Abstract
High-grade prostatic intraepithelial neoplasia is considered the most likely precursor of prostatic carcinoma. The only method of detection is biopsy; prostatic intraepithelial neoplasia (PIN) does not significantly elevate serum prostate-specific antigen concentration and cannot be detected by ultra-sonography. The incidence of PIN in prostate biopsies averages 9% (range, 4%–16%), representing 115,000 new cases of PIN diagnosed each year in United States. PIN has a high predictive value as a marker for adenocarcinoma, and its identification warrants repeated biopsy for concurrent or subsequent invasive carcinoma. Carcinoma will develop in most patients with PIN within 10 years. PIN is associated with progressive abnormalities of phenotype and genotype that are intermediate between normal prostatic epithelium and cancer, indicating impairment of cell differentiation and regulatory control with advancing stages of prostatic carcinogenesis. Androgen deprivation therapy decreases the prevalence and extent of PIN, suggesting that this form of treatment may play a role in chemoprevention.
Key words: Prostatic intraepithelial neoplasia, Prostate cancer, Alpha-methylacyl-CoA racemase, Androgen deprivation therapy
Prostatic intraepithelial neoplasia (PIN) represents the preinvasive end of the continuum of cellular proliferations within the lining of prostatic ducts and acini. The term “PIN” is usually used today as a synonym for high-grade PIN (HGPIN) (formerly PIN grades 2 and 3 on a 1–3 scale). The high level of interobserver variability with low-grade PIN limits its clinical utility, and pathologists do not routinely report this finding except in research studies.1 Interobserver agreement for HGPIN is “good to excellent.”2 Other terms, such as “dysplasia,” “carcinoma in situ,” and “intraductal carcinoma,” are discouraged.
Epidemiology of PIN
In the United States, an estimated 1,300,000 prostate biopsies are performed annually to detect 198,500 new cases of prostate cancer. The incidence of isolated HGPIN averages 9% (range, 4%–16%) of prostate biopsies, representing 115,000 new cases of HGPIN without cancer diagnosed each year (Table 1).
Table 1.
Estimated Frequency of High-Grade PIN in the United States
| Age (y) | No. US Population* | High-Grade PIN (%) |
| 40–49 | 20,550,000 | 3,123,600 (15.2) |
| 50–59 | 14,187,000 | 3,404,880 (24.0) |
| 60–69 | 9,312,000 | 4,404,576 (47.3) |
| 70–79 | 6,926,000 | 4,044,784 (58.4) |
| 80–89 | 2,664,000 | 1,864,800 (70.0) |
| Total | 53,639,000 | 16,842,640 |
1990 US census.
PIN, prostatic intraepithelial neoplasia.
The incidence and extent of PIN appear to increase with patient age (Table 1).3,4 An autopsy study of step-sectioned whole-mount prostates from older men showed that the prevalence of PIN in prostates with cancer increased with age, predating the onset of carcinoma by more than 5 years.4 A similar study of young men revealed that PIN is first seen in men in their 20s and 30s (9% and 22% frequency, respectively), and precedes the onset of carcinoma by more than 10 years.4 Most foci of PIN in young men are low grade, with increasing frequency of HGPIN with advancing age. The volume of HGPIN also increases with patient age.3
Race and geographic location may also influence the incidence of HGPIN.1 For example, African American men have a greater prevalence of HGPIN than whites in the 50- to 60-year age group. In contrast, Japanese men living in Osaka, Japan, have a significantly lower incidence of HGPIN than men residing in the United States, and Asians have the lowest clinically detected rate of prostate cancer.5 Interestingly, Japanese men with HGPIN also had an increased likelihood of prostate cancer developing, suggesting that HGPIN is a precursor of clinical prostate cancer in Asian men, too. Thus, the differences in the frequency of HGPIN in the 50- to 60-year age group across races essentially mirror the rates of clinical prostate cancer observed in the 60- to 70-year age group.
The causal association of HGPIN with prostatic adenocarcinoma is based on the fact that the prevalence of both HGPIN and prostate cancer increases with patient age and that HGPIN precedes the onset of prostate cancer by less than 1 decade (Table 1). The severity and frequency of HGPIN in prostates with cancer are greatly increased (73% of 731 specimens) compared with that of prostates without cancer (32% of 876 specimens).3,6
Incidence of PIN
The incidence of PIN varies according to the population of men under study (Table 2). The lowest likelihood is in men participating in PSA screening and early detection studies, with an incidence of PIN detected on biopsy ranging from 0.7% to 20%. Men seen by urologists in practice show PIN in 4.4% to 25% of contemporary needle biopsy samples. Those undergoing transurethral resection have the highest likelihood of PIN, from 2.8% to 33% (Table 3).
Table 2.
