Abstract
Nonenzymatic glycosylation and cross-linking of proteins by glucose contributes to an age-associated increase in vascular and myocardial stiffness. Some recently sythesized thiazolium compounds selectively break these protein cross-links, reducing collagen stiffness. We investigated the effects of 3-phenacyl-4,5-dimethylthiazolium chloride (ALT-711) on arterial and left ventricular (LV) properties and their coupling in old, healthy, nondiabetic Macaca mulatta primates (age 21 ± 3.6 years). Serial measurements of arterial stiffness indices [i.e., aortic pulse wave velocity (PWV) and augmentation (AGI) of carotid arterial pressure waveform] as well as echocardiographic determinations of LV structure and function were made before and for 39 weeks after 11 intramuscular injections of ALT-711 at 1.0 mg/kg body weight every other day. Heart rate, brachial blood pressure, and body weight were unchanged by the drug. PWV and AGI decreased to a nadir at 6 weeks [PWV to 74.2 ± 4.4% of baseline (B), P = 0.007; AGI to 41 ± 7.3% of B, P = 0.046], and thereafter gradually returned to baseline. Concomitant increases in LV end diastolic diameter to 116.7 ± 2.7% of B, P = 0.02; stroke volume index (SVindex) to 173.1 ± 40.1% of B, P = 0.01; and systolic fractional shortening to 180 ± 29.7% of B, P = 0.01 occurred after drug treatment. The LV end systolic pressure/SVindex, an estimate of total LV vascular load, decreased to 60 ± 12.1% of B (P = 0.02). The LV end systolic diameter/SVindex, an estimate of arterio-ventricular coupling, was improved (decreased to 54.3 ± 11% of B, P < 0.002). Thus, in healthy older primates without diabetes, ALT-711 improved both arterial and ventricular function and optimized ventriculo-vascular coupling. This previously unidentified cross-link breaker may be an effective pharmacological therapy to improve impaired cardiovascular function that occurs in the context of heart failure associated with aging, diabetes, or hypertension, conditions in which arterial and ventricular stiffness are increased.
Long-lived proteins (e.g., vascular and myocardial collagen) undergo continual cross-linking during aging (1) because of the formation of advanced glycosylation end-products (AGEs). In individuals with diabetes mellitus, these reactions occur at an accelerated rate (2). AGE cross-links generally impair the normal function of proteins, cells, and organs. In the cardiovasculature, their presence within the vascular wall and myocardium is an early event in the development of the age-associated increases in vascular and ventricular stiffness (3). Myocardial stiffness is manifested as an alteration of ventricular diastolic filling properties, whereas alterations in arterial stiffness associated with advancing age or diabetes are clinically manifested as progressive increases in systolic blood pressure, pulse pressure, and aortic pulse wave velocity (PWV). These changes occur concomitantly with a late augmentation index (AGI) of the carotid pressure pulse. An increase in arterial stiffness increases the vascular load on the heart, resulting in adverse changes in left ventricular (LV) structure and function (4), in part, by altering coupling efficiency between the vasculature and the heart (5). Epidemiologic studies in humans have shown that elevated measures of arterial stiffness are reliable predictors of cardiovascular morbidity and mortality (6).
Aminoguanidine, an inhibitor of AGE formation, has been shown to prevent increases in aortic and carotid arterial wall stiffness in diabetic rats (7). The more recent development of a class of thiazolium derivatives that catalytically break existing glucose cross-links between proteins has enabled a more direct assessment of the contribution of protein cross-linking to the magnitude of age- or disease-associated changes in arterial and ventricular stiffness (8). Thus, treatment of rats with one such cross-link-breaker, 3-phenacyl-4,5-dimethylthiazolium chloride (ALT-711), for 1–3 weeks reversed the diabetes-induced increase of large artery stiffness, as measured in vivo or in vitro as systemic arterial compliance and aortic input impedance (9). Studies examining the effects of ALT-711 on LV compliance in a cohort of nondiabetic, aged mongrel dogs indicated that this agent produced a significant increase in LV diastolic compliance, associated with an increase in LV diastolic volume and improved LV systolic function (10). These results indicate that ALT-711 improves both arterial and ventricular function in older or diabetic animals and suggest that this drug may improve the impaired coupling between the vasculature and heart that accompanies aging, diabetes, and hypertension. To test this hypothesis, we assessed the effects of ALT-711 on measures of arterial and ventricular function and their coupling over a prolonged period, after a 3-week dosing regimen with the drug. The study was implemented in aged rhesus monkeys to determine whether the demonstrated effectiveness of ALT-711 in rats and dogs could be extended to primates and to assess the duration of any observed drug effect.
