Abstract
OBJECTIVE
The complement system plays a central role in the first line of defense against invading pathogens, and its activation involves the release of potent pro-inflammatory mediators such as anaphylatoxins C3a, C4a and C5a. The aim of this study was to determine whether differences existed in maternal plasma anaphylatoxin concentrations between patients with term and preterm parturition.
STUDY DESIGN
A cross-sectional study was designed to determine the plasma anaphylatoxin concentrations in 296 pregnant women in the following groups: 1) normal pregnancy between 20–36 6/7 weeks (n=64); 2) term not in labor (n=70); 3) term in labor (n=60); and 4) preterm labor with intact membranes (n=102). Women with preterm labor were classified into: a) term delivery (n=24); b) preterm delivery without intra-amniotic infection (IAI) (n=62); and c) preterm delivery with IAI (n=16). Concentrations of C3a, C4a and C5a were determined by ELISAs. Statistical analysis was conducted with non-parametric methods.
RESULTS
1) The median plasma C5a concentration was lower in women at term in labor than in those not in labor (p<0.001). In contrast, there were no differences in plasma C3a and C4a concentrations between the two groups (p>0.05). 2) Among patients with preterm labor, those with IAI had a higher median plasma C5a concentration than those without IAI and those who delivered at term (post-hoc tests p<0.001 and p=0.01, respectively). When comparing the preterm labor subgroups with normal pregnancy, only women with preterm delivery and IAI had a median plasma C5a concentration higher than that of normal pregnant women (Kruskal-Wallis p<0.001, post hoc test p<0.001) There was no difference in the plasma C4a concentration among patients with preterm labor. The median plasma C3a concentration in patients with preterm labor with IAI was significantly higher than in those without IAI (Kruskal-Wallis p=0.01, and post-hoc test p=0.005). There was no difference in the plasma C3a concentrations between women with preterm labor who delivered at term and those with preterm delivery, with or without IAI. In addition, no differences were observed in the median plasma C3a concentration between women with normal pregnancy and those in each of the preterm labor subgroups.
CONCLUSIONS
The maternal plasma concentration of anaphylatoxin C5a is increased in women with preterm labor and IAI, but not in spontaneous labor at term.
Keywords: Parturition, human labor, preterm labor, intra-amniotic infection, anaphylatoxin, C3a, C4a, C5a
INTRODUCTION
The complement system is a key component of the innate immunity,[15,56] and is also involved in the regulation of the adaptive immune response.[5] This system is formed by a group of plasma proteins with catalytic properties that react in a sequential manner, yielding active biological mediators and lytic components to clear microorganisms and “non-self” cells.[15,34,56] Activation of complement through “the classical,” “the alternative,” and “the mannan binding lectin” (MBL) pathways[55,56] leads to the generation of complement split products (C3a, C4a and C5a).[11] These bioactive fragments, known as anaphylatoxins, can induce smooth muscle contraction,[6,9,21] enhance vascular permeability,[6,21,46] and attract white blood cells.[8,24,47] Apart from their role in host defense, uncontrolled or excessive production of anaphylatoxins have been implicated in the pathogenesis of inflammatory diseases including sepsis,[7,36,57] asthma,[28,37] rheumatoid arthritis,[22,35] adult respiratory distress syndrome,[50] ischemia reperfusion injury,[59] and pregnancy loss.[18]
Preterm birth is one of the leading causes of neonatal mortality and morbidity.[14,33,49] A solid body of clinical and epidemiologic evidence implicates systemic and intrauterine infection in the etiology of premature labor and delivery.[4,20] However, other processes associated with the preterm parturition syndrome include utero-placental ischemia, uterine over-distention, allergy, cervical insufficiency, endocrine diseases, and stress disorders.[43]
Phenotypic and metabolic changes of monocytes and granulocytes consistent with intravascular inflammation have been demonstrated in women with preterm labor and intact membranes,[16] as well as in those with preterm premature rupture of membranes.[17] Activated neutrophils and monocytes can generate C5a[26,53,54,58] and this, in turn, may exacerbate the inflammatory response.[57] There is a paucity of information regarding the behavior of anaphylatoxins during human parturition. This study was conducted to determine whether maternal plasma concentrations of anaphylatoxins change during spontaneous preterm and term labor.
