Abstract
Background
People from British South Asian communities have an increased risk of mortality from coronary heart disease (CHD). Doxazosin, a selective α1-adrenergic blocker, in addition to lowering blood pressure, has been shown to have positive effects on glucose metabolism and lipid profiles in patients with hypertension.
Aim
We studied doxazosin (1–8 mg) and bendrofluazide (2.5 mg) in patients of British South Asian origin with existing mild to moderate hypertension (doxazosin n = 78; bendrofluazide n = 82), to compare their effects on glucose and lipid metabolism in this group.
Design of study
A 34-week randomised, double-blind, parallel-group, multicentre study.
Setting
Primary care in the UK.
Method
All doxazosin patients started with an initial dose of 1 mg once daily, titrated to a maximum 8 mg once daily if diastolic blood pressure was >90 mmHg or was not <5 mmHg of the baseline value. The primary efficacy variables were mean glucose and total cholesterol concentrations at week 21.
Result
Doxazosin reduced glucose, total cholesterol, low-density lipoprotein-cholesterol and triglycerides and increased high-density lipoprotein-cholesterol. There were significant differences between doxazosin and bendrofluazide for glucose concentrations at week 21 (P = 0.029) and week 34 (P = 0.015), total cholesterol at week 21 (P = 0.048) and triglycerides at week 21 P = 0.047) and week 34 (P = 0.009). There was no significant difference in blood pressure lowering between the two treatments.
Conclusion
Doxazosin exhibits beneficial effects on glucose concentrations and lipid profile, in particular in lowering triglyceride concentrations in British South Asians. Whether these desirable characteristics translate to improved overall cardiovascular risk requires formal evaluation.
Keywords: bendrofluazide, doxazosin, ethnic groups, glucose, hypertension, lipids
INTRODUCTION
British South Asians with hypertension have an increased risk of coronary heart disease (CHD).1 The National Service Framework for Coronary Heart Disease in England highlights that the death rate from heart disease is 38% higher in men and 43% higher in women born in the Indian subcontinent than in England as a whole.2 Traditional risk factors do not fully explain the higher prevalence of CHD, but this ethnic group has a high prevalence of diabetes, hypertension, hyperinsulinaemia and dyslipidaemia. These risk factors, combined with central obesity, point to an insulin resistance or metabolic syndrome as a possible explanation for the higher prevalence of CHD in this group.3 Comparative studies of Asians in India and the UK suggest that their predisposition to insulin resistance, associated metabolic abnormalities, type 2 diabetes and CHD is genetically determined.4,5
Doxazosin, a selective α1-adrenergic blocker, has been shown to have positive effects on lipid profiles, along with blood pressure reductions, in patients with hypertension.6-10 In short-term and long-term studies, doxazosin was associated with reduced total cholesterol, low-density-lipoprotein cholesterol (LDL-C) and triglycerides, with a slight increase in high-density-lipoprotein cholesterol (HDL-C), both as monotherapy and as combination therapy. Furthermore, doxazosin has been shown to increase insulin sensitivity,11 although studies in hypertensive patients with type 2 diabetes are conflicting.12-14 There are no data on its use in ethnic minority populations at enhanced risk of CHD, but, given its effects on lipids and insulin in mainly white populations, it would appear to be a good candidate for consideration in these ethnic groups.
How this fits in
British South Asians with hypertension are believed to have an increased risk of coronary heart disease because of the reported higher incidence of death from heart disease in British South Asians relative to the general UK population. Doxazosin, a selective α1-adrenergic blocker, has been shown to have effects on glucose metabolism and lipid profiles, along with blood pressure reductions, in patients with hypertension. This study demonstrates that doxazosin has a beneficial effect on glucose metabolism and lipid profiles in British South Asians with hypertension, compared with the thiazide diuretic bendrofluazide, confirming the improvements in glycaemic control and lipid profiles with doxazosin previously reported in mainly white populations.
We undertook this study to determine whether doxazosin has beneficial effects on glucose and lipid metabolism in British South Asian patients with existing mild to moderate hypertension. Bendrofluazide was chosen as the comparator agent because it is widely recommended as first-line treatment for hypertension, although, as a thiazide diuretic, it may tend to lower carbohydrate tolerance and insulin sensitivity, while increasing LDL-C and triglycerides.