Methods
Six male, normotensive, nondiabetic rhesus monkeys (Macaca mulatta), aged 21 ± 3.6 years and weighing 8.6 ± 2.4 kg, were provided a 2-week adaptation period within the vivarium at the National Institutes of Health Primate Unit of the Poolesville Animal Center, an American Association for the Accreditation of Laboratory Animal Care-accredited center. Study protocols were approved by the Gerontology Research Center Animal Care Committee.
All cardiovascular evaluations were conducted in anesthetized animals
without the use of endotracheal intubation, following standard
protocols for sedation (Telazol, 3–5 mg per kg of body weight
intramuscularly, after a 12-h fast). Systolic and diastolic arterial
blood pressures (SBP and DBP) were recorded noninvasively with an
automated sphygmomanometer cuff on the right upper extremity (Dinamap,
Critikon, Tampa, FL). Mean arterial pressure (MBP) was estimated as
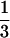 (SBP − DBP) + DBP. Heart rate was recorded by using
continuous electrocardiogram recordings. Three serial measurements of
arterial stiffness indices (aortic PWV and carotid pressure pulse
tracing) and two echocardiographic measurements of LV function were
made during a 1-month period before drug administration (two animals
underwent only one echocardiographic assessment at baseline).
Subsequently, ALT-711 was administered once every other day over a
3-week period as 11 intramuscular injections, each 1.0 mg per kg of
body weight. The study was of a single-arm, double-crossover (no drug,
drug, no drug) design with baseline (predrug) measures as controls.
(SBP − DBP) + DBP. Heart rate was recorded by using
continuous electrocardiogram recordings. Three serial measurements of
arterial stiffness indices (aortic PWV and carotid pressure pulse
tracing) and two echocardiographic measurements of LV function were
made during a 1-month period before drug administration (two animals
underwent only one echocardiographic assessment at baseline).
Subsequently, ALT-711 was administered once every other day over a
3-week period as 11 intramuscular injections, each 1.0 mg per kg of
body weight. The study was of a single-arm, double-crossover (no drug,
drug, no drug) design with baseline (predrug) measures as controls.
Arterial stiffness indices were measured again at 4, 6, 8, 11, 15, and 39 weeks after the last dose of ALT-711. Echocardiographic measurements were repeated at 4, 6, 11, and 39 weeks after the last dose of ALT-711. Arterial pressure waveforms were obtained from the right common carotid artery by applanation tonometry with a pencil-sized probe (Millar Instruments, Houston, TX) on the maximal pulsation of the artery as described (11). The carotid pressure pulse augmentation index (AGI) was determined from the average of 10 simultaneously recorded pressure waves by a custom-designed computer algorithm (12). As described (12), PWV was derived from simultaneous recordings of arterial flow waves from the right common carotid artery and the right femoral artery by using nondirectional transcutaneous Doppler flow probes (model 810A, 10 and 9 MHz, Parks Medical Electronics, Aloha, OR).
M-mode echocardiograms were obtained from two-dimensional guided images
by using a 3.5-MHz transducer recording at 50 mm/sec. LV cavity end
systole diameter (ESD), end diastole diameter (EDD), and septal and
posterior wall thickness were measured with electronic calipers after
the digitalization of individual frames in accordance with established
guidelines (13). Several additional cardiovascular parameters were
derived from the measurements of blood pressure and LV dimensions. LV
fractional shortening (LVFS), a measure of myocardial contractility,
and stroke volume index (SVindex) were estimated
as follows: LVFS = (EDD − ESD)/EDD;
SVindex = EDD − ESD (the term
SVindex refers to an estimate of stroke volume on
the basis of a one-dimensional change in LV size between diastole and
systole). End systolic pressure (ESP) was approximated as ESP =
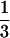 (2SBP + DBP). The effective arterial elastance
(Eaindex = ESP/SVindex),
a measure of total (i.e., both compliance and resistance components) LV
vascular load (14–16), and ESP/ESD, a reflection of LV myocardial
function, were calculated. Furthermore,
ESD/SVindex, a measure of ventriculo-vascular
coupling (5), cardiac output index (COindex)
estimated as COindex =
SVindex × heart rate (beats per minute), and
total systemic vascular resistance index
(TSRindex), derived from
COindex and MBP, were calculated.
