Abstract
Urinary incontinence following prostatectomy is usually due to intrinsic sphincter deficiency and is often referred to as post-prostatectomy incontinence (PPI). The male sling is an effective minimally invasive procedure for low volume PPI. Although the male sling procedure is becoming increasingly popular, the artificial urinary sphincter (AUS) remains the gold standard. Placement of the AUS cuff using the transcorporal technique salvages patients with urethral atrophy as well as prior AUS erosion or infection. As the surgical options for PPI expand, it is important to analyze the outcomes with contemporary surgical techniques and to develop an algorithm for procedure selection.
Key words: Urinary incontinence, Prostatectomy, Male sling, Artificial urinary sphincter
Up to 40% of patients complain of persistent long-term urinary incontinence after radical prostatectomy.1 Although the incontinence is mild in most instances, 4% of patients complain of significant leakage that compromises their lifestyle by necessitating pads.1 Although detrusor overactivity may occur after radical prostatectomy, urine incontinence is mainly a result of intrinsic sphincter deficiency (ISD).2 Preventive measures have been described to decrease the risk of incontinence after radical prostatectomy including preoperative physical therapy, meticulous apical dissection, preservation of the bladder neck, neurovascular bundles, and seminal vesicles. Despite an anatomic dissection, postoperative incontinence can still occur, significantly affecting quality of life.1–3 The rates of incontinence after radiation therapy (external beam radiation or brachytherapy) or cyrotherapy are not as well defined but are generally thought to be lower than with radical surgery. Transurethral resection of the prostate as well as prostatectomy for benign disease can also diminish sphincteric function.
Evaluation and Initial Treatment
Initial evaluation includes medical and surgical history, current stage of prostate cancer, prior surgeries, radiation and comorbid conditions, incontinence questionnaire, physical examination, and laboratory tests, including serum prostate-specific antigen (PSA) level measurement, urine analysis, and culture. A bladder diary, detailing the voided volume, number of voids, and incontinent episodes (eg, severity, triggers) in a 3-day period will help characterize the type of incontinence. A 24-hour pad test, which is a simple collection and weighing of the sanitary pads for 24 hours preceding the appointment, is an objective measure of incontinence that quantifies the severity of urine loss, thereby directing appropriate therapy. Further evaluation before proceeding with surgical therapy includes cystoscopy to assess for urethral vascularity and integrity at the potential cuff site, as well as an analysis of bladder pathology that to implantation. Multichannel urodynamics will determine bladder capacity, compliance, stability, leak point pressures, and the pressure/flow relationship. Urodynamic assessment permits an accurate evaluation of the type of incontinence and its severity while assessing other factors that may adversely affect the surgical outcome, such as poor bladder compliance, detrusor instability, or hypocontractility.2,4
The efficacy of pharmacotherapy and physiotherapy for ISD is similar to placebo and has limited value after the first postoperative year. Surgical therapy is considered in men with persistent post-prostatectomy incontinence (PPI), incontinence that persists beyond the first year. Although bulking agents are safe and well tolerated, their effect on postoperative continence is limited and decreases with time.5 Furthermore, we have noted a detrimental effect in sphincteric function in a number of patients who have undergone repetitive transurethral injections. The best results are achieved by implanting an artificial urinary sphincter, but there continue to be reservations about this therapy due to the perception that cuff placement is difficult and the device is prone to complications. As a result, there has been a reemergence of the bulbourethral sling in addition to novel techniques for cuff implantation, including the transcorporal and transscrotal approach.
Male Sling
The male sling is a bulbourethral sling based on the surgical techniques first described by Kaufman in 1970 as a treatment for PPI.6,7 The technique required passage of a needle carrier retropubicly and was effective in only 56% of patients, with as many as 27% of patients requiring at least 1 “tightening” procedure, and 52% complaining of chronic perineal numbness.6 The recently described bone-anchored sling is performed entirely through the perineum, which results in decreased operative time and absence of perineal pain.8–9 Two to 3 titanium bone-anchors, with attached prolene sutures, are placed into inferior pubic rami bilaterally. This provides a stable point of fixation and obviates the need for blind passage of the suspension sutures retropubicly to achieve urethral compression. Early results by Comiter8 and Madjar and colleagues9 are encouraging, with an overall cure/improved rates of 87% to 90% at a mean follow-up of 12 months. Patients who refuse the artificial urinary sphincter (AUS) due to the fear of infection, erosion, or mechanical failure or those with limited physical or mental capacity and low-volume incontinence are ideal candidates for this procedure.