PATIENTS AND METHODS
Study design
A cross-sectional study was conducted by searching our clinical database and bank of biological samples. This study included 296 women in the following four groups: 1) normal pregnancy between 20–366/7 weeks (n=64); 2) normal pregnancy at term not in labor (n=70); 3) normal pregnancy at term in labor (n=60); and 4) preterm labor with intact membranes (n=102). The inclusion criteria for normal pregnancy consisted of: 1) no medical, obstetrical or surgical complications; 2) no labor; and 3) delivery of a neonate (≥37 weeks) with a birthweight appropriate for gestational age (10th and 90th percentile).[3] Normal pregnant women were enrolled from either a labor/delivery unit (in cases of scheduled cesarean section) or an antenatal clinic. All were followed until delivery. Normal pregnant women at term had a gestational age ≥ 37 weeks. Women with normal pregnancy at term in labor were defined with similar criteria to those of normal pregnant women at term, but with spontaneous labor. Preterm labor was characterized by the presence of regular uterine contractions occurring at a frequency of at least 2 every 10 minutes, with cervical changes that led to delivery at <37 completed weeks of gestation. This group included only patients who had transabdominal amniocentesis performed within 24 hours of maternal blood sampling. Women with preterm labor and intact membranes were classified into: a) term delivery without intra-amniotic infection (IAI) (n=24); b) preterm delivery without IAI (n=62); and c) preterm delivery with IAI (n=16). IAI was defined as a positive amniotic fluid culture for microorganisms. All women provided written informed consent prior to the collection of the plasma sample. The collection of samples was approved by the IRBs of both Wayne State University and the National Institute of Child Health and Human Development (NIH). Many of these samples have been used in previous studies.
Blood Collection
Samples of peripheral blood were collected in tubes containing EDTA (ethylene diamine tetraacetic acid). The samples were centrifuged and stored at −70º C. Specific and sensitive enzyme-linked immunoassays (ELISAs) were used to determine the concentrations of complement C3a, C4a and C5a. Immunoassay systems for C3a and C4a were obtained from Assay Designs, Inc. (Ann Arbor, MI). C5a immunoassays were obtained from American Laboratory Products Company (Windham, NH). Complement C3a, C4a and C5a assays were performed following the manufacturers’ recommendations. Briefly, maternal plasma samples were incubated in duplicate wells of microtiter plates, which had been pre-coated with antigen specific (C3a, C4a or C5a) antibodies. Complement C3a, C4a or C5a present in the standards or maternal plasma samples were immobilized by their specific pre-coated antibodies (forming antigen antibody complexes) during this incubation. Repeated washing and aspiration were conducted to remove unbound materials from the assay plates. This step was followed by incubation with a specific antibody-enzyme reagent. Following a wash step to remove excess unbound materials, a substrate solution was added to the wells of the microtiter plates, and color developed in proportion to the amount of antigen bound in the initial step of the individual assay. The color development was stopped with the addition of an acid solution, and the intensity of color was read using a programmable microtiter plate spectrophotometer (Ceres 900 Microplate Workstation, Bio-Tek Instruments, Winooski, VT). The concentrations of complement C3a, C4a or C5a in maternal plasma samples were determined by interpolation from individual standard curves composed of purified human C3a, C4a or C5a. The calculated inter-assay coefficients of variation (CV) for C3a, C4a and C5a immunoassays in our laboratory were 5.4%, 6.1% and 4.0%, respectively. Calculated intra-assay CV for C3a, C4a and C5a were 6.6%, 6.9% and 2.3%, respectively. The detection limit (sensitivity) was 0.13 ng/ml for C3a, 0.32 ng/ml for C4a and 0.06 ng/ml for C5a.
Statistical Analysis
Shapiro-Wilk tests were used to test for normal distribution of the data. A Kruskal-Wallis test was utilized for multiple comparisons. Mann-Whitney U tests were used for post-hoc comparisons with Bonferroni correction of the alpha in order to maintain the overall probability of a type I error at 0.05. Chi square was used to compare proportions. The statistical package employed was SPSS 12 (SPSS Inc., Chicago, IL). A p value of <0.05 was considered significant.
RESULTS
The clinical and obstetrical characteristics of women with normal term pregnancies are displayed in Table 1. Normal pregnant women at term in labor (20 – 36 6/7 weeks) had a median gestational age at blood sampling and delivery higher than those without labor (p<0.001 and p=0.008, respectively). In contrast, maternal age was significantly higher in women at term gestation and no labor than those in labor (p=0.01).
Table 1.
Clinical and obstetrical characteristics of women at term gestation not in labor and in labor
| Term gestation no labor | Term gestation in labor | P | |
|---|---|---|---|
| n = 70 | n = 60 | ||
| Maternal age (y) | 27 (17–40) | 24 (15–36) | 0.01* |
| Nulliparity | 14 (20) | 18 (30) | 0.2 |
| Smoking | 11 (15.7) | 15 (25) | 0.2 |
| Gestational age at venipuncture (wks) | 39.1 (37–41.7) | 40 (37–41.8) | <0.001* |
| Gestational age at delivery (wks) | 39.2 (37.1–42.4) | 40 (37.1–41.8) | 0.008* |
| Birthweight (g) | 3365 (2850–4080) | 3335 (2800–3950) | 0.2 |
Values are expressed as median (range) or number (percent)
Statistically significant, P < 0.05
Labor did not have a significant effect on maternal plasma concentrations of C3a and C4a in normal women delivering at term (p=0.8 and p=0.1, respectively; Figures 1A and B). Pregnant women at term in labor, however, had a lower median plasma C5a concentration than those at term not in labor (p<0.001; Figure 1C).
Figure 1.