Incidence of Isolated High-Grade PIN in Prostatic Needle Biopsies
| Reference | Patient Population | Men, N | Incidence of PIN (%) |
| Screening Programs | |||
| Mettlin et al, 199137 | American Cancer Society National Prostate Cancer Detection Project | 330 | 5.2 |
| Feneley et al, 199738 | Screening population in Gwent, England, 1991–1993 | 212 | 20 |
| Hoedemaeker et al, 199939 | PSA screening study in Rotterdam, The Netherlands | 1824 | 0.7 |
| Urology Practice | |||
| Lee et al, 198940 | Consecutive biopsies of hypoechoic lesions at St. Joseph Mercy Hospital | 256 | 11 |
| Bostwick et al, 199541 | Consecutive biopsies at Mayo Clinic | 200 | 16.5 |
| Bostwick et al, 199541 | Consecutive biopsies at Glendale Hospital, Calif | 200 | 10.5 |
| Langer et al, 199642 | Consecutive biopsies at University of Pennsylvania Medical Center | 1275 | 4.4 |
| Wills et al, 199743 | Consecutive biopsies at Johns Hopkins Hospital | 439 | 5.5 |
| Feneley et al, 199738 | Consecutive biopsies at University College London Hospitals, 1988–1994 | 1205 | 11 |
| O'Dowd et al, 200044 | Consecutive biopsies at UroCor Labs, Okla, 1994–1998 | 132,426 | 2.3 |
| Fowler et al, 200145 | Consecutive biopsies of men with suspected carcinoma at the Veterans Affairs Medical Center, Miss, 1992–1998 | 1050 | 8.9 |
Note: Table 2 is restricted to larger studies, with an arbitrary cutoff of N ≥ 200.
PIN, prostatic intraepithelial neoplasia; PSA, prostate-specific antigen
Table 3.
Incidence of Isolated High-Grade PIN in Prostatic Transurethral Resections
| Reference | Patient Population | Men, n | Incidence of PIN (%) |
| Gaudin et al, 199746 | Consecutive TURPs without cancer at Johns Hopkins Hospital | 158 | 3.2 |
| Pacelli and Bostwick, 199747 | Consecutive TURPs without cancer at Mayo Clinic | 570 | 2.8 |
| Skjorten et al, 199748 | Consecutive TURPs from 1974–1975 at Ullevaal and Lovisenberg Hospitals, Oslo, Norway | 731 | 33 |
PIN, prostatic intraepithelial neoplasia; TURP, transurethral resection of the prostate.
Diagnostic Criteria for PIN
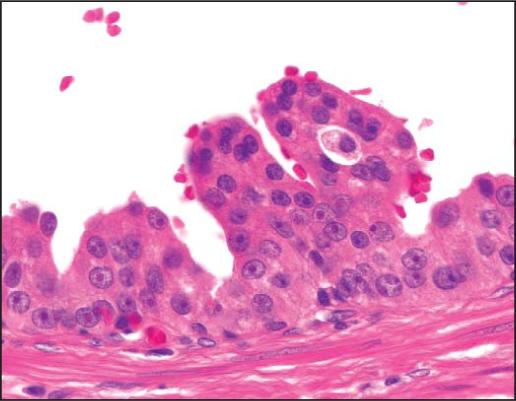
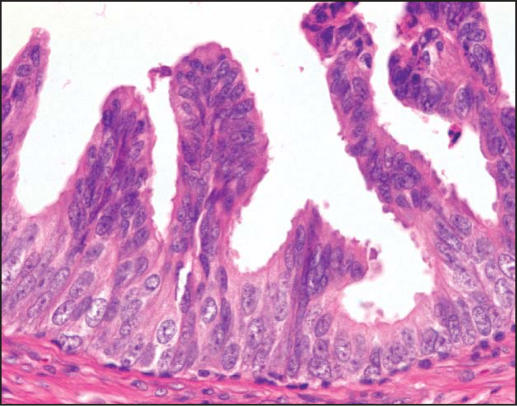
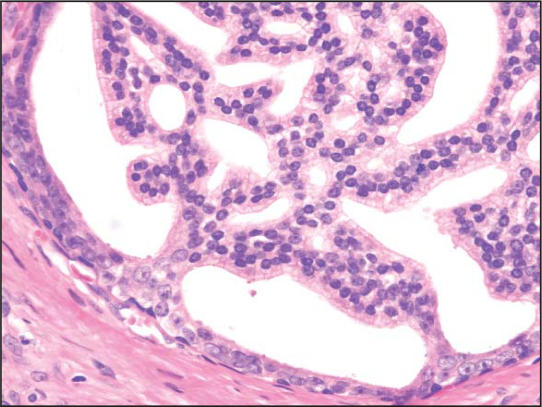
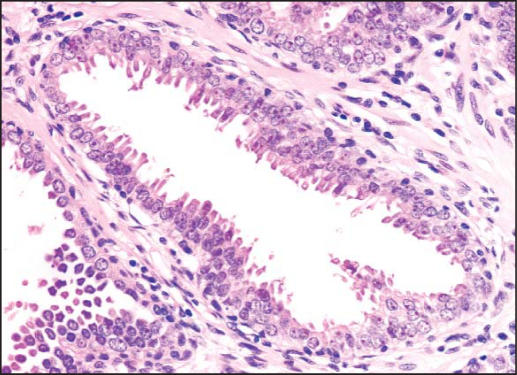
PIN is characterized by cellular proliferations within preexisting ducts and acini with cytologic changes mimicking cancer, including nuclear and nucleolar enlargement. There is inversion of the normal orientation of epithelial proliferation with PIN from the basal cell compartment to the luminal surface, similar to adenomas in the colon and other sites. Four main patterns of HGPIN have been described: tufting, micropapillary, cribriform, and flat7 (Figures 1–4). There are no known clinically important differences between the architectural patterns, and their recognition appears to be only of diagnostic utility. Other unusual patterns of PIN include the signet ring cell pattern, small cell neuroendocrine pattern, mucinous pattern, and microvacuolated (foamy gland) pattern, and inverted (hobnail) pattern.1
Figure 1.
High-grade prostatic intraepithelial neoplasia, tufting pattern (hematoxylin & eosin, × 400).
