Abstract
Background
Experimental and observational data suggest that micronutrients with antioxidant capabilities may retard the development of age-related cataract.
Objective
To evaluate the effect of a high-dose anti-oxidant formulation on the development and progression of age-related lens opacities and visual acuity loss.
Design
The 11-center Age-Related Eye Disease Study (AREDS) was a double-masked clinical trial. Participants were randomly assigned to receive daily oral tablets containing either antioxidants (vitamin C, 500 mg; vitamin E, 400 IU; and beta carotene, 15 mg) or no antioxidants. Participants with more than a few small drusen were also randomly assigned to receive tablets with or without zinc (80 mg of zinc as zinc oxide) and copper (2 mg of copper as cupric oxide) as part of the age-related macular degeneration trial. Baseline and annual (starting at year 2) lens photographs were graded at a reading center for the severity of lens opacities using the AREDS cataract grading scale.
Main Outcome Measures
Primary outcomes were (1) an increase from baseline in nuclear, cortical, or posterior subcapsular opacity grades or cataract surgery, and (2) at least moderate visual acuity loss from baseline (≥15 letters). Primary analyses used repeated-measures logistic regression with a statistical significance level of P = .01. Serum level measurements, medical histories, and mortality rates were used for safety monitoring.
Results
Of 4757 participants enrolled, 4629 who were aged from 55 to 80 years had at least 1 natural lens present and were followed up for an average of 6.3 years. No statistically significant effect of the antioxidant formulation was seen on the development or progression of age-related lens opacities (odds ratio=0.97, P=.55). There was also no statistically significant effect of treatment in reducing the risk of progression for any of the 3 lens opacity types or for cataract surgery. For the 1117 participants with no age-related macular degeneration at baseline, no statistically significant difference was noted between treatment groups for at least moderate visual acuity loss. No statistically significant serious adverse effect was associated with treatment.
Conclusion
Use of a high-dose formulation of vitamin C, vitamin E, and beta carotene in a relatively well-nourished older adult cohort had no apparent effect on the 7-year risk of development or progression of age-related lens opacities or visual acuity loss.
The fact that oxidative damage of lens proteins is a prominent feature of cataract development1,2 has led to speculation that micro-nutrients with antioxidant capabilities, such as vitamin C (ascorbic acid), vitamin E, and the carotenoids, may retard cataract development.3 However, retrospective, cross-sectional, and prospective epidemiological studies of cataract and intake or blood levels of antioxidant nutrients have not produced consistent results.4–27 Most studies with published findings have noted protective associations for various nutrients, but there is no consensus about the specific nutrient(s) that may be involved or the specific type of cataract(s) that might be affected. A major concern in interpreting the results of observational epidemiological studies of micronutrient intake and cataract risk is the possibility of unadjusted confounding. A high degree of correlation between intake levels of various nutrients makes it difficult to identify which of many candidate nutrients might “explain” any observed associations. Confounding could also result if persons with better nutritional status are different from others in unrecognized ways that affect the risk of cataract.
Problems caused by confounding and bias are of less concern in randomized clinical trials, but only limited and inconsistent data are available from such trials about the effect of nutritional supplements on cataract development. In 2 cancer prevention trials of nutritional supplements, end-of-study eye examinations were conducted to assess the effect of the supplements on cataract prevalence.28,29 One noted no effect of either vitamin E or beta carotene on cataract prevalence after a median supplementation time of 6.6 years28; the other, conducted in a nutritionally deprived population, noted a beneficial effect for nuclear cataract of multivitamin and mineral supplements and of niacin and riboflavin after 5 to 6 years of supplementation.29 A large randomized trial of US male physicians noted no effect on cataract incidence or cataract extraction after 13 years of beta carotene use.30 A smaller population-based randomized trial found no effect of vitamin E on the 4-year progression of nuclear or cortical lens opacities or cataract extraction.31 Given the inherent limitations of observational studies and the scarcity of available clinical trial data, clinical trials of sufficient size and duration are needed before recommendations can be made about the effect of nutritional supplements on the risk of cataract. Recommendations from clinical trials about the use of high-dose supplements would be especially useful because such supplements are readily available, increasingly used for many conditions including cataract, and mostly untested for safety and efficacy.32
The Age-Related Eye Disease Study (AREDS) is an ongoing multicenter study of the natural history of age-related cataract and macular degeneration (AMD).33 The study includes a completed randomized clinical trial to evaluate the effect of the antioxidants vitamin C, vitamin E, and beta carotene in combination on the development or progression of age-related lens opacities, and the effect of both the antioxidants and high doses of zinc on the progression to advanced AMD. The vitamins were tested because of preliminary data suggesting that micronutrients with antioxidant characteristics might protect against both cataract and AMD. Zinc was included because of its hypothesized effect on the progression of AMD, but its inclusion in the trial also permits an evaluation of its effect on cataract development. This article reports whether high-dose supplementation with vitamins having antioxidant characteristics (vitamin C, vitamin E, and beta carotene) affected the development or progression of age-related lens opacities in AREDS participants.
RESULTS
ENROLLMENT AND PARTICIPANT CHARACTERISTICS
Thirty-three of the 4629 participants enrolled in the clinical trial of cataract had no annual photographic or visual acuity follow-up after randomization in an AREDS clinic. There is a good balance of characteristics between treatment groups (Table 2). Fifty-six percent of the participants were female, 96% were white, and the median age was 68 years. At baseline 8% were current cigarette smokers and 66% chose to take Centrum. After accounting for age, sex, and race, participants in AREDS had higher or similar dietary intake of vitamins A, C, and E and zinc than the general population sample from the Third National Health and Nutrition Examination Survey (data not shown).42 Baseline dietary intake of the study nutrients was balanced by treatment.
Table 2.
Baseline Characteristics by Treatment Group*
|
Participants Who Received |
|||
|---|---|---|---|
| Participant Characteristics | No Antioxidants, % (n = 2310) | Antioxidants, % (n = 2286) | Total, % (n = 4596) |
| Age, y | |||
| 55–64 | 22 | 24 | 23 |
| 65–74 | 63 | 63 | 63 |
| 75–80 | 14 | 13 | 14 |
| Median | 69 | 68 | 68 |
| Female | 56 | 55 | 56 |
| Race | |||
| White | 96 | 96 | 96 |
| Black | 4 | 4 | 4 |
| Other | < 1 | < 1 | < 1 |
| AMD Category | |||
| 1 and 2 | 48 | 46 | 47 |
| 3 | 33 | 34 | 34 |
| 4 | 19 | 20 | 19 |
| Nuclear opacity score | |||
| < 2.0 | 35 | 39 | 37 |
| 2.0–3.9 | 49 | 47 | 48 |
| ≥ 4.0 | 15 | 15 | 15 |
| Cortical opacity, % of area | |||
| < 0.1 | 47 | 49 | 48 |
| 0.1–4.9 | 42 | 40 | 41 |
| ≥5.0 | 12 | 11 | 11 |
| PCS opacity, % of area | |||
| < 0.1 | 90 | 90 | 90 |
| 0.1–4.9 | 8 | 7 | 8 |
| ≥5.0 | 2 | 2 | 2 |
| Currently smoking | 7 | 8 | 8 |
| Former smoker | 48 | 47 | 48 |
| Chose to take Centrum at enrollment† | 67 | 65 | 66 |
| Taking multivitamins or a supplement containing a study medication | 56 | 54 | 55 |
| Taking insulin or pills for diabetes mellitus | 6 | 6 | 6 |
| Taking medication to control cholesterol and/or lipid levels | 9 | 9 | 9 |
| Taking aspirin | 40 | 37 | 38 |
| Taking antacids | 12 | 11 | 11 |
| Taking medication for hypertension | 32 | 32 | 32 |
| Diagnosis of angina | 10 | 10 | 10 |
| Prior diagnosis of cancer | 18 | 18 | 18 |
Data are given as a percentage unless otherwise indicated for all participants with follow-up information. AMD indicates age-related macular degeneration; PSC, posterior subcapsular.
The multivitamin and mineral supplement was Centrum (Whitehall-Robins Healthcare, Madison, NJ).
DATA QUALITY
About 2.3% of participants were lost to follow-up (missed at least their last 2 consecutive visits). The rate of participant withdrawal from study medication was 14% by 60 months and 15% by the end of the trial. These rates include participants lost to follow-up and current smokers, 24% of whom withdrew from study medication after the results from the clinical trials of beta carotene and lung cancer were announced.40,41 Figure 3 shows the number of participants with follow-up and adherence to the study medication regimen by year of follow-up. Overall, adherence was estimated to be 75% or greater (ie, participants took ≥ 75% of their study tablets) for 70% of the participants at 5 years. At 60 months, 20% of the participants (20% both for current smokers and former or nonsmokers) reported taking some multivitamin supplement containing at least 1 of the study medication ingredients in addition to their assigned study medication and Centrum. Less than 0.1% of the participants were reported to have been unmasked during the trial. About 1 (15%) of 7 participants did not have a set of photographs taken in the last year of the trial, and only 1 of every 11 opportunities for annual photographs (starting at the second annual visit) did not yield any photographs. Of more than 62000 possible follow-up visits, 9% were missed. Mean follow-up time (6.3 years) did not differ by treatment. Most participants (90%) had at least 5 years of follow-up.
