Abstract
Nuclear receptors (NRs) are transcription factors whose activity is modulated by ligand binding. These receptors are at the core of complex signaling pathways and act as integrators of many cellular signals. In the last decade our understanding of NRs has greatly evolved. In particular, regulation of NR subcellular dynamics has emerged as central to their activity. Research on the subcellular distribution of the thyroid hormone receptor (TR) has revealed new dimensions in the complexity of NR regulation, and points to the possibility that NR mislocalization plays a key role in oncogenesis. For many years, TR was thought to reside exclusively in the nucleus. It is now known that TR is a dynamic protein that shuttles between the nucleus and cytoplasm. TR is localized to the nucleus in a phosphorylated form, suggesting that compartment-specific phosphorylation mediates cross-talk between multiple cell signaling pathways. The oncoprotein v-ErbA, a viral-derived dominant negative variant of TR is actively exported to the cytoplasm by the CRM1 export receptor. Strikingly, the oncoprotein causes mislocalization of cellular TR and some of its coactivators by direct interaction. Here, we offer some perspectives on the role of subcellular trafficking in the oncogenic conversion of TR, and propose a new model for oncoprotein dominant negative activity.
Introduction
Members of the nuclear receptor (NR) superfamily are ligand-regulated transcription factors that play crucial roles in cell growth, differentiation, and proliferation. After translation in the cytoplasm, NRs must enter the nucleus to carry out their functions. In recent years, there has been a tremendous increase in our mechanistic understanding of the intricacies of subcellular trafficking of NRs. The classical “two-step” model in which cytoplasmic receptors, following hormone binding, acquire the capacity to import into the nucleus and bind DNA, has undergone a dramatic evolution that helped define two classes of nuclear receptors. For class one receptors, the ligand acts as a switch that allows their nuclear import, while class two receptors are constitutively nuclear at steady state. Moreover, the intranuclear mobility of NRs and their interactions with target genes is much more dynamic than originally envisioned [McNally et al., 2000]. Particularly striking is the shift in our view of the intracellular mobility of the thyroid hormone receptor α 1 (TRα), a class two receptor.
The dual role of TRα, which can modulate transcription whether it is bound to thyroid hormone (T3) or not [Nagl et al., 1995], was long assumed to imply constitutive nuclear localization. This dogma was overturned by the discovery that, although TRα accumulates in the nucleus at steady state, the receptor shuttles rapidly between the cytoplasm and the nucleus [Bunn et al., 2001]. Although the normal function of receptor nucleocytoplasmic shuttling remains unclear, this capacity for bidirectional transport between cellular compartments could represent an Achilles heel for the receptor by allowing its mislocalization. We propose that cytoplasmic mislocalization of TRα by dominant negative variants may contribute to the progression of some forms of cancer [Bonamy et al., 2005].
Shuttling of TRα – sampling of signaling pathways?
The functional significance of TRα shuttling remains elusive, but could reflect a process that is cell-type specific and/or dependent on the cellular environment. Shuttling of TRα may serve to mediate receptor cross-talk signaling pathways between cellular compartments, or may play a role in receptor turnover via the ubiquitin-proteasome degradation pathway [Dace et al., 2000; Kenessey and Ojamaa, 2005]. However, it is not clear from these studies whether T3-mediated degradation of ubiquitinylated TR occurs in the nucleus or in the cytoplasm. Phosphorylation events are thought to play an important role in receptor action at the molecular level [Nicoll et al., 2003]. For instance, changes in TR phosphorylation state alters its transcriptional activity [Jones et al., 1994]. Furthermore, phosphorylation of TRα and TRβ by mitogen–activated protein kinase appears to increase TR stability by shielding the receptor from proteasome degradation [Chen et al., 2003]. The additional checkpoint in receptor control of T3-responsive gene expression provided by shuttling potentially could be regulated by compartment-specific phosphorylation. A phosphorylated form of TRα is present in the nucleus, whereas unphosphorylated TRα remains cytoplasmic [Nicoll et al., 2003]. Changes in the phosphorylation state of TRα occur rapidly and phosphorylation appears to take place in the nucleus. These findings point to the interesting possibility that specific kinase pathways may be linked to NR trafficking. However, the specific role of TR shuttling, whether it is to integrate messages in the cytoplasm or mediate cytoplasmic degradation, remains to be determined.
