Abstract
The use of total prostate-specific antigen (tPSA) measurement has dramatically improved the ability to detect prostate cancer at earlier stages. However, as the number of men presenting with advanced disease (and high tPSA levels) has decreased, and given the fact that tPSA is highly reflective of benign prostatic hyperplasia, the need has emerged for novel biomarkers specifically associated with prostate cancer in order to improve predictive models. Several new biomarkers have shown promise, and studies continue to investigate the role of these markers in the detection, staging, and prognosis of prostate cancer. As new useful biomarkers continue to emerge, guidelines for their employment, as well as coordination of further research studies, are needed; a systematic, phased, nomogram-based model is a rational way to manage these efforts.
Key words: Biomarkers, Insulin-like growth factor, Interleukin-6, Nomograms, Prostate cancer, Prostate-specific antigen, Transforming growth factor ß1, Urokinase plasminogen activator
The discovery of total prostate-specific antigen (tPSA) and its entry into broad clinical use in the late 1980s and early 1990s had a profound impact on the diagnosis and management of prostate cancer. Since the Food and Drug Administration approved the tPSA test for prostate cancer screening in 1994, its widespread use in early detection programs has drastically reduced the number of patients who are found at initial diagnosis to have metastatic disease. In addition, it is likely that prostate cancer screening has contributed to the recent decrease in prostate cancer mortality rates in the United States and around the world. Furthermore, tPSA testing has been found to be an effective staging and prognostic tool for prostate cancer, with higher levels of tPSA being associated with more advanced stages of disease and more adverse clinical outcomes. Lastly, tPSA has become an indispensable marker for monitoring disease status in patients after therapy.
Despite this remarkable performance, there has always been a nagging concern, voiced even during the early phases of the “PSA revolution,” regarding the utility of tPSA as a marker for prostate cancer. First and foremost, tPSA is not a “classic” tumor marker whose levels are directly correlated with increasing stage and grade of prostate cancer. In fact, PSA is organ-specific but not cancer-specific. Normal, hyperplastic, and neoplastic prostate epithelial cells all produce PSA, with the highest levels found in the prostatic transition zone of patients with benign prostatic hyperplasia (BPH). The lower levels per cell of PSA produced by prostate cancer cells compared with those produced by BPH cells are compensated for by the increased amount of PSA that enters the circulation, presumably because of the disordered ductal structure within primary and metastatic prostate cancer lesions. Interestingly, PSA expression decreases with increasing Gleason grade.1,2
Pretreatment PSA level, which is the primary parameter used in most predictive tools (eg, Partin Tables, Kattan-Scardino Nomograms), has been shown to provide less reliable predictive information about prostate cancer as the proportion of men with more advanced prostate cancer and with higher tPSA levels at presentation continues to decrease. Conversely, the link between tPSA and pathologic and clinical outcomes of BPH—a link supported by the high cellular production of tPSA seen in benign prostatic epithelium—has grown stronger. For example, in men without prostate cancer, tPSA level has been shown to be a strong predictor of BPH-related prostate volume.3–6 tPSA level in men without prostate cancer has also been shown to be the strongest predictor of prostate growth and BPH-related outcomes.7,8
Furthermore, in men who have prostate cancer, tPSA levels appear, to a large extent, to be reflective of often-coexistent BPH rather than features of the prostate cancer; this is especially the case in patients with tPSA levels in the lower range (2.5–10 ng/mL) at diagnosis. For example, Stamey and colleagues9,10 recently reported that, for patients with preoperative tPSA levels between 2 ng/mL and 9 ng/mL, PSA level had a poor relationship with cancer volume and grade in radical prostatectomy specimens and a limited relationship with tPSA level progression after radical prostatectomy. However, tPSA level was significantly correlated with the overall volume of the radical prostatectomy specimen—a direct reflection of the degree of BPH present.9,10 In addition, although tPSA level is an excellent predictor of pathologic stage when patients with high levels are evaluated, more than 50% of patients in whom prostate cancer is diagnosed today have a tPSA level below 10 ng/mL—a range for which tPSA level alone is less informative.10–12
Studies have also found a decrease in the value of tPSA measurement for predicting disease progression in more modern cohorts of patients with clinically localized prostate cancer undergoing radical prostatectomy.13,14 These patients had lower median tPSA levels than patients in most older series. Therefore, there is an imminent need for novel biomarkers that have a stronger association with prostate cancer in order to extend and perhaps even preserve the clinical performance of predictive models, which are currently strongly reliant on tPSA testing. Specifically, markers associated with the biologic aggressiveness of prostate cancer may allow improved prediction of outcomes in patients with clinically localized prostate cancer, especially those with lower tPSA levels. The emergence of new therapeutic approaches for prostate cancer, such as chemoprevention, gene therapy, and adjuvant therapies, cannot flourish without a more reliable set of markers to serve as prognosticators, targets, and/or intermediate end points of disease progression and response to therapy.
Despite nearly 20 years of advances in molecular biology, only tPSA and free PSA (fPSA) measurements have found a relatively broad and growing clinical role in the management of prostate cancer patients. Indeed, there are a variety of issues and barriers that affect the transition of clinical tests from research to clinical practice (Table 1). This paucity of new, widely accepted markers continues despite the fact that both physicians and the lay public are now faced almost daily with reports of newly discovered diagnostic and therapeutic genes and molecular markers, often with inflated claims that they provide new information important for determining prognosis or improving cancer treatment. Because the number of these putative markers is likely to increase dramatically in the near future, there is a need for appropriate clinical guidelines and protocols formulated to ensure a systematic and critical evaluation of these markers by multidisciplinary groups of experts before their introduction into patient care.
Table 1.
Challenges and Advances in the Development of Clinically Useful Prostate Cancer Biomarkers
| Challenges |
|---|
| Biologic factors |
|
| Clinical pathologic factors |
|
| Analytic sensitivity and detection limit |
|
| Health service factor |
|
| Factors That Support Advances |
| Defining the biology of prostate cancer and its processes with precision |
|
| Defining host biology: pharmacogenomics and pharmacoproteinomics |
|
| Defining biomarkers and surrogate end points |
|
| Creating guidelines for appropriate clinical employment of each biomarker |
|
|
| Standardization and stringency of analytic technology |
|
| High-quality specimen and clinical data repository |
|
Protocols delineating hierarchical scaling have been proposed for evaluating the weight of available evidence supporting the clinical value of any new marker under investigation.15 However, such a system has not yet been widely implemented by investigators assessing the qualitative strength of new prostate cancer biomarkers. In this article, using our own experiences, we first illustrate the translational mechanism we have used to advance several exciting novel biomarkers from observations in the laboratory to testable hypotheses for evaluation in human clinical trials. Next, we attempt to evaluate the level of evidence supporting the use of current established and novel prostate cancer blood biomarkers to refine clinical decisions at various stages of cancer screening, diagnosis, prognosis, treatment, detection of early relapse, and monitoring of the disease course (Table 2).
Table 2.
Clinical Performance of Selected, Promising Serologic Prostate Cancer Biomarkers
| Aid to | ||||||||
|---|---|---|---|---|---|---|---|---|
| Diagnosis/ | Pathologic | Predicting | Distant | Follow-up/ | Selection for | |||
| Future | Case- | Staging/ | PSA | Metastasis | Monitoring | Targeted | ||
| Biomarker | Risk | Screening | Finding | Prognosis* | Recurrence | Staging | Therapy | Therapy† |
| PSA | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Free PSA | - | No | Yes | Yes | No | No | No | - |
| Complexed PSA | - | No | Yes | Yes | No | No | - | - |
| hK2 | - | - | Yes | Yes | - | - | - | - |
| BPSA | - | No | - | - | - | - | - | - |
| ProPSA | - | No | Yes | Yes | - | - | - | - |
| isoforms | ||||||||
| IGF-I | Yes | No | No | No | No | No | No | - |
| IGFBP-2 | - | - | Yes | Yes; not | Yes | No | No | Yes |
| lymph nodes | ||||||||
| IGFBP-3 | Controversial | No | No | No | Yes | Yes | No | Yes |
| TGF-β1 | - | No | No | Yes | Yes | Yes | Yes | Yes |
| IL-6 | - | No | No | Only | Yes | Yes | No | Yes |
| lymph nodes | ||||||||
| IL-6sR | - | No | No | Only | Yes | Yes | No | Yes |
| lymph nodes | ||||||||
| uPA | - | No | Yes | Yes; not | Yes | Yes | - | - |
| lymph nodes | ||||||||
| uPAR | - | No | Yes | Yes; not | Yes | Yes | - | - |
| lymph nodes | ||||||||
| VEGF | - | No | Yes | Yes | Yes | Yes | - | - |
| Osteoprotegerin | - | No | No | - | - | Yes | - | - |
| RT-PCR/PSA | - | No | No | No | No | No | Yes | - |
| RT-PCR/hK2 | - | No | No | Yes | Yes | No | - | - |
Including metastases to regional pelvic lymph nodes.