Figure 3.
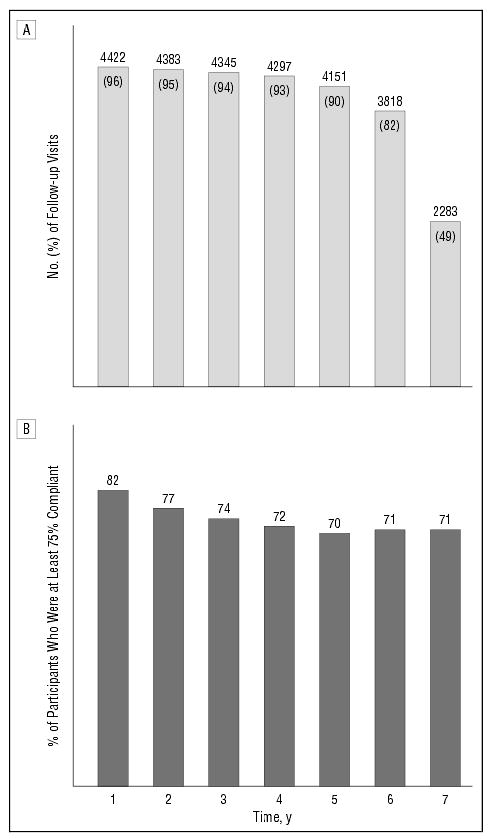
Participant follow-up and adherence, by year in study. A, Number of participants with follow-up visits and percentage of total enrolled (n=4629). B, Percentage of participants taking at least 75% of their study tablets.
The network of collaborating physicians provided data for 50 annual visits and 11 nonannual visits made by 34 participants. The results reported do not include these data, although inclusion of this information had no discernible effect on results.
PHOTOGRAPHIC QUALITY
Slitlamp and retroillumination photographs of the lens taken during the clinical trial were judged by the reading center to be of gradable quality 98% and 99.3% of the time, respectively, during the entire study period.
PRIMARY OUTCOMES
Progression of Lens Opacity or Cataract Surgery
Figure 4 shows repeated-measures estimates of the probability of any lens event over time by treatment. The estimated probability of an event at 5 years is 30% for participants regardless of treatment. Of the 2286 participants with follow-up assigned to an antioxidant treatment, 756 (33%) had a primary lens event within 5 years. First events included 127 nuclear opacity only events, 17 cortical opacity only events, 12 PSC opacity only events, 113 cataract surgical procedures, and 487 events of mixed type. Of the 2310 participants with follow-up assigned to a nonantioxidant treatment, 785 (34%) had a lens event by 5 years. First events included 124 nuclear opacity only events, 17 cortical opacity only events, 12 PSC opacity only events, 147 cataract surgical procedures, and 485 events of mixed type.
Figure 4.
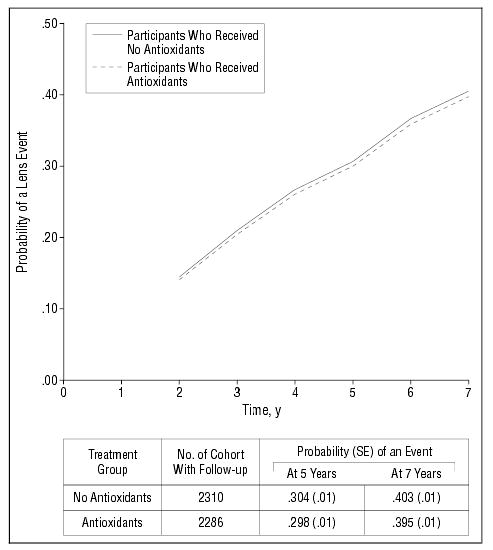
Repeated-measures logistic regression estimates of the probability of any lens event in at least 1 study eye by antioxidant-treated group (all participants). Study eye is an eye without cataract surgery at baseline. Persons with bilateral aphakia are excluded from this analysis. Events before year 2 reflect only cataract surgery.
Treatment effects, estimated by repeated-measures logistic regression, on an increase in lens opacity grade (nuclear, cortical, or PSC opacities) or cataract surgery are listed in Table 3. Participants taking antioxidant treatments did not differ in the risk of developing a lens event from participants not taking antioxidant treatments (odds ratio [OR]=0.97, P=.55). An analysis adjusted for age, sex, race, smoking status, and AMD category did not materially alter the size or direction of these estimates. The results from the Cox proportional hazards survival model are consistent with the repeated-measures analysis (data not shown).
Table 3.
Effect of Treatment on Risk of Any Lens Event*
| Treatment Group | No. of Participants | No. of Events | OR (99% CI) | PValue |
|---|---|---|---|---|
| Antioxidants vs no antioxidants | 4596 | 2230 | ||
| Unadjusted | . . . | . . . | 0.97 (0.84–1.11) | .55 |
| Adjusted† | . . . | . . . | 1.00 (0.87–1.15) | .96 |
Any lens event indicates cataract surgery or change in opacity from baseline of 1.5 U (nuclear), 10% area of a standard central 5-mm lens (cortical), or 5% area of a standard central 5-mm lens (posterior subcapsular). OR indicates odds ratio; CI, confidence interval; and ellipses, does not apply. Analysis using repeated-measures logistic regression.
Value is adjusted for age group, race, sex, baseline smoking status, and age-related macular degeneration category.
Analyses of a possible zinc effect were done on persons enrolled in the AMD trial. In unadjusted and adjusted repeated-measures analyses, participants taking zinc did not differ in risk of developing a lens event from participants not taking zinc (data not shown).
Visual Acuity
Figure 5 shows repeated-measures estimates of the probability of visual acuity loss of 15 letters or more in at least1 eye over time, by treatment, for the 1117 participants without AMD (AMD Category 1) at enrollment. Restriction to these participants should avoid any confounding effect of AMD on visual acuity. Treatment effects are tested using repeated-measures logistic regression (Table 4). No difference was noted between the groups for loss of 15 or more letters in visual acuity score compared with base-line measurement (OR=1.03, P=.89). Results from an analysis of mean change in visual acuity (data not shown) are consistent with results from the repeated-measures analysis.
Figure 5.
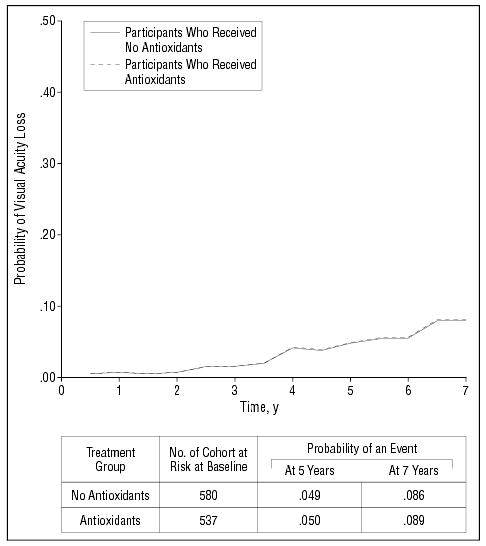
Repeated-measures logistic regression estimates of the probability of a loss in visual acuity score of at least 15 letters in at least 1 study eye by antioxidant-treated group (AMD Category 1 participants only).
Table 4.
Effect of Treatment on Risk of Loss of Visual Acuity Score of 15 Letters or More From Baseline (Participants Without Age-Related Macular Degeneration Only)*
| Treatment Group | No. of Participants | No. of Events | OR (99% CI) | PValue |
|---|---|---|---|---|
| Antioxidants vs placebo | 1111 | 172 | ||
| Unadjusted | . . . | . . . | 1.03 (0.63–1.66) | .89 |
| Adjusted† | . . . | . . . | 1.07 (0.66–1.72) | .73 |
OR indicates odds ratio; CI, confidence interval; and ellipses, does not apply. Analysis using repeated-measures logistic regression.
Value is adjusted for age group, race, sex, and smoking status.
SECONDARY OUTCOMES
Nuclear Opacity
Analyses of each type of a lens event—nuclear opacity, cortical opacity, PSC opacity, and cataract surgery—were performed. Because cataract surgery ends type-specific follow-up of progression of opacity (informative censoring), lens events for each opacity type included cataract surgery. The results are summarized in Table 5. Participants assigned to antioxidants treatment were as likely to experience a nuclear opacity event as participants assigned to no antioxidant treatments (OR=0.98, P=.71). Participants taking the antioxidants-only treatment were also as likely to experience a nuclear opacity event as those taking placebo (OR = 1.00, P = .97). An analysis of mean change in nuclear opacity score, unadjusted for informative censoring, finds results consistent with the repeated-measures analysis. The same analysis was performed for cortical and PSC opacity scores and also found no treatment differences.
