Abstract
This article provides a historical perspective on the evolution of theories regarding the pathophysiology of stress urinary incontinence (SUI). The progression of these theories has followed the development of the diagnostic technologies that have provided insight into different aspects of urethral dysfunction. The earliest theories tied SUI to anatomic failure of urethral support. Recognition that anatomic failure impacted the interplay of intra-abdominal pressure and the bladder and urethra led to theories focused on the dynamic interaction between the bladder and urethral pressures. Investigators then began to recognize the importance of urethral sphincteric dysfunction. More recently, investigators have attempted to combine the anatomic and functional etiologies into a consolidated theory. These efforts point to a multi-factorial etiology of SUI. Continuing research has provided new insight into the neurophysiology of urethral function, opening new avenues for tailoring therapy for SUI.
Key words: Stress urinary incontinence, Pathophysiology, Intrinsic sphincteric deficiency
The evolution of our understanding of the pathophysiology of stress urinary incontinence (SUI) has followed on the heels of new diagnostic modalities for the disorder. Frequently, new diagnostic tools have revealed novel pathologic evidence that has served to modify prevailing theories regarding the etiology of SUI. In some cases, interpretation of the new information has appeared to contradict previous theories.
These theories on the etiology of SUI have largely fallen into 2 categories: those focused on pathologic support of the anterior vaginal wall and those focused on pathologic function of the urethra. Combining these theories into a cohesive understanding has historically been challenging, as expressed by Aldridge in 19461: “Unfortunately, we do not yet have complete knowledge of the anatomy of the urethra and surrounding structures or an entirely satisfactory understanding of the physiology of the delicate sphincter mechanism by which urination is controlled. For this reason it has been difficult to evaluate the importance of various anatomic changes which are usually observed in cases of urinary stress incontinence …” In spite of remarkable advances in our understanding of the pathophysiology of SUI, Aldridge’s sentiments apply as well today as they did in his time. New studies into the neurophysiologic function of the continence mechanism may provide a bridge between the anatomic and functional theories, but an understanding of the evolution of these theories is useful in consolidating them.
Evolution of the Theories on the Pathophysiology of SUI
Early Anatomic Theories
Medicine in the 19th century largely focused on anatomic abnormalities, reflecting the nascent nature of physiology during this era, as well as a diagnostic armamentarium that was chiefly limited to physical diagnosis. Childbirth not only was a common cause of death among women but also frequently resulted in injury to the pelvic floor. For the physician focused on maladies of the pelvic floor, the principal concerns were procidentia and fistulae. Compared with these conditions, the symptom of stress incontinence was a minor problem that was easily overlooked.
Such opinions are reflected in the textbooks of the time. Mann’s American System of Gynecology, for example, dedicated one of its limited number of plates to an illustration of a urethrocele, described as a “dislocation of the urethra,” in which the urethra “no longer retains its normal course and position.” The author hypothesized that “the cause … is usually a prolapse of the anterior vaginal wall,” and as an afterthought notes that “incontinence is also of common occurrence.”2
Technologic advances of the early 20th century, including electric light, significantly broadened the diagnostic modalities available to physicians and emboldened them to tackle previously overlooked maladies. An example of this phenomenon is the Kelly cystoscope and its role in the workup of SUI. Although Kelly was certainly not the first to describe endoscopy of the lower urinary tract, his notoriety, combined with the simplicity of his cystoscope compared with earlier endoscopes, made this tool much more accessible to surgeons of the time.3
Kelly used his cystoscope to describe SUI, reporting that “the cystoscopic picture presents a gaping internal sphincter orifice which closes sluggishly.”4 He attributed SUI to “vesical neck funneling,” which he hypothesized was caused by loss of elasticity or normal tone of the urethral and vesical sphincter. Kelly’s description of vesical neck funneling was a logical progression of the previous anatomic observation of loss of anterior vaginal wall support, but his insight into the involvement of sphincter tone was the predecessor of future functional theories of SUI pathophysiology.
In 1923, Bonney5 read his paper “On Diurnal Incontinence of Urine in Women” before the Royal Society of Medicine. He introduced his topic by noting that the subject had been neglected by urologists and gynecologists. A full decade after Kelly’s description of the condition, SUI remained an understudied and undertreated complaint. Bonney’s work attempted to correct this oversight by defining the symptom complex and describing its epidemiology and pathophysiology. In his definition, he noted that this form of incontinence only occurs when a woman makes some effort (such as coughing or sneezing) that produces sudden violent abdominal strain. Foreshadowing future epidemiologic studies, he observed, “Its occurrence is practically limited to women who have had children, though it does not as a rule begin until several years after the labor … between forty and fifty years of age.” His paper, which was largely based on surgical anatomy, sought to explain the etiology of SUI in terms of failure of anatomic support: “Incontinence appears to be due to laxity of the front part of the pubo-cervical muscle-sheet, so that it yields under sudden pressure and allows the bladder to slip down behind the symphysis pubis and the urethra to carry downwards and forwards by wheeling round the sub-pubic angle.”5
Bonney differentiated between different sites of loss of anterior vaginal wall support—including the superior portion, the mid portion of the pubocervical muscle sheet, and the distal portion—but carefully pointed out that only the loss of distal support results in incontinence. He hypothesized that incontinence was not caused by intravesical pressure forcing the sphincter muscle but by an interference with the sphincter mechanism due to sagging of the pubocervical muscle sheet. His careful and detailed descriptions of the loss of anatomic support became the foundation of the subsequent theories attributing incontinence to anatomic failure.