In experimental prostate cancer models; no published results from phase 3 human clinical trials.
No, not useful; Yes, useful; –, application is not considered and/or association has not been investigated; PSA, prostate-specific antigen; hK2, human glandular kallikrein 2; BPSA, “benign” PSA; IGF, insulin-like growth factor; IGFBP, insulin-like growth factor binding protein; TGF-β1, transforming growth factor β1; IL-6, interleukin-6; IL-6sR, interleukin-6 soluble receptor; uPA, urokinase plasminogen activator; uPAR, urokinase plasminogen activator receptor; VEGF, vascular endothelial growth factor; RT-PCR, reverse transcriptase polymerase chain reaction.
Determining when a marker is clinically useful can be a difficult task in that a marker may be useful in only one or two of the clinical categories mentioned above. Given the plethora of candidate prostate cancer biomarkers, we have chosen to discuss only a select group of novel blood-based biomarkers that have been shown to be independent diagnostic and/or prognostic factors in multivariate analyses from more than one single-institution study. However, the evidence suggests that several markers already in phase 3 evaluation demonstrate properties that may eventually usher out tPSA as the primary marker for prostate cancer detection, staging, and prediction of prognosis.
From Molecular Characteristic to Useful Clinical Cancer Test
At Baylor College of Medicine, we have developed a process to systematically identify, validate, and translate to the clinic the prostate cancer biomarkers that are associated with biologically and clinically aggressive prostate cancer. We have established a formal structure of defined phases of marker development (Figure 1), much like that used for decades in the development of novel drugs.16
Figure 1.
Baylor College of Medicine strategic approach to testing and validating blood-based biomarkers.
First, we identified a host of prostate cancer biomarkers selected on the basis of promising findings in pre-clinical exploratory studies or in hypothesis-generating clinical studies performed in limited numbers of human participants by our group or reported in peer-reviewed journals by others. Next, we evaluated and validated the sensitivity, sensibility, reliability, and accuracy of the assay in the laboratory (generally using commercially available quantitative sandwich enzyme immunoassays). The aim of this phase of investigation was to refine and standardize the assay and sample acquisition protocols.
To date, the performance of all biomarker assays that we evaluated complied with validation criteria appropriate for analytic techniques, including acceptable linearity over the concentration ranges expected clinically and inter- and intra-assay variability, with exception of the assays for three biomarkers that did not allow a sufficiently accurate limit of quantitation (interleukin [IL]-8, plasminogen activator inhibitor-2, endothelin-1). In pilot clinical studies involving synchronously drawn blood specimens obtained from healthy men attending our prostate cancer screening clinic, we assessed in a blinded fashion the effect of different collection formats and sampling procedures on biomarker levels to clarify reliability and validity and to determine in which blood compartment (citrate plasma, EDTA plasma, or serum) the candidate marker provided the most clinically relevant information. Because some biomarkers, such as transforming growth factor ß1 (TGF-ß1) and vascular endothelial growth factor (VEGF), are present in platelet granules and are released on platelet activation, quantification of non-platelet-derived levels of these biomarkers are less accurate in serum. For these biomarkers, we used plasma as the sampling compartment and ensured complete platelet removal by performing an additional centrifugation.17
The next step was to assay these novel markers in serum or plasma specimens obtained from a relatively small cohort of consecutively treated, completely characterized patients. These studies included retrospective pilot studies involving a consecutively treated cohort of 120 to 228 well-characterized patients with at least 5 years of follow-up who underwent radical prostatectomy for clinically localized prostate cancer, a cohort of healthy men with no clinical evidence of any cancer and no history of cancer (n = 44), a cohort of patients with prostate cancer metastases to regional lymph nodes (n = 19), and a cohort of patients with untreated bone scan-proven distant prostate cancer metastases (n = 10).
In some cases, no clinical utility for a putative biomarker was identified and no further studies were performed.18 In other cases, however, preliminary evidence regarding clinical and biologic value were promising, and we conducted confirmatory retrospective clinical studies involving larger cohorts of consecutive patients who had undergone prostatectomy for clinically localized disease to provide a reasonable assurance of the prognostic effectiveness of the biomarker and to further elucidate the origin of the changes associated with different prostate cancer disease states. To further test the strength of evidence and assess clinical relevance, we determined whether the candidate biomarkers could improve the level of accuracy achieved by standard, externally validated preoperative and postoperative nomograms incorporating standard clinical and pathologic predictors.
The next phase of this investigation consists of external confirmation of our findings in large, retrospective, single-institution studies conducted at Memorial Sloan-Kettering Cancer Center in New York to independently verify the reproducibility of the biomarker findings. Several of the investigated biomarkers are currently at this stage of development. If their clinical value is verified, we will move toward validation in large, prospective, multicenter collaborative trials.
Nomograms
In some studies of new markers, the unanswered question is “Does the new marker significantly improve our ability to predict X, given all the other known clinical parameters?” The answer to this question requires more than conventional univariate and multivariate analyses with their associated hazard rates and P values. Predictive models (eg, Partin Tables), including or excluding any new putative biomarker, need to be shown to provide a clinically significant improvement in our predictive ability in order to claim any real benefit.
Nomograms are tools used to predict outcome probabilities for individual patients. Several pretreatment and posttreatment nomograms have been developed19–23 and validated24,25 to predict risk of prostate cancer progression after attempted curative therapy (Figure 2). These tools are unique in that they were developed in a different manner than traditional approaches to prognostic modeling. Because nomograms simultaneously consider multiple aspects of a patient’s cancer (eg, stage, grade, serum PSA level, novel biomarkers), a more accurate prediction for the individual patient is obtained. Nomograms can be used for patient counseling, follow-up scheduling, and clinical trial design and analysis. In our opinion, for new prostate cancer biomarkers to be clinically useful, they must add unique predictive information, improving the performance of a nomogram constructed without the new biomarker by a significant margin as measured by the concordance index, which ranges from 0.5 (when the predictive value is no better than that of a “flip of a coin”) to 1.0 (perfect predictive value) (Figure 3).
Figure 2.
Nomogram software screenshot. TGF ß1, transforming growth factor ß1; IL-6 sR, interleukin-6 soluble receptor; PSA, prostate-specific antigen.
Figure 3.
Levels of discrimination for some nomograms. LN, lymph node; OC, organ confined; IL-6sR, interleukin-6 soluble receptor; TGF-ß1, transforming growth factor ß1.
Promising New Markers: Molecular Forms of PSA
PSA circulates in the serum in multiple molecular forms of both free (unbound) and complexed (bound to protease inhibitors) forms (Figure 4).26,27 Approximately three fourths of the PSA found in serum is irreversibly bound to the protease inhibitor α1-antichymotrypsin (PSA-ACT) in a covalent 1:1 molar catalytically inactive complex. A lesser fraction of serum PSA is bound to either α2-macroglobulin (PSA-A2M) or α1-protease inhibitor (PSA-API, also called α1-antitrypsin or AAT). The complex formation with A2M, contrary to the complex formation with ACT, blocks access to the catalytic cleft of PSA for larger-sized protein substrates but not for small-sized peptide substrates. However, the PSA-A2M complex is difficult to measure, because it appears to be present at very low levels in vivo and because the steric conformation of the A2M molecule blocks access to the PSA epitopes that are the targets of currently available monoclonal antibody-based PSA assays. Low concentrations of PSA in complex with API have also been detected in blood.28
Figure 4.
