Abstract
During the past 15 years, permanent seed brachytherapy for prostate cancer has advanced significantly in all areas, including patient selection, treatment planning, technique, and technology (eg, seeds stranded in Vicryl suture). These improvements have made transperineal seed implantation an accurate and practical treatment option for men with low-, intermediate-, and high-risk disease. Because of the evidence that the various treatment options for prostate cancer are likely to be equally successful in terms of long-term cancer control, continuing efforts focus on enhancing the quality of life of implant patients.
Key words: External beam radiotherapy, Iodine-125, Palladium-103, Prostate cancer, Seed brachytherapy, Transperineal approach
Permanent seed brachytherapy has become an important treatment modality for prostate cancer. Currently, it is estimated that 30% to 40% of all patients with prostate cancer receive seed implantation as part of their treatment.1 Significant advances in patient selection, treatment planning, technique, and technology (eg, connected seeds) have made transperineal seed implantation an accurate, practical treatment option for patients with low-, intermediate-, and high-risk disease.
Modern Permanent Seed Implantation
In the 1960s, Drs Scardino and Carlton2 at Baylor College of Medicine, Houston, TX began modern-day permanent prostate brachytherapy using 198-Au interstitial implantation or iodine-125 (I-125) either alone or in combination with external beam radiotherapy (EBRT).2 In the 1980s, new brachytherapy approaches to the treatment of prostate cancer were initiated.3 Martinez and colleagues4 treated patients with EBRT combined with temporary seeds inserted using a transperineal approach. Dr Puthawala and associates5 pioneered a temporary seed technique of placing the needles while visualizing them through an open laparotomy. At about the same time, Dr Whitmore and colleagues6 at Memorial Sloan-Kettering Cancer Center (MSKCC) also began to insert I-125 seeds as a sole treatment through an open incision.
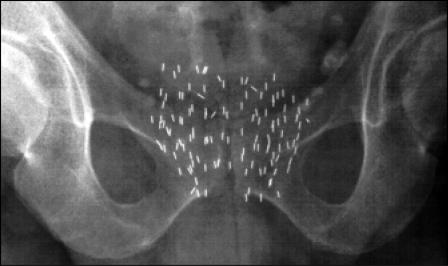
Significant limitations, such as the requirement for an open incision, dosimetry issues, and poor outcomes, prevented the adoption of these techniques. Lack of prostate-specific antigen (PSA) screening (for ideal patient selection) also contributed to high recurrence rates. However, some important information was obtained from these early seed implantation approaches. Local control was better in patients who received high-quality implants and who had low-grade and early-stage cancer.7–9 The group from MSKCC reported a 60% local control rate in patients who received prescription doses of > 140 Gy versus 20% if the dose was < 140 Gy. The 15-year survival was 70% in patients with stage B1 prostate cancer receiving high-quality I-125 seed brachytherapy.10 These results suggested that quality seed placement and proper patient selection were important determinants of cancer control. The subsequent development of the transperineal, ultrasound-guided approach provided a theoretical means to more accurately place seeds and to improve dose coverage (Figure 1).
Figure 1.
Transperineal ultrasound-guided implant with seeds in Vicryl suture (RAPID Strand™, Oncura, Inc., Plymouth Meeting, PA). Image courtesy of Oncura, Inc.
Advances in the Transperineal Approach
Since the mid 1980s, the transrectal ultrasound-guided, template-guided I-125 implantation procedure has become the primary technique of permanent seed implantation. In 1983, Dr Holm11 introduced the use of transrectal ultrasound to visualize the permanent placement of I-125 seeds via needles inserted through the perineum directly into the prostate. In 1985, Drs Blasko and Ragde12 began the first transperineal ultrasound-guided approach in the United States. The transperineal ultrasound-guided, approach resulted in increased accuracy of needle and seed placement and relatively even distribution of seeds throughout the prostate. It marked a major advance in prostate brachytherapy in that it allowed computerized treatment planning of the implant rather than the use of simple nomograms, thus ensuring the proper number, strength, and positioning of radioactive sources. Permanent seed implantation has subsequently evolved over the past 19 years to become an efficient procedure, suitable for outpatient and ambulatory surgical centers.