Table 5.
Effect of Treatment on Risk of a Lens Event by Type of Event*
| Treatment Group† | No. of Participants | No. of Events | OR (99% CI) | PValue |
|---|---|---|---|---|
| Nuclear event | ||||
| Antioxidants vs no antioxidants | 4331 | 1674 | 0.98 (0.84–1.14) | .71 |
| Antioxidants only vs placebo | 2715 | 1027 | 1.00 (0.82–1.22) | .97 |
| Cortical event | ||||
| Antioxidants vs no antioxidants | 4329 | 1058 | 0.99 (0.82–1.19) | .84 |
| Antioxidants only vs placebo | 2715 | 625 | 0.91 (0.71–1.15) | .29 |
| Posterior subcapsular event | ||||
| Antioxidants vs no antioxidants | 4329 | 888 | 0.94 (0.78–1.14) | .39 |
| Antioxidants only vs placebo | 2715 | 535 | 0.91 (0.70–1.17) | .33 |
| Cataract surgery | ||||
| Antioxidants vs no antioxidants | 4596 | 675 | ||
| Unadjusted | . . . | . . . | 0.94 (0.77–1.14)‡ | .41 |
| Adjusted§ | . . . | . . . | 0.97 (0.80–1.19)‡ | .74 |
Analysis using repeated-measures logistic regression, except by Cox proportional hazards survival analysis for cataract surgery. OR indicates odds ratio; CI, confidence interval; RR, relative risk; and ellipses, does not apply.
Nuclear event indicates a change in opacity from baseline of 1.5 U; cortical event, a change from baseline of 10% of the area of a standard central 5-mm circle; posterior subcapsular event, a change from baseline of 5% of the area of a standard central 5-mm circle.
Value is given as relative risk (99% CI).
Value is adjusted for age group, race, sex, baseline smoking status, and age-related macular degeneration.
Cortical and PSC Opacities
Participants taking antioxidants were as likely to experience a cortical event as were those assigned to no antioxidant treatments (OR=0.99, P=.84). Restricting the analysis to antioxidants only vs placebo, the risk of cortical opacity decreased for antioxidants relative to placebo, but not significantly (OR=0.91, P=.29).
Participants taking antioxidants showed no significant change in the risk of a PSC event (OR=0.94, P=.39). Restricting the analysis to antioxidants only vs placebo yielded similar results.
Cataract Surgery
No significant difference was noted between persons taking and not taking antioxidants in the incidence of cataract surgery by Cox proportional hazards survival analysis (relative risk=0.94, P=.41).
More Severe Lens Opacity Progression or Cataract Surgery
An analysis of a more severe lens event (≥ 2.5-U increase for nuclear, ≥ 20% increase for cortical or PSC opacities, or cataract surgery) is presented in Table 6. Participants assigned to antioxidant treatments did not differ significantly in the risk of experiencing a more severe lens event from participants not assigned to anti-oxidant treatments (OR=0.92, P=.27).
Table 6.
Effect of Treatment on Risk of Any Severe Lens Event*
| Treatment Group | No. of Participants | No. of Events | OR (99% CI) | PValue |
|---|---|---|---|---|
| Antioxidants vs no antioxidants | 4596 | 991 | . . . | . . . |
| Unadjusted | . . . | . . . | 0.92 (0.76–1.12) | .27 |
| Adjusted† | . . . | . . . | 0.95 (0.78–1.15) | .48 |
Any severe lens event indicates cataract surgery or a change in opacity from baseline of 2.5 U or more (nuclear), of 20% or more of the area of a standard central 5-mm circle (cortical), or of 20% or more of the area of a standard central 5-mm circle (posterior subcapsular). Analysis using repeated-measures logistic regression. OR indicates odds ratio; CI, confidence interval; and ellipses, does not apply.
Value is adjusted for age group, race, sex, baseline smoking status, and age-related macular degeneration.
Lens Events in Eyes Without Opacities
In the subset of 823 participants with no or minimal opacity in at least 1 eye at baseline (nuclear, < 1.5 U; cortical, ≤ 5%; and PSC, ≤ 5%), there was no significant effect of treatment on risk of developing lens events in these eyes (OR=0.85, 99% confidence interval, 0.55–1.33). Results were similar in the smaller subset of 338 participants with no or minimal opacity in both eyes at baseline, OR=0.66 (99% confidence interval, 0.33–1.33). (Data not shown.)
ADHERENCE
Serum Levels
Serum levels of micronutrients were measured at 3 AREDS clinics to monitor adherence to the treatment regimens. Table 7 provides median baseline values and median percentage of change from baseline at year 1 and year 5 for up to 906 participants (86% of those alive at 5 years) for each of the study ingredients and also for vitamin A, α-carotene, β-cryptoxanthin, lutein and zeaxanthin, and lycopene. Serum levels of each are presented separately for the antioxidant-treated and no antioxidant-treated groups.
Table 7.
Serum Values at Baseline and Median Percent Change at Follow-up Years 1 and 5
|
Median % Change |
|||||||
|---|---|---|---|---|---|---|---|
|
Baseline Median |
At Year 1 |
At Year 5 |
|||||
| Specimen | No. of Participants | No Antioxidants | Antioxidants | No Antioxidants | Antioxidants | No Antioxidants | Antioxidants |
| Vitamin C, mg/dL*† | 879 | 1.1 | 1.0 | −9 | 25 | −10 | 14 |
| Vitamin E–cholesterol ratio‡ | 900 | 6.5 | 6.2 | −1 | 83 | 5 | 82 |
| Beta carotene, μg/dL* | 900 | 24 | 27 | 4 | 496 | 0 | 355 |
| Zinc, μg/dL* | 852 | 82 | 84 | 8 | 4 | 8 | 6 |
| Copper, μg/dL* | 850 | 116 | 116 | −1 | −1 | 0 | 0 |
| Lutein and zeaxanthin, μg/dL | 900 | 24 | 25 | −4 | −16 | −17 | −26 |
| Vitamin A, μg/dL* | 901 | 64 | 62 | 2 | 3 | 5 | 8 |
| β-Cryptoxanthin, μg/dL | 899 | 11 | 12 | 0 | 0 | −17 | −16 |
| Lycopene, μg/dL | 901 | 20 | 20 | 0 | −8 | −21 | −20 |
| α-Carotene, μg/dL | 897 | 6.0 | 6.0 | 0 | 40 | −20 | 0 |
| Triglycerides, mg/dL§ | 906 | 133 | 128 | 1 | 8 | −3 | 2 |
| Cholesterol, mg/dL§ | |||||||
| Total | 906 | 223 | 219 | −1 | 2 | −3 | −2 |
| HDL-C | 878 | 50 | 51 | 3 | 0 | 3 | 0 |
| LDL-C|| | 877 | 138 | 139 | −9 | 3 | −5 | −5 |
| Hematocrit, %¶ | 3490 | 42 | 42 | 0 | 0 | −2 | −2 |
To convert to micromoles per liter in Système International Units multiply by the following conversion factors: vitamin C, 56.78; beta carotene, 0.0186; zinc, 0.153; copper, 0.1574; vitamin A, 0.0349.
Value is the total ascorbate level.
Value is the ratio of vitamin E and total cholesterol levels adjust for potential differences in vitamin E.
To convert to millimoles per liter in Système International Units multiply by the following conversions factors: triglycerides, 0.01129; total cholesterol, 0.02586; high-density lipoprotein cholesterol (HDL-C), 0.02586; and low-density lipoprotein (LDL-C), 0.02586.
Value calculated as (total cholesterol - HDL-C)-(triglycerides/5).
Hematocrit was measured at all clinical centers.
Changes in Serum Levels of Antioxidants
Participants assigned to medications containing antioxidants had large and statistically significant increases in median serum levels from baseline to year 1: 25% for vitamin C, 83% for vitamin E–cholesterol ratio, and 496% for beta carotene. These increases lessened slightly over the 5-year period. Participants assigned to study medications not containing antioxidants (placebo and zinc treatment arms) experienced modest median level changes over the 5-year period: a decrease of 10% for vitamin C, an increase of 5% for vitamin E–cholesterol ratio, and no change for beta carotene.
Changes in Other Serum Levels
Only one of the other serum levels had a statistically significant change during follow-up by treatment arm. Participants assigned to medications containing antioxidants showed a statistically significant increased median percent change in serum levels from baseline at year 1 of 40% for α-carotene, compared with no change for participants taking nonantioxidant medications. This increase was not seen at year 5, but the difference between treatment groups remained. Serum levels of lutein and zeaxanthin decreased over the 5-year period, with decreases of 17% in the nonantioxidant arms and 26% in the antioxidant arms; however, these changes were not significantly different by treatment (P > .20). The effect of Centrum on serum levels of antioxidants in this population was negligible.
