Abstract
Contrast sensitivity is known to be strongly influenced by the target surround, yet the role of the surround interaction in visual processing remains unclear. Previously, we have shown that the surround strongly suppresses contrast sensitivity in the periphery when the surround spatial frequency and orientation match those of the target (Petrov, Carandini, & McKee, 2005). Here, we explore how various spatial characteristics of the iso-oriented and frequency-matched surround, such as surround phase and spatial layout, affect suppression. We manipulated surround geometry (annulus ring, half annulus, and bow tie) and its separation from the target (both laterally and in depth) and varied the position of the half-annulus and bow-tie surrounds with respect to Gabor target’s orientation and with respect to its location in the visual field (i.e., radial vs. tangential surrounds). We also compared monoptic, dichoptic, and binocular surround suppression. Except for a significant radial–tangential anisotropy, only the area of the surround and the lateral separation between the surround and target had a significant effect on the magnitude of suppression. We showed that, although suppression amplitude remains constant with stimulus eccentricity, the lateral extent of suppression scales in proportion to the eccentricity. The most surprising finding was that the extent of surround suppression does not scale with stimulus size or spatial frequency. We suggest that the properties of surround suppression are best explained by a mechanism that selects salient targets for subsequent saccades.
Keywords: psychophysics, surround suppression, contrast detection, saccades
Introduction
Some 40 years ago, Hubel and Wiesel (1965) noticed that neurons in Visual areas 18 and 19 of cats responded much more weakly if the otherwise optimal stimulus was extended beyond the neuron’s receptive field. Since then, this type of inhibition termed surround suppression has been commonly observed in early visual areas of both cats and primates (for a review, see Allman, Miezin, & McGuinness, 1985, and also Carandini, 2004; Cavanaugh, Bair, & Movshon, 2002a; Hubel & Wiesel, 1968). Overall, the physiological data show that surround suppression is strongest when the surround has higher contrast than the center, but otherwise it has matching power spectrum parameters (i.e., orientation, spatial frequency, and direction of motion). In our recent psychophysical study, we found these same properties of the surround suppression in human observers (Petrov, Carandini, & McKee, 2005).
Despite years of research, there is still no clear understanding of the role that surround suppression plays in visual processing. An effect of such magnitude (up to 300%) and spatial extent (many degrees of visual angle in the periphery) has to play a major role. The explanations suggested so far (e.g., Schwartz & Simoncelli, 2001) tend to focus on one aspect of the suppression (usually, its orientation tuning), while ignoring others. In addition, many relevant properties have not been studied. Spatial aspects are of particular interest here because the proposed models make specific assumptions about how the location of the surround mask affects suppression.
The effect of spatial layout was first addressed in the original Hubel and Wiesel (1965) study in cats and was later elaborated by DeAngelis, Freeman, and Ohzawa (1994) and Walker, Ohzawa, and Freeman (1999, 2002). Walker et al. (1999) found that the locus of suppression varies between cells; it can be distributed over the whole surround area or it can be quite localized. There was a slight bias for suppression to occur at the end zones of the receptive field. No significant effect of eye of origin or surround disparity was found (DeAngelis et al., 1994).
Recent studies of primate V1 (Cavanaugh, Bair, & Movshon, 2002b; Webb, Tinsley, Barraclough, Parker, & Derrington, 2003) show that the spatial layout of surround suppression is similar to that observed in cat cortex. When averaged across the whole population of V1 cells, suppression was shown to be isotropic with only a small bias toward larger suppression from the end zones of the receptive fields. A smaller proportion of primate V1 cells showed a significant surround anisotropy compared with cells in the cat area 17.
Psychophysical studies of the spatial aspects of surround suppression in humans have produced less consistent results. In contrast matching tasks, the effects of surround on the perceived contrast of the target were, overall, in agreement with the cat and monkey neurophysiological data: (a) the effect of the surround was to suppress perceived contrast, (b) the suppression was strongest when the target and the surround carriers had the same orientation, and (c) the strength of the suppression did not change significantly between collinear and flanking surround layouts (i.e., the end and side zones of a cell’s receptive field; Cannon & Fullenkamp, 1991; Ejima & Takahashi, 1985; Xing & Heeger, 2001). Although these studies show a qualitative agreement with the neural suppression observed in primary visual cortex of cats and monkeys, it is unclear how to relate the reduction of perceived contrast to the physiological suppression in a quantitative fashion. Xing and Heeger (2001) have proposed a quantitative model linking their data to the underlying neurophysiology, but the model is based on the assumption that perceived contrast is proportional to the responses of V1 neurons. Indeed, this assumption would greatly simplify the interpretation of contrast matching results, but to the best of our knowledge, there is no evidence to support it.
The effect of surrounds on contrast detection thresholds varies from facilitation (Polat & Sagi, 1993, 1994; Yu, Klein, & Levi, 2002; Zenger-Landolt & Koch, 2001) to suppression (Solomon & Morgan, 2000; Williams & Hess, 1998), depending on the surround area, orientation, and phase with respect to the target, as well as the stimulus eccentricity (Andriessen & Bouma, 1976; Petrov et al., 2005; Snowden & Hammett, 1998; Zenger-Landolt & Koch, 2001). Recently, Petrov, Verghese, and McKee (2006) showed that the facilitation observed in contrast detection thresholds is primarily due to a reduction in uncertainty about target location; hence, care should be taken to avoid uncertainty confounds in detection tasks.
A few studies have compared contrast detection and contrast matching experiments (Meese & Hess, 2004; Snowden & Hammett, 1998). Generally, contrast matching shows less specificity regarding the surround parameters than detection measurements. For example, contrast detection measurements show that surround suppression is strong only in the periphery (larger than 1 deg eccentricity) and almost disappears in the fovea (Petrov et al., 2005; Snowden & Hammett, 1998). However, perceived contrast is reduced by an optimal surround even in the fovea, at least when the test contrast is low (Cannon & Fullenkamp, 1991; Xing & Heeger, 2000, 2001). Similarly, Cannon and Fullenkamp (1991) and Xing and Heeger (2001) showed that the perceived contrast was reduced most by surrounds of the same orientation, but in the fovea, suppression remained strong even when the surround was orthogonal to the target. Conversely, in detection threshold measurements, suppression disappears completely once the relative orientation between the target and the annulus exceeds 45 deg (Petrov et al., 2005).
As argued by Snowden and Hammett (1998), the differences might arise because the surround effectively overlaps the skirts of the mechanism responding to the target in the contrast matching studies. Typically, the test targets were suprathreshold sinusoidal grating disks, which were surrounded by a mask with little or no intervening blank space. This overlap could produce suppressive overlay masking because the surround is effectively superimposed on the target. Overlay masking is not tuned to stimulus eccentricity and is only weakly tuned to mask orientation (see Petrov et al., 2005), which would explain the relative lack of specificity in the contrast matching results.
There are also conflicting results reported in the contrast matching studies. Thus, Ejima and Takahashi (1985) and Olzak and Laurinen (1999, 2005) found that suppression depends strongly on the surround phase, whereas Cannon and Fullenkamp (1991) and Xing and Heeger (2001) showed the opposite. Interocular suppression was found to be weak by Chubb, Sperling, and Solomon (1989) but very strong by Meese and Hess (2004).
The goal of the present work is to carry out a comprehensive analysis of the spatial aspects of surround suppression. Given the lack of psychophysical and physiological data on the role of surround disparity, stimulus position in the visual field, and scarce or conflicting evidence on the role of the surround phase and eye of origin, we will also examine these surround parameters on contrast detection thresholds.
To evoke strong surround suppression, we used peripheral stimuli. We estimated the strength of surround suppression by measuring contrast detection thresholds for a target with and without the surrounding mask. Unlike perceived contrast, contrast thresholds have a straightforward interpretation in terms of neurophysiological response functions. This relationship makes it possible to compare human psychophysical results directly to the results of single-cell recordings in cats and primates. Special care has been taken to avoid spurious overlay masking and the effects of uncertainty about target location (see Methods section).