(2SBP + DBP). The effective arterial elastance
(Eaindex = ESP/SVindex),
a measure of total (i.e., both compliance and resistance components) LV
vascular load (14–16), and ESP/ESD, a reflection of LV myocardial
function, were calculated. Furthermore,
ESD/SVindex, a measure of ventriculo-vascular
coupling (5), cardiac output index (COindex)
estimated as COindex =
SVindex × heart rate (beats per minute), and
total systemic vascular resistance index
(TSRindex), derived from
COindex and MBP, were calculated.
Arterial wave forms, pressure contours, and echocardiograms were evaluated by a single observer blinded with respect to the identity of the data. The reliability of waveform and pressure contour analyses was established by sequential measurements of the right carotid contour by the same examiner and reader on differing occasions in all monkeys. The repeated measurements were highly correlated (mean coefficient of variation 5%).
Statistical Analyses.
All results were expressed as mean ± the SEM, unless otherwise noted. Serial measurements were analyzed by using one-way ANOVA for repeated measures. The level of significance was set at P < 0.05, two-tailed.
Results
Values of parameters measured at selected time points are listed in Table 1, whereas changes from baseline values of selected parameters are illustrated in the figures. Body weight and routine laboratory parameters, including chemistry and hematology, were unremarkable (data not shown).
Table 1.
Effect of ALT-711 on vascular and cardiac measures
| Variable | Measure (mean ±
SD)
|
P* | ||||
|---|---|---|---|---|---|---|
| Baseline | Week 4 | Week 6 | Week 11 | Week 39 | ||
| SBD, mmHg | 117 ± 13 | 124 ± 27 | 118 ± 20 | 119 ± 8 | 128 ± 21 | 0.59 |
| DBP, mmHg | 72 ± 10 | 72 ± 17 | 75 ± 18 | 76 ± 10 | 79 ± 19 | 0.63 |
| MBP, mmHg | 88 ± 114 | 82 ± 20 | 89 ± 19 | 91 ± 9 | 95 ± 19 | 0.69 |
| Heart rate, beats per minute | 74 ± 8 | 78 ± 11 | 72 ± 9 | 72 ± 7 | 72 ± 3 | 0.18 |
| AGI, % | 8.8 ± 3.5 | 5.6 ± 3.4 | 4.1 ± 2.8† | 7.5 ± 2.5 | 8.8 ± 6.8 | 0.05 |
| PWV, cm/sec | 1221 ± 263 | 1041 ± 286 | 891 ± 140† | 983 ± 237 | 1281 ± 458 | 0.01 |
| TSRindex | 245 ± 113 | 121 ± 25† | 150 ± 43 | 123 ± 10† | 156 ± 49 | 0.01 |
| LVEDD, cm | 1.68 ± 0.3 | 1.89 ± 0.4 | 1.76 ± 0.3 | 1.95 ± 0.4† | 1.87 ± 0.4 | 0.03 |
| LVESD, cm | 1.25 ± 0.4 | 1.13 ± 0.5 | 1.12 ± 0.4 | 1.21 ± 0.5 | 1.23 ± 0.5 | 0.31 |
| LV mass, g | 21.5 ± 9.1 | 24.8 ± 12.6 | 23.2 ± 9.5 | 25.1 ± 12.6 | 23.5 ± 12.6 | 0.35 |
| SVindex | 0.42 ± 0.2 | 0.75 ± 0.2 | 0.63 ± 0.2 | 0.74 ± 0.1† | 0.65 ± 0.2 | 0.01 |
| LVFS | 26.9 ± 14.3 | 42.2 ± 15.4† | 37.8 ± 18.8 | 40.6 ± 15.8† | 37.3 ± 18.6 | 0.01 |
| COindex | 31.6 ± 12.9 | 57.8 ± 10.2† | 45.3 ± 15.6 | 53.6 ± 10.4† | 46.4 ± 12.3 | 0.01 |
| Eaindex | 296 ± 139 | 151 ± 33† | 181 ± 56 | 145 ± 11† | 189 ± 59 | 0.01 |
| ESD/SVindex | 3.71 ± 2.5 | 1.63 ± 0.9† | 2.04 ± 1.0† | 1.72 ± 0.8† | 2.09 ± 1.1† | 0.01 |
| PW relaxation rate,‡ mm/sec | 1.4 ± 0.3 | 2.1 ± 1.3 | 2.0 ± 0.9 | 1.9 ± 0.6 | 1.6 ± 0.3 | 0.21 |
*, P value for drug effect by one-way ANOVA for repeated measures.