The sling may be composed of a synthetic material (silicone mesh or polypropylene mesh), biologic materials such as allografts (fascia lata or dermis), or xenografts (porcine dermis). In some instances a composite sling such as porcine dermis, to pad the urethra, and silicone mesh, to provide a backboard of support, is utilized. Because the sling provides a constant pressure of 60 to 90 cm H2O to the bulbar urethra, the patient must be able to generate a vesical pressure (detrusor plus abdominal) greater than this to empty the bladder, unless he is committed to clean intermittent catheterization.
Our utilization of the bone-anchored sling is primarily in patients with low-volume PPI due to ISD, which we define as a 24-hour pad weight of 150 grams or less. We do not offer the male sling to patients with a prior history of radiation therapy or prior urethral erosion as the degree of urine loss that exists in this group usually exceeds the limits of this procedure. Additionally, our own experience in this population has been disappointing.
Artificial Urinary Sphincter
The AUS was introduced by Scott in 1973 and is the gold standard for men with incontinence due to ISD.10 PPI is the most common indication for implantation of the AUS. After several evolutions of the device, the current model, the AMS 800, was introduced in 1983. The AMS 800 consists of a cuff, pump, and pressure-regulating balloon. The cuff can be placed around the bladder neck or bulbar urethra, while the pump is placed in the scrotum and the pressure-regulating balloon in a preperitoneal location.
In 2000, Montague and Angermeier reviewed the results of the AUS for PPI in 286 patients from 5 centers.11 They noted that 76% of patients were dry (0–1 pads/day) and 13% were improved, for an overall success rate of 89% at a mean follow-up of 18 to 44 months.11–15 Revision of the device occurred in 39% of patients, urethral erosion in 5%, AUS infection in 3%, and mechanical failure in 15%.11–15 Hajivassiliou recently analyzed a series of published results and noted continence in 73% of cases with improvement in 88%.16 Elliott and Barrett reviewed the long-term durability of the AMS 800 in 323 patients who received the device at the Mayo Clinic between 1983 and 1994.17 In 1988, they noted a significant reduction in reoperation after they changed to the narrow backing occlusive cuff.17
The narrow-backed cuff is designed to provide a more even distribution of occlusive pressure on the urethra.17 This change in design resulted in a decrease in nonmechanical failure from 17% to 9%, primarily due to a reduction in urethral atrophy, and a decrease in mechanical failure from 21% to 7.6%, primarily due to a reduction in cuff leak.17 Additionally, the reduction in mechanical failure was also attributed to improvements in the synthetic material, which lessened the risk of fracture or kinking of the device. Overall, 72% of patients in their series had the original sphincter in place and functioning at a mean follow-up of 68.8 months.17 Similarly, Venn and colleagues noted a 92% continent rate and a 66% device survival rate (excluding removal due to infection or erosion) in their bulbar cuffs at 10 years.18 These results demonstrate that the technologic advances in design and construct of the AMS 800 have significantly decreased the reoperation rate due to mechanical and nonmechanical failure. However, inherent to any mechanical device is its propensity to breakdown and eventually failure requiring revision. In general, this revision is a minor surgical procedure and occurs more than 5 years after primary implantation.
Management of Urethral Atrophy
Due to improvements in the AMS 800, nonmechanical causes now account for the majority of revisions. In our most recent series, 88 of the 119 AUS revisions (74%) at Duke University were for nonmechanical indications.19 Moreover, urethral atrophy accounted for 63 of the 88 nonmechanical revisions (72%).19 Urethral atrophy is the reduction in urethral size that can occur as a result of compression of the spongy tissue that is encircled by the occlusive cuff. Traditional treatment options have included down-sizing the cuff in the same location, moving the cuff to a more proximal or distal location, and placement of a second cuff in tandem. If the existing cuff is 4.5 cm or greater it can be simply downsized in the same location. Saffarian and colleagues reported their experience in down-sizing the cuff in 17 patients with urethral atrophy.20 They noted a mean decrease in daily pad usage after revision from 3.9 to 0.5 and only 1 subsequent removal for infection.20 Couillard and colleagues reported successful repositioning of the AUS cuff to a more proximal location in 5 of 6 patients.21
Our approach is to downsize the cuff in the same location when possible; if the existing cuff is 4.0 cm, then we elect to reposition the cuff more proximally when feasible. If the cuff is at its proximal limits, then distal cuff placement either in tandem or single using a perineal or a transscrotal incision is the usual solution. If the AMS 800 is more than 3 years old at the time of revision, we elect to replace all the components in an effort to reduce the number of subsequent revisions.