Radiography was the dominant diagnostic innovation of the 1930s, and medicine was significantly impacted by novel applications of this new tool. The watch-chain cystogram was such an innovation. Stevens and Smith6 described this predecessor of the bead-chain cystogram in 1937. The investigators improved on the cystogram by adding a transurethral watch chain to the intravesical contrast. The resulting sagittal image demonstrated “funneling of the bladder floor toward the urethra” and flattening of the urethrovesical angle.
Although this new diagnostic modality offered minimal modification to the anatomic descriptions of Bonney, Stevens and Smith arrived at the opposite conclusion of Bonney regarding the importance of the urethral sphincter. They hypothesized that the anatomic abnormalities were a reflection of a weak sphincter: “Funneling of the bladder floor toward the urethra indicates that mere increase in intravesical pressure … has forced fluid through the internal sphincter and denotes a weakening of this sphincter.”6
Pressure Transmission Theories
It is possible that Stevens’ and Smiths’ elevation of the role of the sphincter in the etiology of SUI was influenced by the writings of Kennedy, another prominent surgeon of the time.7 Kennedy also suggested injury to the urethral sphincter as the principal etiology of SUI, although his hypothesis seems to have been based more on a justification of his modifications of the then-dominant surgery for SUI, the Kelly plication, than on histologic evidence. However, he did use roentgenograms from patients who had undergone plication of the proximal urethra with silver wire to justify his theory.
Kennedy hypothesized that fibers of the levator ani muscles posterior to the symphysis pubis join in a median raphe beneath the urethra. He suggested that peripartum injury to this support and the innervation of the voluntary sphincter compromised this aspect of the continence mechanism. The continence mechanism was further compromised following delivery by “cicatricial bands between the urethra and the rami.” Kennedy suggested that these adhesions distorted the normally circular form of the involuntary sphincter causing “the folds of the urethral mucosa [to] no longer completely fill the urethral canal.”7
Barnes8 added a new diagnostic modality, manometry, to watch-chain cystography, creating the ancestor of a later diagnostic tool, video urodynamics. The manometric measurements allowed Barnes to investigate the role of urethral function in the etiology of urinary incontinence. He also sought to explain his observation that SUI did not always result from peripartum injuries. He noted that “theories concerning the nature of damage to the urethral sphincters … fall principally into two groups: those maintaining the primary damage to be on the urethra or its sphincters, and those stating that the essential changes involve peri-urethral or supporting tissues.” Based on his observations, and in an attempt to explain these inconsistencies, he suggested: “Since urinary incontinence, regardless of exact etiology, represents a momentary increase in the forces of urinary expulsion over the powers of urethral resistance, it would appear that incontinence could result from (a) an increase in urinary expulsive forces or intravesical pressure, (b) a lowering of the powers of resistance or urethral sphincter action, or (c) a combination of (a) and (b).” This influential theory set the stage for the future direction of the functional theories of SUI.
Barnes’ early manometric measurements became more sophisticated with the development of solid-state pressure transducers. These instruments were initially used to describe normal bladder and urethral function9 but were soon utilized in investigations of SUI. Enhörning10 developed a urethral catheter with 2 pressure transducers 5 cm apart, which permitted simultaneous measurement of vesical and urethral pressures. Using this apparatus, he showed that, in continent subjects, urethral pressure exceeded vesical pressure, both at rest and during increases in intra-abdominal pressure. He hypothesized that this equal rise in vesical and urethral pressure was due to transmission of intra-abdominal pressure to the bladder and the part of the proximal urethra above the pelvic floor. The transmitted intra-abdominal pressure maintained continence by augmenting the pressure resulting from sphincteric function. Conversely, “In cases of stress incontinence this upper part of the urethra is often relaxed into a funnel and has then functionally become part of the bladder. If muscles with sphincteric function do not compensate for the incompleteness in transmittance of intra-abdominal pressure to the remaining lower part of the urethra, closure pressure decreases during a cough and incontinence may be manifested.”11 This poor transmission could be demonstrated on the physiograph as pressure equalization of the urethral pressure measurement during cough.