SAFETY OUTCOMES
There were no significant differences from baseline measurement in serum cholesterol levels or hematocrit over the 5-year period (Table 7). Self-reported use of lipid-lowering medications at 5 years was more frequent among those in the antioxidant treatment arms than in the no antioxidant treatment arms (23.5% vs 20.9%, P = .04, data not shown). Other safety outcomes were examined for all participants, regardless of cataract status, to describe and contrast the potential adverse events experienced by the entire exposed population. Table 8 summarizes the statistically significant differences in safety outcomes (reported cause of hospitalizations, adverse experiences, and self-reported conditions) of nearly 50 antioxidants vs no antioxidants comparisons. The analyses were for all participants who had follow-up examinations.
Table 8.
Participants Reporting at Least One Hospitalization, Adverse Experience, or Condition During Follow-up by Treatment*
|
No. (%) of Participants Who Received |
|||
|---|---|---|---|
| Variable | No Antioxidants (n = 2377) | Antioxidants (n = 2357) | Total (n = 4734) |
| Primary hospitalization cause† | |||
| Mild/moderate symptoms‡ | 221 (9.3) | 173 (7.3) | 394 (8.3) |
| Primary adverse experience cause† | |||
| Skin, subcutaneous tissue§ | 21 (0.9) | 56 (2.4) | 77 (1.6) |
| Follow-up condition|| | |||
| Change in skin color§ | 146 (6.1) | 203 (8.6) | 349 (7.4) |
| Chest pain‡ | 541 (22.8) | 467 (19.8) | 1008 (21.3) |
All participants with follow-up data are included in this comparison. Of almost 50 comparisons, only causes and conditions significantly different by treatment are presented.
Causes were classified by using the International Classification of Diseases, Ninth Revision codes.
P< .05.
P< .01.
Self-reported by the participant in response to a predefined list of potential signs and symptoms suggesting an adverse event.
Potential Adverse Effects
At the time of enrollment, participants were informed of possible adverse effects from or contraindications to the use of study medications: vitamin C (kidney stones), vitamin E (fatigue, muscle weakness, decreased thyroid gland function, and increased hemorrhagic stroke), and beta carotene (yellow skin). Among participants in the antioxidant treatment arms, there was an observed excess of self-reports of yellow skin (8.6% vs 6.1%, P=.001). No differences were seen for the other conditions of pre-study concern. All other statistically significant safety measures found during the course of this study are summarized in the following sections.
Hospitalizations
Participants in the antioxidant treatment arms were hospitalized less frequently for reasons in the category “mild/moderate symptoms,” eg, chest pain or discomfort, abdominal pain, vasovagal episode, and fever (7.3% vs 9.3%, P=.01).
Adverse Experiences
Adverse experiences reported by participants were assigned International Classification of Diseases, Ninth Revision codes. Skin and subcutaneous tissue conditions were more frequent in the antioxidant treatment arms (2.4% vs 0.9%, P < .001); most participants with these conditions also self-reported yellow skin.
Conditions Reported at Follow-up
Participants in the antioxidant treatment arms less frequently reported chest pains (19.8% vs 22.8%, P=.01).
Mortality
Table 9 provides the relative risk estimates from the Cox proportional hazards survival model for treatment with antioxidants. Figure 6 shows the Kaplan-Meier estimates of the probability of death for each treatment. The antioxidant treatment does not statistically significantly reduce or increase risk of mortality (P > .50).
Table 9.
Effect of Treatment on Risk of Mortality*
| Treatment Group | No. of Participants | No. of Events | RR (99% CI) | PValue |
|---|---|---|---|---|
| Antioxidants vs no antioxidants | 4757 | 491 | 1.06 (0.84–1.33) | .53 |
| Antioxidants only vs placebo | 2965 | 313 | 1.05 (0.78–1.40) | .68 |
Values were calculated using the Cox proportional hazards survival analysis, unadjusted model. RR indicates relative risk; CI, confidence interval.
Figure 6.
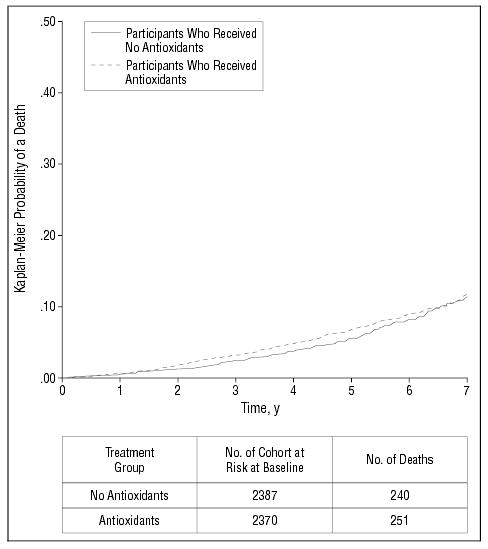
Kaplan-Meier estimates of the probability of death by treatment group among all participants enrolled. P= .53, unadjusted comparison across treatments.
COMMENT
Dietary supplementation with high doses of vitamin C, vitamin E, and beta carotene for an average duration of 6.3 years had no statistically significant effect on the development or progression of age-related lens opacities in AREDS participants. No effect of the antioxidants was noted for the combined opacity group (nuclear, cortical, PSC, or cataract surgery), for the individual types of opacity, or for cataract surgery. For participants with no AMD at baseline no difference was noted between treatment groups for a visual acuity decrease of 15 or more letters compared with the baseline measurement. The 5-year event rate for the primary opacity outcome was 30%, consistent with pretrial estimates of at least 90% power to detect a 25% treatment effect.
Several features of the AREDS design need to be considered in interpreting the null findings for cataract development and progression. First, as is often the case in prevention studies, the population participating in the this study may differ from the general population. The AREDS participants were relatively well nourished compared with the general population, and the effect of this and other differences on the generalizability of AREDS findings is unknown. Second, only a select few antioxidants were studied in AREDS. At the time AREDS was planned, basic science investigations and animal research had suggested an antioxidant hypothesis, and a very limited amount of epidemiological data suggested that cataract occurrence might be inversely associated with use of multivitamins or intake or blood levels of vitamin C, vitamin E, and/or carotenoids.32 In the absence of any proven medical treatment for cataract and the absence of any therapy for most patients with AMD combined with the perception that toxic effects from vitamin usage were low, the use of supplements was being increasingly promoted for both conditions, even in the absence of any convincing efficacy data. During the AREDS planning period a panel of expert nutritionists, ophthalmologists, and biochemists reviewed the basic science and epidemiological data and recommended the AREDS formulation. Two carotenoids, lutein and zeaxanthin, were strong candidates for inclusion in the formulation mainly because they are concentrated in the central retina43 and it was thought that supplementation with these carotenoids might be of benefit in preventing the development of AMD. At the time there were no reports of associations between lutein and zeaxanthin and cataract; there were no commercial preparations available of lutein and zeaxanthin. Beta carotene, another carotenoid with antioxidant properties, was chosen for use in the study because the manufacturers of ophthalmic nutritional supplements were then promoting its effectiveness because of its antioxidant properties, because clinical trials of heart disease and cancer were studying it, and because it was commercially available.33
Since the start of AREDS many observational epidemiological studies have reported associations between the intake or blood levels of various nutrients and cataract.4–27 While almost all retrospective and cross-sectional studies have reported a lower prevalence of cataract in persons who choose to take various supplements or have a higher intake of selected nutrients, the results have been inconsistent in identifying a specific nutrient or cataract type that is affected.
Clinical trials and prospective epidemiological studies have provided little support for a beneficial effect on cataract development of the antioxidant nutrients included in the AREDS formulation. A large randomized trial of US male physicians reported no effect on incident cataract or cataract extraction after 13 years of beta carotene use.3 In 2 cancer prevention trials, treatment with beta carotene had no effect on end-of-study cataract prevalence.28,29 Several prospective epidemiological studies have raised the possibility that lutein and zeaxanthin, the only carotenoids that have been identified in the lens,44 may be better candidates for retarding cataract development than beta carotene. Two prospective studies of the association between dietary intake of antioxidant nutrients and subsequent cataract surgery reported beneficial effects for lutein and zeaxanthin intake but not for intake of other carotenoids.18,20 A third prospective study found a lower incidence of nuclear cataracts with higher levels of intake of lutein and zeaxanthin but again no effect from beta carotene intake.21
Prospective studies evaluating the effect on cataract of higher levels of intake of vitamin E or of higher plasma levels of vitamin E have produced inconsistent results.17,19,21,22,26 Neither of the 2 cancer prevention trials with end-of-study eye examinations showed a statistically significant protective effect for interventions that included vitamin E.28,29 Prospective studies have provided little support for an association between vitamin C intake and the risk of cataract, though one reported that the risk of cataract was lower in women who had used vitamin C supplements for 10 years or longer.17 While the cumulative evidence from AREDS and other studies does not support a beneficial role of vitamin C, vitamin E, or beta carotene in preventing cataract development or progression, questions about a possible role for other micronutrients with or without antioxidant properties remain unanswered.