Management of AUS Infection and Urethral Erosion
Erosion and infection are the 2 most serious complications of the AUS. Management involves removal of the cuff, which allows for healing, and replacement at a later date. Our practice is to remove all components in cases of infection and erosion, as these conditions are usually indistinguishable. Additionally, preservation of a particular component does not significantly decrease the operative time of subsequent reimplantation. In our experience, this approach reduces the frequency of ensuing surgeries and thereby avoids a delay in reimplantation. We place a 12-French urethral catheter after explantation for erosion and leave it indwelling for approximately 3 weeks to limit urinary extravasation and lessen the risk of future urethral stricture at the site of erosion. We perform a retrograde urethrogram to confirm urethral integrity before removing the catheter. Reimplantation occurs a minimum of 3 months after explantation after all infection and inflammation have resolved and urethral patency has been confirmed. Successful reimplantation rates of greater than 80% have been achieved.22–24
In reimplanting an AUS cuff following prior removal for infection/erosion, it is generally advised that the new cuff be placed at a site proximal or distal to the previous site, as the prior site is considered compromised and therefore susceptible to future erosion.23–25 Despite this approach, Frank and colleagues noted an 8.7% urethral erosion rate in patients who were reimplanted after prior urethral erosion or infection.24 Consequently, in cases of prior erosion or infection we prefer the transcorporal technique for cuff placement at a distal site to salvage continence and avert recurrent urethral erosion or infection.25
Double-Cuff Artificial Urinary Sphincter
PPI to ISD is successfully treated with a single cuff AMS 800 in 89% of patients.11 However, approximately 11% of the patients are still significantly wet despite improvements with this device. These patients usually have near total urinary incontinence before AUS implantation. Brito and colleagues have reported on double-cuff implantation in 15 such patients.26 In their report, 80% of patients were rendered satisfactorily dry by adding a second cuff around the bulbar urethra.26 This double-cuff technique increased their success rate with the AMS 800 sphincter to greater than 95%.26 The same group reported successful primary placement of the double-cuff AUS in 84 of 95 patients.27 However, their rate of urethral erosion was 10.5%, which is twice the incidence reported in contemporary reviews of primary single-cuff AUS.11,27,28 Consequently, we do not recommend double-cuff implantation as a primary procedure. Rather, it should be reserved for cases of recurrent or persistent incontinence due to ISD. Additionally, the introduction of the transcorporal technique of cuff placement has further diminished our use of the double-cuff AUS.25
Transcorporal AUS
Management of urethral atrophy after AUS can be a challenge in cases where a 4.0 cm cuff is in place around the proximal bulbar urethra. Simply downsizing the cuff or moving it to a more proximal location is not an option. Relocating the cuff to a more distal urethral site or adding a second cuff in tandem is problematic, because often the more distal bulbar urethra has an inadequate circumference to permit sufficient coaptation. Furthermore, dissection of the urethra from the corporal bodies at this location often leaves the roof of the urethra precariously thin. To resolve this predicament, Webster has developed a transcorporal technique for distal cuff placement.25,29
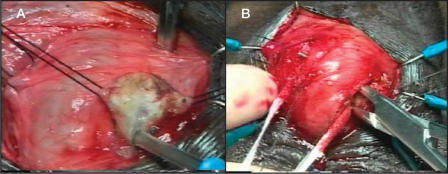
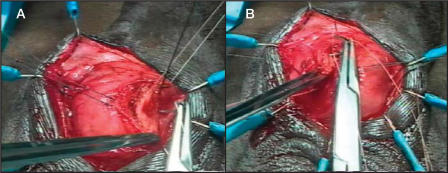
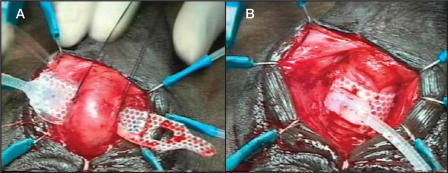
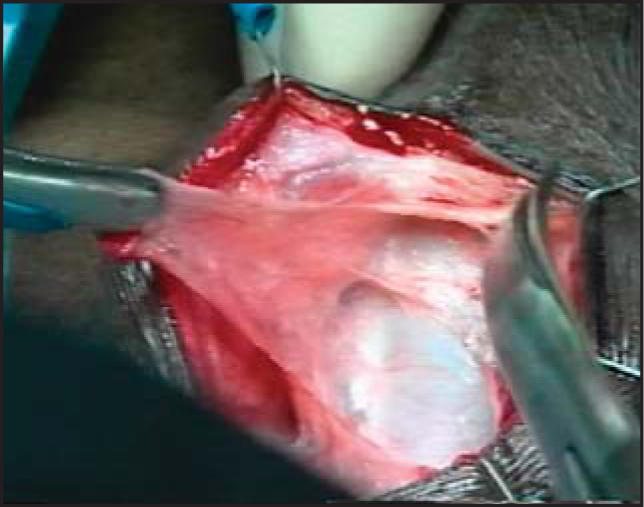