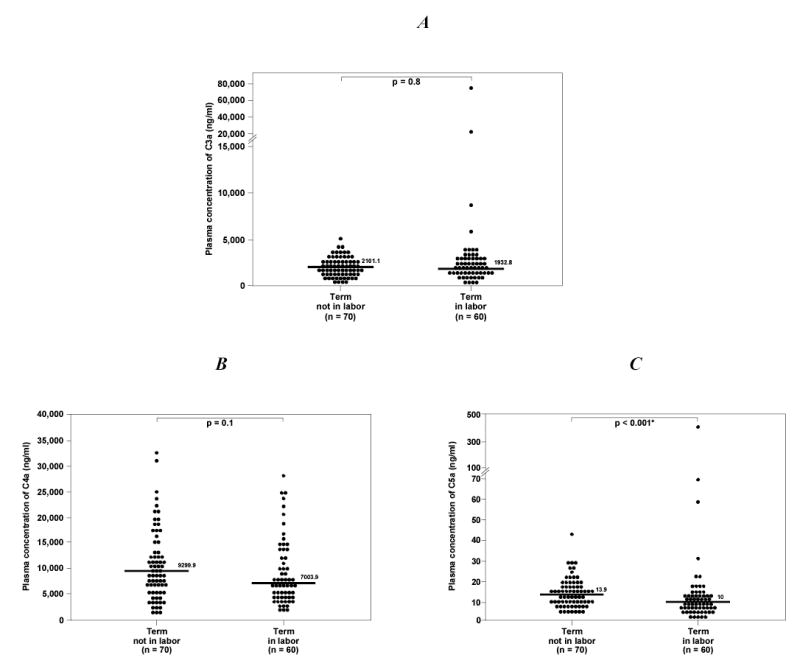
Plasma anaphylatoxin concentrations of normal pregnant women at term. A. There was no difference in the median plasma C3a concentration between women at term in labor and those not in labor [median: 1932.8 ng/ml (range 337.9 – 74300) vs. median: 2101.1 ng/ml (range 582.8 – 5103.6), p=0.8]. B, Similarly, the plasma C4a concentration did not differ between pregnant women at term in labor and those not in labor [median: 7003.9 ng/ml (range 2038.9 – 27910) vs. median: 9299.9 ng/ml (range 1284.1 – 32640), p=0.1]. C, In contrast, the median plasma C5a concentration of normal pregnant women at term in labor was lower than those not in labor [median: 10 ng/ml (range 1.9 – 428.3) vs. median: 13.9 ng/ml (range 5.4 – 43), p<0.001].
The clinical and obstetrical characteristics of women with normal pregnancy not in labor and those with preterm labor and intact membranes are displayed in Table 2. According to the study design, gestational age at delivery and neonatal birthweight were significantly different between the two groups (p<0.001 for each). No differences were observed in the median plasma C3a and C4a concentrations between patients with preterm labor and those with normal pregnancy (p=0.4 and p=0.08, respectively; Figures 2A and B). In contrast, women with preterm labor had a median plasma C5a concentration higher than normal pregnant women (p=0.003; Figure 2C).
Table 2.
Clinical and obstetrical characteristics of normal pregnant women without labor and patients with preterm labor and intact membranes
| Normal pregnancy no labor n = 64 | Preterm labor n = 102 | P | |
|---|---|---|---|
| Maternal age (y) | 23 (17–34) | 23 (13–39) | 0.4 |
| Nulliparity | 23 (35.9) | 41 (40.2) | 0.6 |
| Smokinga | 12 (19) | 30 (29.7) | 0.1 |
| Gestational age at venipuncture (wks) | 30.7 (20–36.8) | 29.8 (20.1–33.7) | 0.1 |
| Gestational age at delivery (wks) | 39.5 (37–42) | 32.8 (21–41.4) | <0.001* |
| Birthweight (g)b | 3325 (2610–4030) | 1870 (400–3750) | <0.001* |
Values are expressed as median (range) or number (percent)
Statistically significant, P < 0.05
Normal pregnancy no labor (n=63); preterm labor (n=101)
Preterm labor (n=100)
Figure 2.