Interpreting the AREDS results also requires a consideration of the timing and duration of use of the anti-oxidant intervention. All AREDS participants were 55 years or older at enrollment in the study. The median age was 68 years. At baseline 15% of the participants already had a nuclear grade of at least 4.0 U, 52% had some cortical opacities, and 10% had some PSC opacities. Even for many participants with no clinically apparent lens opacities, it is likely that cataracts had probably already started to develop. It may be that, for many, the AREDS intervention was started too late in the process for it to be effective. The AREDS was not designed to determine whether earlier intervention with the micronutrients and/or a longer period of treatment would have been effective. Following the unmasking of study participants, all consenting participants will be followed up for at least another 5 years.
A modification of the Wisconsin System for Classifying Cataracts34 was used in AREDS. Lens photographs were taken in a standardized fashion by certified photographers at the 11 clinical centers and graded at a reading center by specially trained and certified observers. A quality control program included masked replicate gradings of samples of photographs to assess contemporaneous and temporal grading reliability. Replicate gradings of photographs showed a high degree of reliability,35 but our ability to reliably detect change using serial photographs taken at yearly intervals could have been affected by factors such as changes in the characteristics of the film available for purchase, the film development processes, and aging of the photographic equipment. To increase the probability that lens events reflected “true” progression, we performed secondary analyses in which a greater amount of change was required than in the primary analyses. For the primary analyses, as described in the protocol, lens events were defined as a 1.5-U increase in nuclear opacity, a 10% increase in cortical opacity, or a 5% increase in PSC opacity. With events defined as a 2.5-U increase in nuclear opacity or a 20% increase in cortical or PSC opacity, the null findings were repeated. Moreover, no statistically significant treatment effect was noted for cataract surgery, an event with little or no misclassification. Any apparent regression of events was also considered in the primary analyses, which used repeated-measures logistic regression that allows for event determination at each visit, compared with models that are more appropriate for irreversible and error-free events.
Fifty-five percent of the AREDS participants were taking dietary supplements of a multivitamin or at least 1 of the ingredients in the AREDS formulation prior to joining the study. About half (55%) of those taking a dietary supplement were taking RDA dosages rather than the 5- to 15-fold higher dosages of the AREDS ingredients. To accommodate these persons and to standardize the use of nonstudy supplements, a daily dose of Centrum without lutein, a widely available multivitamin and mineral preparation with RDA-level dosages, was provided to each participant who wanted to take or continue taking a multivitamin. Approximately 66% of participants chose to take Centrum; use was balanced across treatment groups. Thus, in addition to their dietary intake of vitamins C and E and beta carotene, these persons whether assigned to placebo or “active” intervention had an increase in their intake by approximately 100% of the RDA amount of each of the study ingredients. The statistical power of the study to test its primary hypothesis about high doses of the study ingredients might have been reduced to the extent that prior use or the continued use of RDA-type doses of these nutrients or other nutrients in the Centrum formulation affected the risk of cataract development. Analyses of the primary opacity outcome stratified by Centrum use showed no differential effect from the antioxidant treatment (data not shown).
Few possible adverse effects of prestudy concern were associated with the use of high doses of the 3 antioxidants. Yellowing of the skin, a well-known adverse effect of large doses of beta carotene, was noted more commonly by participants in the antioxidant treatment arms. During the course of the trial concerns were raised about the potential risk of antioxidants on mortality. There was no significant deleterious effect of antioxidants on mortality although the relative risk estimate is in the direction of harm (relative risk=1.06; 99% confidence interval, 0.84–1.33).
After an average treatment time of 6.3 years in this cohort of relatively well-nourished older adults, the AREDS antioxidant formulation containing high doses of vitamins C and E, beta carotene, and/or zinc had no statistically significant effect on the development or progression of age-related lens opacities or cataract surgery. In addition, there was no statistically significant effect on retarding visual acuity loss in the participants without AMD. Ongoing clinical trials evaluating the effect of nutritional supplements on cataract development will provide additional data about whether the ingredients in the AREDS formulation and other nutrients can affect the risk of cataract development.45–49
PARTICIPANTS AND METHODS
STUDY POPULATION
Details of the study design and methods presented elsewhere33 are briefly summarized herein. Eleven retinal specialty clinics enrolled 4757 participants aged 55 to 80 years from November 13, 1992, through January 15, 1998, and followed them up in the clinical trial until April 16, 2001. Potential participants were identified from the following sources: medical records of patients being seen at AREDS clinics, referring physicians, patient lists from hospitals and health maintenance organizations, public advertisements, friends and family of study participants and clinical center staff, and screenings at malls, health fairs, senior citizens centers, and other gathering places.
The ocular eligibility criteria were largely determined by requirements for the study of AMD. Except for the requirement that the media be sufficiently clear in a study eye to obtain quality stereoscopic fundus photographs of the macula, lens opacity status itself was not considered in selecting participants. All participants had a best-corrected visual acuity of 20/32 or better (visual acuity score of ≥74 letters on the ETDRS logMAR chart) in at least 1 eye. Persons were enrolled in 1 of 4 AMD categories determined by the size and extent of drusen and retinal pigment abnormalities in each eye, the presence of manifestations of advanced AMD (determined from photograph grades at a reading center), and visual acuity as described previously.33 Macular status ranged from essentially no macular abnormality in either eye (AMD Category 1), to mild or borderline AMD features (AMD Category 2: many small or few intermediate drusen, or pigment abnormalities), to at least 1 large druse, extensive intermediate drusen, or noncentral geographic atrophy (AMD Category 3), to advanced AMD or lesions of AMD with visual acuity less than 20/32 in only 1 eye (AMD Category 4). Persons aged 55 to 59 years were enrolled only if eligible for AMD Categories 3 and 4.
At least 1 eye of each participant was free from eye disease that could complicate assessment of AMD, lens opacity progression, or visual acuity (eg, optic atrophy or acute uveitis), and that eye could not have had previous ocular surgery (other than cataract surgery). Persons who underwent cataract surgery were eligible for the study to facilitate recruitment in the AMD component of the trial and because their inclusion had little effect on the power of the cataract component of the study to detect differences between the treatment groups. Potential participants were excluded for illness or disorders (eg, history of cancer with a poor 7-year prognosis, major cardiovascular or cerebrovascular event within the last year, or hemachromatosis) that would make long-term follow-up or compliance with the study protocol unlikely or difficult. Persons bilaterally aphakic or pseudophakic were ineligible for AMD Category 1.
Of the 4757 study participants, all but 3 met the study eligibility and exclusion criteria. The 3 exceptions, all in AMD Category 1, were found after randomization to be technically ineligible because 2 were 58 years old at randomization and 1 exceeded by 2 weeks the 4-month allowable time between qualification and randomization visits. All 3 participants remained in the trial and in their assigned treatment group.
Prior to study initiation, the protocol was approved by an independent data and safety monitoring committee and by the institutional review board for each clinical center. Written informed consent was obtained from all participants before enrollment.
STUDY DESIGN
Interventions
The clinical trial component of AREDS consists of 2 trials—AMD and cataract—generally sharing 1 pool of participants (Figure 1). The 4 treatment interventions were double masked and given as an oral total daily supplementation of antioxidants (500 mg of vitamin C, 400 IU of vitamin E, and 15 mg of beta carotene) or zinc (80 mg of zinc as zinc oxide, 2 mg of copper as cupric oxide to prevent potential anemia), or the combination of antioxidants and zinc, or placebo.
Figure 1.
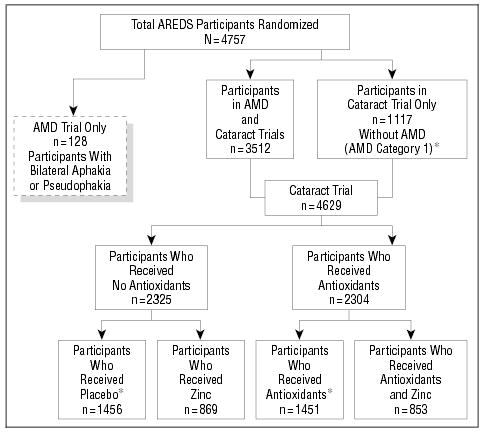
Age-Related Eye Disease Study (AREDS) randomization schema. AMD indicates age-related macular degeneration; asterisks, includes participants in AMD Category 1 (580 placebo-treated subjects and 537 antioxidant-treated subjects).
As in all vitamin products, some ingredients degrade somewhat during the life of the product (ie, prior to an expiration date). The manufacturer formulated each product with slightly different amounts of ingredients than listed above in an effort to achieve appropriate potency at an expiration date.*
Two study medication tablets were to be taken each morning and 2 each evening, to meet the total daily dose requirement. Tablets were to be taken with food to avoid potential irritation of an empty stomach by zinc.