METHOD
Participants and interventions
Patients were eligible for inclusion in the study if they were aged 18–80 years, of South Asian origin (defined as both parents originating from the Indian subcontinent), and were known to have mild to moderate hypertension (defined as having a sitting diastolic blood pressure of 90–114 mmHg and systolic blood pressure of <180 mmHg). Patients were excluded if they had type 1 or type 2 diabetes mellitus, hypercholesterolaemia or established CHD (that is, multiple transient ischaemic attacks, angina, treated heart failure, myocardial infarction within the previous 3 months or stroke in the previous year).
Before entering the study, all eligible patients gave written, witnessed informed consent, having been provided with information sheets in English, Bengali, Gujerati, Hindi, Punjabi or Urdu, as appropriate.
Before starting active treatment, patients underwent the following examinations: demographic details, medical history (including previous and concurrent treatments), height, weight, waist/hip ratio, sitting blood pressure, heart rate, 2-hour oral glucose tolerance test, fasting, 30-minute and 2-hour insulin and proinsulin, fasting lipids, fasting, 30-minute and 2-hour non-esterified fatty acid (NEFA) lipid particles, urate, haematology, electrolytes and liver function tests. Blood pressure was measured locally using a mercury sphygmomanometer, following standard practice procedures. All blood and urine tests were performed by a central laboratory.
After the initial assessment, blood pressure, heart rate and adverse events were recorded at each subsequent visit throughout the study (that is, at weeks 0, 2, 4, 6, 8, 14, 21 and 34). Weight, waist:hip ratio, glucose, insulin, proinsulin, lipids, NEFA, urate, haematology, electrolytes and liver function tests were recorded again at weeks 21 and 34.
Patients entered a 2-week placebo run-in (weeks −2 to 0) to ensure that they were moderately hypertensive. At week 0, eligible patients were randomised to take one capsule of doxazosin (1 mg) or bendrofluazide (2.5 mg) each morning. The doxazosin dose could be titrated to the next highest dose (2, 4 or 8 mg once daily) at weeks 2, 4 and 6 if the patient's diastolic blood pressure was more than 90 mmHg or had not fallen by at least 5 mmHg since the previous visit. Patients receiving either treatment could be co-prescribed open-label amlodipine (5 mg) from week 8 onwards if diastolic blood pressure was at or above 90 mmHg.
Blinding was maintained by having four packs (A, B, C, D) for each drug. For doxazosin, packs A, B, C and D contained doxazosin 1, 2, 4 and 8 mg, respectively. For bendrofluazide, packs A, B, C and D were identical, with each containing bendrofluazide (2.5 mg). Placebo, doxazosin and bendrofluazide were presented as identical-sized dark-grey capsules. Amlodipine tablets were provided from commercial stocks.
Aims
The primary objective determined in the statistical analysis plan was to evaluate the comparative effects of doxazosin and bendrofluazide on glucose and lipid metabolism.
The secondary objectives were to evaluate the comparative efficacy of the two agents on blood pressure and to compare their safety and tolerability.
Efficacy and safety variables
The primary efficacy variable in assessing the effects of doxazosin and bendrofluazide on glucose metabolism was the change in glucose concentrations from baseline (week −2) to week 21, measured by a 2-hour oral glucose tolerance test. The primary variable in assessing the effects on lipid metabolism was the change in total cholesterol concentrations from baseline (week −2) to week 21.
Secondary efficacy variables were the changes in insulin, proinsulin, LDL-C, HDL-C, triglycerides, NEFA and sitting blood pressure from baseline to weeks 21 and 34. Baseline was week −2 for all variables apart from blood pressure, when it was week 0.
Safety variables included clinical and laboratory adverse events (defined as any untoward medical occurrence, regardless of cause, in a patient administered a pharmaceutical product). If an adverse event was reported the investigator recorded the intensity (mild, moderate or severe, defined on subjective criteria), the relationship to the study drug and the outcome.
Power calculations and statistical methods
Sample size was calculated assuming a 5% improvement in LDL-C in patients treated with doxazosin compared with those treated with bendrofluazide. This was based on a baseline LDL-C of 4.5 mmol/l with a standard deviation of 0.614 mmol/l. The correlation between LDL-C concentrations at baseline and after 12 months was 0.8 in a previous, unpublished study of doxazosin (Pfizer Inc, data on file). A sample of 100 patients (50 per treatment group) was sufficient to result in a power of 80% at a significance level of 0.05.
Randomisation was performed centrally, so at week 0, the site telephoned the randomisation centre, with a minimisation code based on age (18–59 years or 60–80 years) and sex for each eligible patient. This code was used to assign a treatment number from the batch that had been issued to the site so that the overall stratification for age and sex was balanced between treatment groups.