Although our findings agree with most of the earlier psychophysical and neurophysiological results, we found some new and unexpected aspects of surround suppression. Using both the new and existing evidence, we discuss possible functions of surround suppression in visual processing and suggest that its main purpose is to determine salient saccadic targets.
Methods
Apparatus
Stimuli were displayed on a gray background (42 cd/m2) and viewed through a Wheatstone stereoscope on a pair of linearized 17-in. Sony Trinitron G220 monitors. The display was 1400 × 1050 pixels; viewing distance was 65 cm. The video signal was rendered with (nominal) 8-bit precision, but an additional factor of 4 increase in precision was attained using 2 × 2 block pixel ordered dithering (analogous to the classical newspaper halftone technique). To illustrate the ordered dithering, suppose that the nominal 8-bit look-up table values range from 0 to 255. Three extra levels of gray for value 127 (for example) are represented by the following 2 × 2 pixel patterns:
| 127 | 127.25 | 127.5 | 127.75 |
|---|---|---|---|
| 127 127 | 128 127 | 128 127 | 128 128 |
| 127 127 | 127 127 | 127 128 | 127 128 |
The resulting effective pixel subtended 2.4 arcmin, whereas the dithering artifacts (0.8% contrast modulation at 22 cpd) were approximately 30 times below the detection threshold. The effective luminance resolution of the screen at the background level (after γ correction) was confirmed to be 0.2% (9 bits) by counting the number of gray levels in the stimulus screenshots and to be better than 0.3% with a Pritchard Photometer.
Subjects
Five observers with normal or corrected visual acuity were tested. Three of the observers were naive to the purpose of the study; all five were experienced psychophysical observers. Observers were trained for a short time (2–5 min) to get acquainted with the stimuli and the task.
Psychometric procedure
We used a two-alternative forced-choice procedure (2AFC), in which the test target appeared at one of two locations, located above and below the fixation point at equal eccentric loci (6 deg, except in Experiment 6, where stimulus eccentricity was varied). A surround mask and a faint dark circle identifying each location were presented at both locations on all trials. The task was to indicate with a button press which location contained the test. Stimulus duration was 150 ms. A fixation pattern comprised of two low-contrast concentric circles and a pair of nonius lines was displayed at the center of the screen at the beginning of each trial. The target location circles in the periphery and the fixation mark in the center were shown binocularly and were continuously visible throughout fixation and target presentation. We used a slightly modified version of the adaptive staircase algorithm, devised by Kontsevich and Tyler (1999), to estimate thresholds and steepness of psychometric functions. The modifications were merely technical (information maximization was replaced by variance minimization at each algorithm step) and were shown to produce a slightly faster estimates of both parameters of the psychometric function. Threshold contrast corresponded to 76% of the correct responses as estimated from the psychometric function. After several preliminary runs, the steepness (slope) parameter of the psychometric function was fixed at 1.5, which was found to be the typical value for all tested observers. Note that the steepness parameter in the Kontsevich algorithm is similar but numerically different from the Weibull exponent β. Normally, observers carried out three blocks of 150 trials per block for each condition. Variability of the psychometric thresholds was taken as the maximum of these two estimates: (a) threshold variation calculated from the resulting probability distribution in the adaptive algorithm and (b) threshold variation among the three experimental blocks.
The test target was a standard cosine phase Gabor (σ = λ/√2) in which ~1.5 periods (1.1 deg) of the sinusoidal pattern were visible, as shown in Figure 1a. The Gabor spatial frequency was 1.3 cpd in all of the experiments, except Experiment 6, in which 2.7 cpd targets were also tested. The Gabor was slanted 45 deg right from the vertical in all of the experiments except Experiment 5, where a vertical target was used instead. Faint thin circles that are 15% in contrast, 1 pixel (1.2 arcmin) wide, and 2.5λ (0.83 deg) in diameter surrounded the 2AFC target regions at all times to reduce the observer’s uncertainty about the regions’ locations, which was particularly important for targets presented without the surround mask (Petrov et al., 2006).
Figure 1.
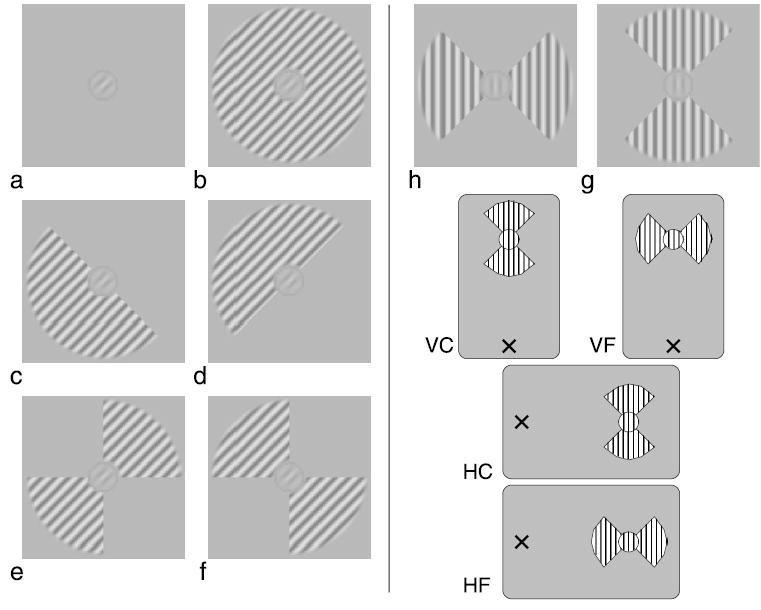
An illustration of the stimuli used in this study. (a) No surround condition. A cosine phase Gabor target with 45 deg orientation and a thin localizer circle is shown. (b) The Gabor target surrounded by the full-annulus mask. The same-phase surround condition is shown. (c) The half-annulus mask positioned at the “end” of the Gabor target. (d) The half-annulus mask positioned at the “side” of the target. (e) The bow-tie mask “collinear” with the target. (f) The bow-tie mask “flanking” the target. (g and h) The bow-tie stimuli with vertical sine grating carrier were used for the visual field anisotropy experiments. The four visual field layouts used in these experiments are illustrated below: vertical collinear (VC), vertical flanking (VF), horizontal collinear (HC), and horizontal flanking (HF). A cross marks the fixation point.
Five spatial surround layouts, shown in Figure 1, were tested: a grating annulus around the target (panel b); a half annulus positioned at one end of the target (panel c); a half annulus positioned at the side of the target (panel d); a bow tie made of two annulus quadrants positioned at the ends of the target (panel e); and a bow tie with the annulus quadrants positioned at the sides of the target (panel f). The circular annulus had an inner radius of 2λ (1.5 deg) and an outer radius of 8λ (6 deg). It contained a sinusoidal grating of the same orientation and spatial frequency as the target. The grating contrast was 10%. The phase of the grating was the same as that of the target, except in Experiment 1, where it was varied over 180 deg. To ensure that no overlay masking was present, a blank region (at the background luminance) approximately 1 period wide (0.75 deg) separated the target from the mask. The separation was only varied in Experiment 6.
Most of the parameters for this study were set to maximize the effect of surround suppression. Thus, we used a surround carrier (with a 10% contrast) that matched the target’s spatial frequency and orientation to produce the maximum suppression for the detection task (Petrov et al., 2005).
The experiments in the Results section are ordered from local surround manipulations (the carrier phase) to global manipulations (location of the surround mask with respect to the visual field). The experimental data are shown in Figures 2–8. The suppression factor, defined as the ratio of the masked to unmasked thresholds, was used as a measure of the surround suppression strength in all figures except Figure 7, where threshold elevation was used instead. The unmasked thresholds (not shown) varied among subjects from 1% to 2.5%.
Figure 2.
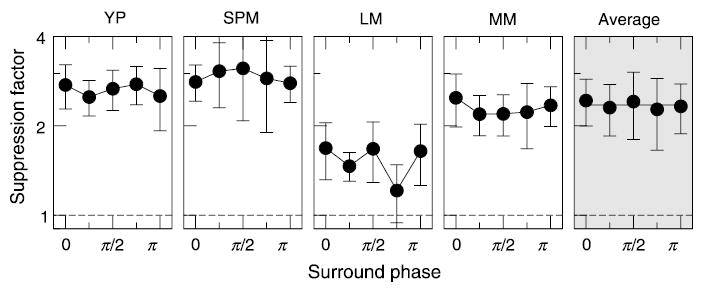
Surround suppression as a function of the surround phase. The target phase is taken as zero here. Results for four observers are arranged in columns; data averaged between the observers are shown in the last column. Dashed lines indicate no suppression.
