Abstract
Prostate cancer recurrence affects up to 50% of men in the first 10 years after radical prostatectomy for clinically localized disease. New treatment approaches that reduce this risk are needed. Current published data on the use of adjuvant hormonal therapy in the post-radical prostatectomy setting are limited. New data from the bicalutamide (Casodex) Early Prostate Cancer program show that bicalutamide 150 mg/d, given as immediate adjuvant treatment, produces clear benefits with respect to both progression-free survival and prostate-specific antigen progression when compared with standard management with radical prostatectomy. The greatest improvement of progression-free survival was observed in patient subgroups at highest risk.
Key words: Prostate cancer, Bicalutamide, Radical prostatectomy, Adjuvant hormonal therapy
Prostate cancer is one of the leading causes of morbidity and cancer-related death among the elderly male population.1 In its advanced stages, prostate cancer can only be treated palliatively. The disease is potentially curable, however, either by radical prostatectomy or radiotherapy if detected while still clinically localized (organ-confined). Radical prostatectomy is generally recommended for men with no contraindications to surgery who have a life expectancy of at least 10 years, and its use is increasing because prostate cancer is being diagnosed at earlier stages and in younger men.2 Indeed, radical prostatectomy is now the most widely employed primary therapeutic strategy for prostate cancer in the United States.2
Radical prostatectomy is associated with high disease-specific survival rates, at least 90% at 10 years.3,4 However, cancer recurrence (biochemical, local, or systemic) is a significant problem, affecting up to 50% of men within 10 years.3,5,6 Many, but not all, of these treatment failures occur in patients found to have locally advanced disease, with or without lymph node involvement, when staged pathologically.3,6,7 Other risk factors for disease recurrence are a high Gleason score (≥ 7), a high prostate-specific antigen (PSA) level at diagnosis (> 4 ng/mL), and multiple positive surgical margins.3,7,8
New treatment approaches that reduce the risk of disease recurrence and potential disease-related morbidity after radical prostatectomy are therefore needed. The recent increase in the number of men diagnosed with and treated for clinically localized prostate cancer9,10 underscores this need. Options being explored to optimize the outcome of radical prostatectomy include refined surgical techniques, neoadjuvant therapy, and adjuvant therapy (both hormonal therapy and chemotherapy). This article reviews the current published data on the use of adjuvant hormonal therapy—treatment given after therapy of primary curative intent, post-radical prostatectomy—and presents new data from the bicalutamide (Casodex) Early Prostate Cancer (EPC) program on the efficacy and tolerability of adjuvant bicalutamide 150 mg/d in this setting.
Current Evidence Supporting the Use of Adjuvant Hormonal Therapy After Radical Prostatectomy
Recurrence of prostate cancer after radical prostatectomy is due to the growth of residual cancer cells, either outside the surgical margins and/or at distant sites, that were either not removed or were undetectable at the time of surgery. The aim of adjuvant hormonal therapy is to target these residual cells.
Despite increasing interest in the treatment of early stage prostate cancer, only three relatively small, randomized studies have published data on the efficacy of adjuvant hormonal therapy in the radical prostatectomy setting (Table 1).11–13
Table 1.
Summary of Published Studies of Hormonal Therapy as Adjuvant to Radical Prostatectomy
| Disease-free Survival | Overall Survival | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adjuvant | Disease | Median | Adjuvant | Adjuvant | ||||||
| Study | Therapy | n | Stage | Follow-up (y) | + RP | RP Alone | P Value | + RP | RP Alone | P Value |
| Messing et al12 | Goserelin | 98 | cT1–T2, | 10 | Data not available for 10 years* | 72% | 49% | .025 | ||
| or BO | pN1-N3 | |||||||||
| Prayer-Galetti | ||||||||||
| et al13 | Goserelin | 201 | pT3 | 5 | 25.2% difference favoring adjuvant | NA | NA | NA | ||
| Wirth et al11 | Flutamide | 365 | pT3 | 4 | 90%† | 69%† | .0029 | NA | NA | NA |
BO, bilateral orchiectomy; NA, not available; RP, radical prostatectomy.