Survey of the research development of the molecular forms of prostate-specific antigen (PSA): Approximate years of discovery are indicated on the left. Each box represents a different molecular form of PSA. The Bayer cPSA assay measures PSA bound to α1-antichymotrypsin (PSA-ACT) and PSA bound to α1-protease inhibitor (PSA-API). BPSA, BPH-associated free PSA; proPSA, precursor form of free PSA; “intact” PSA, other inactive and intact PSA, which also detects proPSA; PSA-A2M, PSA bound to α2-macroglobulin. Reprinted, with permission, from Stephan C et al. Urology. 2002;59:2–8.86
Between 5% and 45% of measured serum PSA exists in free, non-complexed forms (fPSA). The free non-complexed PSA is most likely catalytically inactive, since it remains either slowly reactive or unreactive. The composition of fPSA in blood manifests considerable structural heterogeneity and, as discussed below, novel fPSA isoforms seem to be the most promising candidate markers for improvement of tPSA’s clinical performance.
Free PSA
Since FDA approval of the Hybritech® Tandem-R fPSA test (Beckman Coulter, Fullerton, Calif) as an adjunct to tPSA testing in men with a serum tPSA concentration between 4 ng/mL and 10 ng/mL, requests for %fPSA (ie, [fPSA/tPSA] × 100) determination have likely increased. In men with a tPSA level between 4 ng/mL and 10 ng/mL, a higher %fPSA value indicates a lower probability of finding prostate cancer on biopsy and raises the likelihood that the elevation in tPSA is due to the presence of BPH.29,30
Using the Hybritech Tandem tPSA and fPSA assays, Catalona and colleagues29 reported findings of a multi-center (7 university medical centers) prospective trial using %fPSA to improve the specificity of tPSA testing. Using an fPSA-tPSA ratio cut-point of less than 25% for triggering a sextant prostate biopsy yielded a 95% sensitivity for prostate cancer detection and increased the specificity by 20% over PSA measurement alone.29 Thus, at the expense of missing 5% of the prostate cancer cases, 20% of the unnecessary biopsies could be avoided. In their receiver operating characteristic curves, the area under the curve (AUC) for %fPSA (0.72) was significantly higher than that for tPSA (0.53). However, in response to the realization that sextant biopsies misclassify up to one third of patients who have prostate cancer as being without cancer, a more recent evaluation of the utility of %fPSA in patients undergoing extended 10- or 12-core biopsy has suggested a lower diagnostic efficiency of %fPSA.31
Prostate biopsy is generally not recommended for patients with a PSA level less than 4 ng/mL, unless the patient is younger than 60 years or has abnormal findings on digital rectal examination. However, 13% to 20% of men with tPSA levels between 2.6 ng/mL and 4.0 ng/mL will have cancer detected in 3 to 5 years.32,33 Several authors have reported that %fPSA measurement allows the detection of prostate cancer in men with tPSA levels below 4 ng/mL.34,35 Catalona and colleagues34 reported on 914 consecutive male volunteers older than 50 years with tPSA levels of 2.6 ng/mL to 4.0 ng/mL. Among these men, 332 underwent a biopsy of the prostate, and cancer was detected in 22% of them. The authors determined that, with a %fPSA cutoff of 27% or less for performing a prostate biopsy, they were able to obtain a sensitivity of 90% and avoid 18% of unnecessary biopsies. In addition, 83% of the cancers detected were clinically significant.
Moreover, Catalona and colleagues36 developed models for identifying prostate cancer in men with tPSA levels between 2.51 ng/mL and 4.0 ng/mL in a retrospective analysis of archived serum samples. By choosing a %fPSA cutoff value between 10% and 15%, these authors demonstrated that a sensitivity of 30% to 54% could be achieved, with prostate biopsy recommended for only 9% to 36% of men in this group.
In summary, most investigators agree that %fPSA can improve the sensitivity and specificity of tPSA measurement in identifying men with prostate cancer when the tPSA concentration is between 4 ng/mL and 10 ng/mL. However, they do not agree on the most appropriate %fPSA cutoff value. In addition, there is no agreement on the range of tPSA values such that any value within this range would automatically trigger fPSA testing and determination of the %fPSA.
Data on the utility of %fPSA for the prediction of pathologic grade and stage of prostate cancer are inconclusive. The hypothesis is that lower %fPSA values are associated with more aggressive prostate cancers and metastasis. Several large studies have analyzed the potential role of %fPSA in the staging of prostate cancer. Carter and colleagues37 found that %fPSA was significantly lower in men who had aggressive disease (ie, stage T3, presence of bone or nodal metastases, positive surgical margins, or Gleason score 7 or greater) than in men who had nonaggressive disease. On the other hand, tPSA values were not associated with features of aggressive prostate cancer.
Several other studies confirmed the association between %fPSA and pathologic stage.38 For example, in a multicenter study involving 268 men with tPSA values between 4 ng/mL and 10 ng/mL who underwent radical prostatectomy, Southwick and colleagues38 found that %fPSA was a stronger predictor of postoperative pathologic outcome than Gleason score. In this study, a %fPSA cutoff value of 15% was found to discriminate optimally between favorable and unfavorable pathologic outcome. Seventy-five percent of men with a %fPSA value greater than 15% had organ-confined cancer, a Gleason score less than 7, and small tumors; these favorable pathologic characteristics were found in only 34% of men with a %fPSA value of 15% or less. Unfortunately, other studies have failed to validate these findings, demonstrating that when %fPSA values were adjusted for the effects of tPSA, Gleason score, and clinical stage, they did not provide additional staging or prognostic information.39,40
Although these divergent results remain unexplained, one possible explanation is that the staging utility of %fPSA, like its utility in discriminating between benign and malignant prostate disease, is highly dependent on multiple parameters, such as age, race, distribution of tPSA levels, study design, PSA assay manufacturer, and sample handling. Nevertheless, it is our view that fPSA measurement for the detection of prostate cancer enhances the specificity of the tPSA value while reducing the number of unnecessary prostate biopsies, thus subsequently reducing morbidity and cost to the health care system. However, fPSA is not an ideal marker for staging or prediction of prostate cancer progression.
Complexed PSA
PSA-ACT, which is the predominant form of complexed PSA (cPSA) in patients with prostate cancer,27 can be measured by an assay available from Roche Diagnostics (Mannheim, Germany). The Bayer Diagnostics (Tarrytown, NY) cPSA assay measures both PSA-ACT and PSA-API. Both markers have been studied in the setting of prostate cancer screening, and the results are similar to those of %fPSA when either of the following ratios are used: cPSA/tPSA or PSA-ACT/tPSA.41,42 However, no study has shown a clear advantage to measuring the PSA-ACT level alone or calculating the PSA-ACT/tPSA ratio compared with %fPSA to enhance the specificity of prostate cancer detection.41
Molecular Forms offPSA: BPSA and ProPSA Isoforms
In response to the quandary that %fPSA measurement was a clinically useful test without a clear understanding of its biologic basis, a series of prostate tissue studies were conducted aimed at better understanding the molecular forms of PSA found in normal peripheral zone, cancerous peripheral zone, and BPH-associated transition zone tissues.43,44 These studies culminated in the discovery of BPSA (“benign” PSA), a novel form of fPSA associated with nodular hyperplasia of the transition zone.45 The studies also demonstrated a clear association of truncated molecular forms of proPSA with the prostate peripheral zone, including prostate cancer.46 More recent studies using serum assays specific for these various molecular forms of fPSA have demonstrated that the majority of fPSA in the blood is made up of BPSA, truncated forms of proPSA, and an additional form of intact, yet inactive, PSA (Figure 5).
Figure 5.
