, 2004], S162, S190 and S294 [Narayanan et al., 2005] were used by immunoblotting to study the ligand independent activation of PR and to correlate PR transcriptional activity and cell-cycle progression. Androgen receptor (AR) phosphoantibody to site S81 was used by immunoblotting to study the mechanism of ligand-dependent arrest of the AR in subnuclear foci [Black et al., 2004]. Phosphoantibodies to ERα sites S118 and S167 [Chen et al., 2002; Shah and Rowan, 2005]were used by immunoblotting to study crosstalk between ERα and several kinases. In addition, these phosphoantibodies have been used by immunohistochemistry to detect ERα phosphorylation in human breast tumors [Murphy et al., 2004; Yamashita et al., 2005].
The following section describes six key steps in development and characterization of phosphoantibodies to study nuclear receptor phosphorylation (Figure 1). The first three steps include preparation, purification and initial testing of phosphoantibodies and are generally performed by commercial vendors. The remaining three steps to validate and confirm specificity of the phosphoantibody for specific phosphorylation sites are performed by the investigator in the laboratory and are the major focus of this review.
Figure 1. Steps in phosphoantibody production and validation.
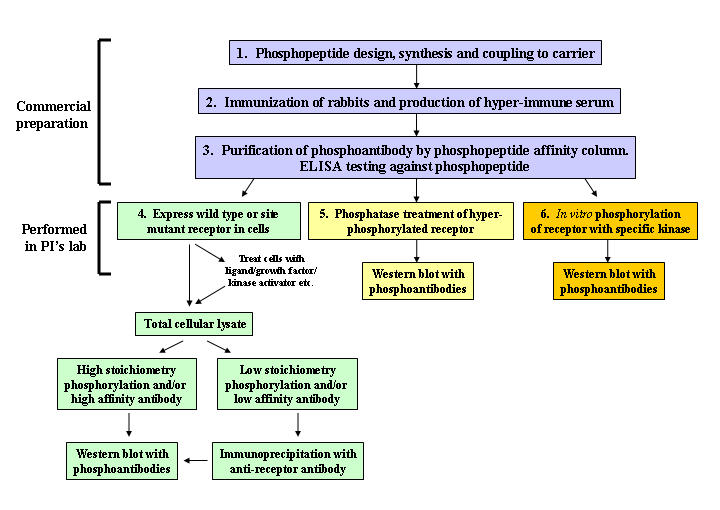
Phosphopeptide design, rabbit immunization, phosphoantibody production and affinity purification (steps 1-3) are performed by commercial vendors. Phosphoantibody specificity experiments (steps 4-6) are performed by the investigator.
Peptide design and immunogen preparation
The synthetic phosphopeptide used for immunization is recommended to be 10-20 amino acids in length and conjugated with a suitable carrier that will solicit a strong immune response and produce a large quantity of the antibody [Eisele et al., 1999; Frank, 1984; Harlow and Lane, 1988]. The most widely used carriers in antibody production are keyhole limpet hemacyanin (KLH) and bovine serum albumin (BSA) [Harlow and Lane, 1988]. It is important to be aware of the conjugated carrier used during immunization to anticipate any false positives that may occur during antibody testing. For example if the conjugated carrier is BSA, a false positive may arise if BSA was used as a blocking agent for Western blotting. Immunogenicity of the peptide-protein carrier can be verified by an enzyme linked immunosorbent assay (ELISA) to identify the most appropriate protein carrier dose.
Producing hyper-immune serum
For broad specificity it is recommended that phosphoantibodies be prepared as polyclonal antibodies [Burns, 2005; Harlow and Lane, 1988; Leenaars and Hendriksen, 2005; Lipman et al., 2005]. Rabbits are the host of choice to avoid self-recognition of the immunogen and since rabbits provide high amounts of sera. The immunogen is mixed with adjuvant prior to immunizing animals. The immunogen mixture is injected subcutaneously and re-administered at day 14 and 44 post immunization. At day 54 sera is collected by bleeding of the marginal ear vein. Bleeding is repeated on day 60 and then every 4 weeks until the antibody titer has declined. A booster dose of immunogen should be administered to re-enhance animal immunity and produce more sera [Burns, 2005]. After each bleeding, antibody titer should be measured by ELISA.