The goal of any implant is to achieve the prescribed dose throughout the prostate. Several studies have documented better biochemical control in patients treated with I-125 monotherapy who achieved a dose > 130–140 Gy as compared with patients whose dose fell below this range.13,14 Research at the Seattle Prostate Institute15 has shown that monotherapy patients treated between 1988 and 1990 achieved significantly better 10-year biochemical control than did identical patients treated at the Institute by the same physicians between 1986 and 1987. The only factor identified as explaining the difference was the quality of the implant. These studies supported the hypothesis that higher-quality implants result in better cancer advances since this early experience has improved the quality of permanent seed implantation.
As brachytherapy has become more popular, many technical improvements have been added to increase the consistency and quality of the procedure.11,12,16–18 Slight differences in technique are in practice as more and more physicians perform this procedure and technical advances are made; however, the basic approach is quite similar, and it remains to be determined whether any single technique will prove superior in controlling the cancer. Virtually all studies are in agreement that the keys to successful outcomes are appropriate patient selection and a high-quality implant.13,14,19–21 Fundamental improvements have evolved in patient selection, treatment planning, dosimetry, imaging, operating room techniques, seed technology, and quality assurance programs.
Patient Selection
The stage of cancer, technical suitability, and toxicity issues are the three key considerations involved in the selection of patients for ultrasound-guided implantation.
Stage and Extent of Cancer
Patients with a high likelihood of disease in the prostate and immediate surrounding area can be treated with seeds alone. Patients with a higher likelihood of microscopic disease (intermediate and high risk) beyond the implant volume are generally treated with a combination of EBRT and seed implantation. Patients with advanced disease—ie, stage ≥ T3—or distant metastatic disease are typically not appropriate candidates for seed implantation.
The Partin tables22–24 and other predictive algorithms have allowed for better patient selection of therapy. In early-stage, low-risk patients, there is a very low risk of disease in the seminal vesicles or lymph nodes and only a modest risk of disease that extends through the outer wall, or capsule of the prostate. Fortunately, the disease that goes through the capsule is almost always within several millimeters of the prostate and is easily covered by the implant volume.25,26 The risk of disease outside the capsule can be estimated by looking at the Partin tables, which correlate the risk of extracapsular penetration (ECP), seminal vesicle (SV) involvement, and lymph node (LN) involvement with the biopsy Gleason score, clinical stage, and pretreatment PSA.24
Typically, surgical and radiation margins are 4 to 15 mm beyond the prostate. Disease that is beyond the margin of surgery or implantation can be roughly estimated from the Partin tables by the following formula: LN + SV + ECP (X). X is 25% if the Gleason score is 6 and 50% if the Gleason score is 7. This calculation is based on a study that showed that for patients with early-stage disease with evidence of ECP only, 25% would fail radical prostatectomy if the Gleason score was 6 or below and approximately 50% would fail if it was 7 or higher.27
Evidence that patients with favorable (low-risk) disease have a high likelihood of disease confined to the implant margin comes not only from pathologic studies but also from clinical studies showing excellent PSA control with seed implantation alone with either palladium-103 (Pd-103) or I-125.15,19,28–34 Some centers perform combined EBRT and implantation on all patients, even those with low-risk disease, but to date, the long-term clinical results of combined treatment have not been shown to be superior to those of implantation as the sole treatment.35–37 For the majority of patients, implant alone causes fewer side effects and is less expensive than EBRT plus seeds.
Other factors in low-risk patients, such as the number of positive biopsy cores, are considered in determining whether these patients with favorable disease require EBRT in addition to implantation. The number of positive cores has been demonstrated in surgical series to correlate with prognosis. In patients treated with radical prostatectomy, those with > 50% of cores were found to have a worse prognosis than those with < 50%.38 Brachytherapy series, however, did not show a statistical difference in patients with low-risk disease and higher-percentage positive biopsies.39
For intermediate-risk patients, the choice of treatment is between implantation alone and EBRT plus seeds. A common definition of intermediate risk is the presence of one of several unfavorable risk factors: PSA > 10 ng/mL, Gleason score ≥ 7, or clinical stage ≥ T2c disease by digital rectal examination (DRE). This intermediate group is a broad group with a significant range of risk of disease beyond the prostate. Some of the more favorable intermediate-risk patients—eg, those with stage T1c, Gleason score < 7, PSA between 10 ng/mL and 15 ng/mL, and a low percentage of positive biopsies—have a relatively low risk of disease beyond the margin and are often treated with implant alone. Other intermediate-risk patients with worse prognostic factors are probably served best by EBRT plus implantation, but further studies are necessary.