Comparison of the enzymatically active prostate-specific antigen (PSA) in tissues and seminal plasma with the inactive forms of free PSA found in serum: Active PSA contains no internal peptide bond cleavages and forms a complex with α1-antichymotrypsin (PSA-ACT) in serum. proPSA is a precursor form of PSA that is expressed with a 7-amino acid N-terminus leader peptide but is found in serum containing from 1 to 7 amino acids. “Benign” PSA (BPSA) contains 2 internal peptide bond cleavages. The remainder of the inactive PSA in serum (iPSA) appears to be composed largely of intact, denatured PSA, although it may contain lesser amounts of internal or N-terminus cleavages. BPH, benign prostatic hyperplasia. Adapted, with permission, from Mikolajczyk SD et al. Urology. 2002;59:797–802.87
BPSA, which is elevated in the transition zone epithelium of prostates with nodular BPH, is predominantly clipped at amino-acid residues lysine 145–146 and lysine 182–183.45 Recent studies have shown that BPSA is also present in seminal plasma.47 A dual-monoclonal antibody assay for BPSA (detection limit of 0.06 ng/mL) has been evaluated in men with symptomatic BPH and those without clinical BPH, as well as in healthy men.48 The median BPSA level in patients with symptomatic BPH was significantly higher than that in patients without BPH symptoms. In the healthy control group, BPSA was almost undetectable.48
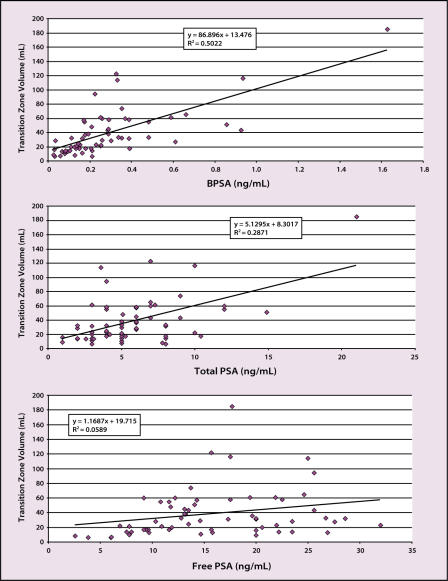
In a preliminary study involving a limited cohort of men with and without prostate cancer, Shariat and colleagues49 found that the serum BPSA level was highly correlated with transition zone and total prostate volume and increased with age (Figure 6). However, because men with prostate cancer may also have enlarged prostates and coexisting BPH, BPSA level alone would not be expected to distinguish accurately between prostate cancer and BPH.
Figure 6.
Correlation of prostate-specific antigen (PSA) forms with transition zone volume in 63 men (27 men with and 36 men without prostate cancer) (unpublished data). BPSA, “benign” PSA.
Ratios of BPSA to fPSA or BPSA to tPSA may prove to be useful prostate cancer staging tools, although they have not yet been studied as such; however, these measurements currently appear most promising as more specific serum markers for BPH.
Like most secreted peptide enzymes, PSA is produced initially as an inactive proPSA molecule that includes a 7-amino acid leader peptide sequence. Human kallikrein 2 (hK2) activates this proPSA molecule by clipping off the 7-amino acid leader peptide sequence. Prostate cancer tissues have been shown to contain levels of a truncated version of proPSA, containing either 2 ([-2]pPSA) or 4 ([-4]pPSA) unclipped amino acids from its leader sequence, higher than those in BPH-associated transition zone epithelium.46 The proPSA/fPSA ratio has demonstrated improved performance in differentiating prostate cancer from BPH in men with modestly elevated PSA levels.49 Studies have yet to be conducted to test the ability of any of the fPSA molecular forms to aid in the staging of prostate cancer. Nevertheless, the various molecular forms of fPSA and their respective ratios hold great promise as biochemical markers for prostate cancer diagnosis, staging, prediction, and monitoring.
The third form of fPSA found in the blood appears to be composed largely of nonclipped fPSA, called intact PSA, that is similar to native PSA except that it is enzymatically inactive.50–52 Nurmikko and colleagues52 recently reported on a newly developed assay that measures intact PSA and proPSA but not BPSA (detection limit, 0.035 ng/mL). Although the absolute levels of the marker detected by this monoclonal assay did not differentiate between the presence or absence of cancer in 383 patients, the ratio of this marker to fPSA was significantly higher in patients with cancer.52
In summary, the area of fPSA molecular isoforms holds the promise to provide powerful new tools for detection, staging, prediction of prognosis, and monitoring of prostate cancer. In addition, BPSA level alone or in combination with fPSA or tPSA level may be useful in studying the development and clinical progression of BPH, as well as response to therapy.
Human Glandular Kallikrein
The human kallikrein family of proteases consists of 15 members, 12 of which have been characterized only recently.53 Structurally, hK2 and PSA (hK3) share the highest homology, with 78% and 80% sequence identity at the amino acid and DNA levels, respectively. Like PSA, hK2 is expressed in various tissues, but its highest level of expression is found in the prostate.53 However, hK2 and PSA differ in their enzymatic activity, with hK2 manifesting trypsin-like substrate specificity. hK2 can activate the zymogen form of urokinase and can generate enzymatically active PSA from the full-length [-7]pPSA.54
In seminal plasma, hK2 cleaves the gel-forming proteins semenogelin I, semenogelin II, and fibronectin.55 hK2 protein levels in both seminal plasma and serum are less than 3% of that of tPSA; however, at the mRNA level, hK2 expression is only about half that of PSA expression.56 Like PSA, hK2 forms complexes with various plasma protease inhibitors, such as α1-antichymotrypsin, α2-antiplasmin, antithrombin III, plasminogen activator inhibitor-1, α2-macroglobulin, and protease inhibitor 6. However, unlike PSA, most of the hK2 in serum is found in the free, unbound form. hK2 bound to α1-antichymotrypsin represents only 4% to 19% of the total hK2.57
The ratio of serum levels of hK2 to fPSA has been shown to enhance prostate cancer detection in patients with serum tPSA concentrations of 2 ng/mL to 4 ng/mL, as well as 4 ng/mL to 10 ng/mL.58 This has been confirmed in studies conducted by Magklara and colleagues59 showing that circulating levels of hK2 enhance the biochemical detection of prostate cancer when combined with fPSA and tPSA measurements. The utility of hK2 level in the preoperative staging of clinically localized prostate cancer remains controversial. In a multi-institutional study, Haese and colleagues60 found that the AUC for the algorithm of (hK2) × (tPSA/fPSA) was significantly superior to that of PSA, which was not different from that of hK2 alone. Whereas mean tPSA and fPSA levels did not differ between patients with stage pT2a/b cancer and those with stage pT3a or greater, both mean hK2 levels and the mean of the results of the algorithm did.
Insulin-Like Growth Factor Family
The local expression of insulin-like growth factors (IGFs) and IGF-binding proteins (IGFBPs) has been associated with tumor grade, pathologic stage, and disease progression in patients with prostate cancer.61–63 Epidemiologic studies have found high circulating IGF-I levels and, in some studies, low IGFBP-3 levels to be associated with an increased risk of prostate cancer.64,65
Using serum from a case-control cohort in the Baltimore Longitudinal Study on Aging population, Harman and colleagues66 found a marginally significantly increased risk of prostate cancer associated with higher serum IGF-I levels. However, tPSA was a far more powerful predictor of prostate cancer than IGF-I, and IGF-I measurement did not add significantly to the diagnostic accuracy of tPSA measurement. Numerous studies have found no difference in IGF-I levels between men with prostate cancer and cancer-free controls.18,67 Furthermore, circulating levels of IGF-I were not associated with established markers of biologically aggressive disease, disease progression, or metastasis in patients with clinically localized prostate cancer.18,68
In contrast, circulating levels of IGFBP-2, the main IGFBP produced by prostate epithelial cells, are significantly elevated in patients with prostate cancer.68–70 However, in men with clinically localized prostate cancer, IGFBP-2 levels were inversely associated with prostatic tumor volume and with features of advanced disease (eg, higher final Gleason score, extraprostatic extension, and seminal vesicle involvement) but remained higher than in men without prostate cancer.68
Circulating levels of IGFBP-3, the primary carrier for IGF-I in the blood, have been shown to be lowest in patients with bony metastases but no different in men with non-metastatic prostate cancer versus healthy men.68–70 Lower preoperative IGFBP-2 and IGFBP-3 levels were associated with a higher risk of disease progression when adjusted for the effects of preoperative PSA level, biopsy-determined Gleason score, and clinical stage in consecutive patients undergoing radical prostatectomy for clinically localized prostate cancer.68
In summary, whereas the major significance of IGF-I appears to be restricted to its association with cancer development during subclinical disease stages, the IGF binding proteins appear to play a more direct role in prostate cancer detection and prognosis. Specifically, IGFBP-2 levels appear to be directly associated with the presence of prostate cancer and inversely associated with the progression from early to more advanced stages of disease. IGFBP-3 levels appear to be inversely associated with the establishment and progression of prostate cancer skeletal metastases.