Figure 8.
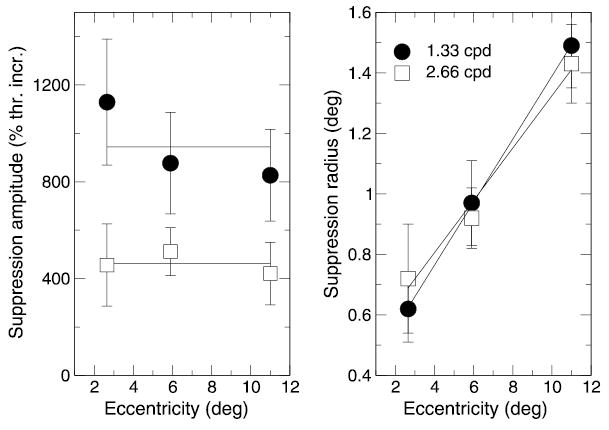
Amplitude and radius of surround suppression. The suppression amplitude A is plotted versus stimulus eccentricity on the right, and the suppression radius ρ (a reciprocal of the slopes taken from Figure 7) on the right. Data for 1.33 and 2.66 cpd stimuli are plotted with filled circles and open squares, respectively. Solid lines show constant fits for the left panel and linear fits for the right panel.
Figure 7.
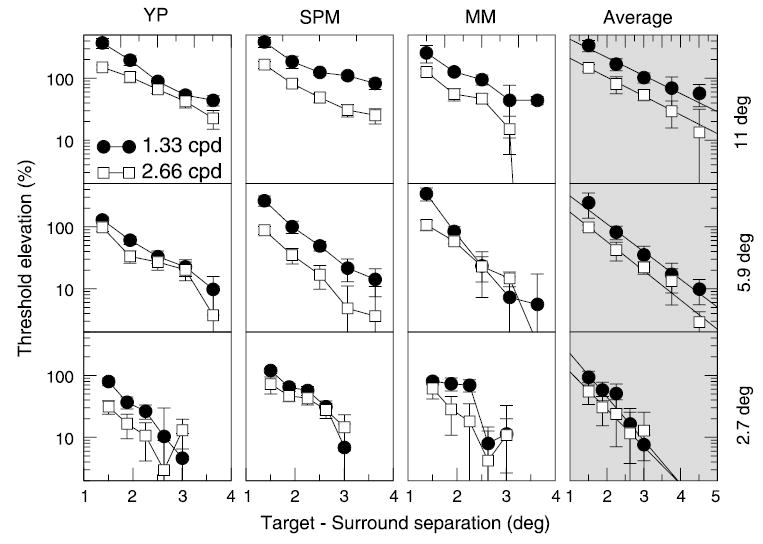
Surround suppression (shown as threshold elevation here) as a function of spatial separation between the target and the surround mask. Results for three observers are arranged in columns; the last column shows data averaged between the observers. Rows correspond to three stimulus eccentricities. Target mask separation is plotted along the X-axis in each plot; 1.33 and 2.66 cpd data are shown with filled circles and open squares, respectively. Solid lines drawn through the averaged data represent two-parameter exponential fits, as described in the text.
Results
Experiment 1: Surround phase
We began by testing surround suppression strength as a function of the phase of the surround carrier. The surround was the full annulus of sinusoidal grating shown in Figure 1b. The carrier phase was varied by the same amount in both eyes. Five different phases ranging from 0 to 180 deg were tested in consecutive blocks, one at a time.
The results for four observers are shown in Figure 2; the carrier phase is plotted along the x axis relative to the target phase: the target (cosine) phase was defined as zero. Data for individual observers are shown in the first four columns; data averaged over the four observers are shown in the last column. Error bars (1 SD) for individual data were calculated as described in the Methods section; error bars for the average data show variation among the subjects.
Suppression did not change significantly as the phase of the surround was varied. To illustrate this point, a constant fit is shown by a solid black line in the plot of the average data.
Experiment 2: Surround disparity
We next explored the effect of surround disparity. The whole annulus (both carrier and annulus boundaries) was shifted horizontally by 3/16 of the carrier period λ in opposite directions in the two eyes. This introduced a disparity of 17 arcmin between the target and the annulus mask. We did not use larger disparities because they could result in ambiguous carrier matches. Subjects were fixating at the target depth before each trial. Crossed, uncrossed, and zero disparities were randomly interleaved within each experimental run. The results for four subjects are presented in Figure 3. Clearly, no significant change of suppression strength was observed when disparity of the surround was varied.
Figure 3.
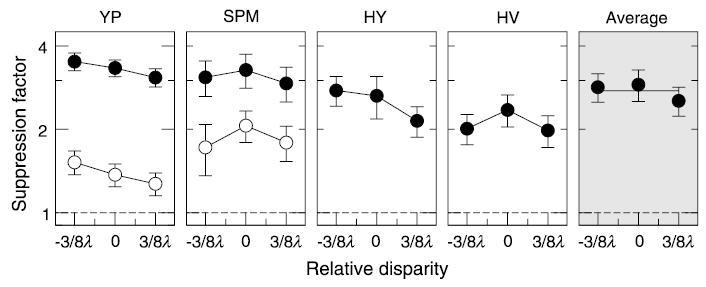
Surround suppression as a function of relative disparity between the Gabor target and the annulus surround (filled circles). Dashed lines indicate no suppression. Disparity of the surround is plotted along the X-axis in terms of the carrier period λ = 0.75 deg. Control results for a modified surround in the form of two sine grating bars above and below the target are shown by open circles for Y.P. and S.P.M.
One possible concern in this experiment was that when the surround mask was shifted, a part of the annulus mask moved closer to the target, whereas the other part moved away. Because the suppression strength is a nonlinear function of separation between the target and the surround (see Experiment 6), the effects of disparity change and the separation change could cancel each other. To test this possibility, the stimulus was modified by using a surround mask composed of two long horizontal bars (5 deg in length and 2 deg in height, with the same carrier as for the annulus mask) positioned above and below the target. For this stimulus, a small horizontal shift of the bars does not modify the separation from the target, yet disparity information from both the carrier and the mask boundary is still readily available. The results for the modified stimulus are shown by open symbols in Figure 3 for two observers. One can see that suppression dropped significantly as compared with that from the annulus surround. This reduction is expected because roughly half of the surround was removed. More importantly, the disparity of the modified surround did not affect the suppression strength, which shows that the suppression mechanism is not tuned to the surround disparity.
Experiment 3: Surround eye of origin
The absence of tuning for disparity suggests that surround suppression operates at an early monocular processing stage before signals from the two eyes are combined. If so, there should be no interocular transfer of surround suppression. We tested this hypothesis by presenting the target and the annulus mask either to the same eye (monoptic masking) or to opposite eyes (dichoptic masking). The four tested conditions were as follows: mask and target were both shown to the left eye (Ll); mask and target were both shown to the right eye (Rr); mask was shown to the right eye, whereas target was shown to the left eye (Rl); and mask was shown to the left eye, whereas target was shown to the right eye (Lr). The four conditions were tested in consecutive runs, one at a time.
The results shown in Figure 4 indicate that, although individual observers had significant differences in the amount of suppression among conditions, there was no consistent pattern across observers. Three of the four observers showed slightly weaker dichoptic suppression, but the difference from monocular suppression was quite small (except for SPM, right eye).
Figure 4.