Disease-free survival at 7.1 years’ follow-up was 77% for adjuvant + RP, 43% for RP alone; P<.001.14
Including freedom from biochemical recurrence in addition to local or systemic recurrence
All three studies enrolled patients identified as having locally advanced disease at radical prostatectomy; the patients of Messing and colleagues12 had lymph node metastases (and therefore also underwent bilateral pelvic lymphadenectomy). The adjuvant therapies used were castration (the luteinizing hormone-releasing hormone [LHRH] agonist goserelin [Zoladex] or bilateral orchiectomy) in two studies12,13 and the nonsteroidal antiandrogen flutamide in one study.11
All three studies report significant differences in disease-free survival favoring adjuvant therapy after a median follow-up of 4–10 years (Table 1). So far, overall and disease-specific survival data have only been presented by Messing and colleagues (Table 1).12 At a median 10 years’ follow-up, overall mortality was significantly higher in the control group than in the adjuvant group (51% vs 28%; P = .025). Disease-specific mortality was also greater in the control group compared with the adjuvant group (43% vs 13%; P = .001).
Thus, the available evidence, although from relatively small studies, suggests that the use of adjuvant hormonal therapy can reduce the risk of prostate cancer recurrence in men with adverse pathological findings at radical prostatectomy. In addition to these findings in prostate cancer, adjuvant hormonal therapy has a well established role in breast cancer, a hormone-responsive tumor that can be considered analogous to prostate cancer. A meta-analysis of randomized controlled trials shows that 5 years’ adjuvant therapy with the antiestrogen tamoxifen, given in addition to radical therapy for early disease, reduces the risk of disease recurrence at 10 years by 47% and corresponding mortality by 26%.15
In two of the above-described studies of adjuvant hormonal therapy in the radical prostatectomy setting, the hormonal therapy used was castration (LHRH agonist therapy or bilateral orchiectomy). These therapies generally cause loss of libido, impotence, and fatigue.16 Alternative adjuvant hormonal strategies are needed for men unwilling to consider castration as adjuvant therapy. In the third study the hormonal therapy used was the nonsteroidal antiandrogen flutamide. Nonsteroidal antiandrogens antagonize the actions of androgens, irrespective of their source, at the receptor level, and do not suppress testosterone production. These agents may offer quality-of-life benefits over castration. However, flutamide has been associated with a high incidence of diarrhea, which appears to be more common, and sometimes more severe, than with other nonsteroidal antiandrogens.17
The Bicalutamide Early Prostate Cancer (EPC) Program
Rationale and Design
The success of the antiestrogen tamoxifen in early breast cancer provides a rationale for assessing the potential role of antiandrogens as adjuvant therapy in early prostate cancer.18 Accordingly, the bicalutamide EPC program, the largest prostate cancer treatment trial program ever conducted, was initiated to evaluate the benefits of the nonsteroidal antiandrogen bicalutamide 150 mg/d given in addition to standard care of radical prostatectomy, radiotherapy, or “watchful waiting” in men with localized or locally advanced disease.
This ongoing international trial program consists of three prospective, randomized, double-blind, placebo-controlled trials being conducted in distinct geographic areas: North America (Trial 23); Europe, South Africa, Israel, Australia, and Mexico (Trial 24); and Scandinavia (Trial 25).19 Although the three trials are of generally similar design, entry criteria did differ reflecting local clinical practices; candidates for “watchful waiting” and patients known to have lymph node metastases were excluded from participation in Trial 23, whereas in Trials 24 and 25 patients were included irrespective of lymph node status and could either have received therapy of primary curative intent or were considered appropriate for “watchful waiting.”
Overall, a total of 8113 men with T1b-4NxM0 prostate cancer have been randomized to receive bicalutamide 150 mg/d or placebo, with randomized treatment scheduled for 2 years in Trial 23 and at least 5 years in the other two trials. Of these patients, 55% underwent radical prostatectomy and 17% underwent radiotherapy in the 16 weeks prior to randomization, while the remaining 28% were considered suitable candidates for “watchful waiting.” All patients are being followed for objective progression and survival. The findings from the first scheduled analysis, conducted after a minimum of 2 years’ follow-up (median 3 years), are summarized here, with the focus on the subgroup of patients given radical prostatectomy.