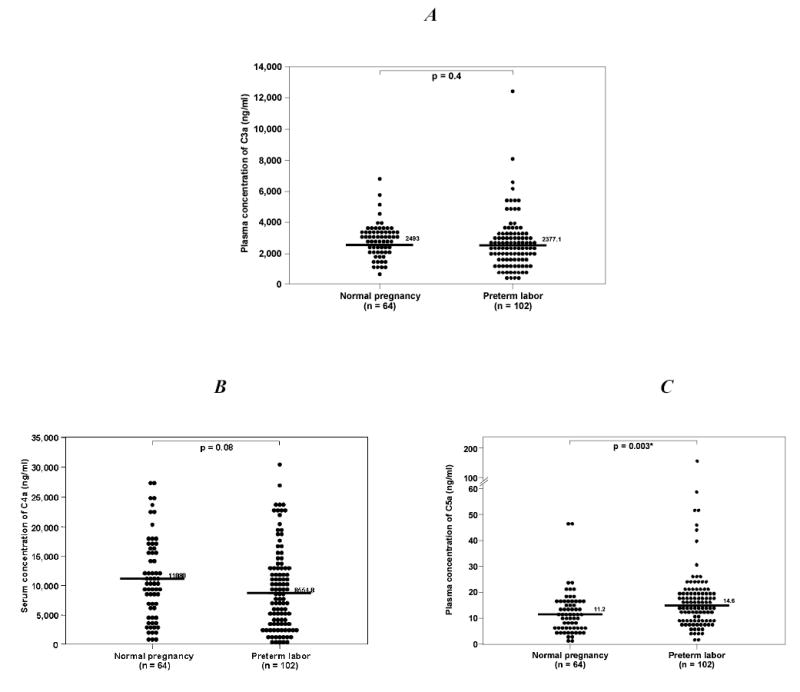
Plasma anaphylatoxin concentrations of normal pregnant women (20–36 6/7 weeks) and patients with preterm labor. A. There was no difference in the median plasma C3a concentration of normal pregnant women and those with preterm labor [median: 2493 ng/ml (range 557.9 – 6642.7) vs. median: 2377.1 ng/ml (range 436.1 – 12690), p=0.4]. B, Similarly, no difference in the median plasma C4a concentration was observed between normal pregnant women and those with preterm labor [median: 11080 ng/ml (range 850.7 – 27850) vs. median: 8554.8 ng/ml (range 436.2 – 30560), p=0.08]. C, In contrast, the median plasma C5a concentration of patients with preterm labor was higher than that of normal pregnant women [median: 14.6 ng/ml (range 1.9 – 166.8) vs. median: 11.2 ng/ml (range 1.2 – 87.1), p<0.003].
Among patients with preterm labor, those with IAI had a higher median plasma C5a concentration than those without IAI who delivered preterm, as well as those who delivered at term (Kruskal-Wallis p<0.001; post-hoc tests p<0.001 and p=0.01, respectively; Figure 3C). No difference in the plasma C5a concentration was observed between patients with preterm delivery without IAI and those with preterm labor who delivered at term (p=0.4; Figure 3C). Comparisons between the three subgroups of preterm labor and the group with normal pregnancy showed that only patients with preterm delivery and IAI had a median plasma C5a concentration higher than that of normal pregnant women (Kruskal-Wallis p<0.001, post hoc test p<0.001; Figure not shown). The maternal plasma C4a concentration was similar in all subgroups of patients with preterm labor (Figure 3B). The median plasma C3a concentration of patients with preterm delivery with IAI was higher than that of those with preterm delivery without IAI (Kruskal-Wallis p=0.01 and post-hoc test p=0.005; Figure 3A). No difference in the median plasma C3a concentration was found between women with preterm labor who delivered at term and those who delivered preterm, with or without IAI (all post-hoc tests p=0.1; Figure 3A). Similarly, no differences were observed in the plasma C3a concentrations between women with normal pregnancy and those in each of the preterm labor subgroups.
Figure 3.
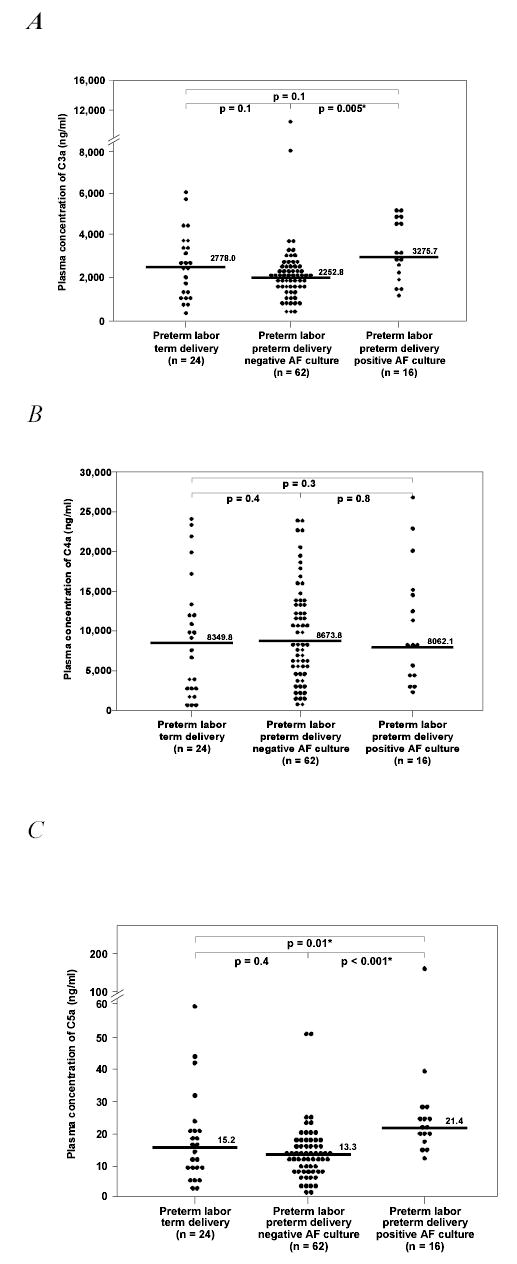
Plasma anaphylatoxin concentrations among patients with preterm labor. A, Patients with preterm labor and IAI had a median plasma C3a concentration higher than those without IAI [median: 3275.7 ng/ml (range 1305.8 – 5541.9) vs. median: 2252.8 ng/ml range 436.1 – 12690), Kruskal-Wallis p=0.01, post hoc p=0.005]. There was no difference in the median C3a concentration between patients with preterm labor who delivered at term and those who delivered preterm, whether or not they had IAI. B, There was no difference in the median plasma C4a concentration among patients with preterm labor (Kruskal-Wallis p=0.6). C, Patients with preterm labor and IAI had a median plasma C5a concentration higher than those without IAI [median: 21.4 ng/ml (range 12.5 – 166.8) vs. median: 13.3 ng/ml (range 1.91 – 51.9) Kruskal-Wallis p<0.001 post hoc p<0.001] and patients with preterm labor who delivered at term [median: 15.2 ng/ml (range 2.2 – 59.1), post hoc p=0.01]. There was no difference in the median plasma C5a concentration between patients with preterm labor who delivered at term and those who delivered preterm without IAI.