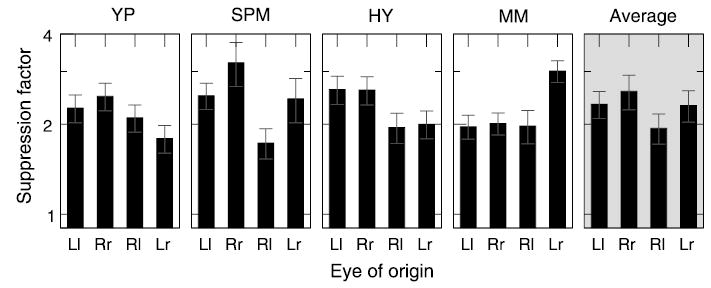
Surround suppression as a function of the eye of origin. Eye of origin (X-axis) is coded by uppercase letters (L or R) for the surround and by lowercase letters (l or r) for the center. Rr and Ll correspond to monocular viewing conditions (right and left eye, respectively), whereas Rl and Lr correspond to dichoptic viewing conditions in which the center and the surround were shown to opposite eyes.
Although the strong dichoptic transfer suggests that suppression occurs at a binocular stage, it is possible that surrounds from both eyes suppress each monocular center before binocular combination of the centers, that is, still at a monocular stage. If this were true, binocular suppression would have been much stronger than either dichoptic or monocular suppression because for the binocular stimulus, each center would have been suppressed by two surrounds, whereas for dichoptic and monocular stimuli, each center is suppressed by only one surround. Yet, the average amount of suppression in monocular and dichoptic conditions, as shown in Figure 4, was roughly the same as that in binocular viewing condition, as shown in Figure 2. Therefore, surround suppression probably occurs after both center and surround signals are combined binocularly.
Experiment 4: Spatial layout around the target
Besides the phase and disparity of the surround mask, its spatial layout relative to the target could be an important factor. In this experiment, the five different layouts illustrated in Figures 1b–f were used to test for the three aspects of the surround geometry: (a) surround area, (b) surround collinearity with the target, and (c) surround symmetry (unilateral vs. bilateral). To test the effect of area, surround suppression from the full annulus (Figure 1b) was compared with that from one half of the surround (partitioned in four different ways, as shown Figures 1c–f). To test for collinearity effects, suppression from the bow-tie mask collinear with the target, C (Figure 1e), was compared with suppression from the bow-tie mask flanking the target, F (Figure 1f). Also, suppression from the half-annulus mask shown near the end of the target, E (Figure 1c), was compared with that from the half-annulus mask shown near the side of the target, S (Figure 1d). Finally, to test the effects of symmetry, suppression from the two unilateral masks, E and S, was compared with that from the two bilateral masks, C and F.
The five surround layouts were tested in consecutive experimental runs. The results for four subjects are shown in Figure 5. One can see that the only important aspect of the surround was its area (A vs. C, F, E, and S). On average, the amount of surround suppression dropped by a factor of 2.7 when the surround was halved. The suppression was unaffected by either the surround collinearity (C vs. F and E vs. S) or its symmetry (E and S vs. C and F).
Figure 5.
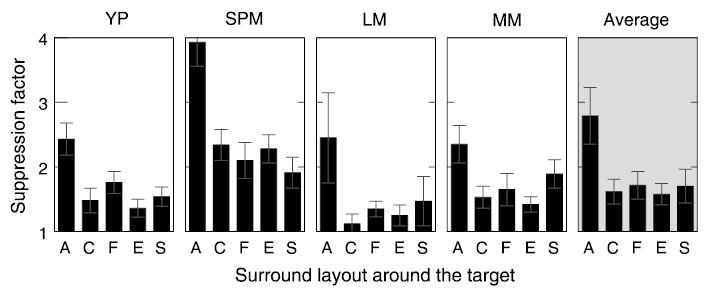
Surround suppression as a function of the surround layout around the target. The tested geometries are shown in Figures 1b–f and are marked along the X-axis as follows: A—full annulus; C—”collinear” bow-tie mask; F—”flanking” bow-tie mask; E—”end” half-annulus mask; S—”side” half-annulus mask.
Experiment 5: Spatial layout with respect to the visual field
In the previous section, we saw that the position of the surround relative to the target was immaterial as long as the separation between the two remained constant and the suppression area was equivalent. However, in the previous experiment, we were careful to lay out the stimulus in such a way that the surround shapes in comparison were equivalent to each other with respect to radial and tangential directions of the visual field. For example, because the stimuli were positioned along the vertical meridian, the layout of the collinear bow-tie C was equivalent to the layout of the flanking bow-tie F in the sense that the two surround shapes could be obtained from each other by reflections along either radial or tangential directions of the visual field (compare Figures 1e and f). In this way, the possible differences between radial and tangential surrounds were removed.
In this experiment, we explored the relationship between surround suppression and retinal geometry. To this end, the bow-tie stimuli C and F were rotated 45 deg counterclockwise, as shown in Figures 1g and h, respectively. If, as before, these stimuli were positioned along the vertical meridian, the two surround patterns would now differ not only by the position of the surround with respect to the target (collinear vs. flanking) but also by the surround layout with respect to the visual field. The surround shown in Figure 1g would extend in the radial direction, whereas the surround shown in Figure 1h would extend in the tangential direction. This radial–tangential organization of the surround would swap between the two stimuli if the same stimuli were positioned along the horizontal meridian. Thus, we studied four possible surround layouts: stimulus on the vertical meridian, collinear surround (VC); stimulus on the vertical meridian, flanking surround (VF); stimulus on the horizontal meridian, collinear surround (HC); and stimulus on the horizontal meridian, flanking surround (HF). The four surround layouts were tested in consecutive experimental runs. As can be seen from Figure 6, despite individual differences between subjects, it appears than on average the radial surround patterns (VC and HF) produced a stronger suppression (by a factor of 1.7) than the tangential surround patterns (VF and HC, respectively). The results of this experiment extend the results of the previous experiment. Although the surround interaction is locally isotropic, it has a significant global anisotropy between radial and tangential directions.
Figure 6.

Surround suppression as a function of the surround spatial layout within the visual field. The bow-tie stimuli used in this experiment are shown in Figures 1g and h. Results for the four tested layouts are plotted along the X-axis in the following order: VC—stimulus was positioned along the vertical meridian, “collinear” surround (Figure 1g) was used; VF—stimulus was on the vertical meridian, “flanking” surround (Figure 1h) was used; HC—stimulus was positioned along the horizontal meridian, “collinear” surround was used; HF—stimulus was on the horizontal meridian, “flanking” surround was used.
Experiment 6: Target-surround separation
In the experiments described above, the separation between the target and the inner radius of the surround was kept at a constant value (1.5 deg). This approach ensured that suppression was strong, yet it precluded any overlay masking. Because Experiment 4 demonstrated that the area of the surround in the proximity of the target is the only critical parameter, we wanted to know how fast surround suppression decreases with increasing separation between the target and the surround, and how that decrease depends on the stimulus size and eccentricity. To this end, we used stimuli of two spatial frequencies: 1.3 and 2.7 cpd. The stimuli were displayed at three eccentricities: 2.7, 5.9, and 11 deg. The Gabor target was scaled with the spatial frequency. The surround mask was somewhat modified: The sinusoidal grating was extended over the whole screen, except for the target area and a blank (background luminance) “moat” region of variable width separating the target from the mask. The radius of the moat varied in the range 1–5 deg depending on the stimulus eccentricity.
Three subjects participated in this experiment. The results are shown in Figure 7. For these measurements, threshold elevation was used instead of suppression factor. The radius of the moat region was plotted along the x axis on a linear scale. As before, results for individual subjects are arranged in columns. The rows correspond to the three tested eccentricities, as marked on the right; 1.3 and 2.7 cpd data are shown with filled circles and open squares, respectively.
The main findings are best appreciated by looking at the averaged data shown in the right column in Figure 7. First, the data very nearly fall on straight lines for all six conditions. This is illustrated by fitting the data with linear regression fits, which are shown by solid lines.
The linearity indicates that surround suppression declined in an exponential fashion, as the separation from the target was increased (note the logarithmic scale along the y axis). This relationship allows us to separate two factors of surround suppression: its strength (amplitude) and its extent (interaction radius). Specifically, given the suppression as a function of separation, r, in the form A × e− r/ρ[or log(A) − r/ρ, when measured in logarithmic units], the slopes of the linear fits in Figure 7 give the reciprocals of the interaction radii ρ, whereas the y intercepts give the logarithm of the interaction amplitudes A. From inspection of the data in the last column in Figure 7, it follows that (a) the interaction amplitude varies little with eccentricity, (b) the interaction amplitude is smaller for the higher frequency stimulus at all eccentricities, (c) the interaction radius increases consistently with the increasing eccentricity, and (d) the interaction radius varies little with the stimulus frequency.