Overall Findings
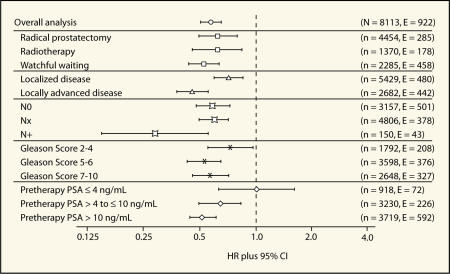
In the overall study population, immediate treatment with bicalutamide 150 mg/d, in addition to standard care, produced a highly significant reduction in the risk of objective progression (defined as progression confirmed by bone scan, magnetic resonance imaging, ultrasound or computed tomography, or death without prior progression) of 42% compared with the placebo controls receiving standard care only (9.0% vs 13.8%; hazard ratio [HR] 0.58, 95% CI, 0.51–0.66, P ≪ .0001).20 Reductions in the risk of objective progression were clearest in patients with poor prognosis (localized [HR 0.72; 95% CI, 0.60–0.86, P < .001]; locally advanced [HR 0.46; 95% CI, 0.38–0.56, P < .001]) and in each of the three standard care groups (Figure 1). Likewise, although the absolute size of the difference varied, statistically significant differences in favor of bicalutamide 150 mg/d therapy were also seen irrespective of lymph node status, across all Gleason grades, and in patients with a pretherapy PSA ≥ 4 ng/mL (Figure 1).
Figure 1.
Forest plot of analysis of objective progression by risk factor in the overall bicalutamide EPC program.
Bicalutamide 150 mg/d given in addition to standard care also reduced the risk of PSA progression (defined as the earliest of PSA doubling relative to the prerandomization value, objective progression, or death in the absence of prior progression) by 59% relative to placebo (16% vs 33%; HR 0.41, 95% CI, 0.38–0.45; P ≪ .0001).20 So far, less than 2% of patients have died of prostate cancer; overall mortality is 6% with no significant difference between the bicalutamide 150 mg/d and placebo groups (HR 0.93, 95% CI, 0.79–1.11; P = .43).20
The tolerability profile of bicalutamide 150 mg/d was closely related to its pharmacology, with gynecomastia and breast pain the most frequently reported side effects (66% and 73%, respectively).20 Other adverse events occurred in 10% or fewer of patients in both groups. Overall, 15.6% of patients in the bicalutamide 150 mg/d group withdrew from randomized treatment due to gynecomastia and/or breast pain compared with 0.5% of the placebo group. However, only 2.6% of the bicalutamide 150 mg/d group withdrew due to objective progression compared with 9.3% of the placebo group.
Bicalutamide 150 mg/d as Adjuvant to Radical Prostatectomy
A total of 4454 patients in the bicalutamide EPC program received randomized therapy as adjuvant to radical prostatectomy, with the majority enrolled in Trials 23 and 24 (59% and 37%, respectively) (Table 2).21 Thus, the bicalutamide EPC program is the largest trial program to evaluate whether adjuvant hormonal therapy improves the outcome of radical prostatectomy. The program included patients with pathologically confirmed organ-confined disease (n = 2734; 61% of the radical prostatectomy population), a subgroup in whom adjuvant therapy has not previously been studied.
Table 2.
Demography and Tumor Characteristics of the Radical Prostatectomy (RP) Population in the Bicalutamide EPC Program21
| % Patients* | ||
|---|---|---|
| Bicalutamide | ||
| 150 mg/d + RP | Placebo + RP | |
| (n = 2236) | (n = 2218) | |
| Mean age (range), y | 63.6 (42–89) | 66.9 (38–93) |
| Disease stage (pathological) | ||
| T1/T2 | 61 | 62 |
| T3 | 37 | 37 |
| T4 | 2 | 1 |
| Nodal status | ||
| N0 | 90 | 89 |
| Nx | 9 | 10 |
| N+ | 2 | 2 |
| Gleason score (tumor grade) | ||
| 7–10 (Poorly differentiated) | 45 | 43 |
| 5–6 (Moderately differentiated) | 45 | 46 |
| 2–4 (Well differentiated) | 10 | 11 |
| Trial | ||
| 23 | 59 | 60 |
| 24 | 37 | 37 |
| 25 | 4 | 4 |
Unless otherwise stated.
The two randomized treatment groups in the radical prostatectomy population (bicalutamide 150 mg/d, n = 2236; placebo, n = 2218) were well balanced in terms of demographic and tumor characteristics (Table 2). Only 2% of patients in each group were node-positive; however, 45% of the bicalutamide 150 mg/d group and 43% of the placebo group had poorly differentiated disease (Gleason score ≥ 7). In terms of PSA levels, 87% had PSA levels > 4 ng/mL at diagnosis (i.e., pretherapy), whereas 85% had a nonquantifiable postprostatectomy PSA (< 0.2 ng/mL in Trial 23, < 1.0 ng/mL in Trials 24 and 25; data not shown).