Transforming Growth Factor ß1
Increased local expression of TGF-ß1 has been associated with higher tumor grade, tumor invasion, and metastatic progression in patients with prostate cancer.71–73 Higher circulating TGF-ß1 levels have been associated with established markers of biologically aggressive cancer (ie, higher preoperative PSA, extracapsular extension, seminal vesicle involvement, and lymph node involvement),13,74,75 clinically evident metastases13,17,74 and occult metastases,13,75 and biochemical progression.13,75 However, circulating levels of TGF-ß1 did not differ between healthy persons and prostate cancer patients.13
Elevated plasma levels of TGF-ß1 in patients with clinically evident or occult metastatic prostate cancer seem to result either from direct production from foci of metastatic tumors or from the host’s response to cancer invasion and dissemination, and not necessarily as the result of production by the primary tumor. Taken together, these data suggest that preoperative and early postoperative plasma TGF-ß1 measurements could be used in combination with standard preoperative and postoperative parameters to improve the accuracy of nomograms designed to predict pathologic stage and disease progression after primary therapy for prostate cancer. Therefore, we developed and internally validated a prognostic model that adds plasma TGF-ß1 and IL-6 soluble receptor (see below) to standard clinical predictors to determine whether we could improve on the level of accuracy achieved by our standard, externally validated pretreatment nomogram.22
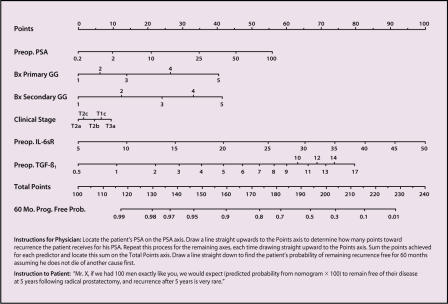
The new nomogram was a better predictor of the risk of disease progression 5 years after radical prostatectomy for clinically localized prostate cancer (Figure 7). Addition of pretreatment TGF-ß1 and IL-6 soluble receptors to the nomogram improved the prediction of biochemical recurrence by a statistically and prognos-tically substantial margin over our previously published nomogram,21 increasing the bootstrap-corrected concordance index from 0.75 to 0.84. After successful external validation, this nomogram could become a valuable tool for counseling patients who are considering radical prostatectomy. The incorporation of these molecular markers may improve prognostic tools for other prostate cancer treatment modalities as well.
Figure 7.
Improved preoperative nomogram including 2 molecular biomarkers-interleukin-6 soluble receptor (IL-6sR) and transforming growth factor ß1 (TGF-ß1)-to a core group of clinical variables for predicting prostate-specific antigen (PSA) recurrence after radical prostatectomy based on 713 patients. Bx, biopsy; GG, Gleason grade. Adapted, with permission, from Kattan M et al. J Clin Oncol. 2003;21:3573–3579.22
Interleukin-6 and Its Receptor
In vitro and in vivo studies have shown that human prostate cancer expresses both IL-6 and its receptor (IL-6R), allowing for establishment of an autocrine/paracrine loop.76–78 Furthermore, IL-6 protein concentrations are approximately 18 times higher in clinically localized prostate cancers than in normal prostate tissue.77 The concentration of IL-6R is also higher in prostate cancer than in normal prostate tissue.77 Elevated circulating levels of IL-6 and soluble IL-6R have been associated with features of aggressive prostate cancer (higher PSA levels, greater prostatic tumor volume, and higher final Gleason sum),75,79 advanced disease stage,17,79,80 presence of distant metastases and metastasis-related morbidity,17,79–82 overall and aggressive disease progression,79 and decreased survival.80
Similarly to TGF-ß1, circulating levels of IL-6 and soluble IL-6R did not differ between healthy men and prostate cancer patients.79 Unlike preoperative circulating levels of TGF-ß1, which were associated with features of locally invasive disease, preoperative circulating levels of IL-6 and soluble IL-6R were associated with pathologic grade of disease but not with extraprostatic extension or seminal vesicle invasion. This, in aggregate with other findings,75 suggests that, in patients with cancer, the elevated circulating levels of IL-6 and soluble IL-6R are produced primarily by tumor cells in the primary prostate cancer. Furthermore, circulating levels of IL-6 and soluble IL-6R appear to be associated with the potential of prostate cancer to metastasize but not with the metastases themselves.
As mentioned above, Kattan and colleagues22 developed and internally validated a preoperative nomogram that allows accurate prediction of the probability of cancer recurrence after radical prostatectomy for localized prostate cancer using clinical stage, Gleason grade, serum PSA level, and plasma levels of soluble IL-6R and TGF-ß1.
Urokinase System of Plasminogen Activation
Urokinase plasminogen activator (uPA) and its inhibitor PAI-1 are the only novel prognostic biomarkers validated at the highest level of evidence (both prospective randomized trial and pooled analysis) with regard to their clinical utility in breast cancer. Levels of circulating uPA and its receptor were shown to be higher in prostate cancer patients than in healthy persons, and the highest circulating levels of uPA and its receptor were found in patients with metastases to bones but not regional lymph nodes.83–85 When evaluated in preoperative blood of patients undergoing radical prostatectomy, levels of uPA and its receptor were associated with extraprostatic disease, seminal vesicle involvement, prostatic tumor volume and, most important, disease progression.84,85 The association with PSA progression presumably was the result of an association with occult prostate cancer metastases to bone already present at the time of radical prostatectomy.
Conclusions
Over the past 15 years, PSA measurement has revolutionized the diagnosis and management of prostate cancer. However, the changing demographics of prostate cancer make it more likely that testing for markers other than tPSA will be necessary to manage prostate cancer most effectively. Several new markers have shown promise in phase 1 biomarker studies (eg, BPSA, proPSA, uPA and its receptor, and VEGF). Phase 2 biomarker studies (eg, studies of com-plexed PSA, hK2, TGF-ß1, and soluble IL-6R) and phase 3 studies are planned to confirm their performance.
There is an urgent need to establish national multidisciplinary initiatives for coordinating the activities of prostate cancer biomarker research, developing laboratory quality-control programs for the analysis of cancer biomarkers, and producing guidelines for appropriate clinical employment of each biomarker. The adoption of a systematic, phased, and nomogram-based model is a rational way to manage the evaluation of the plethora of newly proposed biomarkers for prostate cancer, especially as this list continues to grow.
Main Points.
Nomograms predict outcome probabilities for a patient by simultaneously considering multiple aspects of the patient’s cancer. They also can be used for patient counseling, follow-up scheduling, and clinical trial design and analysis.
Free PSA (fPSA) measurement can enhance the specificity of the total PSA (tPSA) value for detection of prostate cancer, while reducing the number of unnecessary prostate biopsies; fPSA is not, however, an ideal marker for staging or prediction of prostate cancer progression.
BPSA, or “benign” PSA, is a recently discovered form of fPSA associated with nodular hyperplasia of the transition zone. Ratios of BPSA to fPSA or BPSA to tPSA could prove to be useful prostate cancer staging tools; however, they currently appear most promising as more specific serum markers for benign prostatic hyperplasia.
Levels of the insulin-like growth factor-binding protein IGFBP-2 appear to be directly associated with the presence of prostate cancer and inversely associated with the progression from early to advanced disease. IGFBP-3 levels appear to be inversely associated with the establishment and progression of prostate cancer skeletal metastases.
By adding plasma transforming growth factor ß1 and interleukin-6 soluble receptor measurements to standard clinical predictors, researchers at Baylor College of Medicine demonstrated improved performance of an existing nomogram in predicting biochemical recurrence of prostate cancer.