Altered nuclear export of dominant negative variants of TR
Dominant negative mutants of TR with defects in DNA binding and transactivation accumulate in the cytoplasm at steady state, illustrating that even single amino acid changes in functional domains may lead to a dramatic shift in the subcellular distribution of TR [Bunn et al., 2001]. One such variant, the oncoprotein v-ErbA, is found in the nucleus, but is mainly cytoplasmic at steady state. v-ErbA is an oncogenic derivative of TRα (c-ErbA) carried by the avian erythroblastosis virus (AEV). It contains several alterations including the fusion of a portion of AEV Gag to its N-terminus, N- and C-terminal deletions, and 13 amino acid substitutions. Although v-ErbA has retained the capacity to bind corepressors, it has lost hormone-binding and transactivation activity and has altered DNA binding specificity [Urnov et al., 2000]. The oncoprotein interferes with the action of TRα in mammalian and avian cancer cells. Like TRα, v-ErbA is also a shuttling protein. v-ErbA follows a CRM1-mediated export pathway that is sensitive to leptomycin B, a CRM1-specific inhibitor [Bunn et al., 2001; DeLong et al., 2004]. CRM1 is a highly conserved member of the importin-β family and a receptor for leucine-rich nuclear export sequences (NESs). Study of the subcellular trafficking of green fluorescent protein (GFP)-tagged mutant and chimeric v-ErbA proteins in mammalian cells showed that a NES resides within a 70 amino acid domain in the C-terminal portion of the p10 region of Gag, and in vitro binding assays demonstrated that Gag interacts directly with CRM1. Thus, the fusion of the viral gag sequence with c-erbA was a crucial step in the activation of the oncoprotein.
The role of various export receptors in carrying NRs from the nucleus to the cytoplasm has been controversial. Several lines of evidence implicate calreticulin as the mediator of NR nuclear export, while other studies suggest a CRM1-mediated pathway [Shank and Paschal, 2005]. In heterokaryon assays, in which the cell fusion step may induce release of calreticulin from the endoplasmic reticulum [Shank and Paschal, 2005], rapid TRα and TRβ1 shuttling is CRM1-independent [Bunn et al., 2001; DeLong et al., 2004]. Heterokaryon assays conducted with v-ErbA show that the oncoprotein has lost the ability to use this alternate, calreticulin-mediated export pathway [Bunn et al., 2001; DeLong et al., 2004].
Subcellular mislocalization of the oncoprotein v-ErbA
The growth-promoting properties of v-ErbA have traditionally been linked to dominant repression of the anti-mitogenic roles of TR and the retinoic acid receptor (RAR). However, another aspect of v-ErbA’s role in cell cycle induction is its ability to induce ultrastructural changes characteristic of early and intermediate events of meiotic maturation in Xenopus oocytes [Nagl et al., 1997]. v-ErbA-induced maturation events, which are not mediated by dominant repression of endogenous TR, occur without activation of the cAMP/maturation-promoting factor signal pathway, and arrest prior to meiotic spindle formation. These studies suggest that v-ErbA, acting as a transcriptional activator, can contribute to cell cycle reentry by interference with regulatory pathways distinct from those involving TR. A portion of v-ErbA expressed in Xenopus oocytes localizes to the cytoplasmic fibrils of the nuclear pore complexes [Nagl et al., 1997], thus suggesting the interesting possibility that in addition to its intranuclear function, v-ErbA may modulate nucleocytoplasmic transport.
The acquisition of altered nuclear export capabilities contributes to the oncogenic properties of v-ErbA by adding yet another level of dominant negative activity. v-ErbA oncogenic activity has long been attributed to competition with TRα for T3-responsive DNA elements and/or auxiliary factors involved in the transcriptional regulation of T3-responsive genes. However, competition models do not address the altered subcellular localization of v-ErbA as a mode of oncogenic action. In situ protein-protein interaction assays, and colocalization studies in transiently transfected cells indicate that v-ErbA dimerizes with TRα and the retinoid X receptor (RXR) and sequesters a significant fraction of the two NRs in the cytoplasm [Bonamy et al., 2005]. Notably, recruitment of TRα to the cytoplasm by v-ErbA can be partially reversed in the presence of ligand, and when chromatin is disrupted by the histone deacetylase inhibitor trichostatin A. T3 favors TR heterodimer formation with RXR, which would prevent its recruitment by v-ErbA; while trichostatin A decondenses the chromatin, which increases the number of response elements accessible to TR and enhances its nuclear retention.