Later investigators utilizing urodynamics suggested that the pathophysiology of SUI included more than just poor pressure transmission to the urethra. Other urodynamic parameters observed with SUI were a low maximum urethral closure pressure and short functional urethral length.12–14 In addition, the recognition of the shortcomings of the “all or none” cough pressure equalization interpretation led to efforts to quantify the deterioration of pressure transmission to the urethra, through the calculation of a pressure transmission ratio—the ratio, expressed as a percentile, of the change in urethral pressure to the change in vesical pressure associated with a cough (Figure 1).
Figure 1.
The pressure transmission ratio (PTR) is the ratio, expressed as a percentile, of the change in urethral pressure (Pura) to the change in vesical pressure (Pves) associated with a cough.
Several investigators have used pressure transmission ratios to evaluate the physiologic mechanism of failure and complications following surgery for SUI, as well as to define the pathophysiologic differences between stress-continent and stress-incontinent women.15,16 In seeking a pressure transmission ratio threshold characteristic of SUI, Bump and colleagues17 found that pressure transmission ratios of less than 90% in the proximal urethra had a sensitivity of 97% and a positive predictive value of 98% for urodynamic stress incontinence. However, whereas virtually all women with urodynamic stress incontinence had proximal pressure transmission ratios under 90%, the specificity of this finding was 56%, reflecting the fact that many stress-continent women also have decreased pressure transmission.
Sphincteric Dysfunction Theories
Refinements of the pressure transmission theory were dominant during the 1960s and 1970s, until researchers began to apply the diagnostic capabilities of neurophysiologic testing to the pelvic floor. Snooks and colleagues18 began their studies of pelvic floor denervation in women with fecal incontinence but rapidly applied this technology to women with urodynamic stress incontinence. Using nerve conduction techniques, the investigators demonstrated prolonged conduction in the perineal branch of the pudendal nerve and postulated a neurogenic etiology to SUI. Smith and colleagues19 corroborated these findings by comparing women with urodynamic stress incontinence with continent controls and demonstrating denervation injury to both the striated urethral muscle and the pelvic floor musculature in the stress-incontinent cohort.
The mounting neurophysiologic evidence for denervation of the urethral sphincter in the etiology of SUI initially seemed incompatible with the prevailing pressure transmission theories. Many investigators resolved this contradiction by defining a subset of stress-incontinent women with sphincteric dysfunction. A monograph on urinary incontinence from the Agency for Health Care Policy and Research propagated the concept of a unique subset of SUI by defining the condition “intrinsic sphincteric deficiency”20: “In this condition, the urethral sphincter is unable to generate enough resistance to retain urine in the bladder, especially during stress maneuvers.” The concept was readily adopted, although its clinical definition took different forms based on the modalities used to make the diagnosis. Many risk factors for intrinsic sphincteric deficiency have been proposed based on their potential to compromise the sphincteric mechanism (Table 1); the 2 risk factors that are best supported by data are increasing age and prior pelvic surgery.21
Table 1.
Risk Factors for Intrinsic Sphincteric Deficiency
| Classification of Sphincter Weakness | Mechanism of Sphincter Weakness |
|---|---|
| Congenital | CNS dysfunctions/lesions |
| Smooth muscle disorders | |
| Striated muscle disorders | |
| Acquired | Childbirth |
| Surgical therapy | |
| Radiation therapy | |
| CNS lesions | |
| Peripheral neuropathies | |
| Chronic catheter drainage | |
| Other | Hypoestrogenism |
| Aging |
CNS, central nervous system.
Segmentation of the Pathophysiology of SUI
Several investigators used low urethral closure pressure to define a subset of women who did not respond well to retropubic urethropexy.22,23 Because the mechanism of action of retropubic urethropexy was believed to be correction of the anatomic defect that permitted unequal pressure transmission, patients with a low pressure urethra appeared to suffer from a different mechanism, namely, sphincteric incompetence.24 A somewhat arbitrary urethral closure pressure of less than 20 cm H2O became synonymous with intrinsic sphincteric deficiency.
Other investigators defined decreased urethral resistance using the Valsalva leak point pressure.25,26 Still others added radiographic imaging to the urodynamic parameters to define a subset of women with sphincteric dysfunction. McGuire and colleagues22 proposed a classification system for SUI based on the nature of the vesical neck descent and integrity of the intrinsic sphincteric mechanism derived from fluoroscopic images at rest and with straining. This system, a modification of a previous system proposed by Green,27 was unique in its inclusion of type III stress incontinence, characterized by a proximal urethra that no longer functions as a sphincter. In persons with type III stress incontinence, urethral pressure was markedly decreased and the vesical neck was open at rest.