Affinity Purification
Following harvest of the hyper-immune sera, the phosphoantibody can be enriched by an affinity purification against the phosphorylated peptide [Harlow and Lane, 1988]. The affinity purification is validated by ELISA screening against the phosphopeptide in which phosphoantibody purity should exceed 95%.Numerous commercial suppliers will produce custom, affinity purified phosphoantibodies (Bethyl Laboratories, Invitrogen, Global Peptide, ABGENT, Open Biosystems and others).
Phosphoantibody specificity assessed by receptor phosphorylation site mutations
Affinity purified phosphoantibody must be validated as phosphorylation site specific. In mammalian cells that do not express the receptor of interest, wild type receptor expression plasmids or plasmids containing phosphorylation site mutations (Serine (S) to Alanine (A), Threonine (T) to A, Tyrosine (Y) to Phenylalanine (F)) should be transfected in cells to achieve high level protein expression. Total cell extracts from wild-type and phosphorylation site mutant transfected cells should be prepared for Western blotting with the phosphoantibody and peptide-competed phosphoantibody to assess specificity for the phosphorylation site. High receptor levels may be required to measure phosphorylation sites that exhibit low stoichiometry. For other sites that require an activation event, cells may first need to be incubated with ligand, growth factor, kinase activator, etc. prior to detection of the specific phosphorylation with a phosphoantibody.
The ideal phosphoantibody should recognize receptor from cells transfected with wild type but not the site-specific mutant protein. However depending on cell context, protein expression and stoichiometry, some phosphoantibodies may fail to detect a signal in total cellular extract. If this occurs, enrichment of receptor by immunoprecipitation with a receptor-specific antibody is recommended prior to Western blotting with the phosphoantibody. It is critical that immunoprecipitation be carried out over a brief period (no more than a few hours) since dephosphorylation may occur over longer periods. Regardless, phosphatase inhibitors should always be included in lysis buffers whether receptor is immunoprecipitated or not.
A critical step when first using phosphoantibodies for Western blotting is to empirically determine the optimal phosphoantibody concentration that will: 1) exhibit reactivity with wild type, but not phosphorylation site mutant receptor; and 2) not result in high general background on Western blots. In our experience, some phosphoantibodies exhibit specificity for wild type and not phosphorylation site mutant receptor only at high antibody dilutions. The appropriate antibody dilution must be determined empirically using serial dilutions of the affinity purified antibody in Western blot analysis. We have found that some affinity purified phosphoantibodies (stock concentration of 1 mg/ml) require dilution as high as 1:10,000 to eliminate non-specific reactivity with the phosphorylation site mutant receptor.
Absence of phosphoantibody reactivity with de-phosphorylated receptor
A second step for validation of phosphoantibodies is to confirm the antibody does not react with receptor that has been de-phosphorylated. In this context, the hyper-phosphorylated form of the purified receptor should be incubated in the presence or absence of λ phosphatase, followed by Western blotting with the phosphoantibody. Phosphatase treatment should prevent reactivity of the phosphoantibody with the receptor.
in vitro receptor phosphorylation to measure phosphoantibody reactivity
In some cell/tissue contexts that lack a specific kinase or have inactive kinase, some phosphoantibodies may not react with receptor due to absence or very low stoichiometry of phosphorylation at a particular site. In this scenario, in vitro phosphorylation of purified receptor with a kinase known to phosphorylate the specific site can be used prior to incubation with λ phosphatase. Phosphoantibody reactivity with the purified protein should increase following in vitro phosphorylation and signal should be lost following λ phosphatase treatment. A kinase that is not specific for the site in question should be used as a control for the in vitro phosphorylation.
Reactivity and specificity: phosphoantibodies to ERα as an example
In this study, phosphoantibodies developed against eight different phosphorylation sites of ERα were characterized (Figure 2). ERα is phosphorylated upon ligand binding and/or crosstalk with kinases. Although there are eight identified phosphorylation sites in ERα, (S104, S106, S118, S167, S236, S305, T311, and Y537 [Lannigan, 2003; Michalides et al., 2004], only phosphoantibodies against S118 and S167 have been widely applied for receptor functional studies. Phosphoantibodies directed against S104, S106 and Y537 are also commercially available although application of these antibodies to receptor functional studies is limited. This may be due to lower stoichiometry of phosphorylation at these sites, rapid turnover of tyrosine phosphorylation sites and/or poorer affinity of these antibodies compared to the S118 and S167 phosphoantibodies.
Figure 2. Schematic representation of ERα phosphorylation sites.