Significant difference from baseline control by Bonferroni/Dunn post hoc analysis.
Rate of thinning of the LV posterior wall by two-dimensional echocardiogram.
Heart Rate and Blood Pressure.
No changes were observed in heart rate, SBP, DBP, MBP, or—consequently—ESP, at any time point after treatment with ALT-711 (Table 1).
Vascular Parameters.
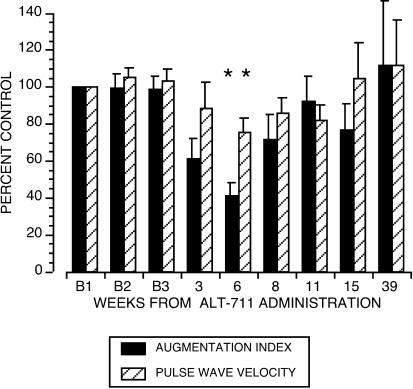
PWV and AGI were directly measured as independent correlates of vascular compliance. A decrease in PWV or AGI has been closely linked to a decrease in vascular wall stiffness, and vice versa. Fig. 1 and Table 1 show that, beginning with the first set of measurements at 4 weeks after the end of exposure to drug, PWV and AGI progressively decreased, both reaching a nadir at 6 weeks [PWV to 74.2 ± 4.4% of baseline (B), P = 0.007; AGI to 41 ± 7.3% of B, P = 0.046]. Thereafter, both parameters gradually increased and had returned to baseline values at 39 weeks. There were no significant differences among the three baseline serial determinations of PWV or AGI, demonstrating the reproducibility of these measurement techniques (Fig. 1). The magnitude of the drug-induced reduction of the PWV and AGI was proportional to the baseline measurement (PWV, r = 0.80, P < 0.05; AGI, r = 0.75, P = 0.07).
Figure 1.
ALT-711 significantly decreased the AGI of the carotid artery and PWV. Baseline measures before drug are indicated as B1, B2, and B3. Values postdrug are depicted as percent of average baseline value ± SE. The overall drug effect assessed by one-way ANOVA for repeated measures was P = 0.007 for the changes from the average baseline value in PWV and P = 0.046 for AGI. *, significant difference from control by Bonferroni/Dunn post hoc analysis.
Cardiac Parameters.
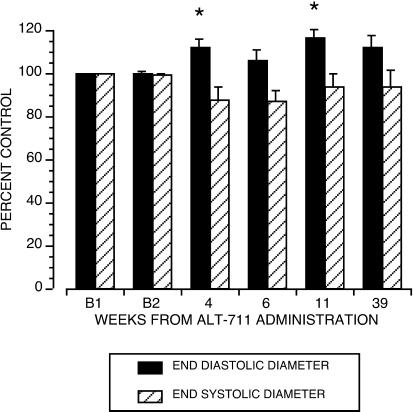
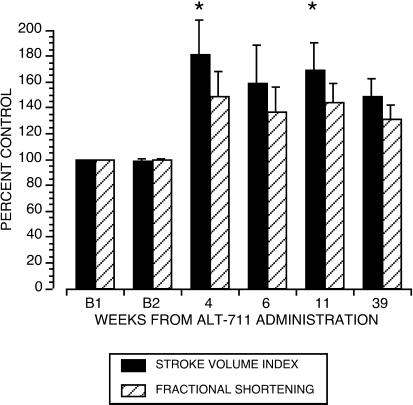
The cardiac parameters LV EDD and ESD, estimates of the pre- and postejection LV chamber volumes, were directly measured by using echocardiography. From these two measurements, the cardiac parameters SVindex, COindex, and LVFS were derived. After exposure to drug, EDD increased to 116.7 ± 2.7% of B, P = 0.02 (Fig. 2). ESD showed a trend toward a reduction (to 93.7 ± 6.9% of B), but this did not reach statistical significance (Fig. 2). After treatment, SVindex increased to 173.1 ± 40.1% of B, P = 0.01 (Fig. 3), and LVFS increased to 180 ± 29.7% of B, P = 0.01 (Fig. 3). With no change in heart rate and blood pressure, treatment with ALT-711 was also associated with an increase in COindex (P = 0.01) and a reduction in TSRindex (P = 0.01, see Table 1).