A new model for dominant negative action of the oncoprotein v-ErbA
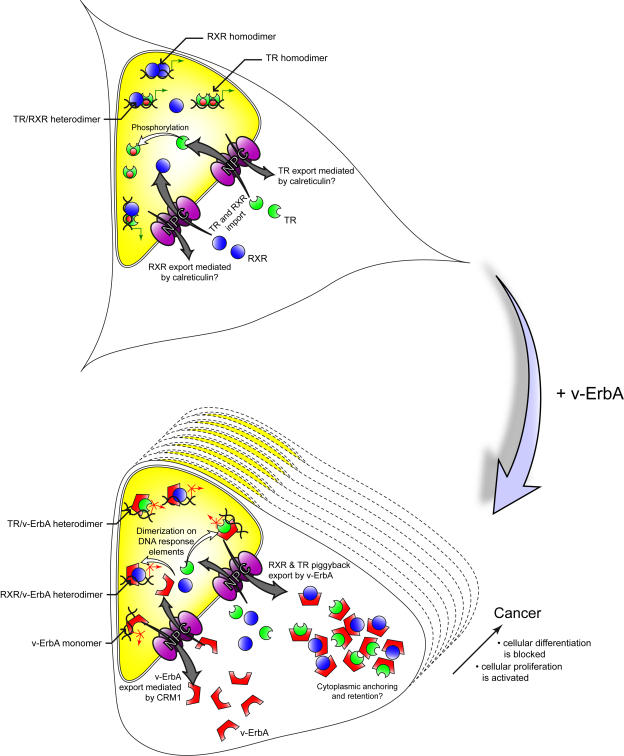
Our current model for the dominant negative activity of v-ErbA highlights the importance of its aberrant mislocalization of NRs in oncogenesis (Figure 1). In the absence of v-ErbA, unliganded and liganded TRα, along with RXR, repress or enhance the transcription of specific genes. In the presence of the oncoprotein, the normal transcriptional activity of TRα and RXR is antagonized by competition with v-ErbA for DNA response elements and/or cofactors, and mislocalization of TRα and RXR to the cytoplasm. Because unliganded TRα is more sensitive to mislocalization, in the absence of T3 v-ErbA dominant negative activity may be mediated to a greater degree by cytoplasmic mislocalization. This mislocalization is most likely due to the CRM1-mediated nuclear export of TRα and RXR, which are “piggybacked” to the cytoplasm by v-ErbA after formation of the dimers in the nucleus, and to the anchoring of these receptors by v-ErbA in the cytoplasm.
Figure 1. Model for v-ErbA dominant negative activity.
In cells not expressing the oncoprotein v-ErbA (top), TR (green) and RXR (blue) are actively imported into the nucleus through the nuclear pore complexes (NPC). In the nucleus, TR is phosphorylated (red circle), which may serve to modify its activity and enhance nuclear retention. In the nucleus, TR and RXR bind to DNA response elements as homodimers or heterodimers, and modulate transcription of T3-responsive genes (green arrows). Although TR and RXR are shuttling proteins, the balance between nuclear import, nuclear retention, and nuclear export is such that these two receptors are almost entirely localized in the nucleus at steady state. Their nuclear export may be mediated by calreticulin, but this requires further evaluation. In contrast, in the presence of the oncoprotein v-ErbA (red symbol) (bottom), RXR and TR either dimerize with v-ErbA on some response elements, or are blocked from DNA binding sites by competition with v-ErbA monomers. In both cases, this interferes with the normal transcriptional activity of T3-responsive genes (red crossed arrows). In addition, a significant fraction of RXR and TR exits the nucleus “piggyback” style by a CRM1-dependent pathway in association with v-ErbA and its viral NES. TR and RXR may be actively anchored in the cytoplasm by the retroviral Gag moiety of v-ErbA. The mislocalization of TR, RXR and possibly other coactivators by v-ErbA blocks these transcription factors from their normal function in the nucleus. Together, all these mechanisms contribute to cellular transformation by v-ErbA, resulting in cancer.