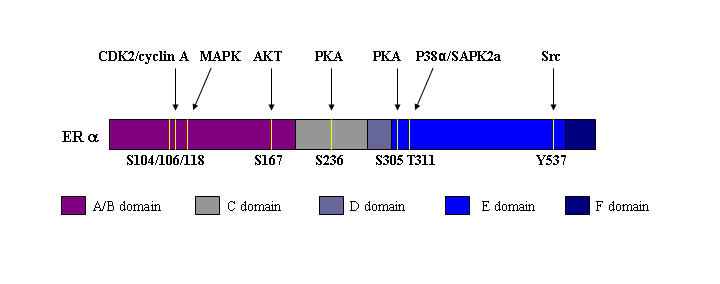
ERα phosphorylation sites that have been confirmed in previous studies by in vivo and/or in vitro phosphorylation are shown.
Reagents
Antibodies
Bethyl Laboratories (Montgomery, TX) provided complimentary phosphoantibodies: ERα ER-P-S104 (Cat. # BL1637), ER-P-S106 (Cat. # BL1638), ER-P-S118 (Cat. # BL1641), ER-P-S167 (Cat. # BL1643), ER-P-S236 (Cat. # 1645), ER-P-S305 (Cat. # 1665), ER-P-T311 (Cat. # BL1667) and ER-P-Y537 (Cat. # BL1647). Antibody D12 for ERα immunopreciptitation (Cat. # sc-8005) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). ERα antibody clone 6F11 for Western blots of total ERα (Cat. # VP-E613), peroxidase conjugated anti-mouse (Cat. # π-2000) and peroxidase conjugated anti-rabbit antibodies (Cat. # π-1000) were purchased from Vector Laboratories (Burlingame, CA).
Purified ERα, phosphatase and kinases
Baculoviral expressed ERα (Cat. # P2187) was purchased from Invitrogen (Carlsbad, CA). λ protein phosphatase (Cat. # P0753S) and active recombinant full length human CDK2-cyclin A complex expressed in E. coli (Cat. # P6025S) were purchased from New England Biolabs (Ipswich, MA). Baculoviral expressed, active recombinant full length human protein kinase A (PKA) catalytic subunit β (Cat. # PPK-448) was purchased from Stressgen (San Diego, CA). Baculoviral expressed, active recombinant full length human Src kinase (Cat. # 14-326) and active recombinant human full length p38α/SAPK2a kinase expressed in E. coli (Cat. # 14-587) were purchased from Upstate (Charlottesville, VA).
Methods
Cell culture and transfection
COS-1 cells were maintained in DMEM phenol red free medium containing 10% Fetal Bovine Serum (FBS), 2% glutamine and 1% penicillin/streptomycin. Cells were plated in 15 cm dishes for 48 hrs in DMEM phenol red-free medium containing 5% FBS that was treated with dextran-coated charcoal to remove endogenous steroids. Wild type or phosphorylation site mutant ERα constructs (6 μg) were transiently transfected in cells for 48 hrs using Fugene 6 (Roche, Indianapolis, IN). Cells were incubated with 17β-estradiol (E2) (10 nM) or Forskolin (10 µM) + IBMX (100 µM) (F/I) for 2 hrs. Cells were harvested and cellular pellets were incubated in high salt lysis buffer (10 mM Tris pH 8.0, 0.4 M NaCl, 2 mM EDTA pH 8.0, 2 mM EGTA, 10 mM β-mercaptoethanol, 0.1% Triton X100, 1 mM sodium orthovanadate, 20 mM β-glycerophosphate, 25 mM sodium fluoride, 0.1 mM PMSF) containing protease inhibitor mixture (σ, St. Louis, MO) for 10 min in ice.
Immunoprecipitation of ERα
Protein A sepharose beads (Amersham Biosciences, Piscataway, NJ) were re-swelled in phosphate buffer saline (PBS) and incubated with ERα antibody (D12, Santa Cruz) by rotating the beads at room temperature for 2 hrs. Beads were washed three times with PBS, followed by incubation with the total COS-1 cellular extract for 3 hrs at 4 C with rotation. Beads were washed three times with PBS, followed by addition of 5X SDS-PAGE loading buffer. Beads were incubated at 100 C for 5 min to elute ERα. Samples were electrophoresed by SDS-PAGE and phosphorylation of ERα was detected by Western blotting using site specific phosphoantibodies. Phosphoantibody signals obtained by Kodak Image analysis of Western blots were normalized to total ERα level by incubating the membrane in stripping buffer (2% SDS, 100 mM β-mercaptoethanol, 62.5 mM Tris HCl pH 6.8) at 55 C for 30 min with rotation and re-probing the membrane by Western blot with antibody against total ERα (clone 6F11, Vector Laboratories).