References
- 1.Darson MF, Pacelli A, Roche P, et al. Human glandular kallikrein 2 (hK2) expression in prostatic intraepithelial neoplasia and adenocarcinoma: a novel prostate cancer marker. Urology. 1997;49:857–862. doi: 10.1016/s0090-4295(97)00108-8. [DOI] [PubMed] [Google Scholar]
- 2.Aihara M, Lebovitz RM, Wheeler TM, et al. Prostate specific antigen and gleason grade: an immunohistochemical study of prostate cancer. J Urol. 1994;151:1558–1564. doi: 10.1016/s0022-5347(17)35302-8. [DOI] [PubMed] [Google Scholar]
- 3.Babaian RJ, Fritsche HA, Evans RB. Prostate-specific antigen and prostate gland volume: correlation and clinical application. J Clin Lab Anal. 1990;4:135–137. doi: 10.1002/jcla.1860040212. [DOI] [PubMed] [Google Scholar]
- 4.Kane RA, Littrup PJ, Babaian R, et al. Prostate-specific antigen levels in 1695 men without evidence of prostate cancer: findings of the American Cancer Society National Prostate Cancer Detection Project. Cancer. 1992;69:1201–1207. doi: 10.1002/cncr.2820690522. [DOI] [PubMed] [Google Scholar]
- 5.Roehrborn CG, Boyle P, Gould AL, Waldstreicher J. Serum prostate-specific antigen as a predictor of prostate volume in men with benign prostatic hyperplasia. Urology. 1999;53:581–589. doi: 10.1016/s0090-4295(98)00655-4. [DOI] [PubMed] [Google Scholar]
- 6.Roehrborn CG, McConnell J, Bonilla J, et al. Serum prostate specific antigen is a strong predictor of future prostate growth in men with benign prostatic hyperplasia. J Urol. 2000;163:13–20. [PubMed] [Google Scholar]
- 7.Roehrborn CG, Malice M, Cook TJ, Girman CJ. Clinical predictors of spontaneous acute urinary retention in men with LUTS and clinical BPH: a comprehensive analysis of the pooled placebo groups of several large clinical trials. Urology. 2001;58:210–216. doi: 10.1016/s0090-4295(01)01155-4. [DOI] [PubMed] [Google Scholar]
- 8.Roehrborn CG, McConnell JD, Lieber M, et al. Serum prostate-specific antigen concentration is a powerful predictor of acute urinary retention and need for surgery in men with clinical benign prostatic hyperplasia. Urology. 1999;53:473–480. doi: 10.1016/s0090-4295(98)00654-2. [DOI] [PubMed] [Google Scholar]
- 9.Noguchi M, Stamey TA, McNeal JE, Yemoto CM. Preoperative serum prostate specific antigen does not reflect biochemical failure rates after radical prostatectomy in men with large volume cancers. J Urol. 2000;164:1596–1600. [PubMed] [Google Scholar]
- 10.Stamey TA, Johnstone IM, McNeal JE, et al. Preoperative serum prostate specific antigen levels between 2 and 22 ng./ml. correlate poorly with post-radical prostatectomy cancer morphology: prostate specific antigen cure rates appear constant between 2 and 9 ng./ml. J Urol. 2002;167:103–111. [PubMed] [Google Scholar]
- 11.Stamey TA, Yang N, Hay AR, et al. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med. 1987;317:909–916. doi: 10.1056/NEJM198710083171501. [DOI] [PubMed] [Google Scholar]
- 12.Partin AW, Carter HB, Chan DW, et al. Prostate specific antigen in the staging of localized prostate cancer: influence of tumor differentiation, tumor volume and benign hyperplasia. J Urol. 1990;143:747–752. doi: 10.1016/s0022-5347(17)40079-6. [DOI] [PubMed] [Google Scholar]
- 13.Shariat SF, Shalev M, Menesses-Diaz A, et al. Preoperative plasma levels of transforming growth factor beta1 (TGF-ß1) strongly predict progression in patients undergoing radical prostatectomy. J Clin Oncol. 2001;19:2856–2864. doi: 10.1200/JCO.2001.19.11.2856. [DOI] [PubMed] [Google Scholar]
- 14.Shariat SF, Gottenger E, Nguyen C, et al. Preoperative blood reverse transcriptase-PCR assays for prostate-specific antigen and human glandular kallikrein for prediction of prostate cancer progression after radical prostatectomy. Cancer Res. 2002;62:5974–5979. [PubMed] [Google Scholar]
- 15.Hayes DF, Bast RC, Desch CE, et al. Tumor marker utility grading system: a framework to evaluate clinical utility of tumor markers. J Natl Cancer Inst. 1996;88:1456–1466. doi: 10.1093/jnci/88.20.1456. [DOI] [PubMed] [Google Scholar]
- 16.Simon R. Design and conduct of clinical trials. In: DeVita V Jr, Hellman S, Rosenberg S, editors. Cancer: Principles and Practice of Oncology. 40th ed. Phildelphia: Lippincott Williams & Wilkins; 1993. pp. 418–440. [Google Scholar]
- 17.Adler HL, McCurdy MA, Kattan MW, et al. Elevated levels of circulating interleukin-6 and transforming growth factor-beta 1 in patients with metastatic prostatic carcinoma. J Urol. 1999;161:182–187. [PubMed] [Google Scholar]
- 18.Shariat SF, Bergamaschi F, Adler HL, et al. Correlation of preoperative plasma IGF-I levels with pathologic parameters and progression in patients undergoing radical prostatectomy. Urology. 2000;56:423–429. doi: 10.1016/s0090-4295(00)00648-8. [DOI] [PubMed] [Google Scholar]
- 19.Kattan MW, Wheeler TM, Scardino PT. Postoperative nomogram for disease recurrence after radical prostatectomy for prostate cancer. J Clin Oncol. 1999;17:1499–1507. doi: 10.1200/JCO.1999.17.5.1499. [DOI] [PubMed] [Google Scholar]
- 20.Kattan MW, Potters L, Blasko JC, et al. Pretreatment nomogram for predicting freedom from recurrence after permanent prostate brachytherapy in prostate cancer. Urology. 2001;58:393–399. doi: 10.1016/s0090-4295(01)01233-x. [DOI] [PubMed] [Google Scholar]
- 21.Kattan MW, Eastham JA, Stapleton AM, et al. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. 1998;90:766–771. doi: 10.1093/jnci/90.10.766. [DOI] [PubMed] [Google Scholar]
- 22.Kattan MW, Shariat SF, Andrews B, et al. The addition of interleukin-6 soluble receptor and transforming growth factor beta1 improves a preoperative nomogram for predicting recurrence in patients with clinically localized prostate cancer. J Clin Oncol. 2003;21:3573–3579. doi: 10.1200/JCO.2003.12.037. [DOI] [PubMed] [Google Scholar]
- 23.Kattan MW, Zelefsky MJ, Kupelian PA, et al. Pretreatment nomogram for predicting the outcome of three-dimensional conformal radiotherapy in prostate cancer. J Clin Oncol. 2000;18:3352–3359. doi: 10.1200/JCO.2000.18.19.3352. [DOI] [PubMed] [Google Scholar]
- 24.Graefen M, Karakiewicz PI, Cagiannos I, et al. Validation study of the accuracy of a postoperative nomogram for recurrence after radical prostatectomy for localized prostate cancer. J Clin Oncol. 2002;20:951–956. doi: 10.1200/JCO.2002.20.4.951. [DOI] [PubMed] [Google Scholar]
- 25.Graefen M, Karakiewicz PI, Cagiannos I, et al. International validation of a preoperative nomogram for prostate cancer recurrence after radical prostatectomy. J Clin Oncol. 2002;20:3206–3212. doi: 10.1200/JCO.2002.12.019. [DOI] [PubMed] [Google Scholar]
- 26.Stenman UH, Leinonen J, Alfthan H, et al. A complex between prostate-specific antigen and alpha 1-antichymotrypsin is the major form of prostate-specific antigen in serum of patients with prostatic cancer: assay of the complex improves clinical sensitivity for cancer. Cancer Res. 1991;51:222–226. [PubMed] [Google Scholar]
- 27.Lilja H, Christensson A, Dahlen U, et al. Prostate-specific antigen in serum occurs predominantly in complex with alpha 1-antichymotrypsin. Clin Chem. 1991;37:1618–1625. [PubMed] [Google Scholar]
- 28.Finne P, Zhang WM, Auvinen A, et al. Use of the complex between prostate specific antigen and αl-protease inhibitor for screening prostate cancer. J Urol. 2000;164:1956–1960. [PubMed] [Google Scholar]
- 29.Catalona WJ, Partin AW, Slawin KM, et al. Use of the percentage of free prostate-specific antigen to enhance differentiation of prostate cancer from benign prostatic disease: a prospective multicenter clinical trial. JAMA. 1998;279:1542–1547. doi: 10.1001/jama.279.19.1542. [DOI] [PubMed] [Google Scholar]