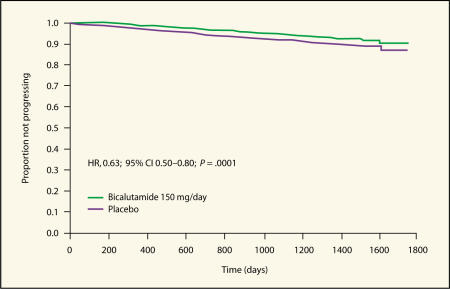
To date, a total of 285 patients (6.4%) in the radical prostatectomy population have experienced objective progression events.22 In the bicalutamide 150 mg/d group, 5.1% of patients showed evidence of objective progression compared with 7.7% in the placebo group (HR 0.63, 95% CI, 0.50–0.80; P = .0001). This represents a reduction of 37% in the risk of objective progression in radical prostatectomy patients treated with adjuvant bicalutamide 150 mg/d compared with those who received placebo.22 These findings are consistent with those of earlier studies of hormonal therapy as adjuvant to radical prostatectomy.11–13 A Kaplan-Meier plot showing the analysis of time to objective progression-free survival is shown in Figure 2.
Figure 2.
Kaplan-Meier plot of time to objective progression-free survival in patients who received radical prostatectomy as standard care.
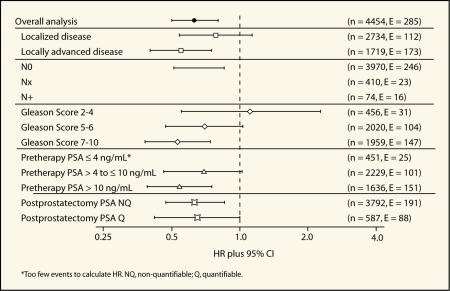
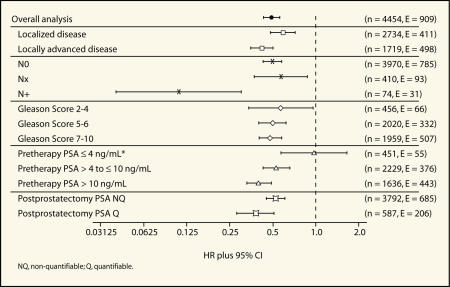
Further exploratory analyses of the objective progression data were performed according to known risk factors for disease progression (disease stage, nodal status, Gleason score, pretherapy PSA level, and postprostatectomy PSA level).21 In general these analyses revealed that adjuvant bicalutamide 150 mg/d reduced the risk of objective progression after radical prostatectomy with the greatest benefits seen in higher risk patients (Figure 3 and Table 3).
Figure 3.
Forest plot of analysis of objective progression by risk factor in patients who received radical prostatectomy as standard care.
Table 3.
Analysis of Objective Progression in the Bicalutamide EPC Program
| Time Taken for First10% | |||||
|---|---|---|---|---|---|
| of Patients to Progress (y) | |||||
| Risk Group | No. Patients | HR (95% CI) | ETR (95% CI) | Bicalutamide 150 mg/d | Placebo |
| Overall population | 4454 | 0.63 (0.50–0.80) | 1.41 (1.18–1.69) | 5.4 | 3.9 |
| Disease stage | |||||
| Localized disease | 2734 | 0.78 (0.54–1.13) | 1.21 (0.91–1.61) | 7.2 | 6.0 |
| Locally advanced disease | 1719 | 0.55 (0.40–0.75) | 1.56 (1.23–1.96) | 3.9 | 2.5 |
| Nodal status | |||||
| N0 | 3970 | 0.66 (0.51–0.85) | 1.36 (1.12–1.64) | 5.4 | 4.1 |
| Nx | 410 | –* | –* | –* | –* |
| N+ | 74 | –* | –* | –* | –* |
| Gleason score | |||||
| 2–4 | 456 | 1.11 (0.55–2.27) | 0.93 (0.56–1.56) | 3.8 | 4.2 |
| 5–6 | 2020 | 0.70(0.47–1.03) | 1.28 (0.98–1.66) | 5.8 | 4.6 |
| 7–10 | 1959 | 0.53 (0.38–0.74) | 1.68 (1.27–2.22) | 5.4 | 3.3 |
| Pretherapy PSA | |||||
| ≤ 4 ng/mL | 451 | –* | –* | –* | –* |
| > 4–10 ng/mL | 2229 | 0.69 (0.46–1.02) | 1.33 (0.98–1.82) | 7.2 | 5.4 |
| > 10 ng/mL | 1636 | 0.54 (0.39–0.75) | 1.56 (1.22–1.99) | 4.1 | 2.7 |
| Postprostatectomy PSA | |||||
| Nonquantifiable | 3792 | 0.63 (0.47–0.85) | 1.36 (1.12–1.66) | 6.2 | 4.6 |
| Quantifiable | 587 | 0.65 (0.42–1.00) | 1.50 (1.00–2.26) | 2.6 | 1.7 |
Too few events to calculate: hazard ratio (HR) and event time ratio (ETR)
Bicalutamide 150 mg/d reduced the risk of objective progression in patients with localized as well as locally advanced disease by 22% and 45%, respectively (HR 0.78, 95% CI, 0.54–1.13 and HR 0.55, 95% CI, 0.40–0.75, respectively; Figure 3).