To better illustrate these findings, Figure 8 shows the interaction amplitude A (shown in the left panel) and the interaction radius ρ (shown in the right panel) plotted as a function of the stimulus eccentricity. The interaction radius corresponds to the distance where suppression has fallen to approximately one third of the estimated amplitude (A). As the data demonstrate, the interaction amplitude does not vary significantly with the eccentricity but is halved when the stimulus frequency is doubled from 1.3 cpd (shown in circles) to 2.7 cpd (shown in squares). However, the interaction radius increases approximately linearly with the stimulus eccentricity (by a factor of 0.1) but is essentially the same for the two tested frequencies.
Discussion
Main results
Our previous study showed that surround suppression, unlike overlay suppression, is tightly tuned to orientation and spatial frequency and is strong only in the periphery. Here, we demonstrate that within these constraints, surround suppression is a robust and ubiquitous phenomenon that does not vary with the shape, phase, or disparity of the surround. We found that the area of the surround in the vicinity of the target is the only important spatial factor. Thus, when the surround was laterally separated from the target, suppression strength decreased exponentially. Also, suppression strength decreased by a factor of 2.7 when the surround area was halved. This indicates that the surround modulates the center response in a nonlinear, accelerating fashion. Although, as we noted above, it is difficult to compare perceived contrast and contrast detection measures, it is nevertheless interesting that Cannon and Fullenkamp (1991) found a similar nonlinear relationship for surround suppression of perceived contrast in the fovea.
Surround phase and layout
Although suppression is tightly tuned to the power spectrum of the surround with respect to the target (its contrast, orientation, and spatial frequency), its phase components are mostly irrelevant. Thus, manipulations of the surround phase and its local spatial layout around the target did not alter suppression strength. In particular, when annulus quadrants were used instead of the full annulus, the position of the quadrants (collinear at Gabor ends vs. parallel at Gabor sides or bilateral bow tie vs. unilateral half annulus) did not have a substantial effect. This result indicates that surround suppression has a simple and locally isotropic spatial organization.
Again, it is difficult to make a direct comparison between our peripheral threshold measures and perceived contrast measurements in the fovea. Nevertheless, both measures show similar spatial properties for surround suppression. The effect of the surround on the perceived contrast is the same for collinear and flanking surrounds (local isotropy; Cannon & Fullenkamp, 1991; Ejima & Takahashi, 1985; Xing & Heeger, 2001). Cannon and Fullenkamp (1991) and Xing and Heeger (2001) found that surround suppression did not depend on the relative phase of center and surround, but Ejima and Takahashi (1985) and Olzak and Laurinen (1999) obtained the opposite result. However, in the latter two studies, there was no gap between the target and the surround. It is likely that a brightness induction effect interferes with contrast judgments for these conditions (for a discussion, see Ejima & Takahashi, 1985; Snowden & Hammett, 1998).
Cavanaugh et al. (2002b) investigated surround suppression in macaque V1 neurons and found that about one third of neurons showed slightly stronger suppression from collinear than from flanking surrounds. However, they also found that these same neurons were the most suppressed. Because we used a detection task, thresholds are likely to reflect the activity of the least suppressed detectors, in which case our data are in good agreement with the suppression properties in macaque V1.
Disparity and eye of origin
Our results demonstrate that the disparity of the surround with respect to the target did not modulate the suppression strength. This result is in agreement with the properties of surround inhibition in cat area 17 (DeAngelis et al., 1994), although in that study, the target patch was monocular; thus, only the surround mask had a well-defined disparity. The insensitivity to relative depth could be explained if the suppression operated monocularly, that is, before signals from the two eyes were combined. Still, the strong interocular suppression revealed in our study, which is also found through single-cell recordings in cats (DeAngelis et al., 1994), argues against such an interpretation. Note that earlier psychophysical studies disagree on the effect of a dichoptic surround. Chubb et al. (1989) found very little dichoptic masking of perceived contrast, whereas Meese and Hess (2004) found the greatest masking in the dichoptic condition.
Global anisotropy
We have presented new experimental evidence for the global anisotropy of surround suppression with respect to the visual field. The radial layout of surround quadrants produced significantly stronger suppression, on average, than the tangential layout. The orientation of the surround mask with respect to the visual field has not previously been considered in physiological and psychophysical studies of surround suppression. For this reason, the global and local aspects of suppression anisotropy could be easily confounded.
It is interesting that Toet and Levi (1992) showed that the same type of anisotropy exists for crowding. Their stimuli consisted of three collinear Ts that were randomly oriented upside down or normal side up. The task was to discriminate the orientation of the middle T. The results show that crowding was significantly stronger when the triad of Ts was oriented radially.
Radial–tangential anisotropies have been found in many psychophysical tasks (e.g., Westheimer, 2005) and, more recently, in fMRI activity in monkeys and humans (Tootell, 2005). Tootell (2005) reported significantly higher fMRI activity in response to radial stimulus orientations as compared with other orientations. Note, however, that it was the orientation of the stimulus that produced the response anisotropy in these studies. In our study, it was the location of the surround with respect to the visual field that modified the suppression strength in Experiment 5, whereas the carrier orientation was the same (vertical) in all conditions.
For both monkeys (Adams & Horton, 2003; Van Essen, Newsome, & Maunsell, 1984) and humans (Schira, Kontsevich, & Tyler, 2005), the cortical magnification factor (cortical distance corresponding to 1 deg of visual field) measured near the vertical meridian is substantially larger when measured radially rather than tangentially. Is it possible that the radial–tangential anisotropy of surround suppression merely reflects the anisotropy of the V1 retinotopic mapping although the surround interactions are actually isotropic in cortical space? If the extent of surround interaction were isotropic in cortex, the observed anisotropy of retinotopic mapping would result in surround interactions being shorter along the radial direction in visual space, which was the opposite of what we found psychophysically. Also, in our data, the radial suppression was stronger along both the vertical and the horizontal meridians, whereas only vertical meridian anisotropy has been observed for cortical mapping. Thus, the anisotropy of surround suppression is not only a mere accident of retinotopic mapping but also a reflection of some important functional properties of surround suppression, as discussed at the end of the Discussion section.
Suppression extent versus eccentricity
Our results show that surround suppression declines exponentially as the separation from the target increases. Additional analysis revealed that whereas suppression amplitude remains constant with stimulus eccentricity, the extent of surround suppression scales in proportion to the eccentricity. Most surprisingly, we discovered that the extent of surround suppression does not scale with respect to stimulus size, spatial frequency, or both (in our experiments, the target’s size and spatial frequency were inversely correlated; thus, size and spatial frequency were confounded).
Although an increase in the extent of suppression with increasing eccentricity is expected from cortical magnification (i.e., assuming the extent to be constant in cortical space), the lack of a similar increase with increasing size is unexpected. Cannon and Fullenkamp (1991) measured suppression produced by a ring-shaped surround grating for various separations between the ring and the test target. Their results also show a sharp decrease in surround suppression with separation, but unlike our results, the extent of suppression was found to scale with the stimulus spatial frequency. However, recall that the Cannon and Fullenkamp study used foveal test targets and perceived contrast measurements, which might account for the difference.
Our results show interesting parallels with crowding, which is known to scale with eccentricity and is similarly unaffected by the stimulus size or spatial frequency (Chung, Levi, & Legge, 2001; Pelli, Palomares, & Majaj, 2004). Note, however, that although the suppression extent did not change, the amplitude of the suppression varied proportionally with the stimulus size (or spatial frequency) in our data. Because a single parameter is used in crowding studies to describe its spatial extent (i.e., critical spacing), it is hard to make a direct comparison here. Still, it is easy to see that because the suppression varies exponentially with extent and linearly with amplitude A, the contribution of the latter to critical spacing is very small.