The primary population for analysis was the intention-to-treat (ITT) population, which included all randomised patients who received at least one dose of the study drug and had baseline efficacy measurements. Missing measurements in the ITT population were treated as missing, as was pre-specified for the primary analysis. The alternative ‘last-observation carried forward’ analysis (when patients withdrew from treatment or had missing measurements, the last observation was carried forward for the analysis) is not presented here, but produced similar results and does not alter conclusions.
The ITT population was analysed for treatment differences (doxazosin–bendrofluazide). The general linear model ‘mean change in parameters = a+b (centre)+f(treatment)+j(sex)+k(age)+w(baseline)' was fitted. Age was considered as a categorical variable and patients were classified as under 60 years or 60 years and over. The model was fitted using type II sums of squares, and least squares means were computed. Data transformations were used on non-normally distributed data. No adjustments were made for multiplicity, because there was only one primary efficacy variable each for glucose metabolism and lipid metabolism. Version 6.12 of SAS for Windows was used for the production of all data summaries and analyses.
Statistical analyses were performed using analysis of covariance, which has less power to detect interaction effects than main effects; hence a higher significance level was used to test for interaction effects. The treatment effect was assessed for statistical significance at the 5% level, and, if the covariates were significant, additional tabular data summaries were generated displaying the primary parameter means by covariate categories. If the treatment effect was not found to be significant, no further models were fitted in the main analysis. If the treatment effect was significant, the model was refitted with terms for treatment by covariate interaction to assess for robustness of the treatment effect across the covariates. The interactions were tested for significance at the 10% level and least squares means computed.
RESULTS
Number of patients and baseline characteristics
Eighteen investigators screened 224 patients in England; of these patients, 160 were randomised from 17 primary care centres. The first patient was enrolled in the study on 4 July 1997 and the last patient completed the study on 12 December 2000. Figure 1 shows the numbers of patients assessed for eligibility; randomised; allocated to each treatment group; withdrawn from the study; and analysed.
Figure 1.
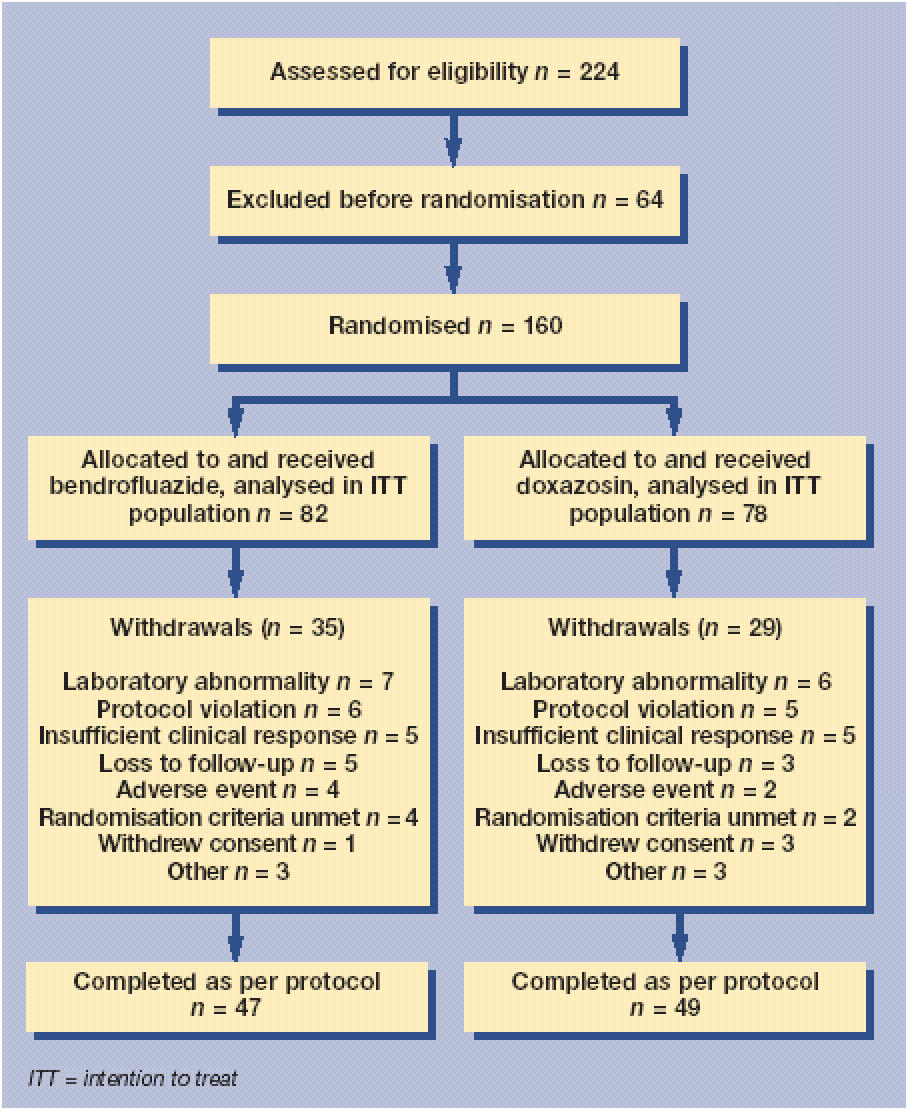
Trial profile.