In patients with negative lymph nodes at study entry (n = 3970) bicalutamide 150 mg/d significantly reduced the risk of objective progression by 34% compared with placebo (HR 0.66, 95% CI, 0.51–0.85; Figure 3). There have been too few events to determine the HR in patients with node-positive or unknown lymph node status (n = 16 and 23, respectively), although the incidence of progression events was lower in the bicalutamide 150 mg/d group than the placebo group for both types of patients. Benefits of bicalutamide 150 mg/d were also seen in patients with moderately and poorly differentiated disease (Gleason score 5–6, HR 0.70, 95% CI, 0.47–1.03 and Gleason score 7–10, HR 0.53, 95% CI, 0.38–0.74, respectively; Figure 3). The reduced risk of objective progression was statistically significant for patients with poorly differentiated disease. In patients with well-differentiated disease (Gleason score 2–4), there was no significant difference in the risk of objective progression between the bicalutamide 150 mg/d and placebo groups (HR 1.11, 95% CI, 0.55–2.27).
Analysis according to pretherapy PSA level revealed a clear, statistically significant benefit for bicalutamide 150 mg/d in patients with a baseline PSA greater than or equal to 10 ng/mL (HR 0.54, 95% CI, 0.39–0.75) and the risk of objective progression was also reduced in those with a PSA greater than 4 ng/mL but less than or equal to 10 ng/mL (HR 0.69, 95% CI, 0.46–1.02; Figure 3). There are still too few events in patients with a baseline PSA less than or equal to 4 ng/mL at diagnosis to calculate a HR as only 25 of the patients in this small subgroup (n = 451) have to date experienced objective progression. Reductions in the risk of progression were apparent in patients with a nonquantifiable as well as a quantifiable postprostatectomy PSA (Figure 3).
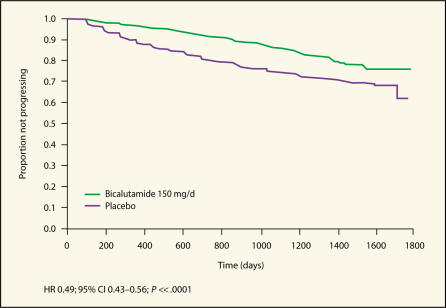
At follow-up, 15.0% of patients in the bicalutamide 150 mg/d group met the trial criteria for PSA progression, compared with 25.8% of patients in the placebo group.21 As shown in Figure 4, adjuvant bicalutamide 150 mg/d significantly reduced the risk of PSA progression relative to standard care with radical prostatectomy (HR 0.49, 95% CI, 0.43–0.56; P ≪ .0001).22 These findings are important as a rising PSA is known to precede objective progression23 and can be a source of anxiety for many patients.
Figure 4.
Kaplan-Meier plot of time to PSA progression-free survival in patients who received radical prostatectomy as standard care.
Exploratory analyses of the PSA progression data show that adjuvant bicalutamide 150 mg/d significantly reduced the risk of PSA progression irrespective of nodal status, Gleason score, disease stage, postprostatectomy PSA, and when the pretherapy PSA exceeded 4 ng/mL, with the greatest reductions in those at highest risk of progression (Figure 5 and Table 4).21 The PSA ≤ 4 ng/mL subgroup, in which there have been relatively few events (55; 12.2%), is the only subgroup in which no significant reduction in risk can be seen at the current time.