Blaivas and Olsson28 modified this classification system still further by adding a type 0 stress incontinence and dividing type II stress incontinence into 2 categories (Table 2). This system differentiated among 5 subsets of incontinence based on fluoroscopic images and included type III stress incontinence of the McGuire classification system. Type III stress incontinence, characterized by a fixed open urethra and vesical neck without descent of the bladder base, became equated with intrinsic sphincteric deficiency. The impetus for all of these classification systems—those of Green and Blaivas and Olsson—was an effort to define subsets of patients best treated with specific surgical techniques. Specifically, Blaivas and Olsson28 suggested that retropubic urethropexy was an ideal treatment of types 0 to II SUI but advocated a suburethral sling for patients with type III SUI, which they believed was inadequately treated with retropubic urethropexy.
Table 2.
Classification of Stress Urinary Incontinence by Blaivas and Olsson
| Classification | Findings | Fluoroscopic Image |
|---|---|---|
| Type 0 | A. Rest: flat bladder base above symphysis | 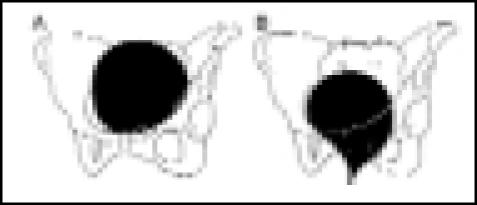 |
| pubis | ||
| B. Cough: rotational descent of urethra | ||
| and bladder base; no leakage | ||
| Type I | A. Rest: flat bladder base above symphysis |  |
| pubis | ||
| B. Cough: bladder base descends; vesical | ||
| neck and urethra open with leakage | ||
| Type IIA | A. Rest: flat bladder base above pubis | 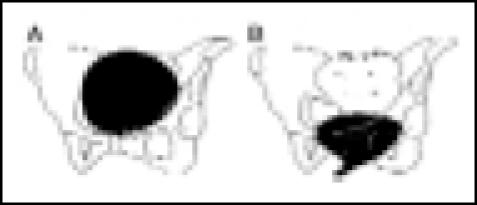 |
| B. Cough: marked descent and rotation | ||
| of bladder and urethra below pubis; | ||
| urethra opens widely with leakage | ||
| Type IIB | A. Rest: flat bladder base below pubis | 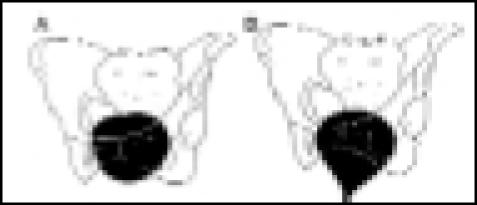 |
| B. Cough: further descent and rotation | ||
| of bladder and urethra below pubis; | ||
| urethra opens widely with leakage | ||
| Type III | A. Rest: bladder base above pubis; |  |
| vesical neck and urethra are open | ||
| B. Cough: bladder base above pubis; | ||
| vesical neck and urethra are open |
Adapted, with permission, from Blaivas JG, Olsson CA. J Urol. 1988;139:727–731.28
The dichotomization of the etiology of SUI into urethral support failure and urethral sphincteric failure seemed to validate both the anatomic and functional theories by making them mutually exclusive. This dichotomization was also compatible with prevailing clinical practice, as it provided a theoretic basis for recommending 1 of the 2 most common categories of surgical techniques for SUI—retropubic urethropexies and suburethral slings. Numerous investigators recommended one of the variations of retropubic urethropexy for women with urethral support failure, while reserving the suburethral sling procedures for patients with sphincteric failure.22–24,28
Controversy remained regarding the best method of diagnosing intrinsic sphincteric deficiency. This dispute was heightened by studies that revealed poor correlation among the different diagnostic modalities.29–31 Although these discrepancies are partly the result of poor reproducibility of the measures, they may also indicate that the various diagnostic modalities measured different aspects of urethral sphincteric function.
The dynamic storage function of the female urethra is dependent on its multiple constituents. This muscular tube is 3 cm to 4 cm in length and is lined by squamous epithelium, supported by a layer of well-vascularized loose connective tissue, which changes to transitional epithelium near the bladder base. The rich blood supply provides for a suburothelial vascular plexus. The smooth muscle sphincter of the urethra lies deep in the urothelium and vascular plexus and includes inner longitudinal and outer circumferential layers.