Seventy-eight patients were randomised to doxazosin and 82 patients were randomised to bendrofluazide. All randomised patients received the allocated study drug and were included in the ITT population. All patients were of South Asian origin. Demographic and baseline characteristics were generally similar in both treatment groups except for the male:female ratio (63% of the bendrofluazide group were men, compared with 56% in the doxazosin group). Arthropathies and related disorders were the most frequent concomitant illnesses reported, and the incidence was similar in both treatment groups. The concomitant medications that were reported most frequently were analgesics.
Primary efficacy measures: glucose and total cholesterol
Doxazosin reduced mean blood glucose concentrations by 0.07 mmol/l at week 21 and by 0.22 mmol/l at week 34; by contrast, bendrofluazide increased blood glucose concentrations at both time points (0.82 mmol/l at week 21; 1.02 mmol/l at week 34). The adjusted mean changes from baseline are shown in Table 1. The adjusted treatment difference between doxazosin and bendrofluazide was −0.14 mmol/l at week 21 (P = 0.029; 95% confidence interval [CI] = −0.26 to −0.01) and −0.17 mmol/l at week 34 (P = 0.015; 95% CI = −0.30 to −0.03) (Table 1).
Table 1.
Change in primary efficacy variables from baseline to weeks 21 and 34, showing means ± standard deviation.
| Absolute change from baseline | ||||
|---|---|---|---|---|
| Bendrofluazide (n) | Doxazosin (n) | Adjusted treatment difference (95% CI) | P-value | |
| Glucose (mmol/l)a | ||||
| Baseline | 5.66±1.814 (81) | 6.08±1.830 (78) | ||
| Week 21 | 0.82±2.254 (62) | −0.07±2.015 (57) | −0.14 (−0.26 to −0.01) | 0.029 |
| Week 34 | 1.02±2.798 (44) | −0.22±1.545 (49) | −0.17 (−0.30 to −0.03) | 0.015 |
| Total cholesterol (mmol/l) | ||||
| Baseline | 5.64±0.874 (81) | 5.30±0.990 (78) | ||
| Week 21 | 0.00±0.610 (64) | −0.11±0.631 (60) | −0.22 (−0.43 to 0.00) | 0.048 |
| Week 34 | −0.05±0.583 (44) | −0.26±0.526 (49) | −0.21 (−0.44 to 0.02) | 0.069 |
Endpoints for glucose were log-transformed before analysis and the adjusted treatment differences are presented on a logscale.
Doxazosin reduced mean total cholesterol concentrations by 0.11 mmol/l at week 21 and by 0.26 mmol/l at week 34; bendrofluazide had a minimal effect on mean total cholesterol concentrations at weeks 21 and 34 (the adjusted mean changes from baseline are shown in Table 2). The adjusted treatment difference was significant at week 21 (−0.22 mmol/l; P = 0.048; 95% CI = −0.43 to 0.00) but not at week 34 (−0.21 mmol/l; P = 0.069; 95% CI = −0.44 to 0.02) (Table 1).
Table 2.
Change in secondary lipid measures from baseline to weeks 21 and 34, showing means ± standard deviation.