λ Phosphatase analysis
200 ng purified ERα was incubated with 200ng λ phosphatase in reaction buffer (50mM Tris-HCl, 100 mM NaCl, 2 mM MnCl2 2mM dithiothreitol (DTT), 0.1 mM EGTA, 0.01 % Brij 35, pH 7.5) for 1 hr at 30 C. To terminate the reaction, 5X SDS-PAGE loading buffer was added and samples were incubated at 100 C for 5 min., followed by SDS-PAGE and Western blots using site specific phosphoantibodies
in vitro phosphorylation
Purified ERα (200 ng) was incubated with the site specific kinase or kinase complex, ATP, and kinase buffer for 1 hr at 30 C in the presence or absence of λ phosphatase. Kinases used were: CDK2-cyclin A (100 ng; (kinase buffer 50 mM Tris-HCl, 10 mM MgCl2, 1 mM EGTA, 2 mM DTT, 0.01% Brij 35, pH 7.5); PKA (100 ng) or inactive PKA (PKA incubated at 100 C for 2 min.; kinase buffer (20 mM MOPS pH 7.0, 1 mM sodium orthovanadate, 25 mM β-glycerophosphate, 1 mM EGTA, 1 mM DTT, 7 mM MgCl2); P38α/SAPK2a (100 ng; (kinase buffer 25 mM Tris-HCl pH 7.5, 0.02 mM EGTA); and Src kinase (100 ng; kinase buffer 8 mM MOPS pH 7.0, 0.2 mM EDTA). in vitro phosphorylation reactions were terminated by addition of 5X SDS-PAGE loading buffer and incubation of samples at 100 C for 5 min. Following SDS-PAGE, phosphorylation of S104, S236, S305, T311, and Y537 were detected by Western blotting with phosphoantibodies.
Results
The initial step for characterization of phosphoantibodies is to prove the antibody is site specific. For this purpose, wild type ERα and site mutant ERα constructs were separately transfected into ERα-negative COS-1 cells. Cells were then incubated with 17β-estradiol (10-8M) or forskolin (10µM) + IBMX (100µM) for 2 hrs and cell extracts were prepared for Western blotting. Phosphorylation sites with low stoichiometry or antibodies with low affinity may not detect receptor in crude cellular extracts. For this reason ERα was first immunoprecipitated with a separate ERα antibody (D12, Santa Cruz) and then the immunopurified receptor was subjected to Western blotting with the phosphoantibodies. Phosphoantibodies to ERα-P-S104 (Figure 3A), ERα-P-S106 (Figure 3B), ERα-P-S118 (Figure 3C), ERα-P-S167 (Figure 3D), ERα-P-S236 (Figure 3E), ERα-P-S305 (Figure 3F), ERα-P-T311 (Figure 3G) and ERα-P-Y537 (Figure 3H) detected wild type ERα, but not phosphorylation site ERα mutants, indicating that antibodies were site specific. For some phosphoantibodies serial dilutions were prepared to determine the optimal antibody concentration for detection of wild type, but not mutant ERα. At high concentration (2 μg/ml) the ERα-P-S167 phosphoantibody recognized both wild-type ERα and mutant S167A (data not shown). This nonspecific interaction with mutant S167A was absent when the antibody concentration was decreased to 0.25 μg/ml (Figure 3D).
Figure 3. Immunoprecipitation of wild type and mutant ERα and Western blotting with phosphoantibodies.