High-risk patients are considered to be those with two or three of the above-mentioned risk factors.29 Some centers define a patient as high risk if one factor is very unfavorable (PSA > 20 ng/mL, DRE stage > T2b, or Gleason score 8–10). Patients in the high-risk group are typically treated with combined therapy, which may also include hormonal therapy. Table 1 shows the current treatment guidelines recommended by the Seattle Prostate Institute, which are a slight modification of those advocated by the American Brachytherapy Society (ABS).
Table 1.
Implant Alone Versus Combination Implant and External Beam Radiotherapy
| Low Risk—Implant Alone | Intermediate and High Risk—EBRT and Implant |
|---|---|
| Clinical stage T1 or T2a, T2b | Clinical stage > T2c |
| Gleason score ≤ 6 | Gleason score ≥ 7 |
| PSA ≤10 ng/mL | PSA > 10 ng/mL |
| Exceptions | Exceptions |
| T2a, T2b | T1c, T2a, Gleason score 7 |
| > 66% biopsies positive | < 34% biopsies positive |
| EBRT and implant | Implant alone |
EBRT, external beam radiotherapy; PSA, prostate-specific antigen
Technical Suitability
Prostate size. If the size of the prostate is much greater than 60 mL, the implant may become technically challenging. The pubic arch can obstruct needle placement, and more swelling can occur during and after the procedure. Most centers set a limit of approximately 60 mL as an upper limit of suitability. For larger glands, either another treatment modality is chosen or the gland size is reduced with hormonal therapy prior to brachytherapy.
Prior prostate surgery. A prior transurethral resection of the prostate (TURP) may sometimes prevent a quality implant. TURPs may leave a large deficit in the central portion of the gland (a “TURP defect”), allowing little room for accurate seed placement. In addition, the early experience noted higher rates of incontinence when TURP patients were treated with implantation.20,40–43 Recent procedural advances that involve placing seeds farther from the TURP defect have decreased this risk of incontinence.44 The current consensus, therefore, is that patients with small TURP defects are eligible for implantation, recognizing that the risk of incontinence may be higher than in non-TURP patients.
Preoperative obstruction. The need for a temporary catheter after implantation increases as the American Urological Association (AUA) score increases. Patients with AUA scores above 15 are at higher risk of needing a temporary catheter after seed implantation. A few of these patients may require treatment, either before the implantation or at a later date, to relieve obstructive problems. Some patients can become candidates for implantation if their urinary symptoms respond well to α-blockers. Another technique, in patients with clear bladder neck-obstruction, is to perform a transurethral incision of the prostate (TUIP) 2 to 3 months prior to implantation. Of note is that patients undergoing hormonal therapy to reduce prostate size may not experience any improvement in their obstructive symptoms. Paradoxically, in some studies, pretreatment with androgen ablation increased the risk of requiring a temporary catheter.45
Treatment Planning
Treatment planning is a three-step process that considers the doses necessary to control the cancer and reduce toxicity to critical structures. The first step is a volume study, followed by outlining an implant volume, followed by a computerized ideal seed placement plan. Computerized planning is now either performed at the time of the procedure (intraoperative) or several weeks prior to the implantation (preplanning). Significant advances have occurred in identifying treatment-planning issues that decrease toxicity.
Volume Study
The basic elements of the volume study are unchanged. A series of cross-sectional ultrasound images of the prostate form the basis for computerized treatment planning and seed placement. Advances in treatment-planning systems, however, have made it possible to conduct the volume study and planning in the operating room; however, most centers still perform the volume study and planning several weeks prior to the procedure.
Seed Distribution
Philosophies of seed distribution have ranged from using high-strength, peripherally positioned seeds to lowenergy, uniformly spaced seeds. Today, almost all centers in the United States have adopted a modification of these techniques, described as modified uniform seed spacing. A few centers, especially in Europe, are still evaluating high-strength seeds. The modified uniform placement of seeds philosophy has been demonstrated to satisfy the dose requirements, to be technically feasible, and to minimize high-dose areas.
Dose
The actual dose delivered to the prostate has remained essentially the same over the years; however, the changes in the formulas of dose calculation for both I-125 and Pd-103 changed the prescription dose.46 Prescribed dose today is determined by the isotope used (eg, Pd-103 or I-125) and whether it is to be used for implantation alone (145 Gy for I-125, 125 Gy for Pd-103) or in combination with EBRT (110 Gy for I-125, 100 Gy for Pd-103).