- 30.Woodrum DL, Brawer MK, Partin AW, et al. Interpretation of free prostate specific antigen clinical research studies for the detection of prostate cancer. J Urol. 1998;159:5–12. doi: 10.1016/s0022-5347(01)63996-x. [DOI] [PubMed] [Google Scholar]
- 31.Singh H, Canto EI, Shariat SF, et al. Systematic 12-core biopsy degrades performance of percent free PSA and PSA density for prostate cancer detection. J Urol. doi: 10.1097/01.ju.0000134619.72675.8d. In press. [DOI] [PubMed] [Google Scholar]
- 32.Gann PH, Hennekens CH, Stampfer MJ. A prospective evaluation of plasma prostate-specific antigen for detection of prostatic cancer. JAMA. 1995;273:289–294. [PubMed] [Google Scholar]
- 33.Smith DS, Catalona WJ, Herschman JD. Longitudinal screening for prostate cancer with prostate-specific antigen. JAMA. 1996;276:1309–1315. [PubMed] [Google Scholar]
- 34.Catalona WJ, Smith DS, Ornstein DK. Prostate cancer detection in men with serum PSA concentrations of 2.6 to 4.0 ng/mL and benign prostate examination: enhancement of specificity with free PSA measurements. JAMA. 1997;277:1452–1465. [PubMed] [Google Scholar]
- 35.Lodding P, Aus G, Bergdahl S, et al. Characteristics of screening detected prostate cancer in men 50 to 66 years old with 3 to 4 ng./ml. prostate specific antigen. J Urol. 1998;159:899–903. [PubMed] [Google Scholar]
- 36.Catalona WJ, Partin AW, Finlay JA, et al. Use of percentage of free prostate-specific antigen to identify men at high risk of prostate cancer when PSA levels are 2.51 to 4 ng/mL and digital rectal examination is not suspicious for prostate cancer: an alternative model. Urology. 1999;54:220–224. doi: 10.1016/s0090-4295(99)00185-5. [DOI] [PubMed] [Google Scholar]
- 37.Carter HB, Partin AW, Luderer AA, et al. Percentage of free prostate-specific antigen in sera predicts aggressiveness of prostate cancer a decade before diagnosis. Urology. 1997;49:379–384. doi: 10.1016/s0090-4295(96)00629-2. [DOI] [PubMed] [Google Scholar]
- 38.Southwick PC, Catalona WJ, Partin AW, et al. Prediction of post-radical prostatectomy pathological outcome for stage T1c prostate cancer with percent free prostate specific antigen: a prospective multicenter clinical trial. J Urol. 1999;162:1346–1351. [PubMed] [Google Scholar]
- 39.Jung K, Brux B, Lein M, et al. Molecular forms of prostate-specific antigen in malignant and benign prostatic tissue: biochemical and diagnostic implications. Clin Chem. 2000;46:47–54. [PubMed] [Google Scholar]
- 40.Graefen M, Karakiewicz PI, Cagiannos I, et al. Percent free prostate specific antigen is not an independent predictor of organ confinement or prostate specific antigen recurrence in unscreened patients with localized prostate cancer treated with radical prostatectomy. J Urol. 2002;167:1306–1309. [PubMed] [Google Scholar]
- 41.Stamey TA, Yemoto CE. Examination of the 3 molecular forms of serum prostate specific antigen for distinguishing negative from positive biopsy: relationship to transition zone volume. J Urol. 2000;163:119–126. [PubMed] [Google Scholar]
- 42.Lein M, Jung K, Elgeti U, et al. Ratio of alpha 1-antichymotrypsin-prostate specific antigen to total prostate specific antigen in prostate cancer diagnosis. Anticancer Res. 2000;20:4997–5001. [PubMed] [Google Scholar]
- 43.Song W, Wheeler TM, Nguyen C, Slawin KM. Western blot and immunohistochemical analysis demonstrates abundant alpha-1-antichymotrypsin protein in both peripheral and transition zone prostate tissue in men with normal sized and enlarged prostates [abstract] J Urol. 1997;157(suppl):436. [Google Scholar]
- 44.Slawin KM, Song W, Bergamaschi F, et al. Quantitative analysis of PSA in normal, hyper-plastic and cancerous prostate tissue. Presented at: American Association for Cancer Research Special Conference: New Research Approaches in the Prevention and Cure of Prostate Cancer; December 2–6, 1998; Indian Wells, Calif.. [Google Scholar]
- 45.Mikolajczyk SD, Millar LS, Wang TJ, et al. “BPSA,” a specific molecular form of free prostate-specific antigen, is found predominantly in the transition zone of patients with nodular benign prostatic hyperplasia. Urology. 2000;55:41–45. doi: 10.1016/s0090-4295(99)00372-6. [DOI] [PubMed] [Google Scholar]
- 46.Mikolajczyk SD, Millar LS, Wang TJ, et al. A precursor form of prostate-specific antigen is more highly elevated in prostate cancer compared with benign transition zone prostate tissue. Cancer Res. 2000;60:756–759. [PubMed] [Google Scholar]
- 47.Mikolajczyk SD, Millar LS, Marker KM, et al. Seminal plasma contains “BPSA,” a molecular form of prostate-specific antigen that is associated with benign prostatic hyperplasia. Prostate. 2000;45:271–276. doi: 10.1002/1097-0045(20001101)45:3<271::aid-pros11>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 48.Marks LS, Llanes AS, Linton HJ, et al. BPSA is a potential serum marker for benign prostatic hyperplasia [abstract] J Urol. 2001;165(suppl):266. [Google Scholar]
- 49.Shariat S, Mikolajczyk S, Singh H, et al. Preoperative serum levels of pro-PSA isoforms are associated with biologically aggressive prostate cancer. Presented at: Society of Urologic Oncology Third Annual Meeting: Extraordinary Opportunities for Discovery; December 13–14, 2002; Bethesda, Md. [Google Scholar]
- 50.Zhang WM, Leinonen J, Kalkkinen N, et al. Purification and characterization of different molecular forms of prostate-specific antigen in human seminal fluid. Clin Chem. 1995;41:1567–1573. [PubMed] [Google Scholar]
- 51.Kumar A, Mikolajczyk SD, Hill TM, et al. Different proportions of various prostate-specific antigen (PSA) and human kallikrein 2 (hK2) forms are present in noninduced and androgen-induced LNCaP cells. Prostate. 2000;44:248–254. doi: 10.1002/1097-0045(20000801)44:3<248::aid-pros10>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 52.Nurmikko P, Pettersson K, Piironen T, et al. Discrimination of prostate cancer from benign disease by plasma measurement of intact, free prostate-specific antigen lacking an internal cleavage site at Lys145–Lys146. Clin Chem. 2001;47:1415–1423. [PubMed] [Google Scholar]
- 53.Yousef GM, Diamandis EP. The new human tissue kallikrein gene family: structure, function, and association to disease. Endocr Rev. 2001;22:184–204. doi: 10.1210/edrv.22.2.0424. [DOI] [PubMed] [Google Scholar]
- 54.Mikolajczyk SD, Millar LS, Kumar A, Saedi MS. Human glandular kallikrein, hK2, shows argi-nine-restricted specificity and forms complexes with plasma protease inhibitors. Prostate. 1998;34:44–50. doi: 10.1002/(sici)1097-0045(19980101)34:1<44::aid-pros6>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 55.Deperthes D, Frenette G, Brillard-Bourdet M, et al. Potential involvement of kallikrein hK2 in the hydrolysis of the human seminal vesicle proteins after ejaculation. J Androl. 1996;17:659–665. [PubMed] [Google Scholar]
- 56.Klee GG, Goodmanson MK, Jacobsen SJ, et al. Highly sensitive automated chemiluminometric assay for measuring free human glandular kallikrein-2. Clin Chem. 1999;45:800–806. [PubMed] [Google Scholar]
- 57.Becker C, Piironen T, Kiviniemi J, et al. Sensitive and specific immunodetection of human glandular kallikrein 2 in serum. Clin Chem. 2000;46:198–206. [PubMed] [Google Scholar]
- 58.Partin AW, Catalona WJ, Finlay JA, et al. Use of human glandular kallikrein 2 for the detection of prostate cancer: preliminary analysis. Urology. 1999;54:839–845. doi: 10.1016/s0090-4295(99)00270-8. [DOI] [PubMed] [Google Scholar]