Surround suppression and crowding
Our objective in this study was not to compare crowding and surround suppression. Nevertheless, the observed similarities merit some discussion. Our present and previous results (Petrov et al., 2005) show that surround suppression and crowding share the following properties: peripheral locus (absent in the fovea), radial–tangential anisotropy, orientation, and spatial frequency tuning, and an extent that depends on eccentricity rather than stimulus size or spatial frequency. Yet, other aspects of surround suppression and crowding do not match. Our results demonstrate that suppression is not tuned to surround phase or to its disparity, whereas Kooi, Toet, Tripathy, and Levi (1994) reported that crowding varies strongly with flankers’ contrast polarity (which corresponds to phase reversal) and to a lesser degree on disparity. Moreover, crowding is commonly observed when test letters and flanking letters have the same contrast, which is at odds with the well-established result showing that surround suppression occurs only when the center is at a lower contrast than the surround (Chubb et al., 1989; Snowden & Hammett, 1998; Xing & Heeger, 2001; Zenger-Landolt & Koch, 2001). Thus, there is more to crowding than surround suppression (for more on this issue, see Levi, Hariharan, & Klein, 2002). Yet, we note that suppression could account for crowding effects reported in studies where a low-contrast target and high-contrast flankers were used.
The role of surround suppression in human vision
The sheer magnitude of surround suppression (up to 300% threshold elevation) makes one wonder what could be its role in visual processing. After all, suppression results in a potential loss of important information. It has been suggested that surround suppression might serve to enhance contrast gain control, contour integration, or boundary detection. We next consider these potential benefits.
Gain control
Local suppression as a contrast gain control mechanism has been proposed not only to explain the nonlinear behavior of cat (Heeger, 1992) and monkey (Carandini, Heeger, & Movshon, 1997) visual neurons but also to account for human contrast sensitivity (Foley, 1994). The standard model assumes that the response of each neuron is divided by a normalizing signal equal to a weighted sum of responses of nearby neurons (divisive normalization). The neurons are usually assumed to have closely positioned receptive fields that can be tuned to different spatial frequencies, orientations, and so forth. Because the model was originally proposed to explain the properties of cross-orientation (overlay) suppression, equal weights were assumed for all the pooled neurons; that is, signals of all orientations would suppress to the same degree.
Later, Schwartz and Simoncelli (2001) proposed a similar rationale for surround suppression, arguing that it would further reduce information redundancy in natural images. In their model, surround suppression acts as a fine-tuned divisive normalization, with larger weights assigned to surround signals having the same orientation as the center. The orientation tuning comes as a result of the model being trained on a set of natural images in which the contrast signal is often locally collinear (along contours). Although the Schwartz and Simoncelli model explains orientation tuning in a satisfactory fashion, it seems to contradict other properties of surround suppression. For example, the model does not explain why suppression is absent in the fovea. If fine-tuned gain control is desirable in the periphery, it is even more useful in central vision, where a significant part visual information is processed. Also, if a collinear signal along the contours is the main reason for orientation tuning, surround suppression should largely come from those segments of the surround that are collinear with the target; that is, it should be locally anisotropic. One would also expect suppression to peak when center and surround have the same disparity because disparity varies smoothly along contours. On the contrary, we found that surround suppression is locally isotropic and does not depend on relative depth.
Contour detection
Unlike gain control, contour detection is a global task (which requires the cooperative activity of many neurons) and therefore is more likely to involve surround interactions. Originally, models based on excitatory interactions were suggested to operate in such a way as to boost the activity of neurons responding to collinear inputs along a contour (Field, Hayes, & Hess, 1993; Parent & Zucker, 1989; Sha’ashua & Ullman, 1988). The interactions flared out along the axis of orientation of a cell satisfying the principle of “good continuation.” Inhibitory interactions suppressing the activity of the cells outside the facilitatory zone were added later to further boost contour detection. Computational models of V1 constructed along these lines used orientation-tuned surround suppression originating from the regions flanking a cell’s receptive field (Li, 1998; Yen & Finkel, 1996). However, the same factors that cast doubt on the gain control explanation of surround suppression apply to contour integration. There is no satisfactory explanation for properties of surround suppression, such as peripheral locus and lack of depth tuning. Besides, the local isotropy of surround suppression strongly contradicts the model’s assumptions. Thus, we saw no sign of facilitation produced either by collinear surrounds or by any surround configuration.
Boundary processing
Like contour integration, boundary processing (texture segmentation) is another basic visual task where the orientation-tuning property of surround suppression could be useful. The reason is that natural boundaries most often occur between textured regions in which a signal of a given orientation is prevalent on either side of the boundary. Boundary detection models that involve surround suppression fall into two classes.
In the first class of models, a kind of iso-oriented “self-suppression” takes place on both sides of the boundary within each orientation channel. Thus, any two iso-oriented inputs (irrespective of their relative strength) positioned within a certain spatial range will evoke inhibitory interactions between corresponding detectors. This will produce response suppression everywhere inside the textured region, yet the net effect will be weaker next to the texture boundary because roughly half of the surround will contain the iso-oriented signal there. Computational modeling shows that such a mechanism will effectively highlight” the boundary with respect to the rest of the stimulus (e.g., Li, 1999).
Because natural boundaries are often associated with an abrupt change in depth, this sort of boundary processing would benefit greatly from surround suppression being tuned to the same disparity as the suppressed signal, which is contrary to our results. Of more importance, mutual suppression in such a model should occur irrespective of signal strength (contrast), which is at odds with the dependence of surround suppression on contrast.
Unlike the first class of models in which the boundary processing operates via a single-layer network of recurrent interactions, models in the second class implement a multilayered feed-forward design (e.g., Malik & Perona, 1990). The essential part of such a model is a two-stage filtering of input signals for each filter channel. The second-order filtering is used to locate the signal discontinuity happening in many channels at the boundary. Surround suppression is used as an auxiliary mechanism that ensures that filters optimally tuned to a given texture (in terms of their orientation, spatial scale, phase, etc.) produce a stronger net response than the remaining (suboptimally tuned) filters. Therefore, in contrast with the class of models discussed above, it is essential for this model that surround suppression operates so that weak, “spurious” responses are inhibited by stronger responses but not vice versa.
In Malik and Perona’s (1990) study, their choice of orientation-tuned suppression was based solely on neuro-physiological evidence, but there is also a functional argument for such specificity. Because the model response to realistic textures involves many different channels, which are all optimal in their own way, indiscriminate suppression between channels would be counterproductive. Note that unlike the first class of models, suppression here is designed to inhibit spurious responses across the texture boundary, instead of operating within each textured region. Accordingly, there is no reason for the surround suppression to be tuned to any particular depth.
Saccades and the range of surround suppression
Following the lines of the Malik and Perona (1990) model, we are proposing that surround suppression can be thought of as a fast “winner-take-all” type of mechanism that participates in the detection of salient features by selectively inhibiting spurious and noisy responses. The suppression would be locally isotropic and phase insensitive, yet would operate narrowly within a given orientation and frequency channel. In addition, we are suggesting that the main purpose of peripheral feature detection is to guide saccadic eye movements. This provides a rationale for the suppression extent to grow with eccentricity, but it should be independent of the target’s spatial frequency. The interplay between salient feature detection and eye movements has been explored in recent computational models of visual attention (for a review, see Itti & Koch, 2001).
If surround suppression was used solely to extract some ecologically important features (such as contours and boundaries), the interaction extent should scale with spatial frequency because natural images are roughly scale invariant (e.g., Ruderman, 1994). Indeed, models of spatial interactions routinely make this assumption. However, for a crude “clean-up” mechanism, spatial frequency becomes immaterial: A narrow range would be ineffective, whereas a broad range would come at a cost of impaired spatial resolution, regardless of the target’s spatial frequency.