| Absolute change from baseline | ||||
|---|---|---|---|---|
| Bendrofluazide (n) | Doxazosin (n) | Adjusted treatment difference (95% CI) | P-value | |
| LDL-C (mmol/l) | ||||
| Baseline | 3.97±0.861 (81) | 3.61±1.072 (78) | ||
| Week 21 | −0.04±0.741 (63) | −0.11±0.757 (60) | −0.22 (−0.47 to 0.03) | 0.083 |
| Week 34 | −0.16±0.883 (47) | −0.31±0.708 (49) | −0.22 (−0.54 to 0.10) | 0.174 |
| HDL-C (mmol/l) | ||||
| Baseline | 1.18±0.295 (81) | 1.17±0.301 (78) | ||
| Week 21 | −0.02±0.196 (64) | 0.04±0.170 (60) | 0.05 (−0.01 to 0.12) | 0.106 |
| Week 34 | 0.00±0.134 (47) | 0.03±0.161 (49) | 0.03 (−0.03 to 0.09) | 0.340 |
| Triglycerides (mmol/l)a | ||||
| Baseline | 2.11±1.487 (81) | 1.81±1.58 (78) | ||
| Week 21 | 0.53±2.545 (64) | −0.03±0.484 (60) | −0.13 (−0.27 to 0.00) | 0.047 |
| Week 34 | 0.33±1.279 (47) | −0.07±0.638 (49) | −0.2 (−0.35 to −0.05) | 0.009 |
| NEFA, fasting (mmol/l)a | ||||
| Baseline | 328.9±142.40 (79) | 389.4±207.36 (76) | ||
| Week 21 | 47.9±181.68 (62) | −21.9±178.29 (56) | −0.04 (−0.23 to 0.15) | 0.686 |
| Week 34 | 3.2±174.00 (47) | −22.6±174.71 (49) | −0.03 (−0.26 to 0.2) | 0.792 |
| NEFA, 30-min (mmol/l)a | ||||
| Baseline | 209.9±134.26 (79) | 218.7±182.35 (76) | ||
| Week 21 | 14.1±157.61 (62) | −6.7±116.83 (56) | −0.25 (−0.46 to −0.03) | 0.028 |
| Week 34 | 14.3±180.17 (46) | 10.6±119.77 (49) | −0.09 (−0.32 to 0.14) | 0.428 |
| aNEFA, 2-hour | ||||
| Baseline | 100.6±138.19 (78) | 90.1±116.17 (75) | ||
| Week 21 | 19.3±143.4 (62) | 4.0±107.04 (55) | −0.32 (−0.63 to −0.02) | 0.038 |
| Week 34 | −16.7±159.28 (46) | −4.6±80.85 (48) | −0.14 (−0.42 to 0.14) | 0.313 |
Endpoints for triglycerides and NEFA were log-transformed before analysis and the adjusted treatment differences are presented on a logscale. HDL-C = high-density-lipoprotein cholesterol. LDL-C = low-density-lipoprotein cholesterol. NEFA = non-esterified fatty acid.
Secondary efficacy variables
The reduction in glucose concentrations was associated with an overall decrease in mean insulin concentrations from baseline with doxazosin at all time points (fasting, 30 minutes and 2 hours) and an increase with bendrofluazide, apart from at week 21 when there was a large decrease in mean insulin concentrations at 30 minutes. However, when the means were adjusted for age, sex, centre and baseline, there was an increase in mean insulin concentrations. The adjusted treatment difference in the insulin response bordered on significance for fasting insulin at week 21 (−11.5 pmol/l; P = 0.053; 95% CI = −23.1 to 0.2) and was significant at week 34 (−20.8 pmol/l; P<0.001; 95% CI = −31.3 to −10.3). At 2 hours, the adjusted treatment difference for insulin bordered significance at week 21 (−10.8 pmol/l; P = 0.052; 95% CI = −21.7 to 0.1) and week at 34 (−9.3 pmol/l; P = 0.054; 95% CI = −18.7 to 0.2).
There was an overall increase in proinsulin concentrations compared with baseline for both treatments, apart from 2-hour proinsulin at week 34, which was reduced with doxazosin, when there was also a statistically significant treatment difference (−0.31 pmol/l; P = 0.042; 95% CI = −0.60 to −0.01). There was no significant treatment difference at any other point.
Both doxazosin and bendrofluazide reduced mean LDL-C at weeks 21 and 34. When the means were adjusted, bendrofluazide reduced mean LDL-C at week 34 only (the adjusted mean changes from baseline are shown in Table 2); the treatment difference was not significant.
Doxazosin increased mean HDL-C at weeks 21 and 34, whereas bendrofluazide increased HDL-C at week 34 only (the adjusted mean changes from baseline are shown in Table 2); the treatment difference was not significant.
Doxazosin decreased mean triglyceride concentrations and bendrofluazide increased mean triglyceride concentrations at weeks 21 and 34 (the adjusted mean changes from baseline are shown in Table 2); the adjusted treatment difference was significant at both week 21 (−0.13 mmol/l; P = 0.047; 95% CI = −0.27 to 0.00) and week 34 (−0.20 mmol/l; P = 0.009; 95% CI = −0.35 to −0.05) (Table 2).