Whereas the longitudinal smooth muscle functions in voiding, the outer circular layer, referred to as the internal urethral sphincter, is important to the continence mechanism. The internal urethral sphincter is innervated by the autonomic nervous system, including sympathetic innervation providing for sphincteric contraction via α-adrenergic receptors, with modulation by parasympathetic innervation via cholinergic receptors. These smooth muscle layers are surrounded by circumferential striated muscles, referred to as the external urethral sphincter. The external urethral sphincter includes the sphincter urethra, a circumferential skeletal muscle surrounding the proximal two thirds of the urethra, and the urethrovaginal sphincter and compressor urethra, both of which originate from the vaginal wall and ischiopubic ramus and envelop the distal third of the posterior urethra.32,33
The external urethral sphincter has somatic innervation via the pudendal nerve. Animal studies have revealed that urethral closure pressure results from the internal urethral sphincter, the external urethral sphincter, and a nonmuscular component that includes the vascular plexus and mucosal coaptation. Each of these components normally contributes roughly one third of urethral closure pressure.34–37 Urethral sphincteric dysfunction could therefore result from compromise of one or more of these components. Moreover, sphincteric deficiency can coexist with poor urethral support; most patients with SUI have a combination of loss of urethral support and compromised sphincteric dysfunction.
The increasing recognition of the limitations of a dichotomous etiology of SUI set the stage for a theory that combined loss of urethral support and sphincteric dysfunction. In 1996, DeLancey38 proposed a consolidated theory of SUI. Using anatomic research, he hypothesized that the pubocervical fascia provides hammock-like support for the vesical neck and thereby creates a backboard for compression of the proximal urethra during increased intra-abdominal pressure. Loss of this support would compromise equal transmission of intra-abdominal pressure. This part of DeLancey’s theory combines the theories of Bonney and Enhörning. However, his theory also accounts for neuromuscular dysfunction. DeLancey’s anatomic observations showed a connection of the pubocervical fascia with the insertion of the levator ani muscles at the symphysis pubis. He hypothesized that this connection with the levator ani muscles permits active elevation of the vesical neck during contraction of the levator ani muscles. This part of the theory provides a mechanism for SUI due to neuromuscular injury.
Petros and Ulmsten39 proposed the integral theory of urinary incontinence. This theory attempts to account for the interplay of the structures involved in female urinary continence, as well as the effects of age, hormones, and iatrogenically induced scar tissue. The investigators hypothesized that stress and urge symptoms both derive, for different reasons, from anatomic laxity in the anterior vaginal wall. The laxity may be caused by defects in the vaginal wall itself or in the ligaments and muscles that support it. According to this theory, the vaginal wall has a structural function that prevents SUI by transmitting the muscle movements involved in bladder neck opening and closing, as well as a function that prevents urgency by supporting hypothesized stretch receptors located in the proximal urethra and bladder neck.
Recent Investigations of Urethral Sphincteric Dysfunction
Ongoing research has provided further insight into urethral sphincteric function. Using a probe that permitted continuous measurement of urethral pressure and cross-sectional area, Lose40 evaluated women with SUI and control subjects. Measurements were made in the proximal, mid, and distal urethra. Results showed a significant decrease in power generation at the bladder neck and mid urethra in the subjects with SUI, suggesting the presence of an active closure mechanism in the mid urethra.
Using a rat model, Kamo and colleagues41 demonstrated a similar dynamic closure. The investigators used microtip transducer catheters in the proximal and mid urethra to evaluate the urethral closure mechanism under stress conditions induced by sneezing. They noted that, during sneezing, pressure readings increased in the proximal and mid urethra but not in the distal urethra. The response in the proximal urethra was almost negligible when the bladder response was subtracted, suggesting that the proximal urethra closed by passive transmission of increased abdominal pressure. Conversely, the mid-urethral response was still observed after subtracting bladder response, suggesting that the mid urethra closed by an active contraction mechanism in addition to the passive mechanism of the proximal urethra. Moreover, in the mid urethra, the active urethral closure pressure was not related to the magnitude of bladder response, and the urethral response began before the bladder response.
Using the same probe employed to measure urethral pressure and cross-sectional area in the previously mentioned study, Thind and Lose42 evaluated urethral pressure and power in 10 healthy women before and after pudendal blockade. These parameters were significantly decreased following pudendal blockade, indicating that the pudendal nerve provided the innervation of the striated external urethral sphincter, which was the site of the active urethral closure mechanism during coughing. The investigators hypothesized that pudendal injury with resulting external urethral sphincter weakness has pathophysiologic significance in SUI. This work is supported by similar findings following pudendal nerve injury in rats.43
There has also been significant progress in elucidating the neurotransmitters responsible for urethral function. Animal studies using retrograde transport of tracer molecules have provided information needed to map out the innervation of the external urethral sphincter. The motor neurons of the external urethral sphincter are located in the ventral horn of the lumbosacral spinal cord in Onuf’s nucleus.44–47 Moreover, anterograde axonal tracing in the cat has revealed neurons that project directly to Onuf’s nucleus from a region in the rostral pons, ventrolateral to the pontine micturition center.48 Stimulation of these neurons evokes contractions of the external urethral sphincter. The external urethral sphincter reflexes are enhanced by serotonin agonists and depressed by serotonin antagonists,49,50 suggesting that the descending serotonergic pathways are responsible for the spinal cord circuitry controlling the closure mechanism of the external urethral sphincter.