The surgical technique was well demonstrated in our video at the 2002 American Urological Association annual meeting.29 Briefly, in cases of atrophy, if the previous cuff is 4.0 cm and is located around the proximal bulbar urethra, a transcorporal cuff is implanted 2 to 3 cm distal to the original cuff site, without mobilizing the intervening urethra. In cases of prior AUS erosion or infection, a transcorporal cuff is placed at a distal site in order to avoid the prior cuff site. Intraoperative urethroscopy is invaluable in detecting urethral atrophy or in determining the extent of urethral scarring. Once an appropriate cuff site has been selected, the skin incision is made and the urethra and adjacent overlying corporal bodies are then exposed (Figure 1). Longitudinal incisions 2 cm in length are made into corpora cavernosa bilaterally and blunt dissection is used to create a tunnel between the 2 corporotomies, leaving a cuff of tunica albuginea attached to the urethra (Figure 2). The amount of bleeding is variable but in most cases it is surprisingly minimal. The corporal bodies are reconstituted dorsally behind the cuff site (Figure 3) and an appropriately sized cuff is positioned (Figure 4). The remaining components are placed in the customary manner and the device is activated 6 weeks later. This technique is currently reserved for impotent men as it may compromise erectile function.26
Figure 1.
Urethral dissection. A midline scrotal incision is made and the dissection is deepened to expose the urethra and corporal bodies.
Figure 2.
Corporal dissection. (A) A 2-cm longitudinal incision is made in the tunica albuginea of each corporal body 5 mm from the urethra and deepened until spongy tissue is encountered. (B) A tunnel is created between the corporal bodies with little difficulty.
Figure 3.
Reconstitution of the corporal bodies. Two mattress sutures of 2-0 polyglycolic acid are used to close the lateral margins of the corpora dorsal to the cuff. (A) Each suture is placed through the lateral edge of one corpora. (B) Each is then transferred through the tunnel and into the lateral edge of the opposite corpora. Each suture is left untied until after the cuff has been placed, as even the small amount of hour-glassing that occurs after tying the mattress sutures can make cuff placement difficult.
Figure 4.
Cuff selection and placement. (A) Due to the inelastic corporal tissue, we select the size of our cuff based on the exact circumference measured. (B) Final appearance of the transcorporal cuff after the previously placed mattress sutures are tied.
In 2002, we reported our initial experience in 31 men who underwent cuff placement utilizing this approach and noted a cure/improved rate (0–1 pad/day) of 84%.25 The fact that 58% of the transcorporally placed cuffs in this series were 4.0 cm attests to the narrowness of the distal bulbar urethra. Remarkably, there were no intraoperative urethral injuries or post-operative urethral erosions at a mean follow-up of 17 months.25 Placement of the distal cuff transcorporally adds an extra degree of protection to the urethra dorsally, lessening the risk of urethral erosion. Conversely, in patients reimplanted after prior erosion using conventional techniques, subsequent erosion occurs in 8.7% in a single cuff AUS and 11% in a double-cuff AUS.24,30
Consequently, in cases of prior erosion or infection that were once considered unsalvageable, we prefer the transcorporal technique for reimplantation at a distal site, as it enables safe and effective placement. In cases of urethral atrophy in patients with an existing 4.0 cm cuff at its proximal limits, distal cuff placement utilizing the transcorporal technique is our preferred approach in impotent men. If potency exists, we recognize the limitations with this approach and consider single or tandem cuff at a more distal site.