Both treatments reduced mean blood pressure at weeks 21 and 34, with no significant treatment differences (Table 3). The average final dose of study drug needed to achieve this reduction was 2.74 mg in the doxazosin treatment group and 2.50 mg in the bendrofluazide treatment group. More patients taking bendrofluazide required additional treatment with amlodipine to control blood pressure (n = 18 [22%] versus n = 12 [15%] with doxazosin).
Table 3.
Change in blood pressure from baseline to weeks 21 and 34, showing means ± standard deviation.
| Absolute change from baseline | ||||
|---|---|---|---|---|
| Bendrofluazide (n) | Doxazosin (n) | Adjusted treatment difference (95% CI) | P-value | |
| Diastolic (mmHg) | ||||
| Baseline | 97.0±5.72 (82) | 98.0±5.85 (78) | ||
| Week 21 | −12.7±8.00 (65) | −14.3±10.09 (60) | −1.2 (−3.9 to 1.6) | 0.407 |
| Week 34 | −13.4±6.88 (48) | −13.8±7.68 (50) | 0.3 (−2.4 to 3.0) | 0.827 |
| Systolic (mmHg) | ||||
| Baseline | 147.7±13.32 (82) | 150.8±13.67 (78) | ||
| Week 21 | −19.1±13.29 (65) | −18.3±15.05 (60) | 1.5 (−3.1 to 6.1) | 0.519 |
| Week 34 | −19.6±14.74 (48) | −15.2±11.81 (50) | 3.5 (−0.8 to 7.8) | 0.106 |
Safety
Both treatments were well tolerated, with a similar number of adverse events reported for each treatment overall: 60 (77%) patients taking doxazosin and 61 (74%) patients taking bendrofluazide reported adverse events.
The most frequent treatment-emergent adverse events in either group were headache (occurring in 17% of doxazosin-treated patients and 13% of bendrofluazide recipients), arthralgia (9% and 11%, respectively) and upper respiratory tract infection (12% in each group). For most body systems, there was no difference in the number of treatment-emergent adverse events. However, almost twice as many patients in the doxazosin group had a generalised increase in various skin disorders (22% versus 11% with bendrofluazide); in contrast, hyperuricaemia, hypokalaemia and accidental injury were reported in 10%, 6% and 6% respectively of patients in the bendrofluazide group, but did not occur in the doxazosin group.
One person in each group reported a serious adverse event. Adverse events were the primary reason for two patients in the doxazosin group and four patients in the bendrofluazide group withdrawing from the study prematurely. No patients who received amlodipine experienced an adverse event that was the primary reason for withdrawal from the study.
DISCUSSION
Summary of main findings
This study demonstrates that doxazosin, an α1-adrenergic blocker, has a beneficial effect on glucose metabolism and lipid profiles in British South Asians with hypertension, compared with the thiazide bendrofluazide.
An important finding was the difference between the two treatments in terms of changes in the mean glucose concentrations from baseline to weeks 21 and 34. In the doxazosin group, the mean glucose concentrations decreased, whereas they increased with bendrofluazide. The continuing increase in glucose concentrations over time in patients treated with bendrofluazide may indicate the development or worsening of insulin resistance in this treatment group.
In terms of improvement in lipid profile, doxazosin significantly reduced mean total cholesterol concentrations at week 21, compared with bendrofluazide. The most interesting effect was on triglyceride concentrations, which decreased at weeks 21 and 34 with doxazosin, but increased with bendrofluazide. The difference was significant at both time points.
As would be expected, both treatments reduced blood pressure (and to a similar degree); however, fewer patients taking doxazosin required additional treatment with amlodipine (although many required dose titration), suggesting that doxazosin may be more effective as monotherapy in this patient group.
The two treatments had similar safety and tolerability profiles.
Strengths and limitations of the study
This prospective, active-controlled study was designed specifically to look at the comparative effects of doxazosin and bendrofluazide in an ethnic population that is rarely the focus of clinical investigation, even though this group is known to be at particularly high risk of CHD and currently accounts for around 3.5% of the UK population as a whole (and much higher proportions in urban areas such as parts of London and the West Midlands).15 The study was undertaken in a range of centres across England, to provide a representative sample of the British South Asian population, and used a clear definition of ‘British South Asian’ — both parents originating from the Indian subcontinent — to ensure consistency of recruitment across sites. Furthermore, the patient information leaflets were provided in a range of languages to accommodate participants whose first language was not English.