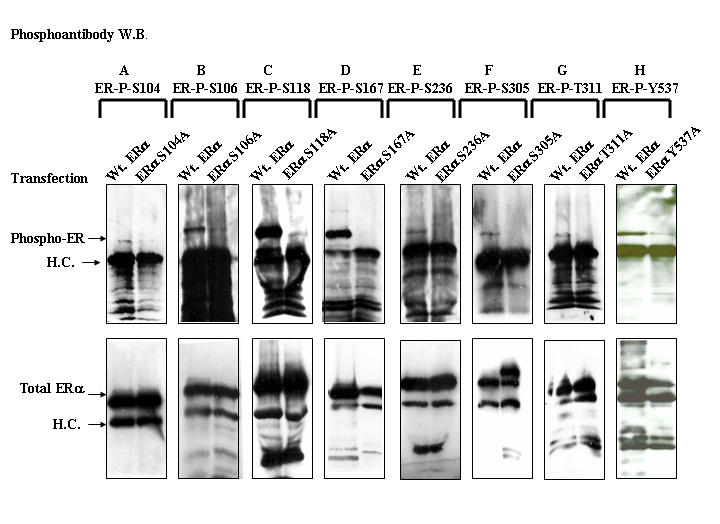
COS-1 cells were plated in DMEM media containing 5% FBS treated with charcoal coated dextran to remove the endogenous steroids. Cells were transfected with wild type ERα (A-H) or ERα phosphorylation site mutants ERαS104A (A), ERαS106A (B), ERαS118A (C), ERαS167A (D), ERαS236A (E), ERαS305A (F), ERαT311A (G), or ERαY537A (H). Cells were incubated with 17β-estradiol (A-D, G, H) or F/I (E, F) for 2 hrs, followed by preparation of cell extracts for immunoprecipitation. Total ERα was immunoprecipitated with ERα antibody (D12, Santa Cruz) and phosphorylation of ERα was detected by Western blot using site specific phosphoantibodies. The immunoblot was stripped and re-probed by Western blot with antibody to total ERα (Clone 6F11, Vector Laboratories).
Specificity of the antibodies was verified by λ phosphatase treatment of the hyper-phosphorylated purified ERα or by in vitro phosphorylation of ERα with specific kinases. Phosphoantibodies to sites S106, S118 and S167 recognized purified, baculoviral expressed ERα, indicating that kinase pathways that phosphorylate these sites are conserved in insect Sf9 cells (Figure 4 A-C, lane 1). A similar conservation between mammalian and Sf9 insect cells for receptor phosphorylation was observed with baculoviral expressed progesterone receptor [Beck et al., 1996]. Purified ERα was incubated with λ phosphatase for 1 hr at 30 C and the phosphorylation of S106, S118, and S167 was assessed by Western blotting with the phosphospecific antibodies. λ phosphatase treatment of ERα resulted in loss of Western blot signal with phosphoantibodies to sites S106 (Figure 4A), S118 (Figure 4B) and S167 (Figure 4C).
Figure 4. Phosphatase treatment of purified ERα and Western blotting with phosphoantibodies.
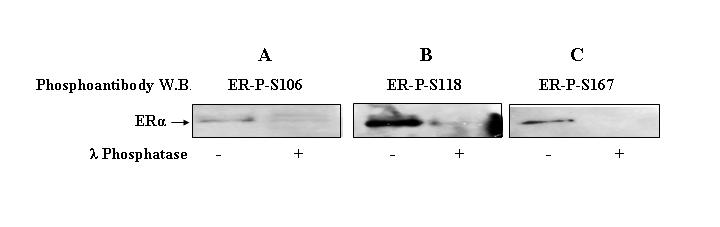
Purified ERα (200ng) was incubated in the presence or absence of phosphatase λ (200ng) at 30 °C for 1 hr and phosphorylation of ERα was detected by Western blotting using ERα-P-S106 (A), ERα-P-S118 (B), or ERα-P-S167 (C) phosphoantibodies.
Unlike phosphoantibodies to S106, S118, and S167, phosphoantibodies to sites S104, S236, S305, T311 and Y537 exhibited no reactivity with purified ERα (data not shown). The antibody specificity was assessed by in vitro phosphorylation of ERα with CDK2-cyclin A, PKA, p38α/SAPK2a and Src kinases followed by Western blotting with phosphoantibodies to sites S236, S305, T311 and Y537.
CDK2-cyclin A significantly induced the phosphorylation of site S104 (Figure 5A, lane 2) and subsequent λ phosphatase treatment (Figure 5A, lane 3) abolished the phosphorylation as assessed by Western blotting with the ERα-P-S104 phosphoantibody. In the absence of kinase, incubation of purified baculoviral ERα with kinase buffer and ATP also resulted in phosphorylation of site S104 (Figure 5A, lane 1), suggesting that purified, baculoviral ERα may be contaminated with a co-purifying kinase.
Figure 5. in vitro phosphorylation of purified ERα and Western blotting with phosphoantibodies.