Figure 2.
High-grade prostatic intraepithelial neoplasia, micropapillary pattern (hematoxylin & eosin, × 400).)
Figure 3.
High-grade prostatic intraepithelial neoplasia, cribiform pattern (hematoxylin & eosin, × 200).)
Figure 4.
High-grade prostatic intraepithelial neoplasia, flat pattern (hematoxylin & eosin, × 200).)
Early stromal invasion, the earliest evidence of carcinoma, occurs at sites of acinar outpouching and basal cell disruption in acini with HGPIN. Such microinvasion is present in about 2% of high-power microscopic fields of PIN and is seen with equal frequency in all architectural patterns.8
HGPIN, like cancer, is usually multicentric3,8,9 and most commonly found in the peripheral zone of the prostate. The volume of HGPIN in prostates with cancer increases with increasing pathologic stage, Gleason grade, positive surgical margins, and perineural invasion.3 These findings underscore the close spatial and biologic relationship between PIN and cancer.
Because of the inability to separate tangential cutting of the larger preexisting acini of PIN (which may appear as small, separate, adjacent acini) from the smaller discrete acini of cancer in needle biopsy specimens, an equivocal diagnosis (atypical small acinar proliferation suspicious for but not diagnostic of malignancy) has to be rendered.
Diagnostic Immunohistochemistry of PIN
Select antibodies such as anti-keratin 34ß-E12 (high molecular weight keratin) or p63 may be used to stain tissue sections for the presence of basal cells, recognizing that PIN retains an intact or fragmented basal cell layer, whereas cancer does not.
Monoclonal basal cell-specific anti-keratin 34ß-E12 is the most commonly used immunostain for prostatic basal cells.10 According to studies utilizing anti-keratin 34ß-E12, increasing grades of PIN are associated with progressive disruption of the basal cell layer. Early invasive carcinoma occurs at sites of glandular out-pouching and basal cell discontinuity in association with PIN.11 Cancer cells consistently fail to react with this antibody, although rare (0.2%) cases of adenocarcinoma that express keratin 34ß-E12 have been reported.12 Thus, immunohistochemical stain for anti-keratin 34ß-E12 plays an important role in separating cancer from its mimics, including cribriform pattern of PIN, basal cell hyperplasia, inflamed acini, atypical adenomatous hyperplasia, post-atrophic hyperplasia, and radiation treatment changes.
Other markers of basal cells include proliferation markers, differentiation markers, and genetic markers. p63 is a recently introduced nuclear marker that may be useful for separating PIN and cancer from benign mimics. Keratins 5, 10, 11, 13, 14, 16, and 19 are immunoreactive at least focally in basal cells; of these, only keratin 19 is also found in secretory cells.13 Keratins found exclusively in the secretory cells include 7, 8, and 18. Basal cells usually do not display immunoreactivity for PSA, prostatic acid phosphatase (PAP), and S-100 protein. Conversely, the normal secretory luminal cells invariably stain with PSA and PAP.
A new molecular marker, alpha-methylacyl-CoA racemase (P504S), was introduced for separating benign and neoplastic acini. Its advantage over anti-keratin 34ß-E12 is its positive cytoplasmic staining in cancer cells, with little or no staining in benign acini. Current reports have substantiated the differential expression of this enzyme protein in benign and cancerous prostate tissues by immunohistochemistry.14
Genetic and Molecular Changes
HGPIN and prostate cancer share similar genetic alterations.15 For example, the frequent 8p12-21 allelic loss commonly found in prostate cancer was also found in microdissected PIN.15 Other examples of genetic changes found in carcinoma that already exist in PIN include loss of heterozygosity at chromosomes 6 and 8, decrease in telomere length,16 and gain of chromosomes 7, 8, 10, and 12.17 Recently, by cDNA microarray containing 8700 features, Calvo and coworkers18 have identified more than 400 genes abnormally expressed in both HGPIN and prostate cancer. In summary, these molecular studies indicate that the presence of HGPIN alerts both the clinician and the patient that progression to clinically significant prostate cancer is likely.
PIN is associated with progressive abnormalities of phenotype and genotype, which are intermediate between normal prostatic epithelium and cancer, indicating impairment of cell differentiation and regulatory control with advancing stages of prostatic carcinogenesis. There is progressive loss of some markers, such as PSA, PAP, cytoskeletal proteins, and annex-in I protein.19 Other markers show progressive increase, such as c-erbB-2 (Her-2/neu) and c-erbB-3 oncoproteins, c-met proto-oncogene, bcl-2 oncoprotein, members of the growth factor family,20 inducible nitric oxide synthase, alpha-methylacyl-CoA racemase,14 glycoprotein A-80,21 and apolipoprotein-D.22 Furthermore, Henshall and associates23 found that overexpression of p16INK4A in HGPIN was an independent predictor of disease relapse, providing the first evidence for a prognostic marker in HGPIN.
Microvessel Density Is Increased in PIN
PIN is virtually always accompanied by a proliferation of small capillaries in the stroma, despite separation from the underlying vasculature by a basal cell layer and basement membrane. The degree of microvessel density in PIN is intermediate between benign epithelium and cancer, lending support to the HGPIN.