Figure 2.
Change from baseline in LV ESD and EDD. Measurements before drug (B) were made on two separate occasions in four monkeys; the remaining two had only one single baseline measure. Baseline echocardiographic data did not differ statistically in the four animals with two predrug assessments (Bland–Altman Test). Values postdrug are depicted as percent of average baseline value ± SE. ALT-711 significantly increased EDD (P = 0.02). The effect on ESD was not significant (P < 0.22).
Figure 3.
Change from baseline in SVindex and LVFS. Baseline echocardiographic data did not differ statistically in the four animals with two predrug assessments (Bland–Altman Test). Values postdrug are depicted as percent of average baseline value ± SE. ALT-711 significantly changed SVindex (P = 0.01) and FS (P = 0.01). *, differs from baseline by Bonferroni/Dunn test.
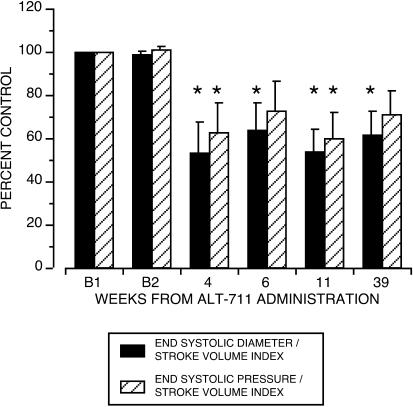
Additional cardiac parameters were derived from the measurements of blood pressure and chamber dimensions, as outlined in Methods. The total vascular load Eaindex (or ESP/SVindex), against which the LV chamber has to eject blood into the circulation, was reduced postdrug to 60 ± 12.1% B, P < 0.01 (Fig. 4). The efficiency of ventriculo-vascular coupling was assessed as ESD/SVindex and was found to be significantly improved after exposure to ALT-711 (reduced to about 50% of B, P = 0.002, Fig. 4). LV function, estimated as ESP/ESD, tended to improve after treatment, but this effect did not reach statistical significance.
Figure 4.
Change from baseline in the effective arterial elastance (ESP/SVindex) and ventriculo-vascular coupling (ESD/SVindex). Baseline derived echocardiographic data did not differ statistically in the four animals with two predrug assessments (Bland–Altman Test). Values are depicted as percent control value ± SE. Exposure to ALT-711 was associated with a significant reduction in ESP/SVindex (P = 0.02) and ESD/SVindex (P = 0.002). *, significantly differs from baseline by Bonferroni/Dunn test.
Of note, the LV septal and posterior wall thicknesses, mass, and rate of thinning of the posterior wall, assessed echocardiographically, were not changed significantly after drug administration (data not shown).
Discussion
The current study was conducted to assess noninvasively the effects of ALT-711 on the cardiovasculature of normotensive, nondiabetic, older rhesus monkeys. ALT-711, a stable 4,5-dimethylthiazolium derivative of the prototype agent N-phenacylthiazolium bromide, belongs to a class of compounds that break glucose-derived cross-links on proteins such as collagen (8). Previous work has demonstrated that improvements in tissue compliance occur rapidly after short-term exposure to this AGE cross-link breaker (9, 10). Although it is likely that cross-link formation continues during and after exposure to a breaker, little is known about the duration of changes in tissue compliance after discontinuation of treatment. Therefore, the study was designed as a single-arm, double-crossover trial to evaluate the rapidity of onset and the duration of any observed effects in the examined cardiovascular tissues.