Randomization
Simple randomization, stratified by clinical center and AMD category, was used to assign treatment (Figure 1). Participants in AMD Categories 2 through 4 were assigned with a probability of one quarter to placebo, antioxidants, zinc, or antioxidants and zinc. Participants in AMD Category 1 were assigned with a probability of one half to placebo or antioxidants. Persons with little or no AMD abnormality (AMD Category 1) were not randomized to zinc treatment (only to antioxidants or placebo) because of no likely effect on lens opacities, no likely benefit to their low risk of developing AMD, and potential toxic effects. Multiple unique bottle codes were randomly assigned to each of the 4 treatments for AMD Categories 2 through 4, and also to each of the 2 treatments for participants in AMD Category 1. A bottle code corresponding to the assigned treatment was randomly selected for each participant.
Masking
Study medication tablets for the 4 treatment groups were identical in external appearance and similar in internal appearance and taste. The coordinating center was custodian of the treatment code. Information documenting unmasking was collected during the study.
PROCEDURES
General physical and ophthalmic examinations at baseline and at annual intervals included standardized measurement of the participant’s height, weight, blood pressure, manifest refraction, best-corrected visual acuity, and intraocular pressure. Slitlamp biomicroscopy and ophthalmoscopy were performed at each examination. Lens photographs were taken at baseline and annually starting with the second annual visit by a specially modified slitlamp (model SL-6E; Top-con Corp, Tokyo, Japan) and retroillumination cameras (Neitz Instruments Co Ltd, Tokyo). The presence and severity of nuclear, cortical, and posterior subcapsular lens opacities were graded at a reading center using standardized grading procedures.34 Demographic information, history of smoking and sunlight exposure, medical history, history of specific prescription drug and nonprescription medication use, and history of vitamin and mineral use were obtained at baseline.
Following determination of participant eligibility by the coordinating center and the reading center and by the successful participation in a 1-month placebo run-in to demonstrate compliance with the treatment regimen (at least 75% of the run-in medication taken, according to pill count), participants were randomly assigned to 1 of the treatment groups and then evaluated every 6 months. Participants supplementing with any of the study medication ingredients prior to randomization must have agreed to permanently stop using supplements during the run-in period and were offered Centrum (Whitehall-Robins Healthcare, Madison, NJ), a multivitamin and mineral supplement with recommended daily allowance (RDA)–level dosages, as a replacement for the duration of the study. Fifty-five percent of the study participants were supplementing their diets with some antioxidant vitamins or zinc prior to joining the study. Almost all of this group chose to take Centrum. In addition, although not encouraged, an additional 13% who were not using supplements prior to the study chose to take Centrum, which the study provided.
At each visit, participants returned their used study medication bottles and any unused tablets and received new tablets. They received an ophthalmic examination every 6 months. In addition to the lens photography that was taken at baseline and at annual visits starting with the second, photographs were also taken when a decrease in visual acuity score of 10 or more letters was first observed at a nonannual visit or at the first annual visit. If any submitted photographs were inadequate to assess lens status, requests were made for those photographs to be retaken. Best-corrected visual acuity was measured according to the ETDRS protocol (AREDS Manual of Operations; The EMMES Corp, Rockville, Md) at every annual visit and whenever a decrease from baseline of 10 or more letters was observed at a nonannual visit using the participant’s previous refraction. Special questionnaires were administered to all or a subset of participants at various times throughout the follow-up period: National Eye Institute Visual Function Questionnaire35; a modified Block Food Frequency Questionnaire, a 24-hour dietary recall questionnaire, and cognitive function tasks (AREDS Manual of Operations); and an ocular sunlight-exposure questionnaire derived from the Melbourne study.36
Four clinical centers (The Johns Hopkins Medical Institutions [Baltimore, Md], Devers Eye Institute [Portland, Ore], National Eye Institute Clinical Center [Bethesda, Md], and the Associated Retinal Consultants, PC [Royal Oak, Mich]) collected blood samples at baseline, which were analyzed at the central laboratory (Centers for Disease Control and Prevention, Atlanta, Ga) for the levels of total cholesterol; high-density lipoprotein cholesterol; triglycerides; vitamins A, C, and E; β-carotene; zinc; copper; α-carotene; lutein and zeaxanthin; β-cryptoxanthin; and lycopene. The first 3 centers also collected blood samples annually during follow-up visits for estimation of adherence to the study medication regimen and to assess the effect of the study medications on serum levels of the parameters measured at baseline. Hematocrit was measured at all centers on all participants at baseline and annually thereafter to monitor for the development of anemia. Safety outcomes included serum levels, adverse events, hospitalizations, and mortality. Participants were also asked at each annual visit if they had experienced any of 19 conditions since the last follow-up visit. These included anemia, gastrointestinal conditions, kidney stones, fatigue, skin conditions, cardiovascular conditions, and thyroid abnormalities. Although individuals could have multiple occurrences of a condition or safety outcome, analyses compared the frequency of those who ever had the event with those who never had the event. Safety outcomes were monitored annually by the data and safety monitoring committee. A network of collaborating physicians from non-AREDS clinics was formed to assist in obtaining visual acuity measurements and fundus photographs and to perform ophthalmic examinations for participants who could not return to an AREDS clinic.
SAMPLE SIZE AND POWER
A total sample size of 4600 was planned. For the cataract trial with an estimated 4500 participants enrolled, power was calculated assuming 7 years of follow-up, during which time 20% were projected to drop out (discontinue study medication) and assume the placebo event rate, 30% would drop in (begin a nonstudy supplement containing study medication ingredients) and assume the full treatment (antioxidants) event rate, and 15% would be lost to follow-up before experiencing an event. For 2-sided α =.05, a projected sample size of 4500 would provide at least 90% power to detect treatment effects of 15%, 25%, and 30% and for placebo event rates of 50%, 30%, and 20%, respectively.33
OUTCOMES
Slitlamp photographs were used to grade nuclear opacities on a decimal scale by comparing photographs of participants with standard stereophotographs of lenses with increasingly severe nuclear opacities; retroillumination photographs were used to estimate the area of involvement for cortical and posterior subcapsular (PSC) opacities.34
Cataract
The protocol defines the lens event outcome in a participant as the occurrence in at least 1 eye (having a natural lens) of cataract surgery or of any of the following changes from baseline in photographic grade: nuclear opacity (a 1.5-U increase on a scale from 0.9–6.1 U); cortical opacity (10% absolute increase in the area of opacity within a standard central 5-mm circle); and PSC (5% absolute increase in the area of opacity within a standard central 5-mm circle). Examples of these changes are shown in Figure 2.
Figure 2.
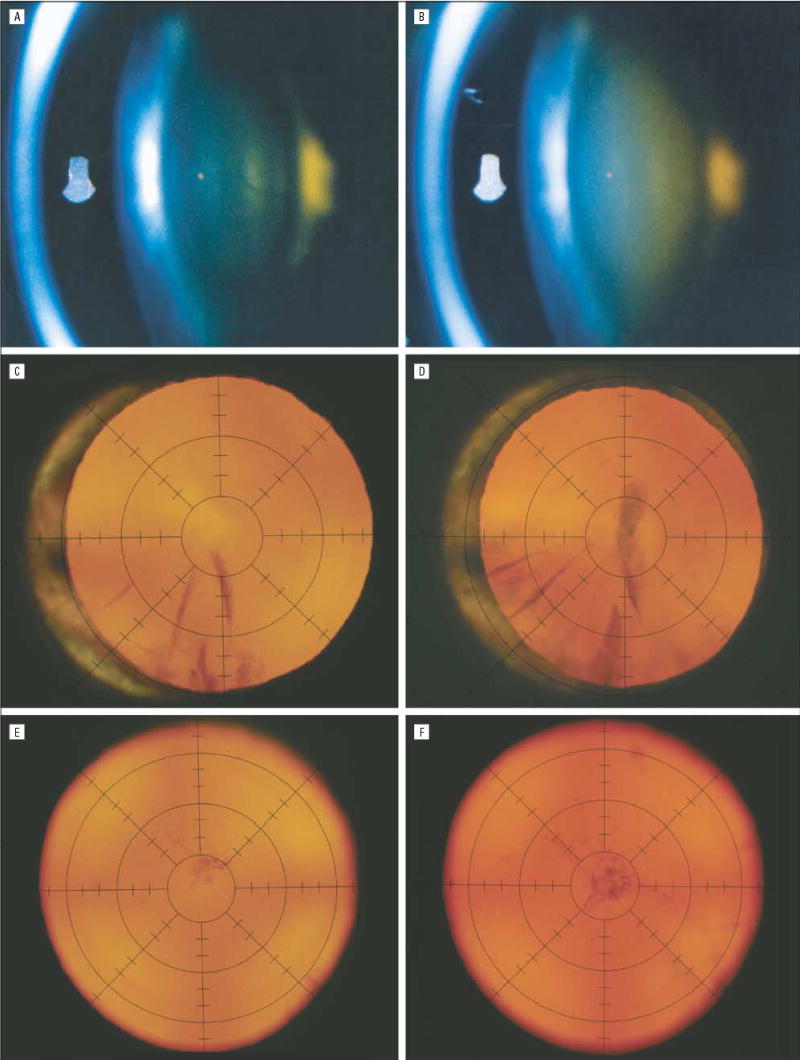
Examples of lens opacity progression in Age-Related Eye Disease Study (AREDS) participants. Nuclear opacity graded from slitlamp photographs (model SL-6E; Topcon Corp, Tokyo, Japan) increased from 2.0 U (equal to AREDS standard photograph 3) at baseline (A) to 3.9 U (approaching standard photograph 5) at the 5-year visit (B). Cortical opacity within 5 mm of the lens center (ie, within the second innermost circle of the grid) graded from retroillumination photographs (Neitz Instruments Co, Ltd, Tokyo) increased from 6% at baseline (C) to 45% at the 6-year visit (D). Posterior subcapsular opacity within 5 mm of the lens center increased from 6% at baseline (E) to 22% at the 5-year visit (F).