Animal Models of PIN and Prostate Cancer
Several different animal models of prostate cancer have demonstrated that HGPIN is in the direct causal pathway to prostate cancer.1 The transgenic mouse model of prostate cancer (TRAMP) has been shown to mimic human prostate cancer. In the TRAMP model, the probasin promoter-SV40 large T antigen (PB-Tag) transgene is expressed specifically in the epithelial cells of the murine prostate under the control of the probasin promoter. The probasin promoter is androgen-dependent. As a result, this model has several advantages over currently existing models: 1) Mice develop progressive forms of prostatic epithelial hyperplasia and HGPIN as early as 10 weeks and invasive prostate adenocarcinoma around 18 weeks of age; 2) the pattern of metastatic spread of prostate cancer mimics that of human prostate cancer, with common sites of metastases being lymph node, lung, kidney, adrenal gland, and bone; 3) the development as well as the progression of prostate cancer can be followed within a relatively short period of 10 to 30 weeks; 4) spontaneous prostate tumors arise with 100% frequency; and 5) animals may be screened for the presence of the prostate cancer transgene before the onset of clinical prostate cancer.
Other specific genes, such as ECO:R1 Nkx3.1, and FGF8b, have been targeted in mouse models resulting in the development of HGPIN.24 The dog is the only nonhuman species in which spontaneous prostate cancer occurs, and, like in humans, the rate of canine prostate cancer increases with aging.25 HGPIN also has been observed in the prostates of these animals, with cytologic features identical to those of the human counterpart.26 Similar to the incidence of prostatic adenocarcinoma, HGPIN incidence also increases with aging.26 Thus, like the transgenic mouse models, the canine model supports HGPIN as part of a continuum in the progression of prostate cancer.
Clinical Significance of PIN
PIN Does Not Elevate PSA
Biopsy remains the definitive method for detecting PIN and early invasive cancer, but noninvasive methods, including serum tests, are being evaluated. Serum PSA concentration may be elevated in patients with PIN, although this has been refuted.27 Elevated PSA levels in patients with HGPIN may have resulted from the undetected cancer.
Transrectal Ultrasound Cannot Detect PIN
On transrectal ultrasonograms, PIN may be hypoechoic like carcinoma, although these findings have not been confirmed.28 Today, most urologists and radiologists do not believe that PIN is detectable by transrectal ultra-sound because PIN is a microscopic finding that is below the detection threshold for this form of imaging.
Prostate Cancer Develops in Men With PIN
The predictive value of HGPIN for cancer was evaluated in a retrospective case-control study of 100 patients with sextant needle biopsy results showing HGPIN and 112 with biopsy results without PIN matched for clinical stage, age, and serum PSA level.29 Adenocarcinoma was identified in 36% of subsequent biopsies from patients with PIN, compared with 13% in the control group. The likelihood of finding cancer increased as the time interval from first biopsy increased (32% incidence of cancer within 1 year compared with 38% in follow-up biopsies performed after more than 1 year).
HGPIN, patient age, and serum PSA concentration were jointly highly significant predictors of cancer, with PIN providing the highest risk ratio (14.9).
Other series have also found a high predictive value of PIN for cancer (Table 4). Kronz and coworkers30 further found that the number of core samples with HGPIN was the only independent histologic predictor of a cancer diagnosis; risk of cancer was 30.2% with 1 or 2 cores with HGPIN, 40% with 3 cores, and 75% with more than 3 cores. These data underscore the strong association of PIN and adenocarcinoma and indicate that vigorous diagnostic follow-up is needed. Our recent study, however, revealed a 23% predictive value of PIN for prostate cancer, which is comparable to the 25%, 27%, and 28% predictive values reported by other groups at the 2003 United States and Canadian Academy of Pathology meeting.
Table 4.
Cancer Detection in Patients With High-Grade PIN
| Reference | Population | Men, N | Patients With Cancer on Repeated Biopsy(%) |
| Davidson et al29 | Two urology practices | 100 | 35 |
| Raviv et al49 | Urology practice | 48 | 47.9 |
| Langer et al42 | Urology practice | 53 | 27 |
| Shepherd et al50 | PSA screening | 66 | 58 |
| Kirschenbaum et al51 | Urology practice | 74 | 31 |
| Park et al52 | Urology practice | 43 | 51 |
| Kronz et al30 | Urology practice | 245 | 32 |
| Igel et al53 | Urology practice | 88 | 43 |
| Vukovic et al16 | Urology practice | 104 | 22 |
| Gokden et al** | Urology practice | 221 | 28 |
| Sakr et al** | Urology practice | 540 | 27 |
| Siever et al** | Urology practice | 145 | 25 |
| Schlesinger & Bostwick** | Urology practice | 335 | 23 |
PIN, prostatic intraepithelial neoplasia; PSA, prostate-specific antigen.
Note: Table 4 is restricted to larger studies, with an arbitrary cutoff of N ≥ 40.
Presented at 2003 United States and Canadian Academy of Pathology meeting.
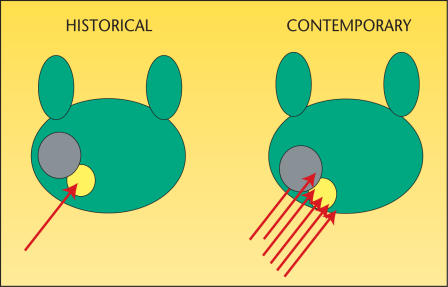
We believe that the following factors account for the decline in predictive accuracies of HGPIN for prostate cancer. The major role is played by use of extended biopsy techniques that result in more thorough prostate sampling and in higher cancer detection rates (Figure 5). Conversely, by these actions there remains a smaller pool of patients to receive isolated diagnoses of PIN. Another contributor is the lower detection rate for and difficulty in the detection of the remaining small cancers; larger significant tumors may also escape detection. These results may reflect a new steady state and a newly reached low plateau in the predictive accuracy of PIN.