Retroviruses can acquire host DNA sequences via conserved mechanisms that result in the fusion of the cellular proto-oncogene to a sequence encoding variable-sized N-terminal fragments of the Gag polyprotein. Because Gag is a cytoplasmic protein, fusion to nuclear proteins may often result in the ectopic expression of a fusion protein localized to the cytoplasm. This raises the interesting possibility that viral oncogenes encoding Gag-fusion proteins could mediate tumorigenesis in part via mislocalization of different cellular factors. Whether the example of v-ErbA represents a widespread mechanism or not remains to be determined.
Cancer and mislocalization of regulatory proteins
Signal-mediated nuclear localization of transcription factors can act as a regulatory pathway to control transcription. Thus, it is not surprising that disruption of the critical balance between nuclear import, nuclear retention, and nuclear export of transcription regulatory proteins is associated with cancer and other diseases [for review, Kau et al., 2004]. For example, mislocalization of INI1/nSNF5 blocks its normal tumor suppression function [Craig et al., 2002], the tumor suppressor p53 is abnormally exported from the nucleus and/or sequestered in the cytoplasm in some transformed cells [Stommel et al., 1999]]. In addition, ectopic expression of the hepatitis B virus X protein sequesters CRM1 in the cytoplasm, suggesting that inactivation of the CRM1-mediated pathway may be an early step during viral hepatitis-mediated liver carcinogenesis [Forgues et al., 2001]. Moreover, a recent report suggests that the hepatitis C virus core protein modulates the retinoid signaling pathway by sequestering Sp110b, a corepressor of RARα [Watashi et al., 2003]. In support of this model, Sp110b is mislocalized by the core protein to the perinuclear region in situ. In addition, the core protein and Sp110b were both enriched in the microsomal membrane fraction after biochemical extraction, suggesting mislocalization to the endoplasmic reticulum.
Other studies have also implicated mislocalization of regulatory proteins in the progression of cancer. For example, cytoplasmic mislocalization of mutant BRCA1 is associated with breast cancer [Rodriguez et al., 2004]. In acute promyelocytic leukemia cells that express chimeric RARα fusion proteins, normal RXRα aberrantly colocalizes with the chimeric protein, and shows decreased intranuclear mobility [Dong et al., 2004]. Further, functional inactivation of the tumor suppressor p27 (kip1) in human thyroid cancers occurs either through loss of expression or through phosphorylation-dependent cytoplasmic sequestration [Motti et al., 2005], and loss of tumor suppressor RUNX3 expression is causally related to gastric cancer and linked to its impaired nuclear localization [Ito et al., 2005]. Finally, a human prostate cancer was linked to a mutant of the androgen receptor, which is aberrantly localized in nuclear foci and subsequently mislocalizes SRC-1 [Nazareth et al., 1999].
Concluding remarks
In summary, oncogenic conversion of TRα into v-ErbA not only involves changes in DNA binding specificity and ligand binding properties, but also the acquisition of altered nuclear export capabilities and subcellular localization [Bonamy et al., 2005]. Other dominant negative variants of TR may be involved in human cancer [Gonzalez-Sancho et al., 2003]. For instance, point mutations in TRα have been linked to pituitary tumors [McCabe et al., 1999], to thyroid papillary cancer [Puzianowska-Kuznicka et al., 2002] and to gastric cancer [Wang et al., 2002]. Thus, it would be interesting to assess the localization of TR variants in cancer cells to determine if there is an altered distribution of mutant and wild-type receptors.
Acknowledgments
This work arose in part from the PhD dissertation research of G. Bonamy under the supervision of A. Guiochon-Mantel and L. A. Allison, and was sponsored by funding from the National Institutes of Health (DK058028-01A1) and the National Science Foundation (MCB0090923) to L.A.A.