Conclusion
Over the past 100 years, much has been elucidated about the pathophysiology of SUI. As improved diagnostic modalities have provided new insight into the function and dysfunction of the urethral continence mechanism, theories have evolved from being purely anatomic to being both functional and anatomic. In reality, there are probably many aspects of the continence mechanism that are vulnerable to injury. When a woman manifests symptoms of SUI, multiple aspects of the continence mechanism may be damaged; consequently, correction of one of the injuries may be sufficient to render the patient asymptomatic. As our knowledge of the neurocircuitry of the urethral continence mechanism expands, new opportunities for intervention become possible, setting the stage for novel innovative prevention and treatment options.
Main Points.
In the early 1900s, Bonney attempted to define the symptom complex, epidemiology, and pathophysiology of stress urinary incontinence (SUI), hypothesizing that it was a result of sagging of the pubocervical muscle sheet, not intravesical pressure on the sphincter, and setting the stage for future anatomic theories of incontinence.
The influential functional theories of Barnes grew from his use of manometry to study urethral function; he posited that incontinence could result from an increase in urinary expulsive forces or intravesical pressure, a lowering of the powers of resistance or urethral sphincter action, or a combination of both.
Evidence of the role of denervation of the urethral sphincter in the etiology of SUI led to the identification of a subset of patients with sphincteric dysfunction. In women with “intrinsic sphincteric deficiency,” the urethral sphincter cannot generate enough resistance to retain urine in the bladder, especially during stress maneuvers.
As new diagnostic methods have been applied to SUI, theories regarding the etiology of this condition have evolved. Current theories integrate anatomic and functional factors, as well as the effects of neuromuscular injury, aging, and hormones.
References
- 1.Aldridge AH. Transplantation of fascia for relief of urinary incontinence. Am J Obstet Gynecol. 1942;3:398–411. [Google Scholar]
- 2.Baker WH. Diseases of the bladder and urethra. In: Mann MD, editor. American System of Gynecology. Philadelphia: Lea Brothers & Co; 1888. p. 475. [Google Scholar]
- 3.Cundiff GW, Bent AE. Endoscopic Diagnosis of the Female Lower Urinary Tract. London: WB Saunders Co; 1998. [Google Scholar]
- 4.Kelly HA, Dumm WM. Urinary incontinence in women, without manifest injury to the bladder. Surg Gynecol Obstet. 1914;18:444–453. doi: 10.1007/BF02001086. [DOI] [PubMed] [Google Scholar]
- 5.Bonney V. On diurnal incontinence of urine in women. J Obstet Gynaecol Br Emp. 1923;30:358–365. [Google Scholar]
- 6.Stevens WE, Smith SP. Roentgenological examination of the female urethra. J Urol. 1937;37:194–201. [Google Scholar]
- 7.Kennedy WT. Incontinence of urine in the female, the urethral sphincter mechanism, damage of function, and restoration of control. Am J Obstet Gynecol. 1937;34:576–589. [Google Scholar]
- 8.Barnes A. A method for evaluating the stress of urinary incontinence. Am J Obstet Gynecol. 1940;40:381–390. [Google Scholar]
- 9.Karlson S. Experimental studies on the functioning of the female urinary bladder and urethra. Acta Obstet Gynecol Scand. 1953;32:285–307. doi: 10.3109/00016345309157582. [DOI] [PubMed] [Google Scholar]
- 10.Enhörning G. Simultaneous recording of intravesical and intra-urethral pressure: a study on urethral closure in normal and stress incontinent women. Acta Chir Scand. 1961;(suppl 276):1–68. [PubMed] [Google Scholar]
- 11.Enhörning G, Miller ER, Hinman F., Jr Urethral closure studied with cineroentgenography and simultaneous bladder-urethra pressure recording. Surg Gynecol Obstet. 1964;118:507–516. [PubMed] [Google Scholar]
- 12.Tanagho EA. Urodynamics of female urinary incontinence with emphasis on stress incontinence. J Urol. 1979;122:200–204. doi: 10.1016/s0022-5347(17)56329-6. [DOI] [PubMed] [Google Scholar]
- 13.Toews H. Intraurethral and intravesical pressures in normal and stress-incontinent women. Obstet Gynecol. 1967;29:613–624. [PubMed] [Google Scholar]