The study was a relatively short-term investigation of the effect of treatment on specific outcomes (glucose, lipids and blood pressure), but was not designed to look at any long-term benefits in terms of reducing the risk of developing CHD. Indeed, the currently available coronary risk calculators would make such an assessment difficult, because, on the whole, they lack weighting for the population being studied here. The most widely used tools are usually based on the Framingham data, derived from a mainly white, middle-class population.16-18 More recently, the UK Prospective Diabetes Study investigators have developed a risk equation based on the data derived from a large, long-term study, but this tool is, as the name implies, most relevant for patients who have already developed diabetes.19
Context
Coronary risk prediction tools, whatever the population that they are based on, are useful to identify people for whom intervention would be appropriate to reduce their risk of future coronary events. Such interventions involve blood pressure reduction, management of lipids, and, in people with diabetes, glucose control, as well as lifestyle changes (including smoking cessation, improved diet, increased physical activity and weight management).2,16,20
It is well known that British South Asians, as an ethnic group, are particularly susceptible to CHD; this is believed to result from a genetic predisposition to insulin resistance, manifesting as type 2 diabetes, hypertension, hyperinsulinaemia, dyslipidaemia (characterised by low HDL-C and high triglyceride concentrations) and central obesity.3 Indeed, some of the patients enrolled in the present study would now be candidates for lipid-lowering therapy under current guidelines.16 The striking effects of doxazosin on glucose and lipids in the present study, in addition to its antihypertensive effect, demonstrate that this agent has a beneficial effect on several of the components of the insulin resistance syndrome to which British South Asians are predisposed.
The results achieved in the present study are similar to those in previous studies of the lipid-lowering properties of doxazosin in mainly white populations — a reduction in total cholesterol, reduction in LDL-C, small increases in HDL-C and reduction in triglycerides in patients with hypertension, both with and without type 2 diabetes.6-10 The results of this study demonstrate a similar improvement in glycaemic control as was reported in earlier studies of doxazosin in patients with hypertension and type 2 diabetes.12,13
Thiazide diuretics are recommended by the British Hypertension Society (BHS) as first-line therapy for the treatment of hypertension.20 However, their effects on morbidity and mortality from CHD have been less than predicted from observational studies. Although the reasons for this are not clear, hypertension is only one of many risk factors for the development of CHD, and concerns have been expressed that thiazides may have a negative effect on hyperlipidaemia and glucose metabolism.21 The BHS guidelines state that dyslipidaemia is a possible contraindication for treatment with a thiazide, whereas it is a possible indication for treatment with an α blocker.20
Implications for future research
This study did not measure the clinical outcomes of both treatments in terms of CHD, so it is not possible to determine whether the beneficial effects of doxazosin on intermediate cardiovascular risk factors, blood pressure, levels of glycaemia, and lipid profile, would have long-term benefits in terms of reducing the risk of developing CHD. Long-term studies in people from the Asian subcontinent are needed to determine this, using coronary risk equations developed specifically for this population.
Acknowledgments
The authors would like to acknowledge the contribution of the following investigators and their patients: A Connolly, Bradford; MB Maltz, London; JE Miller, Cheadle; ID Patchett, Leicester; GU Patel, Sheffield; A Prasad, Bolton; WA Shaida, South Harrow; MR Turner, Heston; SA Zafar, Birmingham; S Ali, Bradford; RK Dutta, Coventry; SU Khan, Oldham; CA Morley, Bradford; VK Rajput, Birmingham; MV Ramarao, Birmingham.