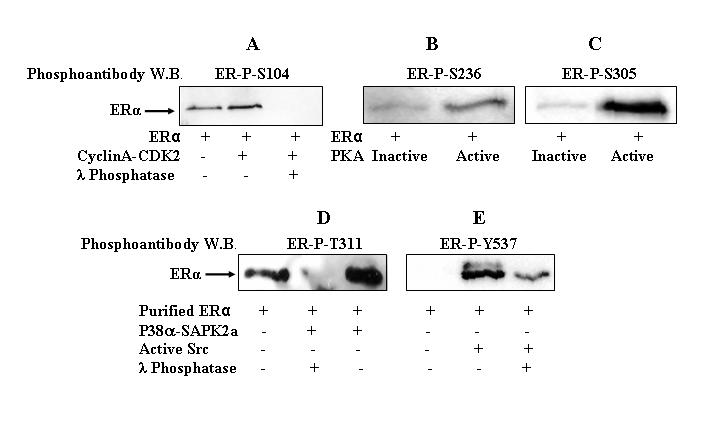
Purified ERα (200 ng) was incubated with or without CDK2-cyclin A complex (100 ng) (A), PKA (100 ng) (B, C), P38α/SAPK2a complex (100 ng) (D), or Src (100 ng) (E) in the presence or absence of λ phosphatase (200 ng) (A, D, E) for 1 hr at 30°C. PKA was inactivated by incubating the kinase at 100°C for 2 min. ERα phosphorylation was detected by Western blot analysis using ERα-P-S104 (A), ERα-P-S236 (B), ERα-P-S305 (C), ERα-P-T311 (D), or ERα-P-Y537 (E) phosphoantibodies.
Incubation of ERα with active PKA, but not inactive PKA, resulted in phosphorylation of ERα at S236 (Figure 5B, lane 2 versus lane 1) and S305 (Figure 5C, lane 2 versus lane 1). in vitro phosphorylation of ERα with P38α/SAPK2a complex resulted in T311 phosphorylation (Figure 5D, lane 3), and this phosphorylation was lost with subsequent λ phosphatase incubation (Figure 5D, lane 2). Incubation of ERα with Src kinase resulted in phosphorylation of ERα at Y537 (Figure 5E, lane 2) and this phosphorylation was markedly reduced with subsequent λ phosphatase incubation (Figure 5E, lane 3).
Discussion
Although there are several different methods to characterize changes in receptor phosphorylation, the phosphoantibody approach is particularly useful for simultaneously measuring each phosphorylation site. In this report the general steps in characterizing and validating phosphoantibodies are described using the example of phosphoantibodies raised against eight phosphorylation sites in ERα. Validation of each phosphoantibody was assessed in two steps; first the site specificity of the phosphoantibody was validated by expressing ERα wild type or site mutant plasmids into receptor negative cell lines. Second, the phosphoantibody specificity was validated by incubating the hyper-phosphorylated purified ERα with λ phosphatase and by performing in vitro phosphorylation with a specific kinase or kinase complex that is known to phosphorylate a specific site.
Although all eight phosphoantibodies raised against each ERα phosphorylation site were site specific, these phosphoantibodies reacted differently with the hyper-phosphorylated, baculoviral expressed ERα. Only phosphoantibodies to sites S106, S118 and S167 recognized the baculoviral expressed ERα. This may have occurred because some sites are not phosphorylated in insect Sf9 cells due to either the absence of kinase activity or the presence of phosphatases that prevent ERα phosphorylation at these sites.
CDK2-cyclin A is a specific kinase complex previously shown to phosphorylate both S104 and S106 of ERα [Rogatsky et al., 1999]. Because of the close proximity of S104 and S106, it is possible that phosphorylation of one site may be required for phosphorylation of the adjacent site (currently under investigation). Serine 236 and Serine 305 are located within consensus sequences for PKA and phosphorylation of these sites by PKA has been demonstrated [Chen et al., 1999; Michalides et al., 2004]. The direct kinase that phosphorylates ERα at T311 has not been identified. Lee and Bai [Lee and Bai, 2002] reported that T311 is phosphorylated by a p38 MAPK kinase complex that contained both p38α and SAPK2a. Since T311 does not lie within a consensus sequence for MAPK phosphorylation (the T residue is not followed by a proline residue), it is highly unlikely that p38 directly phosphorylates T311. A more likely explanation is that the kinase that directly phosphorylates T311 is a kinase that copurified with p38 in the present and previous study. Y537 is present within a Src kinase consensus sequence and is phosphorylated by Src kinase in vitro [Arnold and Notides, 1995; Arnold et al., 1995].