Exposure to ALT-711 in the present study was associated with changes in several cardiac and vascular indices. Compliance of large arteries was significantly increased after treatment with ALT-711, as reflected in the measurements of the independent parameters PWV and AGI. The increase in compliance peaked at the week 6 evaluation, after the end of treatment with drug, and had returned to baseline at the week 39 visit, with both parameters exhibiting essentially the same time course. Serial measurements of LV EDD and ESD demonstrated a sustained increase in EDD and a trend toward a decrease in ESD, consistent with an increased LV chamber compliance. These changes were first noted at week 4 after drug evaluation and, unlike the vascular effects, persisted till the final study visit at week 39. SVindex, estimated on the basis of the measured LV chamber dimensions, almost doubled after exposure to drug. The increase in SVindex was attributable not only to Frank–Starling mechanisms but also to an apparent increase in calculated LVFS. Because LVFS is governed by both vascular and cardiac properties, the increase in LVFS was consistent with the observed reduction in the total vascular load and total systemic resistance. The data also demonstrated that exposure to ALT-711 was associated with a trend toward an increase in ESP/ESD and a substantial reduction in ESD/SVindex, suggesting that the drug improved LV function and optimized the coupling between the heart and vasculature.
These data are similar to results obtained with ALT-711 in studies involving diabetic rats and nondiabetic, aged dogs (9, 10). In these studies, an increase in cardiovascular compliance was accompanied by an increase in cardiac output. Despite the marked and sustained increase in cardiac output in the current study as well as the previously reported trials in rats and dogs (9, 10), it is interesting to note the absence of a change in blood pressure and heart rate. Although it is premature to conclusively explain this observation, it is possibly related to the unique mechanism of action of this compound, which simultaneously affects cardiac and vascular performance and improves ventriculo-vascular coupling. That SVindex increased after ALT-711, without an increase in arterial pressure or a reduction in heart rate, may be interpreted to indicate that ALT-711 alters baroreceptor responses by modifying mechanical properties of the carotid sinus.
Furthermore, the observed marked reduction of AGI may indicate that ALT-711 affects arterial properties in addition to those that determine large vessel stiffness. The AGI is not simply a surrogate marker for enhanced PWV that accompanies reduced compliance of conduit arteries. Sodium nitroprusside or nitroglycerin, which have minor effects on aortic PWV or compliance, significantly attenuate or abolish the late systolic augmentation of the carotid pressure (17). These agents act on distal vessels and appear to have an impact on the genesis of the reflected pulse wave, rather than its propagation velocity. In view of these data, we postulate that ALT-711 also directly affected biomechanical properties of the distal arterial vasculature, contributing to a decrease in total systemic resistance.
The effect of ALT-711 on cardiac indices persisted until the end of the study, in contrast to a more transient drug effect on vascular parameters. This dichotomous activity on the vasculature and the heart is not readily explained, but may be the result of anatomical, functional, and/or biomechanical differences between these two structures. Because nonenzymatic cross-link formation is accelerated in hyperglycemic milieus, the relative high myocardial glucose extraction and resulting low tissue glucose concentrations in the heart, compared with vascular tissues, may delay the formation of new cross-links. Alternatively, these compounds may exert tissue-specific cross-link-breaking activities, possibly on the basis of tissue perfusion and/or protein characteristics.
In summary, the net actions of ALT-711 were to increase venous return into the heart and the ejection of blood from the LV, thus augmenting overall tissue perfusion in the resting state. Further study in animals and humans is needed to better elucidate the mechanism of action of this class of agents on the cardiovasculature. In particular, an understanding of the drug's impact on hemodynamic feedback mechanisms regulating tissue perfusion at rest and during exercise, and the possibility of tissue-specific actions of cross-link breakers, will be essential.
Acknowledgments
P.V.V. is partially supported by the Brookdale Foundation, New York, NY 10022.