Transscrotal AUS
In this technique described by Wilson and colleagues in 2003, all components are placed through a single transverse scrotal incision.31 The reported location of the cuff, as with the perineal approach, is around the proximal bulbar urethra. The pump is placed in a subdartous pouch and the pressure-regulating balloon is placed in the retropubic space under finger guidance analogous to the technique used to place the reservoir for a 3-piece penile prosthesis. Due to urethral atrophy, the transscrotal approach was developed for reimplantation surgery to allow placement of a more distal cuff in tandem.31 This approach has evolved into a primary procedure for PPI because it limits the number of incisions, expedites the procedure, and allows synchronous placement of a penile prosthesis. Additionally, those that favor this approach argue that the circumferential mobilization of the bulbar urethra is facilitated by this approach due to improved exposure and laxity of the bulbar urethra.31 Wilson and colleagues reported a cure (0 pads/day)/improved rate (0–1 pads/day) of 66% and 34%, respectively, in 37 patients at a mean follow-up of 1 year.31 Of the 37 men, 12 had a prior infection or erosion whereas 25 were primary implants. Urethral erosion occurred in 2 patients, both of which were secondary implants in patients who had a prior erosion/infection.31
Skeptics of the transscrotal technique argue that the cuff is not placed around the proximal bulbar urethra as in the perineal approach, but rather at the distal bulbar urethra. This argument is supported by the observation that in the transscrotal technique the bulbospongious muscle is never divided, rather it is displaced with deaver retractors. Our extensive experience with urethral surgery tells us that if the “bulbospongious muscle is not a factor,” the cuff location must be the distal bulbar urethra. Further-more, the urethral caliber becomes progressively smaller from mid-bulb to urethral meatus, thereby necessitating a smaller cuff size at a distal site. In the transscrotal approach, 32 of the 37 cuffs were 4.0 cm and the remaining 5 were 4.5 cm,31 compared with the cuffs placed through a perineal incision at the Mayo Clinic, where 267 of the 272 bulbar cuffs were 4.5 cm and the remaining 5 were 5.0 cm.17 This large disparity in cuff size testifies to the fact that transscrotally placed cuffs are located at the distal, not the proximal, bulbar urethra.
It will be interesting to see if the concern over cuff location with the transscrotal technique translates into an increased revision rate due to a loose-fitting cuff or urethral atrophy. Furthermore, it remains to be seen if placement of the cuff around this relatively thin portion of the urethra, compared with placement around the more robust proximal bulb approached via the perineum, leads to a greater incidence of iatrogenic urethral injury or urethral erosion.
Conclusion
Although the surgical technique for radical prostatectomy continues to improve, persistent urinary incontinence due to ISD still occurs in 4% of patients, significantly compromising their lifestyle. Our evaluation includes history, physical, questionnaire, bladder diary, 24-hour pad test, cystoscopy, and urodynamics. Although the male sling procedure is becoming increasingly popular for PPI, the AUS remains the gold standard. We continue to prefer the perineal approach over the transscrotal approach for primary implants as we are assured of proper cuff placement around the proximal bulbar urethra. Placement of the AUS cuff using the transcorporal technique has been able to salvage patients with urethral atrophy and with prior AUS erosion or infection.
Main Points.
Urinary incontinence following prostatectomy, or post-prostatectomy incontinence (PPI), is the result of intrinsic sphincter deficiency (ISD) and can persist as a chronic ailment in 40% of patients following radical prostatectomy.
ISD is evaluated using medical and surgery history, physical examination, and urodynamic testing. The 24-hour pad test determines the severity of the urine loss in order for the appropriate medical treatment or surgical therapy to be prescribed.
The artificial urinary sphincter (AUS) has proven to be the gold standard procedure for men with incontinence due to ISD. The current model is the AMS 800, first introduced in 1983. Erosion and infection are the 2 most serious complications of the AUS.
Bulbourethral and bone-anchored slings are alternative procedures for patients who refuse AUS, and bone-anchored slings are effective primarily in patients with low volume PPI due to ISD, defined as a 24-hour pad weight of 150 grams or less.