Visual Acuity Loss
The primary visual acuity outcome was a decrease of best-corrected visual acuity score from baseline of 15 or more letters in a study eye (equivalent to a doubling or more of the initial visual angle, eg, 20/20 to ≤ 20/40 or 20/50 to ≤ 20/100). Visual acuity was measured every 6 months.
Secondary Outcomes
Secondary outcomes defined during the design phase of the study included worsening of each opacity type and cataract surgery. In addition, at the time of analysis, a severe lens event was defined as follows: an increase in nuclear opacity of at least 2.5 U, an absolute increase in the area of cortical or PSC opacity of at least 20%, or cataract surgery.
STATISTICAL ANALYSES
All comparisons were made on an intention-to-treat basis. Photographic lens events were determined from photographs taken at annual visits, beginning at year 2. Events of cataract surgery from clinical reports at nonannual visits were attributed to the next annual visit. The primary comparison for lens event and visual acuity event was the overall (main) effect of antioxidants (1+3) vs no antioxidants (2+4) among all participants (Table 1). Analyses of possible zinc effect involving the factorial design (1+2 vs 3+4) were of persons in AMD Categories 2 through 4. Because persons are the units of analysis, no adjustment for correlation between paired eyes is needed.
Table 1.
Treatment Design
| Antioxidants | No Antioxidants | |
|---|---|---|
| Zinc | (1) Antioxidants + zinc | (2) Zinc |
| No zinc | (3) Antioxidants | (4) Placebo |
Primary analysis of treatment effect was by repeated-measures logistic regression, hereinafter referred to as “repeated measures,” using the SAS procedure GENMOD (SAS Institute, Cary, NC), a generalized estimating equations method that allows for determining events at each visit. In repeated-measures logistic regression we model the effect of explanatory variables on the occurrence of an event, considering the correlation of observations at follow-up visits within a patient and the time at which visits occurred. Cox proportional hazards survival analyses for the lens outcomes and repeated-measures analysis of variance of mean change in visual acuity and lens opacity scores were used for comparison with the logistic regression findings. Cox proportional hazards survival analysis, an extension of life-table analysis, is a regression model of the effect of explanatory variables on time to first occurrence of an event. This method is given secondary importance in the primary analyses because it is more appropriate for irreversible and error-free events such as cataract surgery and death, where subsequent observations are not relevant. Analyses were unadjusted and also adjusted for the following baseline covariates: age (55–64, 65–69, and 70–80 years), sex, race, smoking status, and AMD category.
STATISTICAL MONITORING
A data and safety monitoring committee monitored 5 end points from the 2 trials (AMD and cataract) simultaneously for both safety and efficacy.33 Sequential monitoring of end points assumed no interaction between antioxidants and zinc, so that only main effects were analyzed. An α-spending function group–sequential method37 was extended to address multiple time-to-event outcome variables by a Bonferroni adjustment distributing the type I error among the multiple end points. Log rank tests were used to compare the response distributions of the 2 treatment groups with an O’Brien-Fleming boundary.38 A separate monitoring of mortality used a Pocock-type boundary.39 Comparisons were made, with spending of α, when requested by the data and safety monitoring committee. At the end of the trial, treatment effects significant at P=.01 can be considered statistically significant at α =.05 after adjustment for multiple outcomes and interim analyses.
CHANGE IN TREATMENT
In 1994 and 1996, AREDS participants were informed of the results of the Alpha-Tocopherol, Beta Carotene Cancer Prevention Study40 and the Beta-Carotene and Retinol Efficacy Trial41 suggesting potential harmful effects of beta carotene among smokers. Participants who were current cigarette smokers at baseline were contacted in 1996 and offered the option of continuing or discontinuing their masked AREDS study medication. Participants in AMD Categories 2 through 4 who were current or former smokers at baseline were additionally given the opportunity to be reassigned to a masked study medication that excluded any antioxidant component. As a result, 117 (2.5% of all participants and 24% of the current smokers) of the participants stopped taking medications (38 participants or 2.6% in the placebo arm), and 84 participants (1.8%) were reassigned from a study medication containing beta carotene to one without beta carotene. The original treatment group assignments were retained for intention-to-treat analyses.
Footnotes
A complete list of the principal investigators and members of the Age-Related Eye Disease Study (AREDS) Research Group appears in the box on page 1434. The AREDS investigators have no commercial or proprietary interest in the supplements used in this study.
Tablets used in the active treatment arms of these trials were manufactured to have the following minimum contents throughout the shelf life of the product: 7160 IU of vitamin A (beta carotene), 113 mg of vitamin C (ascorbic acid), 100 IU of vitamin E (dl-alpha tocopheryl acetate), 17.4 mg of zinc (zinc oxide), and 0.4 mg of copper (cupric oxide).
This investigation was supported by contracts from the National Eye Institute, National Institutes of Health, Bethesda, Md, with additional support from Bausch & Lomb Inc, Rochester, NY.
References
- 1.Bunce GE, Kinoshita J, Horwitz J. Nutritional factors in cataract [review] Annu Rev Nutr. 1990;10:233–254. doi: 10.1146/annurev.nu.10.070190.001313. [DOI] [PubMed] [Google Scholar]
- 2.Taylor A. Cataract: relationships between nutrition and oxidation [review] J Am Coll Nutr. 1993;12:138–146. doi: 10.1080/07315724.1993.10718294. [DOI] [PubMed] [Google Scholar]
- 3.Christen WG, Glynn RJ, Hennekens CH. Antioxidants and age-related eye disease current and future perspectives [review] Ann Epidemiol. 1996;6:60–66. doi: 10.1016/1047-2797(95)00094-1. [DOI] [PubMed] [Google Scholar]
- 4.Leske MC, Chylack LT, Jr, Wu SY. The Lens Opacities Case-Control Study: risk factors for cataract. Arch Ophthalmol. 1991;109:244–251. doi: 10.1001/archopht.1991.01080020090051. [DOI] [PubMed] [Google Scholar]
- 5.Leske MC, Wu SY, Connell AMS, Hyman L, Schachat AP. Lens opacities, demographic factors and nutritional supplements in the Barbados Eye Study. Int J Epidemiol. 1997;26:1314–1322. doi: 10.1093/ije/26.6.1314. [DOI] [PubMed] [Google Scholar]
- 6.Mares-Perlman JA, Klein BE, Klein R, Ritter LL. Relation between lens opacities and vitamin and mineral supplement use. Ophthalmology. 1994;101:315–325. doi: 10.1016/s0161-6420(94)31333-9. [DOI] [PubMed] [Google Scholar]
- 7.Robertson JM, Donner AP, Trevithick JR. A possible role for vitamins C and E in cataract prevention. Am J Clin Nutr. 1991;53(suppl 1):346S–351S. doi: 10.1093/ajcn/53.1.346S. [DOI] [PubMed] [Google Scholar]
- 8.Cumming RG, Mitchell P, Smith W. Diet and cataract: The Blue Mountains Eye Study. Ophthalmology. 2000;107:450–456. doi: 10.1016/s0161-6420(99)00024-x. [DOI] [PubMed] [Google Scholar]
- 9.Mares-Perlman JA, Brady WE, Klein BE, et al. Diet and nuclear lens opacities. Am J Epidemiol. 1995;141:322–334. doi: 10.1093/aje/141.4.322. [DOI] [PubMed] [Google Scholar]
- 10.Mohan M, Sperduto RD, Angra SK, et al. India-US case-control study of age-related cataracts. Arch Ophthalmol. 1989;107:670–676. doi: 10.1001/archopht.1989.01070010688028. [DOI] [PubMed] [Google Scholar]
- 11.Jacques PF, Chylack LT., Jr Epidemiologic evidence of a role for the antioxidant vitamins and carotenoids in cataract prevention. Am J Clin Nutr. 1991;53(suppl 1):352S–355S. doi: 10.1093/ajcn/53.1.352S. [DOI] [PubMed] [Google Scholar]
- 12.Tavani A, Negri E, La Vecchia C. Food and nutrient intake and risk of cataract. Ann Epidemiol. 1996;6:41–46. doi: 10.1016/1047-2797(95)00099-2. [DOI] [PubMed] [Google Scholar]