Figure 5.
Forest plot of analysis of PSA progression by risk factor in patients who received radical prostatectomy as standard care.
Table 4.
Analysis of PSA Progression in the Bicalutamide EPC Program
| Time Taken for First 10% | |||||
|---|---|---|---|---|---|
| of Patients to Progress (y) | |||||
| Risk Group | No. Patients | HR (95% CI) | ETR (95% CI) | Bicalutamide 150 mg/d | Placebo |
| Overall population | 4454 | 0.49 (0.43–0.56) | 1.94 (1.71–2.21) | 2.0 | 1.1 |
| Disease stage | |||||
| Localized disease | 2734 | 0.59 (0.48–0.72) | 1.65 (1.36–2.00) | 2.6 | 1.7 |
| Locally advanced disease | 1719 | 0.42 (0.35–0.50) | 2.22 (1.87–2.65) | 1.5 | 0.7 |
| Nodal status | |||||
| N0 | 3970 | 0.50 (0.43–0.58) | 1.90 (1.66–2.19) | 2.1 | 1.2 |
| Nx | 410 | 0.57 (0.37–0.87) | 1.65 (1.11–2.44) | 1.9 | 1.1 |
| N+ | 74 | 0.11 (0.04–0.30) | 6.00 (2.64–13.60) | 1.6 | 0.3 |
| Gleason score | |||||
| 2–4 | 456 | 0.57 (0.34–0.96) | 1.56 (1.02–2.38) | 2.7 | 1.7 |
| 5–6 | 2020 | 0.50 (0.40–0.62) | 1.94 (1.56–2.41) | 2.5 | 1.4 |
| 7–10 | 1959 | 0.48 (0.40–0.58) | 1.98 (1.66–2.35) | 1.6 | 0.9 |
| Pretherapy PSA | |||||
| ≤ 4 ng/mL | 451 | 0.97 (0.57–1.65) | 1.03 (0.60–1.76) | 2.5 | 2.6 |
| > 4–10 ng/mL | 2229 | 0.53 (0.43–0.66) | 1.79 (1.46–2.19) | 2.4 | 1.4 |
| > 10 ng/mL | 1636 | 0.40 (0.33–0.49) | 2.31 (1.92–2.79) | 1.6 | 0.7 |
| Postprostatectomy PSA | |||||
| Nonquantifiable | 3792 | 0.52 (0.45–0.61) | 1.78 (1.54–2.05) | 2.3 | 1.4 |
| Quantifiable | 587 | 0.38 (0.28–0.51) | 2.75 (2.02–3.74) | 2.0 | 0.4 |
HR, hazard ratio; ETR, event time ratio
In addition to the conventional Cox regression analysis to estimate the HR, which measures the reduction in the risk of an event (with bicalutamide relative to placebo), the relative increase in the time to progression for each risk group within the radical prostatectomy population was estimated using a new analysis, the Event Time Ratio (ETR).24 An ETR > 1 indicates a benefit for bicalutamide 150 mg/d and a 95% CI excluding 1 indicates that the benefit was statistically significant. This model was used to estimate the time taken for 10% of patients in each treatment group to progress. The results for the overall population and each of the risk subgroups are presented in Table 3 and Table 4.
In the overall population, the estimated time taken for the first 10% of patients to objectively progress was 3.9 years in the placebo group and 5.4 years in the bicalutamide 150 mg/d group, a difference of 18 months at this point on the Kaplan-Meier curve.22 The estimated time taken for 10% of patients to experience PSA progression was 1.1 years in the placebo group and 2.0 years in the bicalutamide 150 mg/d group, a difference at this point on the Kaplan-Meier curve of 10.8 months.22
In line with the findings in the overall study population, the tolerability profile of bicalutamide 150 mg/d given as adjuvant to radical prostatectomy was closely related to its pharmacology.21 Gynecomastia and breast pain were the most frequent adverse events in the bicalutamide 150 mg/d group, reported in 72% and 78% of patients, respectively; the corresponding incidence of these events in the placebo group was 9% and 8%. The incidence of hot flashes was relatively low in both groups (bicalutamide 150 mg/d 10%, placebo 6%). Decreased libido was reported by 4% and 1% of patients, respectively, whereas 6% of men in both groups reported impotence as an adverse event; the frequency of these events was similar or slightly lower than in the study population as a whole. Diarrhea was reported infrequently in both the bicalutamide 150 mg/d and placebo groups and the incidence was comparable in the two groups (both 5%). Likewise, the incidence of abnormal liver function tests, reported as an adverse event, was low in both treatment groups (3% versus 2%). All other adverse events occurred in 11% or fewer patients in both groups; there were no marked differences between the treatment groups and no unexpected adverse events occurred.