Urethral atrophy is the reduction in urethral size that occurs from compression of the tissue encircled by the occlusive cuff. Treatment options include downsizing the cuff, moving the cuff, and placement of a second cuff in tandem. Treatment with a single cuff AMS 800 is successful in 89% of patients.
The transcorporal technique for AUS placement has permitted safe and effective reimplantation in men with prior history of AUS erosion and infection that were once considered unsalvageable.
References
- 1.Litwin MS, Hays RD, Fink A, et al. Quality-of-life outcomes in men treated for localized prostate cancer. JAMA. 1995;273:129–135. doi: 10.1001/jama.273.2.129. [DOI] [PubMed] [Google Scholar]
- 2.Ficazolla MA, Nitti VW. The etiology of post-radical prostatectomy incontinence and correlation of symptoms with urodynamic findings. J Urol. 1998;160:1317–1320. [PubMed] [Google Scholar]
- 3.Peyromaure M, Ravery V, Boccon-Gibod LB. The management of stress urinary incontinence after radical prostatectomy. BJU Int. 2002;90:155–161. doi: 10.1046/j.1464-410x.2002.02824.x. [DOI] [PubMed] [Google Scholar]
- 4.Perez LM, Webster GD. Successful outcome of artificial urinary sphincters in men with post-prostatectomy urinary incontinence despite adverse implantation features. J Urol. 1992;148:1166–1170. doi: 10.1016/s0022-5347(17)36850-7. [DOI] [PubMed] [Google Scholar]
- 5.Kuznetsov DD, Kim HL, Patel RV, et al. Comparison of the artificial urinary sphincter and collagen for the treatment of postprostatectomy incontinence. Urology. 2000;56:600–603. doi: 10.1016/s0090-4295(00)00723-8. [DOI] [PubMed] [Google Scholar]
- 6.Schaeffer AJ, Clemens JQ, Ferrari M, et al. The male bulbourethral sling procedure for post-radical prostatectomy incontinence. J Urol. 1998;159:1510–1515. doi: 10.1097/00005392-199805000-00026. [DOI] [PubMed] [Google Scholar]
- 7.Kaufman JJ. A new operation for male incontinence. Surg Gynecol Obstet. 1970;131:295–299. [PubMed] [Google Scholar]
- 8.Comiter CV. The male sling for stress urinary incontinence: a prospective study. J Urol. 2002;167:597–601. doi: 10.1016/S0022-5347(01)69092-X. [DOI] [PubMed] [Google Scholar]
- 9.Madjar S, Jacoby K, Giberti C, et al. Bone anchored sling for the treatment of post-prostatectomy incontinence. J Urol. 2001;165:72–76. doi: 10.1097/00005392-200101000-00018. [DOI] [PubMed] [Google Scholar]
- 10.Scott FB, Bradley WE, Timm GW. Treatment of urinary incontinence by implantable prosthetic sphincter. Urology. 1973;1:252–259. doi: 10.1016/0090-4295(73)90749-8. [DOI] [PubMed] [Google Scholar]
- 11.Montague DK, Angermeier KW. Prostatectomy urinary incontinence: the case for the artificial urinary sphincter. Urology. 2000;55:2–4. doi: 10.1016/s0090-4295(99)00413-6. [DOI] [PubMed] [Google Scholar]
- 12.Gundian JC, Barrett DM, Parulkar BG. Mayo clinic experience with use of the AMS 800 artificial urinary sphincter for urinary incontinence following radical prostatectomy. J Urol. 1989;142:1459–1461. doi: 10.1016/s0022-5347(17)39126-7. [DOI] [PubMed] [Google Scholar]
- 13.Marks JL, Light JK. Management of urinary incontinence after prostatectomy with the artificial urinary sphincter. J Urol. 1989;142:302–304. doi: 10.1016/s0022-5347(17)38739-6. [DOI] [PubMed] [Google Scholar]
- 14.Singh G, Thomas DG. Artificial urinary sphincter for post-prostatectomy incontinence. Br J Urol. 1996;77:248–251. doi: 10.1046/j.1464-410x.1996.85614.x. [DOI] [PubMed] [Google Scholar]
- 15.Litwiller SE, Kim KB, Fone PD, et al. Post-prostatectomy incontinence and the AUS: a long-term study of patient satisfaction and criteria for success. J Urol. 1996;156:1975–1980. doi: 10.1016/s0022-5347(01)65408-9. [DOI] [PubMed] [Google Scholar]