In summary, development of phosphoantibodies to assess the functional role of nuclear receptor phosphorylation is a powerful approach that has distinct advantages over other more labor intensive and costly approaches. However, rigorous validation experiments must precede use of phosphoantibodies to ascertain both reactivity with the phosphorylated receptor and absence of cross reactivity with non-phosphorylated receptor. Phosphoantibody specificity is determined using several complimentary approaches, including expression of wild type and mutant receptor in cells, phosphatase treatment of receptor, and in vitro phosphorylation of receptor. Using phosphoantibodies that are specific for each identified phosphorylation site will permit investigators to simultaneously measure the entire profile of receptor phosphorylation during studies that probe nuclear receptor mechanisms of action.
Acknowledgments
This work was supported by National Institutes of Health Grant RO1 DK06832 (to B.G.R.) and by a Department of Defense Breast Cancer Research Program Idea Award (DAMD17-02-1-0531) and Career Development Award (DAMD17-02-1-0530) (to B.G.R). We thank Drs. Simak Ali (Imperial College, UK), Rakesh Kumar (University of Texas M.D. Anderson Cancer Center) and Wenlong Bai (University of South Florida) for the plasmids ERαS236A, ERαS305A and ERαT311A, respectively. We thank Drs. Abeer El-Gharbawy and Aninda Basu for technical assistance.
Abbreviations
- A
Alanine
- E2
17β-estradiol
- ERα
Estrogen receptor α
- PKA
Protein Kinase A
- S
Serine
- T
Threonine
- Y
Tyrosine
References
- Arnold S. F., Notides A. C. An antiestrogen: a phosphotyrosyl peptide that blocks dimerization of the human estrogen receptor. Proc Natl Acad Sci U S A. 1995a;92:7475–9. doi: 10.1073/pnas.92.16.7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold S. F., Obourn J. D., Yudt M. R., Carter T. H., Notides A. C. in vivo and in vitro phosphorylation of the human estrogen receptor. J Steroid Biochem Mol Biol. 1995b;52:159–71. doi: 10.1016/0960-0760(94)00166-j. [DOI] [PubMed] [Google Scholar]
- Beck C. A., Zhang Y., Altmann M., Weigel N. L., Edwards D. P. Stoichiometry and site-specific phosphorylation of human progesterone receptor in native target cells and in the baculovirus expression system. J Biol Chem. 1996;271:19546–55. doi: 10.1074/jbc.271.32.19546. [DOI] [PubMed] [Google Scholar]
- Black B. E., Vitto M. J., Gioeli D., Spencer A., Afshar N., Conaway M. R., Weber M. J., Paschal B. M. Transient, ligand-dependent arrest of the androgen receptor in subnuclear foci alters phosphorylation and coactivator interactions. Mol Endocrinol. 2004;18:834–50. doi: 10.1210/me.2003-0145. [DOI] [PubMed] [Google Scholar]
- Burns R. Immunization strategies for antibody production. Methods Mol Biol. 2005;295:1–12. doi: 10.1385/1-59259-873-0:001. [DOI] [PubMed] [Google Scholar]
- Chen D., Washbrook E., Sarwar N., Bates G. J., Pace P. E., Thirunuvakkarasu V., Taylor J., Epstein R. J., Fuller-Pace F. V., Egly J. M., Coombes R. C., Ali S. Phosphorylation of human estrogen receptor α at serine 118 by two distinct signal transduction pathways revealed by phosphorylation-specific antisera. Oncogene. 2002;21:4921–31. doi: 10.1038/sj.onc.1205420. [DOI] [PubMed] [Google Scholar]
- Chen D., Pace P. E., Coombes R. C., Ali S. Phosphorylation of human estrogen receptor α by protein kinase A regulates dimerization. Mol Cell Biol. 1999;19:1002–15. doi: 10.1128/mcb.19.2.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele F., Owen D. J., Waldmann H. Peptide conjugates as tools for the study of biological signal transduction. Bioorg Med Chem. 1999;7:193–224. doi: 10.1016/s0968-0896(98)00204-1. [DOI] [PubMed] [Google Scholar]
- Frank A. W. Synthesis and properties of N-, O-, and S-phospho derivatives of amino acids, peptides, and proteins. CRC Crit Rev Biochem. 1984;16:51–101. doi: 10.3109/10409238409102806. [DOI] [PubMed] [Google Scholar]