Abbreviations
- AEV
Avian erythroblastosis virus
- GFP
Green fluorescent protein
- NES
Nuclear export sequence
- NR
Nuclear receptor
- RAR
Retinoic acid receptor
- RXR
Retinoid X receptor
- T3
Thyroid hormone
- TRα1
Thyroid hormone receptor α1
References
- Bonamy G. M., Guiochon-Mantel A., Allison L. A. Cancer promoted by the oncoprotein v-ErbA may be due to subcellular mislocalization of nuclear receptors. Mol Endocrinol. 2005;19:1213–30. doi: 10.1210/me.2004-0204. [DOI] [PubMed] [Google Scholar]
- Bunn C. F., Neidig J. A., Freidinger K. E., Stankiewicz T. A., Weaver B. S., McGrew J., Allison L. A. Nucleocytoplasmic shuttling of the thyroid hormone receptor α. Mol Endocrinol. 2001;15:512–33. doi: 10.1210/mend.15.4.0619. [DOI] [PubMed] [Google Scholar]
- Chen S. L., Chang Y. J., Wu Y. H., Lin K. H. Mitogen-activated protein kinases potentiate thyroid hormone receptor transcriptional activity by stabilizing its protein. Endocrinology. 2003;144:1407–19. doi: 10.1210/en.2002-220911. [DOI] [PubMed] [Google Scholar]
- Craig E., Zhang Z. K., Davies K. P., Kalpana G. V. A masked NES in INI1/hSNF5 mediates hCRM1-dependent nuclear export: implications for tumorigenesis. Embo J. 2002;21:31–42. doi: 10.1093/emboj/21.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dace A., Zhao L., Park K. S., Furuno T., Takamura N., Nakanishi M., West B. L., Hanover J. A., Cheng S. Hormone binding induces rapid proteasome-mediated degradation of thyroid hormone receptors. Proc Natl Acad Sci U S A. 2000;97:8985–90. doi: 10.1073/pnas.160257997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong L. J., Bonamy G. M., Fink E. N., Allison L. A. Nuclear export of the oncoprotein v-ErbA is mediated by acquisition of a viral nuclear export sequence. J Biol Chem. 2004;279:15356–67. doi: 10.1074/jbc.M308214200. [DOI] [PubMed] [Google Scholar]
- Dong S., Stenoien D. L., Qiu J., Mancini M. A., Tweardy D. J. Reduced intranuclear mobility of APL fusion proteins accompanies their mislocalization and results in sequestration and decreased mobility of retinoid X receptor α. Mol Cell Biol. 2004;24:4465–75. doi: 10.1128/MCB.24.10.4465-4475.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgues M., Marrogi A. J., Spillare E. A., Wu C. G., Yang Q., Yoshida M., Wang X. W. Interaction of the hepatitis B virus X protein with the Crm1-dependent nuclear export pathway. J Biol Chem. 2001;276:22797–803. doi: 10.1074/jbc.M101259200. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Sancho J. M., Garcia V., Bonilla F., Munoz A. Thyroid hormone receptors/THR genes in human cancer. Cancer Lett. 2003;192:121–32. doi: 10.1016/s0304-3835(02)00614-6. [DOI] [PubMed] [Google Scholar]
- Ito K., Liu Q., Salto-Tellez M., Yano T., Tada K., Ida H., Huang C., Shah N., Inoue M., Rajnakova A., Hiong K. C., Peh B. K., Han H. C., Ito T., Teh M., Yeoh K. G., Ito Y. RUNX3, a novel tumor suppressor, is frequently inactivated in gastric cancer by protein mislocalization. Cancer Res. 2005;65:7743–50. doi: 10.1158/0008-5472.CAN-05-0743. [DOI] [PubMed] [Google Scholar]
- Jones K. E., Brubaker J. H., Chin W. W. Evidence that phosphorylation events participate in thyroid hormone action. Endocrinology. 1994;134:543–8. doi: 10.1210/endo.134.2.8299553. [DOI] [PubMed] [Google Scholar]
- Kenessey A., Ojamaa K. Ligand-mediated decrease of thyroid hormone receptor-alpha1 in cardiomyocytes by proteosome-dependent degradation and altered mRNA stability. Am J Physiol Heart Circ Physiol. 2005;288:H813–21. doi: 10.1152/ajpheart.00804.2004. [DOI] [PubMed] [Google Scholar]
- McCabe C. J., Gittoes N. J., Sheppard M. C., Franklyn J. A. Thyroid receptor alpha1 and alpha2 mutations in nonfunctioning pituitary tumors. J Clin Endocrinol Metab. 1999;84:649–53. doi: 10.1210/jcem.84.2.5469. [DOI] [PubMed] [Google Scholar]