Conclusions
Although radical prostatectomy is an effective strategy for managing clinically localized prostate cancer, a significant proportion of patients are at high risk of subsequent treatment failure in terms of biochemical, local, or systemic disease recurrence. Published data evaluating the use of adjuvant hormonal therapy in this setting has been limited. The patients in the bicalutamide EPC program who underwent radical prostatectomy represent the largest group of patients in whom adjuvant hormonal therapy has been evaluated in this setting. At 3-year follow up, bicalutamide 150 mg/d, when given as immediate adjuvant treatment in men with localized or locally advanced disease, demonstrated clear benefits over standard management with radical prostatectomy with respect to progression-free survival and PSA progression. Further exploratory analyses of the results by risk factor show that the benefits of adjuvant bicalutamide 150 mg/d with respect to progression-free survival are clearest in those patients at greatest risk of disease progression after radical prostatectomy. Bicalutamide 150 mg/d therapy adjuvant to radical prostatectomy was not associated with any unexpected tolerability findings.
Main Points.
Recurrence of prostate cancer after radical prostatectomy is due to the growth of residual cancer cells, either outside the surgical margins and/or at distant sites, that were either not removed or were undetectable at the time of surgery. The aim of adjuvant hormonal therapy is to target these residual cells.
The available evidence suggests that the use of adjuvant hormonal therapy can reduce the risk of prostate cancer recurrence in men with adverse pathological findings at radical prostatectomy.
The bicalutamide (Casodex) EPC program, the largest prostate cancer treatment trial program ever conducted, was initiated to evaluate the benefits of the nonsteroidal antiandrogen bicalutamide 150 mg/d given in addition to standard care of radical prostatectomy, radiotherapy, or “watchful waiting” in men with localized or locally advanced disease.
In the overall study population, immediate treatment with bicalutamide 150 mg/d, in addition to standard care, produced a highly significant reduction in the risk of objective progression (defined as progression confirmed by bone scan, magnetic resonance imaging, ultrasound or computed tomography, or death without prior progression) compared with the placebo controls receiving standard care only.
To date, a total of 285 patients (6.4%) in the radical prostatectomy population have experienced objective progression events. In the bicalutamide 150 mg/d group, 5.1% of patients showed evidence of objective progression compared with 7.7% in the placebo group. This represents a reduction of 37% in the risk of objective progression in radical prostatectomy patients treated with adjuvant bicalutamide 150 mg/d compared with those who received placebo.
References
- 1.Prostate Cancer Resource Center. American Cancer Society web site. Available at: http://www.cancer.org/cancerinfo.