DISCUSSION
Our study demonstrates that spontaneous human parturition at term is associated with lower median maternal plasma C5a concentrations than term gestation not in labor. In contrast, women with preterm labor and IAI have higher median maternal plasma C5a concentrations than those with preterm labor without IAI.
The median maternal plasma concentrations of the anaphylatoxins C3a, C4a and C5a are higher than that of non-pregnant women in the secretory phase of the menstrual cycle.[41] Moreover, the plasma concentrations of anaphylatoxins do not change with increasing gestational age (20 weeks to term gestation).[41] We have proposed that these findings may represent a normal adaptation during pregnancy, in which the activity of the innate immune system is enhanced to compensate for the reduced adaptive immunity considered necessary for the tolerance of the conceptus.[41]
A previous study reported no changes in maternal plasma C3 and C4 concentrations through the last trimester of pregnancy and labor.[32] Similarly, in our study, maternal plasma C3a and C4a concentrations did not change during labor. On the other hand, maternal plasma C5a concentration at term was significantly lower in women with labor than in those not in labor. Since human parturition has been linked to an inflammatory process[30,31,52] with an increase in the maternal neutrophil count[48] and proinflammatory cytokine concentrations including IL-1β, IL-6 and IL-8,[25] we expected an increase in the maternal plasma C5a concentration. Surprisingly, the results of the study do not support this view. Further studies will be required to determine both the clinical significance and the mechanisms responsible for the lower maternal plasma C5a concentrations observed in normal human labor at term.
Huffacker et al.[27] reported that the maternal serum complement hemolytic activity (CH50) in women with preterm labor was similar between those who delivered preterm and those who delivered at term. These results, however, should be interpreted with caution since the CH50 assay is an insensitive marker of complement activation.[1]
The current study shows that the median maternal plasma C5a concentration is higher in patients with preterm labor associated with IAI than in those with preterm labor without infection delivering preterm or at term. This finding suggests that the maternal immune system is responding to either microbial products or pro-inflammatory mediators produced in the uterine cavity, and such response can be detected in the maternal compartment. It is possible that enzymes from activated phagocytic cells can cleave C5 and contribute to the increased maternal concentration of C5a.[26,53,54,58] Furthermore, C5a may induce degranulation of inflammatory cells,[8,23,51] enhance respiratory burst with generation of reactive oxygen species in leukocytes,[10,19,60] delay neutrophil apoptosis,[40] and up-regulate the expression of adhesion molecules from granulocytes[29] and endothelial cells[13] to combat intrauterine infection. Another function of C5a is the induction and/or release of inflammatory cytokines from neutrophils, mononuclear and endothelial cells. [2,12,38,39,42,44,45] Altogether, these properties of C5a have been associated with the induction of a potent inflammatory response and the prolongation of the life span of neutrophils in the presence of antigens. The lack of change in C4a suggests that the classical and MBL pathways may not be the main complement routes activated in women presenting with preterm labor and IAI.[1]
In conclusion, term and preterm labor are associated with different profiles of maternal plasma anaphylatoxin concentrations. At term, maternal plasma C5a concentration is lower in patients with spontaneous labor than in those not in labor. In contrast, patients with preterm labor with IAI hade higher plasma C5a concentrations than those without IAI delivering preterm or at term. Our study provides further evidence that maternal intravascular inflammation is present in patients with preterm delivery and IAI.
Acknowledgments
This research was supported by the Intramural Program of the National Institute of Child Health and Human Development, NIH.