- McNally J. G., Muller W. G., Walker D., Wolford R., Hager G. L. The glucocorticoid receptor: rapid exchange with regulatory sites in living cells. Science. 2000;287:1262–5. doi: 10.1126/science.287.5456.1262. [DOI] [PubMed] [Google Scholar]
- Motti M. L., Califano D., Troncone G., De Marco C., Migliaccio I., Palmieri E., Pezzullo L., Palombini L., Fusco A., Viglietto G. Complex regulation of the cyclin-dependent kinase inhibitor p27kip1 in thyroid cancer cells by the PI3K/AKT pathway: regulation of p27kip1 expression and localization. Am J Pathol. 2005;166:737–49. doi: 10.1016/S0002-9440(10)62295-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagl S. B., Nelson C. C., Romaniuk P. J., Allison L. A. Constitutive transactivation by the thyroid hormone receptor and a novel pattern of activity of its oncogenic homolog v-ErbA in Xenopus oocytes. Mol Endocrinol. 1995;9:1522–32. doi: 10.1210/mend.9.11.8584030. [DOI] [PubMed] [Google Scholar]
- Nagl S. B., Bunn C. F., Allison L. A. v-erbA oncogene initiates ultrastructural changes characteristic of early and intermediate events of meiotic maturation in Xenopus oocytes. J Cell Biochem. 1997;67:184–200. [PubMed] [Google Scholar]
- Nazareth L. V., Stenoien D. L., Bingman W. E., 3rd, James A. J., Wu C., Zhang Y., Edwards D. P., Mancini M., Marcelli M., Lamb D. J., Weigel N. L. A C619Y mutation in the human androgen receptor causes inactivation and mislocalization of the receptor with concomitant sequestration of SRC-1 (steroid receptor coactivator 1) Mol Endocrinol. 1999;13:2065–75. doi: 10.1210/mend.13.12.0382. [DOI] [PubMed] [Google Scholar]
- Nicoll J. B., Gwinn B. L., Iwig J. S., Garcia P. P., Bunn C. F., Allison L. A. Compartment-specific phosphorylation of rat thyroid hormone receptor alpha1 regulates nuclear localization and retention. Mol Cell Endocrinol. 2003;205:65–77. doi: 10.1016/s0303-7207(03)00199-0. [DOI] [PubMed] [Google Scholar]
- Puzianowska-Kuznicka M., Krystyniak A., Madej A., Cheng S. Y., Nauman J. Functionally impaired TR mutants are present in thyroid papillary cancer. J Clin Endocrinol Metab. 2002;87:1120–8. doi: 10.1210/jcem.87.3.8296. [DOI] [PubMed] [Google Scholar]
- Rodriguez J. A., Au W. W., Henderson B. R. Cytoplasmic mislocalization of BRCA1 caused by cancer-associated mutations in the BRCT domain. Exp Cell Res. 2004;293:14–21. doi: 10.1016/j.yexcr.2003.09.027. [DOI] [PubMed] [Google Scholar]
- Shank L. C., Paschal B. M. Nuclear transport of steroid hormone receptors. Crit Rev Eukaryot Gene Expr. 2005;15:49–73. doi: 10.1615/critreveukaryotgeneexpr.v15.i1.40. [DOI] [PubMed] [Google Scholar]
- Stommel J. M., Marchenko N. D., Jimenez G. S., Moll U. M., Hope T. J., Wahl G. M. A leucine-rich nuclear export signal in the p53 tetramerization domain: regulation of subcellular localization and p53 activity by NES masking. Embo J. 1999;18:1660–72. doi: 10.1093/emboj/18.6.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urnov F. D., Yee J., Sachs L., Collingwood T. N., Bauer A., Beug H., Shi Y. B., Wolffe A. P. Targeting of N-CoR and histone deacetylase 3 by the oncoprotein v-erbA yields a chromatin infrastructure-dependent transcriptional repression pathway. Embo J. 2000;19:4074–90. doi: 10.1093/emboj/19.15.4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. S., Lin K. H., Hsu Y. C. Alterations of thyroid hormone receptor α gene: frequency and association with Nm23 protein expression and metastasis in gastric cancer. Cancer Lett. 2002;175:121–7. doi: 10.1016/s0304-3835(01)00722-4. [DOI] [PubMed] [Google Scholar]
- Watashi K., Hijikata M., Tagawa A., Doi T., Marusawa H., Shimotohno K. Modulation of retinoid signaling by a cytoplasmic viral protein via sequestration of Sp110b, a potent transcriptional corepressor of retinoic acid receptor, from the nucleus. Mol Cell Biol. 2003;23:7498–509. doi: 10.1128/MCB.23.21.7498-7509.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]