- 59.Magklara A, Scorilas A, Catalona WJ, Diamandis EP. The combination of human glandular kallikrein and free prostate-specific antigen (PSA) enhances discrimination between prostate cancer and benign prostatic hyperplasia in patients with moderately increased total PSA. Clin Chem. 1999;45:1960–1966. [PubMed] [Google Scholar]
- 60.Haese A, Graefen M, Steuber T, et al. Human glandular kallikrein 2 levels in serum for discrimination of pathologically organ-confined from locally-advanced prostate cancer in total PSA-levels below 10 ng/ml. Prostate. 2001;49:101–109. doi: 10.1002/pros.1123. [DOI] [PubMed] [Google Scholar]
- 61.Thrasher JB, Tennant MK, Twomey PA, et al. Immunohistochemical localization of insulin-like growth factor binding proteins 2 and 3 in prostate tissue: clinical correlations. J Urol. 1996;155:999–1003. [PubMed] [Google Scholar]
- 62.Tennant MK, Thrasher JB, Twomey PA, et al. Insulin-like growth factor-binding protein-2 and -3 expression in benign human prostate epithelium, prostate intraepithelial neoplasia, and adenocarcinoma of the prostate. J Clin Endocrinol Metab. 1996;81:411–420. doi: 10.1210/jcem.81.1.8550786. [DOI] [PubMed] [Google Scholar]
- 63.Figueroa JA, De Raad S, Tadlock L, et al. Differential expression of insulin-like growth factor binding proteins in high versus low Gleason score prostate cancer. J Urol. 1998;159:1379–1383. [PubMed] [Google Scholar]
- 64.Wolk A, Mantzoros CS, Andersson SO, et al. Insulin-like growth factor 1 and prostate cancer risk: a population-based, case-control study. J Natl CancerInst. 1998;90:911–915. doi: 10.1093/jnci/90.12.911. [DOI] [PubMed] [Google Scholar]
- 65.Chan JM, Stampfer MJ, Giovannucci E, et al. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279:563–566. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- 66.Harman SM, Metter EJ, Blackman MR, et al. Serum levels of insulin-like growth factor I (IGF-I), IGF-II, IGF-binding protein-3, and prostate-specific antigen as predictors of clinical prostate cancer. J Clin Endocrinol Metab. 2000;85:4258–4265. doi: 10.1210/jcem.85.11.6990. [DOI] [PubMed] [Google Scholar]
- 67.Finne P, Auvinen A, Koistinen H, et al. Insulin-like growth factor I is not a useful marker of prostate cancer in men with elevated levels of prostate-specific antigen. J Clin Endocrinol Metab. 2000;85:2744–2747. doi: 10.1210/jcem.85.8.6725. [DOI] [PubMed] [Google Scholar]
- 68.Shariat SF, Lamb DJ, Kattan MW, et al. Association of preoperative plasma levels of insulin-like growth factor I and insulin-like growth factor binding proteins-2 and -3 with prostate cancer invasion, progression, and metastasis. J Clin Oncol. 2002;20:833–841. doi: 10.1200/JCO.2002.20.3.833. [DOI] [PubMed] [Google Scholar]
- 69.Kanety H, Madjar Y, Dagan Y, et al. Serum insulin-like growth factor-binding protein-2 (IGFBP-2) is increased and IGFBP-3 is decreased in patients with prostate cancer: correlation with serum prostate-specific antigen. J Clin Endocrinol Metab. 1993;77:229–233. doi: 10.1210/jcem.77.1.7686915. [DOI] [PubMed] [Google Scholar]
- 70.Cohen P, Peehl DM, Stamey TA, et al. Elevated levels of insulin-like growth factor-binding protein-2 in the serum of prostate cancer patients. J Clin Endocrinol Metab. 1993;76:1031–1035. doi: 10.1210/jcem.76.4.7682560. [DOI] [PubMed] [Google Scholar]
- 71.Steiner MS, Barrack ER. Transforming growth factor-beta 1 overproduction in prostate cancer: effects on growth in vivo and in vitro. Mol Endocrinol. 1992;6:15–25. doi: 10.1210/mend.6.1.1738367. [DOI] [PubMed] [Google Scholar]
- 72.Truong LD, Kadmon D, McCune BK, et al. Association of transforming growth factor-beta 1 with prostate cancer: an immunohistochemical study. Hum Pathol. 1993;24:4–9. doi: 10.1016/0046-8177(93)90055-l. [DOI] [PubMed] [Google Scholar]
- 73.Shariat SF, Menesses AD, Kim IY, et al. Tissue expression of transforming growth factor beta1 and its receptors: correlation with pathologic features and biochemical progression in patients undergoing radical prostatectomy. Urology. doi: 10.1016/j.urology.2003.12.015. In press. [DOI] [PubMed] [Google Scholar]
- 74.Ivanovic V, Melman A, Davis-Joseph B, et al. Elevated plasma levels of TGF-beta 1 in patients with invasive prostate cancer. Nat Med. 1995;1:282–284. doi: 10.1038/nm0495-282. [DOI] [PubMed] [Google Scholar]
- 75.Shariat SF, Kattan MW, Troxel E, et al. Association of pre- and postoperative plasma levels of transforming growth factor ß1 and interleukin 6 and its soluble receptor with prostate cancer progression. Clin Cancer Res. 2004;10:1992–1999. doi: 10.1158/1078-0432.ccr-0768-03. [DOI] [PubMed] [Google Scholar]
- 76.Hobisch A, Eder IE, Putz T, et al. Interleukin-6 regulates prostate-specific protein expression in prostate carcinoma cells by activation of the androgen receptor. Cancer Res. 1998;58:4640–4645. [PubMed] [Google Scholar]
- 77.Giri D, Ozen M, Ittmann M. Interleukin-6 is an autocrine growth factor in human prostate cancer. Am J Pathol. 2001;159:2159–2165. doi: 10.1016/S0002-9440(10)63067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chung TD, Yu JJ, Spiotto MT, et al. Characterization of the role of IL-6 in the progression of prostate cancer. Prostate. 1999;38:199–207. doi: 10.1002/(sici)1097-0045(19990215)38:3<199::aid-pros4>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 79.Shariat SF, Andrews B, Kattan MW, et al. Plasma levels of interleukin-6 and its soluble receptor are associated with prostate cancer progression and metastasis. Urology. 2001;58:1008–1015. doi: 10.1016/s0090-4295(01)01405-4. [DOI] [PubMed] [Google Scholar]
- 80.Nakashima J, Tachibana M, Horiguchi Y, et al. Serum interleukin 6 as a prognostic factor in patients with prostate cancer. Clin Cancer Res. 2000;6:2702–2706. [PubMed] [Google Scholar]
- 81.Twillie DA, Eisenberger MA, Carducci MA, et al. Interleukin-6: a candidate mediator of human prostate cancer morbidity. Urology. 1995;45:542–549. doi: 10.1016/S0090-4295(99)80034-X. [DOI] [PubMed] [Google Scholar]
- 82.Wise GJ, Marella VK, Talluri G, Shirazian D. Cytokine variations in patients with hormone treated prostate cancer. J Urol. 2000;164:722–725. doi: 10.1097/00005392-200009010-00024. [DOI] [PubMed] [Google Scholar]
- 83.Hienert G, Kirchheimer JC, Pfluger H, Binder BR. Urokinase-type plasminogen activator as a marker for the formation of distant metastases in prostatic carcinomas. J Urol. 1998;140:1466–1469. doi: 10.1016/s0022-5347(17)42074-x. [DOI] [PubMed] [Google Scholar]
- 84.Miyake H, Hara I, Yamanaka K, et al. Elevation of serum levels of urokinase-type plasminogen activator and its receptor is associated with disease progression and prognosis in patients with prostate cancer. Prostate. 1999;39:123–129. doi: 10.1002/(sici)1097-0045(19990501)39:2<123::aid-pros7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 85.Shariat SF, Sadeghi F, Canto EI, et al. Association of the urokinase system of plasminogen activation with prostate cancer presence, invasion, progression, and metastasis. Urology. In press. [Google Scholar]
- 86.Stephan C, Jung K, Diamandis EP, et al. Prostate-specific antigen, its molecular forms, and other kallikrein markers for detection of prostate cancer. Urology. 2002;59:2–8. doi: 10.1016/s0090-4295(01)01449-2. [DOI] [PubMed] [Google Scholar]
- 87.Mikolajczyk SD, Marks LS, Partin AW, Rittenhouse HG. Free prostate-specific antigen in serum is becoming more complex. Urology. 2002;59:797–802. doi: 10.1016/s0090-4295(01)01605-3. [DOI] [PubMed] [Google Scholar]