Clearly, the optimal suppression extent would be determined by the required precision of the task at hand. We suggest that the effect of stimulus eccentricity on surround suppression can be explained if its main purpose is to select salient peripheral targets (e.g., object boundaries) for a subsequent saccade. Then, the extent of suppression should be determined by the precision of a first saccade. Because the inaccuracy of a first saccade is known to be proportional to the target eccentricity (for a review, see Becker, 1991), the optimal suppression extent will also increase proportionally to the target eccentricity, irrespective of the target spatial frequency. In fact, saccade inaccuracy matches the suppression radius measured in Experiment 6 quite well: Both are about 10% of the target eccentricity. Moreover, saccade inaccuracy in the radial direction is about twice as large as in the orthogonal direction (Deubel, 1987; Van Opstal & Van Gisbergen, 1989). This is close to the 1.7 ratio of radial to tangential suppression strengths in our data.
Note that there is no similar reason for the suppression amplitude to vary with eccentricity. Instead, the amplitude might correlate with the visual system’s contrast sensitivity, peaking at the optimal spatial frequency for a given eccentricity, where the signal is likely to be the strongest. The two frequencies tested in Experiment 6 (1.3 and 2.7 cpd) are above the optimal frequency for all three tested eccentricities, which would explain why the suppression amplitude decreased as spatial frequency increased from 1.3 to 2.7 cpd in this experiment.
Conclusions
Various spatial aspects of surround suppression were studied using a contrast detection task. The results demonstrate that the suppression is rather insensitive to the spatial layout of the surround mask around the target. Thus, manipulations with the surround phase, disparity, eye of origin, and the mask shape (collinear vs. flanking, unilateral vs. bilateral) had no significant effect. The only exception was visual field anisotropy: A radial mask suppressed significantly more than a tangential mask. Otherwise, the area of the surround mask and its separation from the target were the only important spatial aspects of the surround interaction. We found that suppression falls exponentially as separation increases and that the extent of suppression grows linearly with stimulus eccentricity. Our most surprising finding was that the stimulus size (or spatial frequency) does not affect the extent of the suppression. Based on these and previous findings, we suggest that surround suppression can be used to determine salient peripheral locations for saccadic eye movements.
Acknowledgments
We would like to thank Dr. Matteo Carandini, Dr. Preeti Verghese, Dr. Loura Renninger, and Dr. Mark Schira for helpful discussions. This work was supported by National Institutes of Health (NIH) National Eye Institute Grant 06644 (S.M.) and by an NIH National Eye Institute Institutional National Service Award (Y.P.).
Footnotes
Commercial relationships: none.
Address: The Smith-Kettlewell Eye Research Institute, San Francisco, CA 94115, USA.
Contributor Information
Yury Petrov, The Smith-Kettlewell Eye Research Institute, San Francisco, CA, USA.
Suzanne P. McKee, The Smith-Kettlewell Eye Research Institute, San Francisco, CA, USA
References
- Adams DL, Horton JC. A precise retinotopic map of primate striate cortex generated from the representation of angioscotomas. The Journal of Neuroscience. 2003;23:3771–3789. doi: 10.1523/JNEUROSCI.23-09-03771.2003. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allman J, Miezin F, McGuinness E. Stimulus specific responses from beyond the classical receptive field: Neurophysiological mechanisms for local-global comparisons in visual neurons. Annual Review of Neuroscience. 1985;8:407–430. doi: 10.1146/annurev.ne.08.030185.002203. [PubMed] [DOI] [PubMed] [Google Scholar]
- Andriessen JJ, Bouma H. Eccentric vision: Adverse interactions between line segments. Vision Research. 1976;16:71–78. doi: 10.1016/0042-6989(76)90078-x. [PubMed] [DOI] [PubMed] [Google Scholar]
- Becker, W. (1991). Saccades. In R. H. S. Carpenter (Ed.), Vision and visual dysfunction: Eye movements (Vol. 8, pp. 95–137). London: The Macmillan Press Ltd.
- Cannon MW, Fullenkamp SC. Spatial interactions in apparent contrast: Inhibitory effects among grating patterns of different spatial frequencies, spatial positions and orientations. Vision Research. 1991;31:1985–1998. doi: 10.1016/0042-6989(91)90193-9. [PubMed] [DOI] [PubMed] [Google Scholar]
- Carandini, M. (2004). Receptive and suppressive fields in the early visual system. In Cognitive Neurosciences (3rd ed.). Cambridge, MA: MIT press.
- Carandini M, Heeger DJ, Movshon JA. Linearity and normalization in simple cells of the macaque primary visual cortex. The Journal of Neuroscience. 1997;17:8621–8644. doi: 10.1523/JNEUROSCI.17-21-08621.1997. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh JR, Bair W, Movshon JA. Nature and interaction of signals from the receptive field center and surround in macaque V1 neurons. Journal of Neurophysiology. 2002a;88:2530–2546. doi: 10.1152/jn.00692.2001. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Cavanaugh JR, Bair W, Movshon JA. Selectivity and spatial distribution of signals from the receptive field surround in macaque V1 neurons. Journal of Neurophysiology. 2002b;88:2547–2556. doi: 10.1152/jn.00693.2001. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Chubb C, Sperling G, Solomon JA. Texture interactions determine perceived contrast. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:9631–9635. doi: 10.1073/pnas.86.23.9631. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung ST, Levi DM, Legge GE. Spatial-frequency and contrast properties of crowding. Vision Research. 2001;41:1833–1850. doi: 10.1016/s0042-6989(01)00071-2. [PubMed] [DOI] [PubMed] [Google Scholar]
- DeAngelis GC, Freeman RD, Ohzawa I. Length and width tuning of neurons in the cat’s primary visual cortex. Journal of Neurophysiology. 1994;71:347–374. doi: 10.1152/jn.1994.71.1.347. [PubMed] [DOI] [PubMed] [Google Scholar]
- Deubel, H. (1987). Adaptivity of gain and direction in oblique saccades. In J. K. O’Regan & A. Levy-Schoen (Eds.), Eye movements. From physiology to cognition (pp. 181–190). Amsterdam: Elsevier.