Figure 5.
Historical biopsy approaches (left) could easily miss invasive cancer (gray) because of undersampling. In modern biopsy approaches (right), with multiple cores being taken, it is unlikely that a concomitant carcinoma in the face of PIN (yellow) will be missed. PIN, prosatic intraepithelial neoplasia.
Androgen Deprivation Therapy Eliminates PIN
There is a marked decrease in the prevalence and extent of HGPIN after androgen deprivation therapy when compared with untreated cases. This decrease is accompanied by epithelial hyperplasia, cytoplasmic clearing, and prominent glandular atrophy, with a decreased ratio of glands to stroma. These findings indicate that the cells of HGPIN are as hormone-dependent as normal secretory epithelium.
Neoadjuvant hormone deprivation with a monthly dose of leuprolide and flutamide, 250 mg PO tid for 3 months, resulted in a 50% reduction in HGPIN. There is also evidence that cessation of flutamide resulted in return of HGPIN.31 Conversely, blockade of 5α-reductase with finasteride appears to have little or no effect on PIN.32
Radiation Therapy Eliminates PIN
The prevalence and extent of PIN are decreased after radiation therapy,33 and PIN retains the features characteristic of untreated PIN. However, 1 study paradoxically noted a higher incidence (70%) of PIN after radiation therapy than expected, but it failed to employ accepted diagnostic criteria for PIN, so its results are not comparable with others.
The long-term efficacy of radiation treatment may depend on eradication of cancer as well as precancerous lesions. The question remains whether recurrent cancer after irradiation is due to regrowth of incompletely eradicated tumor or progression from incompletely eradicated PIN. Further studies of salvage prostatectomy specimens and post-radiation therapy needle biopsies are justified in an attempt to establish the significance of HGPIN as a source of long-term treatment failure among these patients. If PIN is associated with treatment failure, adjuvant chemo-prevention strategies that ablate this lesion may reduce the risk of cancer recurrence.
Should Men With HGPIN Be Treated?
The clinical importance of recognizing PIN is based on its strong association with prostatic carcinoma, so its identification in biopsy specimens warrants further search for concurrent invasive carcinoma. Follow-up biopsy is suggested at 3- to 6-month intervals for 2 years, and thereafter at 12-month intervals for life.29,34 If all procedures fail to identify coexistent carcinoma, close surveillance and follow-up are indicated. As HGPIN progresses, the likelihood of basal cell layer disruption increases, very much like what is observed for carcinoma in situ (CIS) of the urinary bladder. CIS of the urinary bladder, like PIN, may become invasive and is treated aggressively. The standard of care for management of CIS of the bladder is intravesical instillation of chemotherapy or bacillus Calmette Guérin (BCG), and, in some cases, radical cystectomy.
Most authors agree that the identification of PIN in the prostate should not influence or dictate therapeutic decisions.35 We are aware of 21 radical prostatectomies that were purposely (3 cases) or inadvertently (18 cases) performed in patients whose biopsies contained only HGPIN; all but 2 of the cases contained adenocarcinoma in the surgical specimen.
Currently, routine treatment is not available for patients who have HGPIN. Prophylactic radical prostatectomy, radiation, and androgen deprivation are not acceptable treatments for patients who have HGPIN only. The development and identification of acceptable agents to treat HGPIN would fill a therapeutic void. Acapodene, an anti-estrogen, is currently in a phase 2b multicenter, randomized, prospective placebo-controlled human clinical trial to determine if it can treat HGPIN and reduce prostate cancer incidence; preliminary results are encouraging.36
PIN offers promise as an intermediate endpoint in studies of chemo-prevention of prostatic carcinoma. Recognizing the slow growth rate of prostate cancer and the considerable amount of time needed in animal and human studies for adequate follow-up, the noninvasive precursor lesion PIN is a suitable intermediate histologic marker to indicate subsequent likelihood of cancer.
Conclusion
HGPIN is the most likely precursor of prostatic adenocarcinoma, according to virtually all available evidence. PIN is associated with progressive abnormalities of phenotype and genotype, which are intermediate between normal prostatic epithelium and cancer, indicating impairment of cell differentiation and regulatory control with advancing stages of prostatic carcinogenesis.
The clinical importance of recognizing PIN is based on its strong association with prostatic carcinoma. PIN has a high predictive value as a marker for adenocarcinoma, and its identification in biopsy specimens of the prostate warrants further search for concurrent invasive carcinoma. Studies to date have not determined whether PIN remains stable, regresses, or progresses, although the implication is that it can progress.
Main Points.
Isolated high-grade prostatic intraepithelial neoplasia (HGPIN) is detected in an average of 9% of prostate biopsies. The incidence, extent, and volume of HGPIN increase with patient age; its presence precedes the onset of prostatic carcinoma by 5 to 10 years or more.
Prostatic intraepithelial neoplasia (PIN), characterized by cellular proliferations within preexisting ducts and acini with cytologic changes mimicking cancer, has been shown to follow 4 main patterns: tufting, micropapillary, cribriform, and flat. These patterns appear to be of diagnostic utility only.
Immunohistochemical staining for anti-keratin 34ß-E12, the most commonly used staining method for prostatic basal cells, can separate cancer from its mimics. The nuclear marker p63 may be similarly useful, whereas the molecular marker alpha-methylacyl-CoA racemase can separate benign from neoplastic acini.