- 16.Hajivassiliou CA. A review of the complications and results of implantation of the AMS artificial urinary sphincter. Eur Urol. 1999;35:36–44. doi: 10.1159/000019817. [DOI] [PubMed] [Google Scholar]
- 17.Elliott DS, Barrett DM. Mayo Clinic long-term analysis of the functional durability of the AMS 800 artificial urinary sphincter: a review of 323 cases. J Urol. 1998;159:1206–1208. [PubMed] [Google Scholar]
- 18.Venn SN, Greenwell TJ, Mundy AR. The long-term outcome of artificial urinary sphincters. J Urol. 2000;164:702–706. doi: 10.1097/00005392-200009010-00020. [DOI] [PubMed] [Google Scholar]
- 19.Raj GV, Peterson AC, Toh KL, Webster GD. Outcomes following revisions and secondary implantations of the artificial urinary sphincter. J Urol. 2003;169 doi: 10.1097/01.ju.0000152315.91444.d0. abstract 471. [DOI] [PubMed] [Google Scholar]
- 20.Saffarian A, Walsh K, Walsh IK, Stone AR. Urethral atrophy after artificial urinary sphincter placement: is cuff downsizing effective? J Urol. 2003;169:567–569. doi: 10.1097/01.ju.0000046665.89269.f7. [DOI] [PubMed] [Google Scholar]
- 21.Couillard DR, Vapnek JM, Stone AR. Proximal artificial sphincter cuff repositioning for urethral atrophy incontinence. Urology. 1995;45:653–656. doi: 10.1016/s0090-4295(99)80058-2. [DOI] [PubMed] [Google Scholar]
- 22.Motley RC, Barrett DM. Artificial urinary sphincter cuff erosion: experience with reimplantation in 38 patients. Urology. 1990;35:215–218. doi: 10.1016/0090-4295(90)80034-k. [DOI] [PubMed] [Google Scholar]
- 23.Kowalczyk JJ, Nelson R, Mulcahy JJ. Successful reinsertion of the artificial urinary sphincter after removal for erosion or infection. Urology. 1996;48:906–908. doi: 10.1016/s0090-4295(96)00245-2. [DOI] [PubMed] [Google Scholar]
- 24.Frank I, Elliott DS, Barrett DM. Success of de novo reimplantation of the artificial genitourinary sphincter. J Urol. 2000;163:1702–1703. [PubMed] [Google Scholar]
- 25.Guralnick ML, Miller E, Toh KL, Webster GD. Transcorporal artificial urinary sphincter cuff placement in cases requiring revision for erosion and urethral atrophy. J Urol. 2002;167:2075–2078. [PubMed] [Google Scholar]
- 26.Brito CG, Mulcahy JJ, Mitchell ME, Adams MC. Use of a double cuff AMS 800 urinary sphincter for severe stress incontinence. J Urol. 1993;149:283–285. doi: 10.1016/s0022-5347(17)36057-3. [DOI] [PubMed] [Google Scholar]
- 27.Kowalczyk JJ, Spicer DL, Mulcahy JJ. Long-term experience with the double-cuff AMS 800 artificial urinary sphincter. Urology. 1996;47:895–897. doi: 10.1016/S0090-4295(96)00042-8. [DOI] [PubMed] [Google Scholar]
- 28.Kowalczyk JJ, Spicer DL, Mulcahy JJ. Erosion rate of the double cuff AMS 800 artificial urinary sphincter: long-term followup. J Urol. 1996;156:1300–1301. [PubMed] [Google Scholar]
- 29.Flynn BJ, Webster GD, Guralnick ML. Implantation of the artificial urinary sphincter (AUS) using the transcorporal approach. J Urol. 2002;167 abstact V1140. [Google Scholar]
- 30.DiMarco DS, Elliot DS. Tandem cuff artificial urinary sphincter as a salvage procedure following failed primary sphincter placement for the treatment of post-prostatectomy incontinence. J Urol. 2003;170:1252–1254. doi: 10.1097/01.ju.0000085787.21140.db. [DOI] [PubMed] [Google Scholar]
- 31.Wilson SK, Delk JR, Henry GH, Siegel AL. New surgical technique for sphincter urinary control system using upper transverse scrotal incision. J Urol. 2003;169:261–264. doi: 10.1016/S0022-5347(05)64082-7. [DOI] [PubMed] [Google Scholar]