References
- 1.Ahmed AE, Peter JB. Clinical utility of complement assessment. Clin Diagn Lab Immunol. 1995;2:509. doi: 10.1128/cdli.2.5.509-517.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albrecht EA, Chinnaiyan AM, Varambally S, Kumar-Sinha C, Barrette TR, Sarma JV, Ward PA. C5a-induced gene expression in human umbilical vein endothelial cells. Am J Pathol. 2004;164:849. doi: 10.1016/S0002-9440(10)63173-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 4.Amon E: Preterm Labor. In: Reece E, Hobbins J: Medicine of the Fetus and Mother. Lippincontt-Robin, Philladelphia 1999
- 5.Carroll MC. The complement system in regulation of adaptive immunity. Nat Immunol. 2004;5:981. doi: 10.1038/ni1113. [DOI] [PubMed] [Google Scholar]
- 6.Cochrane CG, Muller-Eberhard HJ. The derivation of two distinct anaphylatoxin activities from the third and fifth components of human complement. J Exp Med. 1968;127:371. doi: 10.1084/jem.127.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Czermak BJ, Sarma V, Pierson CL, Warner RL, Huber-Lang M, Bless NM, Schmal H, Friedl HP, Ward PA. Protective effects of C5a blockade in sepsis. Nat Med. 1999;5:788. doi: 10.1038/10512. [DOI] [PubMed] [Google Scholar]
- 8.Daffern PJ, Pfeifer PH, Ember JA, Hugli TE. C3a is a chemotaxin for human eosinophils but not for neutrophils. I. C3a stimulation of neutrophils is secondary to eosinophil activation. J Exp Med. 1995;181:2119. doi: 10.1084/jem.181.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dias DS, Lepow IH. Complement as a mediator of inflammation. II. Biological properties of anaphylatoxin prepared with purified components of human complement. J Exp Med. 1967;125:921. doi: 10.1084/jem.125.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehrengruber MU, Geiser T, Deranleau DA. Activation of human neutrophils by C3a and C5A. Comparison of the effects on shape changes, chemotaxis, secretion, and respiratory burst. FEBS Lett. 1994;346:181. doi: 10.1016/0014-5793(94)00463-3. [DOI] [PubMed] [Google Scholar]
- 11.Ember JA, MA Jagels, TE Hugli: Characterization of Complement Anaphylatoxins and Their Biological Responses. In: Volanakis JE, Frank MM: The Human Complement System in Health and Disease. Marcel Dekker, INC., New York 1998
- 12.Ember JA, Sanderson SD, Hugli TE, Morgan EL. Induction of interleukin-8 synthesis from monocytes by human C5a anaphylatoxin. Am J Pathol. 1994;144:393. [PMC free article] [PubMed] [Google Scholar]
- 13.Foreman KE, Vaporciyan AA, Bonish BK, Jones ML, Johnson KJ, Glovsky MM, Eddy SM, Ward PA. C5a-induced expression of P-selectin in endothelial cells. J Clin Invest. 1994;94:1147. doi: 10.1172/JCI117430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foulder-Hughes LA, Cooke RWI. Motor, cognitive, and behavioural disorders in children born very preterm. Developmental Medicine and Child Neurology. 2003;45:97. [PubMed] [Google Scholar]
- 15.Gasque P. Complement: a unique innate immune sensor for danger signals. Mol Immunol. 2004;41:1089. doi: 10.1016/j.molimm.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Gervasi MT, Chaiworapongsa T, Naccasha N, Blackwell S, Yoon BH, Maymon E, Romero R. Phenotypic and metabolic characteristics of maternal monocytes and granulocytes in preterm labor with intact membranes. Am J Obstet Gynecol. 2001;185:1124. doi: 10.1067/mob.2001.117681. [DOI] [PubMed] [Google Scholar]
- 17.Gervasi MT, Chaiworapongsa T, Naccasha N, Pacora P, Berman S, Maymon E, Kim JC, Kim YM, Yoshimatsu J, Espinoza J, Romero R. Maternal intravascular inflammation in preterm premature rupture of membranes. J Matern Fetal Neonatal Med. 2002;11:171. doi: 10.1080/jmf.11.3.171.175. [DOI] [PubMed] [Google Scholar]
- 18.Girardi G, Berman J, Redecha P, Spruce L, Thurman JM, Kraus D, Hollmann TJ, Casali P, Caroll MC, Wetsel RA, Lambris JD, Holers VM, Salmon JE. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J Clin Invest. 2003;112:1644. doi: 10.1172/JCI18817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldstein IM, Roos D, Kaplan HB, Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975;56:1155. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomez R, Ghezzi F, Romero R, Munoz H, Tolosa JE, Rojas I. Premature labor and intra-amniotic infection. Clinical aspects and role of the cytokines in diagnosis and pathophysiology. Clin Perinatol. 1995;22:281. [PubMed] [Google Scholar]