- Ejima Y, Takahashi S. Apparent contrast of a sinusoidal grating in the simultaneous presence of peripheral gratings. Vision Research. 1985;25:1223–1232. doi: 10.1016/0042-6989(85)90036-7. [PubMed] [DOI] [PubMed] [Google Scholar]
- Field DJ, Hayes A, Hess RF. Contour integration by the human visual system: Evidence for a local “association field.”. Vision Research. 1993;33:173–193. doi: 10.1016/0042-6989(93)90156-q. [PubMed] [DOI] [PubMed] [Google Scholar]
- Foley JM. Human luminance pattern-vision mechanisms: Masking experiments require a new model. Journal of the Optical Society of America A, Optics, Image Science, and Vision. 1994;11:1710–1719. doi: 10.1364/josaa.11.001710. [PubMed] [DOI] [PubMed] [Google Scholar]
- Heeger DJ. Normalization of cell responses in cat striate cortex. Visual Neuroscience. 1992;9:181–197. doi: 10.1017/s0952523800009640. [PubMed] [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields and functional architecture in two nonstriate visual areas (18 and 19) of the cat. Journal of Neurophysiology. 1965;28:229–289. doi: 10.1152/jn.1965.28.2.229. [PubMed] [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields and functional architecture of monkey striate cortex. Journal of Physiology. 1968;195:215–243. doi: 10.1113/jphysiol.1968.sp008455. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itti L, Koch C. Computational modelling of visual attention. Nature Reviews: Neuroscience. 2001;2:194–203. doi: 10.1038/35058500. [PubMed] [DOI] [PubMed] [Google Scholar]
- Kontsevich LL, Tyler CW. Bayesian adaptive estimation of psychometric slope and threshold. Vision Research. 1999;39:2729–2737. doi: 10.1016/s0042-6989(98)00285-5. [PubMed] [DOI] [PubMed] [Google Scholar]
- Kooi FL, Toet A, Tripathy SP, Levi DM. The effect of similarity and duration on spatial interaction in peripheral vision. Spatial Vision. 1994;8:255–279. doi: 10.1163/156856894x00350. [PubMed] [DOI] [PubMed] [Google Scholar]
- Levi DM, Hariharan S, Klein SA. Suppressive and facilitatory spatial interactions in peripheral vision: Peripheral crowding is neither size invariant nor simple contrast masking. Journal of Vision. 2002;2(2):167–177. doi: 10.1167/2.2.3. http://journalofvision.org/2/2/3/, doi:10.1167/2.2.3. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Li Z. A neural model of contour integration in the primary visual cortex. Neural Computation. 1998;10:903–940. doi: 10.1162/089976698300017557. [PubMed] [DOI] [PubMed] [Google Scholar]
- Li Z. Visual segmentation by contextual influences via intra-cortical interactions in the primary visual cortex. Network. 1999;10:187–212. [PubMed] [PubMed] [Google Scholar]
- Malik J, Perona P. Preattentive texture discrimination with early vision mechanisms. Journal of the Optical Society of America A, Optics, and Image Science. 1990;7:923–932. doi: 10.1364/josaa.7.000923. [PubMed] [DOI] [PubMed] [Google Scholar]
- Meese TS, Hess RF. Low spatial frequencies are suppressively masked across spatial scale, orientation, field position, and eye of origin. Journal of Vision. 2004;4(10):843–859. doi: 10.1167/4.10.2. http://journalofvision.org/4/10/2/, doi:10.1167/4.10.2. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Olzak LA, Laurinen PI. Multiple gain control processes in contrast–contrast phenomena. Vision Research. 1999;39:3983–3987. doi: 10.1016/s0042-6989(99)00131-5. [PubMed] [DOI] [PubMed] [Google Scholar]
- Olzak LA, Laurinen PI. Contextual effects in fine spatial discriminations. Journal of the Optical Society of America A, Optics, Image Science, and Vision. 2005;22:2230–2238. doi: 10.1364/josaa.22.002230. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent P, Zucker SW. Trace inference, curvature consistency, and curve detection. IEEE Transactions on Pattern Analysis and Machine Intelligence. 1989;11:823–839. [Google Scholar]
- Pelli DG, Palomares M, Majaj NJ. Crowding is unlike ordinary masking: Distinguishing feature integration from detection. Journal of Vision. 2004;4(12):1136–1169. doi: 10.1167/4.12.12. http://journalofvision.org/4/12/12/, doi:10.1167/4.12.12. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Petrov Y, Carandini M, McKee S. Two distinct mechanisms of suppression in human vision. The Journal of Neuroscience. 2005;25:8704–8707. doi: 10.1523/JNEUROSCI.2871-05.2005. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrov Y, Verghese P, McKee SP. Collinear facilitation is largely uncertainty reduction. Journal of Vision. 2006;6(2):170–178. doi: 10.1167/6.2.8. http://journalofvision.org/6/2/8/, doi:10.1167/6.2.8. [PubMed] [Article]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polat U, Sagi D. Lateral interactions between spatial channels: Suppression and facilitation revealed by lateral masking experiments. Vision Research. 1993;33:993–999. doi: 10.1016/0042-6989(93)90081-7. [PubMed] [DOI] [PubMed] [Google Scholar]
- Polat U, Sagi D. The architecture of perceptual spatial interactions. Vision Research. 1994;34:73–78. doi: 10.1016/0042-6989(94)90258-5. [PubMed] [DOI] [PubMed] [Google Scholar]
- Ruderman DL. The statistics of natural images. Network. 1994;5:517–548. [Google Scholar]
- Schira MM, Kontsevich LL, Tyler CW. Geometric and metric properties of visual areas V1 and V2 in humans [Abstract] Journal of Vision. 2005;5(8):897a. http://www.journalofvision.org/5/8/897/, doi:10.1167/5.8.897. [Google Scholar]
- Schwartz O, Simoncelli EP. Natural signal statistics and sensory gain control. Nature Neuroscience. 2001;4:819–825. doi: 10.1038/90526. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Sha’ashua, A., & Ullman, S. (1988, December 5–8). Structural saliency: The detection of globally salient structures using a locally connected network (pp. 321–327). International Conference on Computer Vision (ICCV), Tampa, Florida.
- Snowden RJ, Hammett ST. The effects of surround contrast on contrast thresholds, perceived contrast and contrast discrimination. Vision Research. 1998;38:1935–1945. doi: 10.1016/s0042-6989(97)00379-9. [PubMed] [DOI] [PubMed] [Google Scholar]
- Solomon JA, Morgan MJ. Facilitation from collinear flanks is cancelled by non-collinear flanks. Vision Research. 2000;40:279–286. doi: 10.1016/s0275-5408(99)00059-9. [PubMed] [DOI] [PubMed] [Google Scholar]
- Toet A, Levi DM. The two-dimensional shape of spatial interaction zones in the parafovea. Vision Research. 1992;32:1349–1357. doi: 10.1016/0042-6989(92)90227-a. [PubMed] [DOI] [PubMed] [Google Scholar]
- Tootell R. A very different slant on orientation sensitivity in human and non-human primates [Abstract] Journal of Visual. 2005;5(12):43a. http://journalofvision.org/5/12/43, doi:10.1167/5.12.43. [Google Scholar]
- Van Essen DC, Newsome WT, Maunsell JH. The visual field representation in striate cortex of the macaque monkey: Asymmetries, anisotropies, and individual variability. Vision Research. 1984;24:429–448. doi: 10.1016/0042-6989(84)90041-5. [PubMed] [DOI] [PubMed] [Google Scholar]
- Van Opstal AJ, Van Gisbergen JA. Scatter in the metrics of saccades and properties of the collicular motor map. Vision Research. 1989;29:1183–1196. doi: 10.1016/0042-6989(89)90064-3. [PubMed] [DOI] [PubMed] [Google Scholar]
- Walker GA, Ohzawa I, Freeman RD. Asymmetric suppression outside the classical receptive field of the visual cortex. The Journal of Neuroscience. 1999;19:10536–10553. doi: 10.1523/JNEUROSCI.19-23-10536.1999. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker GA, Ohzawa I, Freeman RD. Disinhibition outside receptive fields in the visual cortex. The Journal of Neuroscience. 2002;22:5659–5668. doi: 10.1523/JNEUROSCI.22-13-05659.2002. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb BS, Tinsley CJ, Barraclough NE, Parker A, Derrington AM. Gain control from beyond the classical receptive field in primate primary visual cortex. Visual Neuroscience. 2003;20:221–230. doi: 10.1017/s0952523803203011. [PubMed] [DOI] [PubMed] [Google Scholar]
- Westheimer G. Anisotropies in peripheral vernier acuity. Spatial Vision. 2005;18:159–167. doi: 10.1163/1568568053320611. [PubMed] [DOI] [PubMed] [Google Scholar]
- Williams CB, Hess RF. Relationship between facilitation at threshold and suprathreshold contour integration. Journal of the Optical Society of America A, Optics, Image Science, and Vision. 1998;15:2046–2051. doi: 10.1364/josaa.15.002046. [PubMed] [DOI] [PubMed] [Google Scholar]
- Xing J, Heeger DJ. Center-surround interactions in foveal and peripheral vision. Vision Research. 2000;40:3065–3072. doi: 10.1016/s0042-6989(00)00152-8. [PubMed] [DOI] [PubMed] [Google Scholar]
- Xing J, Heeger DJ. Measurement and modeling of center-surround suppression and enhancement. Vision Research. 2001;41:571–583. doi: 10.1016/s0042-6989(00)00270-4. [PubMed] [DOI] [PubMed] [Google Scholar]
- Yen, S.-C., & Finkel, L. H. (1996). Salient contour extraction by temporal binding in a cortically-based network. In D. Touretzky, M. C. Mozer, & M. E. Hasselmo (Eds.), Advances in neural information processing systems Boston: MIT Press.
- Yu C, Klein SA, Levi DM. Facilitation of contrast detection by cross-oriented surround stimuli and its psychophysical mechanisms. Journal of Vision. 2002;2(3):243–255. doi: 10.1167/2.3.4. http://journalofvision.org/2/3/4/, doi:10.1167/2.3.4. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Zenger-Landolt B, Koch C. Flanker effects in peripheral contrast discrimination—Psychophysics and modeling. Vision Research. 2001;41:3663–3675. doi: 10.1016/s0042-6989(01)00175-4. [PubMed] [DOI] [PubMed] [Google Scholar]