- Garcia B. A., Shabanowitz J., Hunt D. F. Analysis of protein phosphorylation by mass spectrometry. Methods. 2005;35:256–64. doi: 10.1016/j.ymeth.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Harlow E., Lane D. Antibodies: A Laboratory Manual . Cold Spring Harbor: CSHL Press; 1988. [Google Scholar]
- Lannigan D. A. Estrogen receptor phosphorylation. Steroids. 2003;68:1–9. doi: 10.1016/s0039-128x(02)00110-1. [DOI] [PubMed] [Google Scholar]
- Leenaars M., Hendriksen C. F. Critical steps in the production of polyclonal and monoclonal antibodies: evaluation and recommendations. Ilar J. 2005;46:269–79. doi: 10.1093/ilar.46.3.269. [DOI] [PubMed] [Google Scholar]
- Lee H., Bai W. Regulation of estrogen receptor nuclear export by ligand-induced and p38-mediated receptor phosphorylation. Mol Cell Biol. 2002;22:5835–45. doi: 10.1128/MCB.22.16.5835-5845.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman N. S., Jackson L. R., Trudel L. J., Weis-Garcia F. Monoclonal versus polyclonal antibodies: distinguishing characteristics, applications, and information resources. Ilar J. 2005;46:258–68. doi: 10.1093/ilar.46.3.258. [DOI] [PubMed] [Google Scholar]
- Michalides R., Griekspoor A., Balkenende A., Verwoerd D., Janssen L., Jalink K., Floore A., Velds A., van't Veer L., Neefjes J. Tamoxifen resistance by a conformational arrest of the estrogen receptor α after PKA activation in breast cancer. Cancer Cell. 2004;5:597–605. doi: 10.1016/j.ccr.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Murphy L., Cherlet T., Adeyinka A., Niu Y., Snell L., Watson P. Phospho-serine-118 estrogen receptor-α detection in human breast tumors in vivo. Clin Cancer Res. 2004;10:1354–9. doi: 10.1158/1078-0432.ccr-03-0112. [DOI] [PubMed] [Google Scholar]
- Narayanan R., Edwards D. P., Weigel N. L. Human progesterone receptor displays cell cycle-dependent changes in transcriptional activity. Mol Cell Biol. 2005;25:2885–98. doi: 10.1128/MCB.25.8.2885-2898.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orti E., Bodwell J. E., Munck A. Phosphorylation of steroid hormone receptors. Endocr Rev. 1992;13:105–28. doi: 10.1210/edrv-13-1-105. [DOI] [PubMed] [Google Scholar]
- Pierson-Mullany L. K., Lange C. A. Phosphorylation of progesterone receptor serine 400 mediates ligand-independent transcriptional activity in response to activation of cyclin-dependent protein kinase 2. Mol Cell Biol. 2004;24:10542–57. doi: 10.1128/MCB.24.24.10542-10557.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogatsky I., Trowbridge J. M., Garabedian M. J. Potentiation of human estrogen receptor α transcriptional activation through phosphorylation of serines 104 and 106 by the cyclin A-CDK2 complex. J Biol Chem. 1999;274:22296–302. doi: 10.1074/jbc.274.32.22296. [DOI] [PubMed] [Google Scholar]
- Rowan B. G., Narayanan R., Weigel N. L. Analysis of receptor phosphorylation. Methods Enzymol. 2003;364:173–202. doi: 10.1016/s0076-6879(03)64011-5. [DOI] [PubMed] [Google Scholar]
- Shah Y. M., Rowan B. G. The Src kinase pathway promotes tamoxifen agonist action in Ishikawa endometrial cells through phosphorylation-dependent stabilization of estrogen receptor (α) promoter interaction and elevated steroid receptor coactivator 1 activity. Mol Endocrinol. 2005;19:732–48. doi: 10.1210/me.2004-0298. [DOI] [PubMed] [Google Scholar]
- Wang Z., Frederick J., Garabedian M. J. Deciphering the phosphorylation "code" of the glucocorticoid receptor in vivo. J Biol Chem. 2002;277:26573–80. doi: 10.1074/jbc.M110530200. [DOI] [PubMed] [Google Scholar]
- Yamashita H., Nishio M., Kobayashi S., Ando Y., Sugiura H., Zhang Z., Hamaguchi M., Mita K., Fujii Y., Iwase H. Phosphorylation of estrogen receptor α serine 167 is predictive of response to endocrine therapy and increases postrelapse survival in metastatic breast cancer. Breast Cancer Res. 2005;7:R753–64. doi: 10.1186/bcr1285. [DOI] [PMC free article] [PubMed] [Google Scholar]