- 14.Öbrink A, Bunne G, Ingelman-Sundberg A. Pressure transmission to the pre-urethral space in stress incontinence. Urol Res. 1978;6:135–140. doi: 10.1007/BF00261313. [DOI] [PubMed] [Google Scholar]
- 15.Bump RC, Copeland WE, Jr, Hurt WG, Fantl JA. Dynamic urethral pressure/profilometry pressure transmission ratio determinations in stress-incontinent and stress-continent subjects. Am J Obstet Gynecol. 1988;159:749–755. doi: 10.1016/s0002-9378(88)80048-6. [DOI] [PubMed] [Google Scholar]
- 16.Hilton P, Stanton SL. Urethral pressure measurement by microtransducer: the results in symptom-free women and in those with genuine stress incontinence. Br J Obstet Gynaecol. 1983;90:919–933. doi: 10.1111/j.1471-0528.1983.tb06764.x. [DOI] [PubMed] [Google Scholar]
- 17.Bump RC, Fantl JA, Hurt WG. Dynamic urethral pressure profilometry pressure transmission ratio determinations after continence surgery: understanding the mechanism of success, failure, and complications. Obstet Gynecol. 1988;72:870–874. doi: 10.1097/00006250-198812000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Snooks SJ, Badenoch DF, Tiptaft RC, Swash M. Perineal nerve damage in genuine stress urinary incontinence: an electrophysiological study. Br J Urol. 1985;57:422–426. doi: 10.1111/j.1464-410x.1985.tb06302.x. [DOI] [PubMed] [Google Scholar]
- 19.Smith AR, Hosker GL, Warrell DW. The role of pudendal nerve damage in the aetiology of genuine stress incontinence in women. Br J Obstet Gynaecol. 1989;96:29–32. doi: 10.1111/j.1471-0528.1989.tb01572.x. [DOI] [PubMed] [Google Scholar]
- 20.Urinary Incontinence in Adults: Clinical Practice Guidelines. Rockville, Md: Agency for Health Care Policy and Research, US Dept of Health and Human Services; 1992. Urinary Incontinence Guidelines Panel. AHCPR publication 9-2-0038. [Google Scholar]
- 21.Horbach NS, Ostergard DR. The pathophysiology of genuine stress incontinence. Int Urogynecol J. 1990;1:12–18. [Google Scholar]
- 22.McGuire EJ, Lytton B, Pepe V, Kohorn EI. Stress urinary incontinence. Obstet Gynecol. 1976;47:255–264. [PubMed] [Google Scholar]
- 23.Sand PK, Bowen LW, Panganiban R, Ostergard DR. The low pressure urethra as a factor in failed retropubic urethropexy. Obstet Gynecol. 1987;69(3 pt 1):399–402. [PubMed] [Google Scholar]
- 24.Summitt RL, Sipes DR, Bent AE, Ostergard DR. Evaluation of pressure transmission ratios in women with genuine stress incontinence and low urethral pressure: a comparative study. Obstet Gynecol. 1994;83:984–988. doi: 10.1097/00006250-199406000-00018. [DOI] [PubMed] [Google Scholar]
- 25.McGuire EJ, Cespedes RD, O’Connell HE. Leak-point pressures. Urol Clin North Am. 1996;23:253–262. doi: 10.1016/s0094-0143(05)70309-8. [DOI] [PubMed] [Google Scholar]
- 26.Theofrastous JP, Bump RC, Elser DM, et al. Correlation of urodynamic measures of urethral resistance with clinical measures of incontinence severity in women with pure genuine stress incontinence. Am J Obstet Gynecol. 1995;173:407–414. doi: 10.1016/0002-9378(95)90260-0. [DOI] [PubMed] [Google Scholar]
- 27.Green TH., Jr Classification of stress urinary incontinence in the female: an appraisal of its current status. Obstet Gynecol Survey. 1968;23:632–634. doi: 10.1097/00006254-196807000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Blaivas JG, Olsson CA. Stress incontinence: classification and surgical approach. J Urol. 1988;139:727–731. doi: 10.1016/s0022-5347(17)42611-5. [DOI] [PubMed] [Google Scholar]
- 29.Bump RC, Coates KW, Cundiff GW, et al. Diagnosing intrinsic sphincteric deficiency: comparing urethral closure pressure, urethral axis, and valsalva leak point pressures. Am J Obstet Gynecol. 1997;177:303–310. doi: 10.1016/s0002-9378(97)70191-1. [DOI] [PubMed] [Google Scholar]
- 30.Swift SE, Ostergard DR. A comparison of stress leak-point pressure and maximal urethral closure pressure in patients with genuine stress incontinence. Obstet Gynecol. 1995;85(5 pt 1):704–708. doi: 10.1016/0029-7844(95)00014-i. [DOI] [PubMed] [Google Scholar]
- 31.Fleischmann N, Flisser AJ, Blaivas JG, Panagopoulos G. Sphincteric urinary incontinence: relationship of vesical leak point pressure, urethral mobility and severity of incontinence. J Urol. 2003;169:999–1002. doi: 10.1097/01.ju.0000051895.28240.12. [DOI] [PubMed] [Google Scholar]