With more than 400 genes abnormally expressed in both prostate cancer and HGPIN, the presence of HGPIN cannot be denied as an important marker of potential progression to cancer and cause for close follow-up.
The transgenic mouse model of prostate cancer has been shown to mimic prostate cancer in humans, including in patterns of metastatic spread, making it and the canine model useful in supporting the role of HGPIN in the continuum to prostate cancer.
Serum prostate-specific antigen measurement and transrectal ultrasound have not been proved to be able to detect PIN; biopsy remains the definitive method.
Androgen deprivation therapy has been shown to reduce HGPIN, as has radiation therapy, whereas 5α-reductase therapy has little to no effect.
Patients with HGPIN should be closely monitored with prostate biopsies at regular intervals for life. Although routine treatment for PIN is not yet available, preliminary results on acapodene, an anti-estrogen, are encouraging.
References
- 1.Argani P, Epstein JI. Inverted (Hobnail) high-grade prostatic intraepithelial neoplasia (PIN): report of 15 cases of a previously undescribed pattern of high-grade PIN. Am J Surg Pathol. 2001;25:1534–1539. doi: 10.1097/00000478-200112000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Epstein JI, Carmichael M, Partin AW. OA-519 (fatty acid synthase) as an independent predictor of pathologic state in adenocarcinoma of the prostate. Urology. 1995;45:81–86. doi: 10.1016/s0090-4295(95)96904-7. [DOI] [PubMed] [Google Scholar]
- 3.Jenkins RB, Qian J, Lieber MM, Bostwick DG. Detection of c-myc oncogene amplification and chromosomal anomalies in metastatic prostatic carcinoma by fluorescence in situ hybridization. Cancer Res. 1997;57:524–531. [PubMed] [Google Scholar]
- 4.Sakr WA, Sarkar FH, Sreepathi P, et al. Measurement of cellular proliferation in human prostate by AgNOR, PCNA, and SPF. Prostate. 1993;22:147–154. doi: 10.1002/pros.2990220207. [DOI] [PubMed] [Google Scholar]
- 5.Hiroi H, Inoue S, Watanabe T, et al. Differential immunolocalization of estrogen receptor alpha and beta in rat ovary and uterus. J Mol Endocrinol. 1999;22:37–44. doi: 10.1677/jme.0.0220037. [DOI] [PubMed] [Google Scholar]
- 6.Bostwick DG. Progression of prostatic intraepithelial neoplasia to early invasive adenocarcinoma. Eur Urol. 1996;30:145–152. doi: 10.1159/000474164. [DOI] [PubMed] [Google Scholar]
- 7.Cheville JC, Reznicek MJ, Bostwick DG. The focus of “atypical glands, suspicious for malignancy” in prostatic needle biopsy specimens: incidence, histologic features, and clinical follow-up of cases diagnosed in a community practice. Am J Clin Pathol. 1997;108:633–640. doi: 10.1093/ajcp/108.6.633. [DOI] [PubMed] [Google Scholar]
- 8.Bostwick DG, Srigley J, Grignon D, et al. Atypical adenomatous hyperplasia of the prostate: morphologic criteria for its distinction from well-differentiated carcinoma. Hum Pathol. 1993;24:819–832. doi: 10.1016/0046-8177(93)90131-y. [DOI] [PubMed] [Google Scholar]
- 9.Bostwick DG. Prostatic adenocarcinoma following androgen deprivation therapy: the new difficulty in histologic interpretation. Anat Pathol. 1998;3:1–16. [PubMed] [Google Scholar]
- 10.Novis DA, Zarbo RJ, Valenstein PA. Diagnostic uncertainty expressed in prostate needle biopsies. A College of American Pathologists Q-probes Study of 15,753 prostate needle biopsies in 332 institutions. Arch Pathol Lab Med. 1999;123:687–692. doi: 10.5858/1999-123-0687-DUEIPN. [DOI] [PubMed] [Google Scholar]
- 11.Bostwick DG, Pacelli A, Blute M, et al. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: a study of 184 cases. Cancer. 1998;82:2256–2261. doi: 10.1002/(sici)1097-0142(19980601)82:11<2256::aid-cncr22>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 12.Yang XJ, Lecksell K, Gaudin P, Epstein JI. Rare expression of high-molecular-weight cytokeratin in adenocarcinoma of the prostate gland: a study of 100 cases of metastatic and locally advanced prostate cancer. Am J Surg Pathol. 1999;23:147–152. doi: 10.1097/00000478-199902000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Nagle RB, Brawer MK, Kittelson J, Clark V. Phenotypic relationships of prostatic intraepithelial neoplasia to invasive prostatic carcinoma. Am J Pathol. 1991;138:119–128. [PMC free article] [PubMed] [Google Scholar]
- 14.Luo J, Zha S, Gage WR, et al. Alpha-methylacyl-CoA racemase: a new molecular marker for prostate cancer. Cancer Res. 2002;62:2220–2226. [PubMed] [Google Scholar]
- 15.Emmert-Buck MR, Vocke CD, Pozzatti RO, et al. Allelic loss on chromosome 8p12-21 in microdissected prostatic intraepithelial neoplasia. Cancer Res. 1995;55:2959–2962. [PubMed] [Google Scholar]