- 21.Gorski JP, Hugli TE, Muller-Eberhard HJ. C4a the third anaphylatoxin of the human complement system. Proc Natl Acad Sci U S A. 1979;76:5299. doi: 10.1073/pnas.76.10.5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grant EP, Picarella D, Burwell T, Delaney T, Croci A, Avitahl N, Humbles AA, Gutierrez-Ramos JC, Briskin M, Gerard C, Coyle AJ. Essential role for the C5a receptor in regulating the effector phase of synovial infiltration and joint destruction in experimental arthritis. J Exp Med. 2002;196:1461. doi: 10.1084/jem.20020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haeger M, Unander M, Norder-Hansson B, Tylman M, Bengtsson A. Complement, neutrophil, and macrophage activation in women with severe preeclampsia and the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Obstet Gynecol. 1992;79:19. [PubMed] [Google Scholar]
- 24.Hartmann K, Henz BM, Kruger-Krasagakes S, Kohl J, Burger R, Guhl S, Haase I, Lippert U, Zuberbier T. C3a and C5a stimulate chemotaxis of human mast cells. Blood. 1997;89:2863. [PubMed] [Google Scholar]
- 25.Hebisch G, Neumaier-Wagner PM, Huch R, von Mandach U. Maternal serum interleukin-1 beta, -6 and -8 levels and potential determinants in pregnancy and peripartum. J Perinat Med. 2004;32:475. doi: 10.1515/JPM.2004.131. [DOI] [PubMed] [Google Scholar]
- 26.Huber-Lang M, Younkin EM, Sarma JV, Riedemann N, McGuire SR, Lu KT, Kunkel R, Younger JG, Zetoune FS, Ward PA. Generation of C5a by phagocytic cells. Am J Pathol. 2002;161:1849. doi: 10.1016/S0002-9440(10)64461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huffaker J, Witkin SS, Cutler L, Druzin ML, Ledger WJ. Total complement activity in maternal sera, amniotic fluids and cord sera in women with premature labor, premature rupture of membranes or chorioamnionitis. Surg Gynecol Obstet. 1989;168:397. [PubMed] [Google Scholar]
- 28.Humbles AA, Lu B, Nilsson CA, Lilly C, Israel E, Fujiwara Y, Gerard NP, Gerard C. A role for the C3a anaphylatoxin receptor in the effector phase of asthma. Nature. 2000;406:998. doi: 10.1038/35023175. [DOI] [PubMed] [Google Scholar]
- 29.Jagels MA, Daffern PJ, Hugli TE. C3a and C5a enhance granulocyte adhesion to endothelial and epithelial cell monolayers epithelial and endothelial priming is required for C3a-induced eosinophil adhesion. Immunopharmacology. 2000;46:209. doi: 10.1016/s0162-3109(99)00178-2. [DOI] [PubMed] [Google Scholar]
- 30.Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and parturition--a review. Placenta. 2003;24(Suppl A):S33. doi: 10.1053/plac.2002.0948. [DOI] [PubMed] [Google Scholar]
- 31.Kelly RW. Inflammatory mediators and parturition. Rev Reprod. 1996;1:89. doi: 10.1530/ror.0.0010089. [DOI] [PubMed] [Google Scholar]
- 32.Kovar IZ, Riches PG. C3 and C4 complement components and acute phase proteins in late pregnancy and parturition. J Clin Pathol. 1988;41:650. doi: 10.1136/jcp.41.6.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathews TJ, Menacker F, MacDorman MF. Infant mortality statistics from the 2002 period linked birth/infant death data set. Natl Vital Stat Rep. 2004;53:1. [PubMed] [Google Scholar]
- 34.Medzhitov R, Janeway CA., Jr Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 35.Moxley G, Ruddy S. Elevated plasma C3 anaphylatoxin levels in rheumatoid arthritis patients. Arthritis Rheum. 1987;30:1097. doi: 10.1002/art.1780301003. [DOI] [PubMed] [Google Scholar]
- 36.Nakae H, Endo S, Inada K, Yoshida M. Chronological changes in the complement system in sepsis. Surg Today. 1996;26:225. doi: 10.1007/BF00311579. [DOI] [PubMed] [Google Scholar]
- 37.Nakano Y, Morita S, Kawamoto A, Suda T, Chida K, Nakamura H. Elevated complement C3a in plasma from patients with severe acute asthma. J Allergy Clin Immunol. 2003;112:525. doi: 10.1016/s0091-6749(03)01862-1. [DOI] [PubMed] [Google Scholar]
- 38.Okusawa S, Dinarello CA, Yancey KB, Endres S, Lawley TJ, Frank MM, Burke JF, Gelfand JA. C5a induction of human interleukin 1. Synergistic effect with endotoxin or interferon-gamma. J Immunol. 1987;139:2635. [PubMed] [Google Scholar]
- 39.Okusawa S, Yancey KB, van der Meer JW, Endres S, Lonnemann G, Hefter K, Frank MM, Burke JF, Dinarello CA, Gelfand JA. C5a stimulates secretion of tumor necrosis factor from human mononuclear cells in vitro. Comparison with secretion of interleukin 1 beta and interleukin 1 alpha. J Exp Med. 1988;168:443. doi: 10.1084/jem.168.1.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perianayagam MC, Balakrishnan VS, King AJ, Pereira BJ, Jaber BL. C5a delays apoptosis of human neutrophils by a phosphatidylinositol 3-kinase-signaling pathway. Kidney Int. 2002;61:456. doi: 10.1046/j.1523-1755.2002.00139.x. [DOI] [PubMed] [Google Scholar]