Abbreviations
- ALT-711
3-phenacyl-4,5-dimethylthiazolium chloride
- PWV
pulse wave velocity
- AGI
augmentation index
- LV
left ventricular
- EDD
end diastolic diameter
- FS
fractional shortening
- ESP
end systolic pressure
- ESD
end systolic diameter
- AGE
advanced glycosylation end-product
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- MBP
mean blood pressure
References
- 1.Bucala R, Cerami A. Adv Pharmacol. 1992;23:1–34. doi: 10.1016/s1054-3589(08)60961-8. [DOI] [PubMed] [Google Scholar]
- 2.Bucala R, Cerami A, Vlassara H. Diabetes Metab Rev. 1995;3:258–268. [Google Scholar]
- 3.Li Y-M, Steffes M, Donnelly T, Liu C, Fuh H, Basgen J, Bucala R, Vlassara H. Proc Natl Acad Sci USA. 1996;93:3902–3907. doi: 10.1073/pnas.93.9.3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lakatta E-G. Physiol Rev. 1993;73:413–467. doi: 10.1152/physrev.1993.73.2.413. [DOI] [PubMed] [Google Scholar]
- 5.Chen C-H, Nakayama M, Nevo E, Fetics B-J, Maughan W-L, Kass D-A. J Am Coll Cardiol. 1998;32:1221–1227. doi: 10.1016/s0735-1097(98)00374-x. [DOI] [PubMed] [Google Scholar]
- 6.O'Rourke M, Frohlich E. Hypertension. 1999;34:372–374. doi: 10.1161/01.hyp.34.3.372. [DOI] [PubMed] [Google Scholar]
- 7.Norton G-R, Candy G, Woodiwiss A-J. Circulation. 1996;93:1905–1912. doi: 10.1161/01.cir.93.10.1905. [DOI] [PubMed] [Google Scholar]
- 8.Vasan S, Zhang X, Zhang S, Kapurniotu A, Bernhagen J, Teichberg S, Basgen F, Wagle D, Shih D, Terlesfky I, et al. Nature (London) 1996;382:275–278. doi: 10.1038/382275a0. [DOI] [PubMed] [Google Scholar]
- 9.Wolfenbuttel B H R, Boulanger C M, Crijns F R L, Huijberts M S P, Poitevin P, Swennen G M N, Vasan S, Egan J J, Ulrich P, Cerami A, Levy B. Proc Natl Acad Sci USA. 1998;95:4630–4634. doi: 10.1073/pnas.95.8.4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asif M, Egan J, Vasan S, Jyothirmayi G-N, Masurekar M-R, Lopez S, Willams C, Torres R-L, Wagle D, Ulrich P, et al. Proc Natl Acad Sci USA. 2000;97:2809–2813. doi: 10.1073/pnas.040558497. . (First Published March 7, 2000; 10.1073/pnas.040558497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly R, Hayward C, Ganis J, Daley J, Avolio A, O'Rourke M. J Vasc Med Biol. 1989;1:142–149. [Google Scholar]
- 12.Vaitkevicius P-V, Fleg J-L, Engel J-H, O'Connor F-C, Wright J-G, Lakatta L, Yin F C, Lakatta E-G. Circulation. 1993;88:1456–1462. doi: 10.1161/01.cir.88.4.1456. [DOI] [PubMed] [Google Scholar]
- 13.American Society of Echocardiography Committee on Standards. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 14.Kelly R, Ting C, Yang T, Lie C, Maughan W-L, Chang A, Kass D-A. Circulation. 1992;86:513–521. doi: 10.1161/01.cir.86.2.513. [DOI] [PubMed] [Google Scholar]
- 15.Sunagawa K, Maughan W-L, Sagawa K. Circ Res. 1985;56:586–595. doi: 10.1161/01.res.56.4.586. [DOI] [PubMed] [Google Scholar]
- 16.Sunagawa K, Maughan W-L, Burkhoff D, Sagawa K. Am J Physiol. 1983;245:H773–H780. doi: 10.1152/ajpheart.1983.245.5.H773. [DOI] [PubMed] [Google Scholar]
- 17.Kelly R P, Gibbs H H, O'Rourke M F, Daley J E, Mang K, Morgan J J, Avolio A P. Eur Heart J. 1990;11:138–144. doi: 10.1093/oxfordjournals.eurheartj.a059669. [DOI] [PubMed] [Google Scholar]