- 16.Vukovic B, Park PC, Al-Maghrabi J, et al. Evidence of multifocality of telomere erosion in high-grade prostatic intraepithelial neoplasia (HPIN) and concurrent carcinoma. Oncogene. 2003;22:1978–1987. doi: 10.1038/sj.onc.1206227. [DOI] [PubMed] [Google Scholar]
- 17.Bostwick DG, Qian J. Atypical adenomatous hyperplasia of the prostate: relationship with carcinoma in 217 whole-mount radical prostatectomies. Am J Surg Pathol. 1995;19:506–518. doi: 10.1097/00000478-199505000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Calvo A, Xiao N, Kang J, et al. Alterations in gene expression profiles during prostate cancer progression: functional correlations to tumorigenicity and down-regulation of selenoprotein-P in mouse and human tumors. Cancer Res. 2002;62:5325–5335. [PubMed] [Google Scholar]
- 19.Kang JS, Calvo BF, Maygarden SJ, et al. Dysregulation of annexin I protein expression in high-grade prostatic intraepithelial neoplasia and prostate cancer. Clin Cancer Res. 2002;8:117–123. [PubMed] [Google Scholar]
- 20.Nakashiro K, Hayashi Y, Oyasu R. Immuno-histochemical expression of hepatocyte growth factor and c-Met/HGF receptor in benign and malignant human prostate tissue. Oncol Rep. 2003;10:1149–1153. [PubMed] [Google Scholar]
- 21.Coogan CL, Bostwick DG, Bloom KJ, Gould VE. Glycoprotein A-80 in the human prostate: immunolocalization in prostatic intraepithelial neoplasia, carcinoma, radiation failure, and after neoadjuvant hormonal therapy. Urology. 2003;61:248–252. doi: 10.1016/s0090-4295(02)02061-7. [DOI] [PubMed] [Google Scholar]
- 22.Hall RE, Horsfall DJ, Stahl J, et al. Apolipoprotein-D: a novel cellular marker for HGPIN and prostate cancer. Prostate. 2004;58:103–108. doi: 10.1002/pros.10343. [DOI] [PubMed] [Google Scholar]
- 23.Henshall SM, Quinn DI, Lee CS, et al. Overexpression of the cell cycle inhibitor p16INK4A in high-grade prostatic intraepithelial neoplasia predicts early relapse in prostate cancer patients. Clin Cancer Res. 2001;7:544–550. [PubMed] [Google Scholar]
- 24.Song Z, Wu X, Powell WC, et al. Fibroblast growth factor 8 isoform B overexpression in prostate epithelium: a new mouse model for prostatic intraepithelial neoplasia. Cancer Res. 2002;62:5096–5105. [PubMed] [Google Scholar]
- 25.Aquilina JW, McKinney L, Pacelli A, et al. High grade prostatic intraepithelial neoplasia in military working dogs with and without prostate cancer. Prostate. 1998;36:189–193. doi: 10.1002/(sici)1097-0045(19980801)36:3<189::aid-pros7>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 26.Walker-Daniels J, Coffman K, Azimi M, et al. Overexpression of the EphA2 tyrosine kinase in prostate cancer. Prostate. 1999;41:275–280. doi: 10.1002/(sici)1097-0045(19991201)41:4<275::aid-pros8>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 27.Alexander EE, Qian J, Wollan PC, et al. Prostatic intraepithelial neoplasia does not appear to raise serum prostate-specific antigen concentration. Urology. 1996;47:693–698. doi: 10.1016/s0090-4295(96)00004-0. [DOI] [PubMed] [Google Scholar]
- 28.Brawer MK, Rennels MA, Nagle RB, et al. Prostatic intraepithelial neoplasia: a lesion that may be confused with cancer on prostatic ultrasound. Urol. 1989;142:1510–1512. doi: 10.1016/s0022-5347(17)39142-5. [DOI] [PubMed] [Google Scholar]
- 29.Davidson D, Bostwick DG, Qian J, et al. Prostatic intraepithelial neoplasia is a risk factor for adenocarcinoma: predictive accuracy in needle biopsies. J Urol. 1995;154:1295–1299. [PubMed] [Google Scholar]
- 30.Kronz JD, Allan CH, Shaikh AA, Epstein JI. Predicting cancer following a diagnosis of high-grade prostatic intraepithelial neoplasia on needle biopsy: data on men with more than one follow-up biopsy. Am J Surg Pathol. 2001;25:1079–1085. doi: 10.1097/00000478-200108000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Steiner MS, Raghow S. Antiestrogens and selective estrogen receptor modulators reduce prostate cancer risk. World J Urol. 2003;21:31–36. doi: 10.1007/s00345-002-0316-x. [DOI] [PubMed] [Google Scholar]
- 32.Yang XJ, Lecksell K, Short K, et al. Does long-term finasteride therapy affect the histologic features of benign prostatic tissue and prostate cancer on needle biopsy? PLESS Study Group. Proscar Long-Term Efficacy and Safety Study. Urology. 1999;53:696–700. doi: 10.1016/s0090-4295(98)00579-2. [DOI] [PubMed] [Google Scholar]
- 33.Bostwick DG, Ramnani D, Cheng L. Treatment changes in prostatic hyperplasia and cancer, including androgen deprivation therapy and radiotherapy. Urol Clin North Am. 1999;26:465–479. doi: 10.1016/s0094-0143(05)70195-6. [DOI] [PubMed] [Google Scholar]
- 34.Bostwick DG. Prostatic intraepithelial neoplasia (PIN): current concepts. J Cell Biochem Suppl. 1992;16H:10–19. doi: 10.1002/jcb.240501205. [DOI] [PubMed] [Google Scholar]