- 41.Richani K, Soto E, Espinoza J, Nien J, Chaiworapongsa T, Edwin S, Mazor M, Romero R. Normal pregnancy is characterized by systemic complement activation of the complement system. Am J Obstet Gynecol. 2004;191:S139. doi: 10.1080/14767050500072722. (abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riedemann NC, Guo RF, Hollmann TJ, Gao H, Neff TA, Reuben JS, Speyer CL, Sarma JV, Wetsel RA, Zetoune FS, Ward PA. Regulatory role of C5a in LPS-induced IL-6 production by neutrophils during sepsis. FASEB J. 2004;18:370. doi: 10.1096/fj.03-0708fje. [DOI] [PubMed] [Google Scholar]
- 43.Romero R, J Espinoza, M Mazor, T Chaiworapongsa: The preterm parturition syndrome. In: Critchley C, Bennet P, Thornton S: Preterm Birth. RCOG Press, London 2004
- 44.Schindler R, Gelfand JA, Dinarello CA. Recombinant C5a stimulates transcription rather than translation of interleukin-1 (IL-1) and tumor necrosis factor translational signal provided by lipopolysaccharide or IL-1 itself. Blood. 1990;76:1631. [PubMed] [Google Scholar]
- 45.Scholz W, McClurg MR, Cardenas GJ, Smith M, Noonan DJ, Hugli TE, Morgan EL. C5a-mediated release of interleukin 6 by human monocytes. Clin Immunol Immunopathol. 1990;57:297. doi: 10.1016/0090-1229(90)90043-p. [DOI] [PubMed] [Google Scholar]
- 46.Schumacher WA, Fantone JC, Kunkel SE, Webb RC, Lucchesi BR. The anaphylatoxins C3a and C5a are vasodilators in the canine coronary vasculature in vitro and in vivo. Agents Actions. 1991;34:345. doi: 10.1007/BF01988727. [DOI] [PubMed] [Google Scholar]
- 47.Shin HS, Snyderman R, Friedman E, Mellors A, Mayer MM. Chemotactic and anaphylatoxic fragment cleaved from the fifth component of guinea pig complement. Science. 1968;162:361. doi: 10.1126/science.162.3851.361. [DOI] [PubMed] [Google Scholar]
- 48.Siegel I, Gleicher N. Peripheral white blood cell alterations in early labor. Diagn Gynecol Obstet. 1981;3:123. [PubMed] [Google Scholar]
- 49.Slattery MM, Morrison JJ. Preterm delivery. Lancet. 2002;360:1489. doi: 10.1016/S0140-6736(02)11476-0. [DOI] [PubMed] [Google Scholar]
- 50.Stevens JH, O'Hanley P, Shapiro JM, Mihm FG, Satoh PS, Collins JA, Raffin TA. Effects of anti-C5a antibodies on the adult respiratory distress syndrome in septic primates. J Clin Invest. 1986;77:1812. doi: 10.1172/JCI112506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takafuji S, Tadokoro K, Ito K, Dahinden CA. Degranulation from human eosinophils stimulated with C3a and C5a. Int Arch Allergy Immunol. 1994;104(Suppl 1):27. doi: 10.1159/000236743. [DOI] [PubMed] [Google Scholar]
- 52.Thomson AJ, Telfer JF, Young A, Campbell S, Stewart CJ, Cameron IT, Greer IA, Norman JE. Leukocytes infiltrate the myometrium during human parturition further evidence that labour is an inflammatory process. Hum Reprod. 1999;14:229. [PubMed] [Google Scholar]
- 53.Vogt W. Complement activation by myeloperoxidase products released from stimulated human polymorphonuclear leukocytes. Immunobiology. 1996;195:334. doi: 10.1016/S0171-2985(96)80050-7. [DOI] [PubMed] [Google Scholar]
- 54.Vogt W. Cleavage of the fifth component of complement and generation of a functionally active C5b6-like complex by human leukocyte elastase. Immunobiology. 2000;201:470. doi: 10.1016/S0171-2985(00)80099-6. [DOI] [PubMed] [Google Scholar]
- 55.Volanakis JE: Overview of the Complement System. In: Volanakis JE, Frank MM: The Human Complement System in Health and Disease. Marcel Dekker, Inc., New York 1998
- 56.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 57.Ward PA. The dark side of C5a in sepsis. Nat Rev Immunol. 2004;4:133. doi: 10.1038/nri1269. [DOI] [PubMed] [Google Scholar]
- 58.Ward PA, Hill JH. C5 chemotactic fragments produced by an enzyme in lysosomal granules of neutrophils. J Immunol. 1970;104:535. [PubMed] [Google Scholar]
- 59.Woodruff TM, Arumugam TV, Shiels IA, Reid RC, Fairlie DP, Taylor SM. Protective effects of a potent C5a receptor antagonist on experimental acute limb ischemia-reperfusion in rats. J Surg Res. 2004;116:81. doi: 10.1016/j.jss.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 60.Wymann MP, Kernen P, Deranleau DA, Baggiolini M. Respiratory burst oscillations in human neutrophils and their correlation with fluctuations in apparent cell shape. J Biol Chem. 1989;264:15829. [PubMed] [Google Scholar]