- 2.Mettlin C. Changes in patterns of prostate cancer care in the United States: results of American College of Surgeons Commission on Cancer studies, 1974–1993. Prostate. 1997;32:221–226. doi: 10.1002/(sici)1097-0045(19970801)32:3<221::aid-pros9>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 3.Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 4.Lai S, Lai H, Krongrad A, Roos BA. Overall and disease-specific survival after radical prostatectomy: geographic variation. Urology. 2001;57:504–509. doi: 10.1016/s0090-4295(00)01035-9. [DOI] [PubMed] [Google Scholar]
- 5.Trepasso JG, deKernion JB, Smith RB, Dorey F. The incidence and significance of detectable levels of serum prostate-specific antigen after radical prostatectomy. J Urol. 1994;152:1821–1825. doi: 10.1016/s0022-5347(17)32394-7. [DOI] [PubMed] [Google Scholar]
- 6.Amling CL, Brute ML, Bergstralh EJ, et al. Long-term hazard of progression after radical prostatectomy for clinically localized prostate cancer: continued risk of biochemical failure after 5 years. J Urol. 2000;164:101–105. [PubMed] [Google Scholar]
- 7.Epstein JI, Partin AW, Sauvageot J, Walsh PC. Prediction of progression following radical prostatectomy. Am J Surg Path. 1996;20:286–292. doi: 10.1097/00000478-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Kupelian PA, Katcher J, Levin HS, Klein EA. Stage T1–2 prostate cancer: a multivariate analysis of factors affecting biochemical and clinical failures after radical prostatectomy. Int J Radiat Oncol Biol Phys. 1997;37:1043–1052. doi: 10.1016/s0360-3016(96)00590-1. [DOI] [PubMed] [Google Scholar]
- 9.Stewart AK, Bland KI, McGinnis LS, et al. Clinical highlights from the National Cancer Database, 2000. CA Cancer J Clin. 2000;50:171–183. doi: 10.3322/canjclin.50.3.171. [DOI] [PubMed] [Google Scholar]
- 10.Cookson MM. Prostate cancer: screening and early detection. Cancer Control. 2001;8:133–140. doi: 10.1177/107327480100800203. [DOI] [PubMed] [Google Scholar]
- 11.Wirth M, Frohmuller H, Marz F, et al. Randomized multicenter trial on adjuvant flutamide therapy in locally advanced prostate cancer after radical surgery [abstract] Br J Urol. 1997;80(suppl 2):263. Abstract 1033. [Google Scholar]
- 12.Messing E, Manola J, Sarosdy M, et al. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node positive prostate cancer: results at 10 years of EST 3886 [abstract] J Urol. 2003;169:396. doi: 10.1056/NEJM199912093412401. Abstract 1480. [DOI] [PubMed] [Google Scholar]
- 13.Prayer-Galetti T, Zattoni F, Capizzi A, et al. Disease-free survival in patients with pathological “C Stage” prostate cancer at radical retropubic prostatectomy submitted to adjuvant hormonal treatment [abstract] Eur Urol. 2000;38:504. Abstract 48. [Google Scholar]
- 14.Messing EM, Manola J, Parody M, et al. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N Engl J Med. 1999;341:1781–1788. doi: 10.1056/NEJM199912093412401. [DOI] [PubMed] [Google Scholar]
- 15.Early Breast Cancer Trialists’ Collaborative Group, authors. Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. 1998;351:1451–1467. [PubMed] [Google Scholar]
- 16.Kirby RS. Treatment options for early prostate cancer. Urology. 1998;52:948–962. doi: 10.1016/s0090-4295(98)00428-2. [DOI] [PubMed] [Google Scholar]
- 17.McLeod DG. Tolerability of nonsteroidal antiandrogens in the treatment of advanced prostate cancer. Oncologist. 1997;2:18–27. [PubMed] [Google Scholar]
- 18.Chodak G, Kolvenbag GJ. Will the experience with tamoxifen in breast cancer help define the role of antiandrogens in prostate cancer? Prostate Cancer Prostatic Dis. 2001;4:72–80. doi: 10.1038/sj.pcan.4500518. [DOI] [PubMed] [Google Scholar]
- 19.See WA, McLeod D, Iversen P, Wirth M. The Bicalutamide Early Prostate Cancer Program. Demography. Urol Oncol. 2001;6:43–47. doi: 10.1016/s1078-1439(00)00118-6. [DOI] [PubMed] [Google Scholar]
- 20.See WA, Wirth MP, McLeod DG, et al. Bicalutamide (Casodex) 150 mg as immediate therapy either alone or as adjuvant to standard care in patients with localized or locally advanced prostate cancer: first analysis of the Early Prostate Cancer Program. J Urol. 2002;168:429–435. [PubMed] [Google Scholar]
- 21. AstraZeneca LP, Wilmington, DE: data on file.
- 22.Klimberg I, See WA, Wirth MP, et al. New analysis shows that bicalutamide 150 mg as adjuvant to radical prostatectomy significantly increases progression-free survival in early prostate cancer [abstract] J Urol. 2003;169(suppl 4):180. Abstract 696. [Google Scholar]
- 23.Pollack A, Zagars GK, Kavadi VS. Prostate specific antigen doubling time and disease relapse after radiotherapy for prostate cancer. Cancer. 1994;74:670–678. doi: 10.1002/1097-0142(19940715)74:2<670::aid-cncr2820740220>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 24.Carroll KJ. On the use and utility of the Weibull model in the analysis of survivial data. Control Clin Trials. 2003;24:682–701. doi: 10.1016/s0197-2456(03)00072-2. [DOI] [PubMed] [Google Scholar]