- 35.Cheng L, Leibovich BC, Bergstralh EJ, et al. p53 alteration in regional lymph node metastases from prostate carcinoma: a marker for progression? Cancer. 1999;85:2455–2459. [PubMed] [Google Scholar]
- 36.Steiner MS. High grade prostatic intraepithelial neoplasia is a disease. Curr Urol Rep. 2001;2:195–198. doi: 10.1007/s11934-001-0078-9. [DOI] [PubMed] [Google Scholar]
- 37.Mettlin C, Lee F, Drago J, Murphy GP. The American Cancer Society National Prostate Cancer Detection Project. Findings on the detection of early prostate cancer in 2425 men. Cancer. 1991;67:2949–2958. doi: 10.1002/1097-0142(19910615)67:12<2949::aid-cncr2820671202>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 38.Feneley MR, Green JS, Young MP, et al. Prevalence of prostatic intra-epithelial neoplasia (PIN) in biopsies from hospital practice and pilot screening: clinical implications. Prostate Cancer Prostatic Dis. 1997;1:79–83. doi: 10.1038/sj.pcan.4500210. [DOI] [PubMed] [Google Scholar]
- 39.Hoedemaeker RF, Kranse R, Rietbergen JB, et al. Evaluation of prostate needle biopsies in a population-based screening study: the impact of borderline lesions. Cancer. 1999;85:145–152. [PubMed] [Google Scholar]
- 40.Lee F, Torp-Pedersen ST, Carroll JT, et al. Use of transrectal ultrasound and prostate-specific antigen in diagnosis of prostatic intraepithelial neoplasia. Urology. 1989;34(6 suppl):4–8. [PubMed] [Google Scholar]
- 41.Bostwick DG, Qian J, Frankel K. The incidence of high grade prostatic intraepithelial neoplasia in needle biopsies. J Urol. 1995;154:1791–1794. [PubMed] [Google Scholar]
- 42.Langer JE, Rovner ES, Coleman BG, et al. Strategy for repeat biopsy of patients with prostatic intraepithelial neoplasia detected by prostate needle biopsy. J Urol. 1996;155:228–231. [PubMed] [Google Scholar]
- 43.Wills ML, Hamper UM, Partin AW, Epstein JI. Incidence of high-grade prostatic intraepithelial neoplasia in sextant needle biopsy specimens. Urology. 1997;49:367–373. doi: 10.1016/S0090-4295(96)00622-X. [DOI] [PubMed] [Google Scholar]
- 44.O’Dowd GJ, Miller MC, Orozco R, Veltri RW. Analysis of repeated biopsy results within 1 year after a noncancer diagnosis. Urology. 2000;55:553–559. doi: 10.1016/s0090-4295(00)00447-7. [DOI] [PubMed] [Google Scholar]
- 45.Fowler JE, Jr, Bigler SA, Lynch C, et al. Prospective study of correlations between biopsy-detected high grade prostatic intraepithelial neoplasia, serum prostate specific antigen concentration, and race. Cancer. 2001;91:1291–1296. doi: 10.1002/1097-0142(20010401)91:7<1291::aid-cncr1131>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 46.Gaudin PB, Sesterhenn IA, Wojno KJ, et al. Incidence and clinical significance of high-grade prostatic intraepithelial neoplasia in TURP specimens. Urology. 1997;49:558–563. doi: 10.1016/s0090-4295(96)00542-0. [DOI] [PubMed] [Google Scholar]
- 47.Pacelli A, Bostwick DG. Clinical significance of high-grade prostatic intraepithelial neoplasia in transurethral resection specimens. Urology. 1997;50:355–359. doi: 10.1016/S0090-4295(97)00249-5. [DOI] [PubMed] [Google Scholar]
- 48.Skjorten FJ, Berner A, Harvei S, et al. Prostatic intraepithelial neoplasia in surgical resections: relationship to coexistent adenocarcinoma and atypical adenomatous hyperplasia of the prostate. Cancer. 1997;79:1172–1179. doi: 10.1002/(sici)1097-0142(19970315)79:6<1172::aid-cncr16>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 49.Raviv G, Janssen T, Zlotta AR, et al. High-grade intraepithelial prostatic neoplasms: diagnosis and association with prostate cancer [in French] Acta Urol Belg. 1996;64:11–15. [PubMed] [Google Scholar]
- 50.Shepherd D, Keetch DW, Humphrey PA, et al. Repeat biopsy strategy in men with isolated prostatic intraepithelial neoplasia on prostate needle biopsy. J Urol. 1996;156(2 pt 1):460–463. doi: 10.1097/00005392-199608000-00038. [DOI] [PubMed] [Google Scholar]
- 51.Kirschenbaum A, Liotta DR, Yao S, et al. Immunohistochemical localization of cyclooxy-genase-1 and cyclooxygenase-2 in the human fetal and adult male reproductive tracts. J Clin Endocrinol Metab. 2000;85:3436–3441. doi: 10.1210/jcem.85.9.6780. [DOI] [PubMed] [Google Scholar]
- 52.Park S, Shinohara K, Grossfeld GD, Carroll PR. Prostate cancer detection in men with prior high grade prostatic intraepithelial neoplasia or atypical prostate biopsy. J Urol. 2001;165:1409–1414. [PubMed] [Google Scholar]
- 53.Igel TC, Knight MK, Young PR, et al. Systematic transperineal ultrasound guided template biopsy of the prostate in patients at high risk. J Urol. 2001;165:1575–1579. [PubMed] [Google Scholar]