- 13.Vitale S, West S, Hallfrisch J, et al. Plasma antioxidants and risk of cortical and nuclear cataract. Epidemiology. 1993;4:195–203. doi: 10.1097/00001648-199305000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Jacques PF, Chylack LT, Jr, McGandy RB, Hartz SC. Antioxidant status in persons with and without senile cataract. Arch Ophthalmol. 1988;106:337–340. doi: 10.1001/archopht.1988.01060130363022. [DOI] [PubMed] [Google Scholar]
- 15.Leske CL, Wu SY, Hyman L, et al. for the Lens Opacities Case-Control Study Group. Biochemical factors in the lens opacities: case-control study. Arch Ophthalmol. 1995;113:1113–1119. doi: 10.1001/archopht.1995.01100090039020. [DOI] [PubMed] [Google Scholar]
- 16.Knekt P, Heliövaara M, Rissanen A, Aromaa A, Aaran RK. Serum antioxidant vitamins and risk of cataract. BMJ. 1992;305:1392–1394. doi: 10.1136/bmj.305.6866.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hankinson SE, Stampfer MJ, Seddon JM, et al. Nutrient intake and cataract extraction in women: a prospective study. BMJ. 1992;305:335–339. doi: 10.1136/bmj.305.6849.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chasan-Taber L, Willett WC, Seddon JM, et al. A prospective study of carotenoid and vitamin A intakes and risk of cataract extraction in US women. Am J Clin Nutr. 1999;70:509–516. doi: 10.1093/ajcn/70.4.509. [DOI] [PubMed] [Google Scholar]
- 19.Seddon JM, Christen WG, Manson JE, et al. The use of vitamin supplements and the risk of cataract among US male physicians. Am J Public Health. 1994;84:788–792. doi: 10.2105/ajph.84.5.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown L, Rimm EB, Seddon JM, et al. A prospective study of carotenoid intake and risk of cataract extraction in US men. Am J Clin Nutr. 1999;70:517–524. doi: 10.1093/ajcn/70.4.517. [DOI] [PubMed] [Google Scholar]
- 21.Lyle BJ, Mares-Perlman JA, Klein BE, Klein R, Greger JL. Antioxidant intake and risk of incident age-related nuclear cataracts in the Beaver Dam Eye Study. Am J Epidemiol. 1999;149:801–809. doi: 10.1093/oxfordjournals.aje.a009895. [DOI] [PubMed] [Google Scholar]
- 22.Leske MC, Chylack LT, Jr, He Q, et al. Antioxidant vitamins and nuclear opacities: the longitudinal study of cataract. Ophthalmology. 1998;105:831–836. doi: 10.1016/s0161-6420(98)95021-7. [DOI] [PubMed] [Google Scholar]
- 23.The Italian-American Cataract Study Group. Risk factors for age-related cortical, nuclear, and posterior subcapsular cataracts. Am J Epidemiol. 1991;133:541–553. [PubMed] [Google Scholar]
- 24.McCarty CA, Mukesh BN, Fu CL, Taylor HR. The epidemiology of cataract in Australia. Am J Ophthalmol. 1999;128:446–465. doi: 10.1016/s0002-9394(99)00218-4. [DOI] [PubMed] [Google Scholar]
- 25.Mares-Perlman JA, Lyle BJ, Klein R, et al. Vitamin supplement use and incident cataracts in a population-based study. Arch Ophthalmol. 2000;118:1556–1563. doi: 10.1001/archopht.118.11.1556. [DOI] [PubMed] [Google Scholar]
- 26.Rouhiainen P, Rouhiainen H, Salonen JT. Association between low plasma vitamin E concentration and progression of early cortical lens opacities. Am J Epidemiol. 1996;144:496–500. doi: 10.1093/oxfordjournals.aje.a008956. [DOI] [PubMed] [Google Scholar]
- 27.Nadalin G, Robman LD, McCarty CA, Garrett SK, McNeil JJ, Taylor HR. The role of past intake of vitamin E in early cataract changes. Ophthalmic Epidemiol. 1999;6:105–112. doi: 10.1076/opep.6.2.105.1561. [DOI] [PubMed] [Google Scholar]
- 28.Teikari J, Virtamo J, Rautalahti M, Palmgren J, Liesto K, Heinonen OP. Long-term supplementation with alpha-tocopherol and beta-carotene and age-related cataract. Acta Ophthalmol Scand. 1997;75:634–640. doi: 10.1111/j.1600-0420.1997.tb00620.x. [DOI] [PubMed] [Google Scholar]
- 29.Sperduto RD, Hu TS, Milton RC, et al. The Linxian Cataract Studies: two nutritional intervention trials. Arch Ophthalmol. 1993;111:1246–1253. doi: 10.1001/archopht.1993.01090090098027. [DOI] [PubMed] [Google Scholar]
- 30.Christen WG. Beta-carotene and age-related cataract in a randomized trial of U.S. physicians [ARVO abstract] Invest Ophthalmol Vis Sci. 2001;42:S518. Abstract 2790. [Google Scholar]
- 31.Robman LD, McCarty CA, Tikellis G, et al. VECAT Study: The effect of vitamin E on the progression of lens opacities: preliminary results [ARVO abstract] Invest Ophthalmol Vis Sci. 2001;42:S508. Abstract 2742. [Google Scholar]
- 32.Sperduto RD, Ferris FL, III, Kurinij N. Do we have a nutritional treatment for age-related cataract or macular degeneration [editorial]? Arch Ophthalmol. 1990;108:1403–1405. doi: 10.1001/archopht.1990.01070120051026. [DOI] [PubMed] [Google Scholar]
- 33.The Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study (AREDS): design implications: AREDS Report No. 1. Control Clin Trials. 1999;20:573–600. doi: 10.1016/s0197-2456(99)00031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.The Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study (AREDS) system for classifying cataracts from photographs: AREDS Report No. 4. Am J Ophthalmol. 2001;131:167–175. doi: 10.1016/s0002-9394(00)00732-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119:1050–1058. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
- 36.McCarty CA, Lee SE, Livingston PM, Bissinella M, Taylor HR. Ocular exposure to UV-B in sunlight: the Melbourne visual impairment project model. Bull World Health Organ. 1996;74:353–360. [PMC free article] [PubMed] [Google Scholar]
- 37.Lan KK, Lachin JM. Implementation of group sequential logrank tests in a maximum duration trial. Biometrics. 1990;46:759–770. [PubMed] [Google Scholar]
- 38.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–556. [PubMed] [Google Scholar]
- 39.Pocock SJ. Group sequential methods in the design and analysis of clinical trials. Biometrika. 1977;64:191–199. [Google Scholar]
- 40.The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta-carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 41.Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta-carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 42.Alaimo K, McDowell MA, Briefel RR, et al. Dietary intake of vitamins, minerals, and fiber of persons ages 2 months and over in the United States: Third National Health and Nutrition Examination Survey, Phase 1, 1988–91. Adv Data. 1994;14:1–28. [PubMed] [Google Scholar]
- 43.Bone RA, Landrum JT, Tarsis SL. Preliminary identification of the human macular pigment. Vision Res. 1985;25:1531–1535. doi: 10.1016/0042-6989(85)90123-3. [DOI] [PubMed] [Google Scholar]
- 44.Yeum KJ, Taylor A, Tang G, Russell RM. Measurement of carotenoids, retinoids, and tocopherols in human lenses. Invest Ophthalmol Vis Sci. 1995;36:2756–2761. [PubMed] [Google Scholar]
- 45.Garrett SK, McNeil JJ, Silagy C, et al. Methodology of the VECAT study: vitamin E intervention in cataract and age-related macular degeneration. Ophthalmic Epidemiol. 1999;6:195–208. doi: 10.1076/opep.6.3.195.1500. [DOI] [PubMed] [Google Scholar]
- 46.Christen WG, Gaziano M, Hennekens CH. Design of Physicians’ Health Study II: a randomized trial of beta-carotene, vitamins E and C, and multivitamins, in prevention of cancer, cardiovascular disease, and eye disease, and review of results of completed trials [review] Ann Epidemiol. 2000;10:125–134. doi: 10.1016/s1047-2797(99)00042-3. [DOI] [PubMed] [Google Scholar]
- 47.Manson JE, Gaziano JM, Spelsberg A, et al. for the WACS Research Group. A secondary prevention trial of antioxidant vitamins and cardiovascular disease in women: rationale, design, and methods. Ann Epidemiol. 1995;5:261–269. doi: 10.1016/1047-2797(94)00091-7. [DOI] [PubMed] [Google Scholar]
- 48.Buring JE, Hennekens CH. The Women’s Health Study: summary of the study design. J Myocardial Ischemia. 1992;4:27–29. [Google Scholar]
- 49.Chylack LT, Jr, Wolfe JK, Friend J, et al. Validation of methods for the assessment of cataract progression in the Roche European-American Anticataract Trial (REACT) Ophthalmic Epidemiol. 1995;2:59–75. doi: 10.3109/09286589509057085. [DOI] [PubMed] [Google Scholar]