- 32.DeLancey JO. Correlative study of paraurethral anatomy. Obstet Gynecol. 1986;68:91–97. [PubMed] [Google Scholar]
- 33.DeLancey JO. Structural aspects of the extrinsic continence mechanism. Obstet Gynecol. 1988;72:296–301. [PubMed] [Google Scholar]
- 34.Raz S, Caine M, Ziegler M. The vascular component in the production of intraurethral pressure. J Urol. 1972;108:93–96. doi: 10.1016/s0022-5347(17)60650-5. [DOI] [PubMed] [Google Scholar]
- 35.Donker PJ, Ivanovici F, Noach EL. Analyses of the urethral pressure profile by means of electromyography and the administration of drugs. Br J Urol. 1972;44:180–193. doi: 10.1111/j.1464-410x.1972.tb10064.x. [DOI] [PubMed] [Google Scholar]
- 36.Mattiason A, Andersson KE, Sjorgen C. Urethral sensitivity to alpha adrenoceptor stimulation and blockade in patients with parasympathetically decentralized lower urinary tract and in healthy volunteers. Neurourol Urodyn. 1984;3:230–234. [Google Scholar]
- 37.Ek A. Innervation and receptor functions of the human urethra. Scand J Urol Nephrol Suppl. 1977;45:1–50. [PubMed] [Google Scholar]
- 38.DeLancey JO. Stress urinary incontinence: where are we now, where should we go? Am J Obstet Gynecol. 1996;175:311–319. doi: 10.1016/s0002-9378(96)70140-0. [DOI] [PubMed] [Google Scholar]
- 39.Petros PE, Ulmsten UI. An integral theory of female urinary incontinence: experimental and clinical considerations. Acta Obstet Gynecol Scand Suppl. 1990;153:7–31. doi: 10.1111/j.1600-0412.1990.tb08027.x. [DOI] [PubMed] [Google Scholar]
- 40.Lose G. Urethral pressure and power generation during coughing and voluntary contraction of the pelvic floor in females with genuine stress incontinence. Br J Urol. 1991;67:580–585. doi: 10.1111/j.1464-410x.1991.tb15219.x. [DOI] [PubMed] [Google Scholar]
- 41.Kamo I, Torimoto K, Chancellor MB, et al. Urethral closure mechanisms under sneeze-induced stress condition in rats: a new animal model for evaluation of stress urinary incontinence. Am J Physiol Regul Integr Comp Physiol. 2003;285:R356–R365. doi: 10.1152/ajpregu.00010.2003. [DOI] [PubMed] [Google Scholar]
- 42.Thind P, Lose G. The effect of bilateral pudendal blockade on the adjunctive urethral closure forces in healthy females. Scand J Urol Nephrol. 1994;28:249–255. [PubMed] [Google Scholar]
- 43.Kerns JM, Damaser MS, Kane JM, et al. Effects of pudendal nerve injury in the female rat. Neurourol Urodyn. 2000;19:53–69. doi: 10.1002/(sici)1520-6777(2000)19:1<53::aid-nau7>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 44.McKenna KE, Nadelhaft I. The organization of the pudendal nerve in the male and female rat. J Comp Neurol. 1986;248:532–549. doi: 10.1002/cne.902480406. [DOI] [PubMed] [Google Scholar]
- 45.Thor K, Morgan C, Nadelhaft I, et al. Organization of afferent and efferent pathways in the pudendal nerve of the female cat. J Comp Neurol. 1989;288:263–279. doi: 10.1002/cne.902880206. [DOI] [PubMed] [Google Scholar]
- 46.Ueyama T, Mizuno N, Nomura S, et al. Central distribution of afferent and efferent components of the pudendal nerve in cat. J Comp Neurol. 1984;222:38–46. doi: 10.1002/cne.902220104. [DOI] [PubMed] [Google Scholar]
- 47.Sasaki M. Morphological analysis of external urethral and external anal sphincter motoneurones of cat. J Comp Neurol. 1994;349:269–287. doi: 10.1002/cne.903490209. [DOI] [PubMed] [Google Scholar]
- 48.Holstege G, Griffiths D, de Wall H, Dalm E. Anatomical and physiological observations on the supraspinal control of bladder and urethral sphincter muscles in the cat. J Comp Neurol. 1986;250:449–461. doi: 10.1002/cne.902500404. [DOI] [PubMed] [Google Scholar]
- 49.de Groat WC, Yoshiyama M, Ramage AG, et al. Modulation of voiding and storage reflexes by activation of alpha1-adrenoceptors. Eur Urol. 1999;36(suppl 1):68–73. doi: 10.1159/000052324. [DOI] [PubMed] [Google Scholar]
- 50.Danuser H, Thor KB. Spinal 5-HT2 receptor-mediated facilitation of pudendal nerve reflexes in the anaesthetized cat. Br J Pharmacol. 1996;118:150–154. doi: 10.1111/j.1476-5381.1996.tb15378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