Funding body
The costs of this study were supported by Pfizer Ltd, Tadworth, Surrey
Ethics committee
South Thames Medical Research Ethics Committee, and relevant local ethics committees
Competing interests
FDR Hobbs has received sonsorship and research funding previously from a variety of pharmaceutical companies, including Pfizer. T Kahn worked at Pfizer UK and B Collins was contracted by Pfizer UK
REFERENCES
- 1.Balarajan R. Ethnic differences in mortality from ischaemic heart disease and cerebrovascular disease in England and Wales. BMJ. 1991;302:560–564. doi: 10.1136/bmj.302.6776.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Department of Health. National Service Framework for coronary heart disease. London: The Stationery Office; 2000. [Google Scholar]
- 3.McKeigue PM, Shah B, Marmot MG. Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. Lancet. 1991;337:382–386. doi: 10.1016/0140-6736(91)91164-p. [DOI] [PubMed] [Google Scholar]
- 4.Dhawan J, Bray CL, Warburton R, et al. Insulin resistance, high prevalence of diabetes, and cardiovascular risk in immigrant Asians. Genetic or environmental effect? Br Heart J. 1994;72:413–421. doi: 10.1136/hrt.72.5.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams B. Westernised Asians and cardiovascular disease: nature or nurture? Lancet. 1995;345:401–402. doi: 10.1016/s0140-6736(95)90394-1. [DOI] [PubMed] [Google Scholar]
- 6.Betteridge DJ. Doxazosin and lipid and lipoprotein metabolism. Rev Contemp Pharmacother. 1992;3:9–21. [Google Scholar]
- 7.Englert RG, Barlage U. The addition of doxazosin to the treatment regimen of patients with hypertension not adequately controlled by beta-blockers. Am Heart J. 1991;121:311–316. doi: 10.1016/0002-8703(91)90864-e. [DOI] [PubMed] [Google Scholar]
- 8.Hoogerbrugge N, de Groot E, de Heide LHM, et al. Doxazosin and hydrochlorothiazide equally affect arterial wall thickness in hypertensive males with hypercholesterolaemia (the DAPHNE study) Neth J Med. 1999;60:354–361. [PubMed] [Google Scholar]
- 9.Ulahannan TJ, Karpe F, Humphreys SM, et al. Effects of acute administration of doxazosin on fasting and postprandial haemodynamics and lipid metabolism in healthy subjects. Horm Metab Res. 2002;34:499–503. doi: 10.1055/s-2002-34789. [DOI] [PubMed] [Google Scholar]
- 10.Courtney CH, McCance DR, Atkinson AB, et al. Effect of the alpha-adrenergic blocker, doxazosin, on endothelial function and insulin action. Metabolism. 2003;52:1147–1152. doi: 10.1016/s0026-0495(03)00190-2. [DOI] [PubMed] [Google Scholar]
- 11.Lithell HOL. Effect of antihypertensive drugs on insulin, glucose, and lipid metabolism. Diabetes Care. 1991;14:203–209. doi: 10.2337/diacare.14.3.203. [DOI] [PubMed] [Google Scholar]
- 12.Giordano M, Matsuda M, Sanders L, et al. Effects of angiotensin-converting enzyme inhibitors, Ca2+ channel antagonists, and alpha-adrenergic blockers on glucose and lipid metabolism in NIDDM patients with hypertension. Diabetes. 1995;44:665–671. doi: 10.2337/diab.44.6.665. [DOI] [PubMed] [Google Scholar]
- 13.Huupponen R, Lehtonen A, Vahatalo M. Effect of doxazosin on insulin sensitivity in hypertensive non-insulin dependent diabetic patients. Eur J Clin Pharmacol. 1992;43:365–368. doi: 10.1007/BF02220610. [DOI] [PubMed] [Google Scholar]
- 14.Maheux P, Facchini F, Jeppesen J, et al. Changes in glucose, insulin, lipid, lipoprotein, and apoprotein concentrations and insulin action in doxazosin-treated patients with hypertension. Comparison between nondiabetic individuals and patients with non-insulin-dependent diabetes mellitus. Am J Hypertens. 1994;7:416–424. doi: 10.1093/ajh/7.5.416. [DOI] [PubMed] [Google Scholar]
- 15.National Statistics Online. Census 2001. http://www.statistics.gov.uk/census2001/default.asp (accessed 23 March 2005)
- 16.Wood D, Durrington P, Poulter N, et al. Joint British recommendations on prevention of coronary heart disease in clinical practice. Heart. 1998;80(Suppl 2):S1–S29. [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson PR. Updated New Zealand cardiovascular disease risk-benefit prediction guide. BMJ. 2000;320:709–710. doi: 10.1136/bmj.320.7236.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wallis EJ, Ramsay LE, Haq IU, et al. Coronary and cardiovascular risk estimation for primary prevention: validation of a new Sheffield table in the 1995 Scottish health survey population. BMJ. 2000;320:671–676. doi: 10.1136/bmj.320.7236.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevens RJ, Kothari V, Adler AI, et al. The UKPDS risk engine: a model for the risk of coronary heart disease in Type II diabetes (UKPDS 56) Clin Sci (Lond) 2001;101:671–679. [PubMed] [Google Scholar]
- 20.Ramsay LE, Williams B, Johnston GD, et al. Guidelines for management of hypertension: report of the third working party of the British Hypertension Society. J Hum Hypertens. 1999;13:569–592. doi: 10.1038/sj.jhh.1000917. [DOI] [PubMed] [Google Scholar]
- 21.Sever PS. Alpha 1-blockers in hypertension. Curr Med Res Opin. 1999;15:95–103. doi: 10.1185/03007999909113369. [DOI] [PubMed] [Google Scholar]