The doses delivered by implantation are significantly higher than those achievable by three-dimensional conformal/intensity-modulated radiotherapy (IMRT), EBRT, or high-dose rate (HDR) brachytherapy. Typical doses for implantation are 125 to 145 Gy. For EBRT, the doses are 70 to 80 Gy. An EBRT dose of 120 Gy is an approximate dose equivalent to that of an implant47,48 and is far beyond the tolerance range for EBRT. EBRT is typically unable to give much more than 80 Gy.
Target Volumes and Critical Structures
Experience with thousands of patients has resulted in identifying doses to critical targets and volumes that minimize side effects and complications while ensuring high control rates. Recommended initial target volumes should include the prostate and a 5 to 10 mm lateral margin, but tight posterior margins. Current regimens are paying closer attention to the margins at the apex because higher doses to the penile-bulb region have been associated with increased erectile dysfunction.49 Acceptable doses are now referenced to the prescription dose and the volume receiving the dose. For example, V100 refers to the volume of the structure receiving 100% of the prescribed dose. The ranges of acceptable doses to the prostate and critical structures used by the physicians at the Seattle Prostate Institute are given in Table 2.
Table 2.
Recommended Brachytherapy Prescription Doses at the Seattle Prostate Institute
| Area | Dose |
|---|---|
| Entire prostate | |
| V100 | 100% |
| V150 | 30%–60% |
| V200 | 10%–20% |
| Urethra point | 110%–120% of prescribed dose |
| Rectum point | < 100% of prescribed dose |
| Penile bulb | 50%, < 40 Gy |
| Rectum | < 40% receiving 60 Gy < 25% receiving 70 Gy |
| Bladder | 70% of gland, < 25% of prescribed dose |
V100, V150, V200, volume receiving 100%, 150%, and 200% of prescribed dose, respectively.
Isotope Selection
I-125 and Pd-103 are the primary isotopes used in permanent seed implantation. I-125 was introduced to the clinical treatment of prostate cancer in 1965 and Pd-103 in 1989. The photon energy of 28 kiloelectron volts (keV) for I-125 and 21 keV for Pd-103 are very similar. The primary difference between the two isotopes is the rate at which they decay. I-125 has a half-life (the time it takes to decrease by one half) of 60 days versus 17 days for Pd-103. The effect is that Pd gives up its energy more quickly. There is no evidence yet that quicker is better for prostate cancer or that one isotope is clearly better than another for each grade of cancer; therefore, selection of the isotope is at the discretion of the brachytherapy team.50,51 The ABS does not recommend one isotope over the other.19
Seed Technology
Stranded seeds and seed migration. Original implant techniques, which are still used in many centers, involve placing individual, or “loose,” seeds into the gland. Spacing is accomplished in preloaded needles by absorbable spacers or, with the Mick applicator (Mick® Radio-Nuclear Instruments, Inc., Mount Vernon, NY) by mechanically depositing the seed at the determined distance from the other seeds. Loose seeds, however, have migrated to the pelvis and lungs in several studies, regardless of technique.52–54 In one study, a loose seed was found in a coronary artery.55 Recent studies demonstrated that 18% to 55% of patients treated with loose seeds via the Mick applicator experienced seed migration to the lungs,56,57 whereas studies of preloaded loose seeds have reported 10% to 22% patients with lung migration.52,53 The mechanism for this migration is likely seed embolization in the venous plexus surrounding the gland or inadvertent deposition in the periprostatic region.52,54
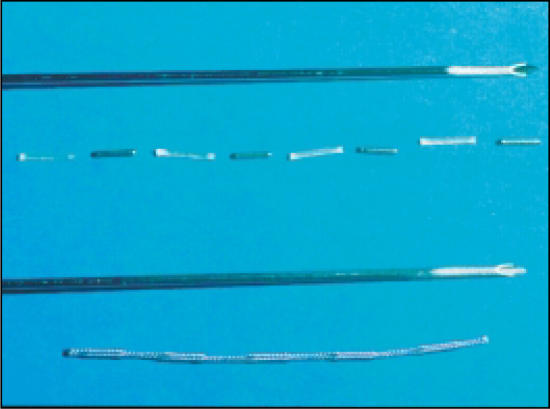
Compared with loose or “free” seeds, seeds stranded in Vicryl suture (RAPID Strand™, Oncura, Inc., Plymouth Meeting, PA) have been demonstrated to substantially lower the incidence of seed migration to the lung (Figure 2).52 Data showing lower seed migration rates with seeds stranded in Vicryl suture were first published in 1998. The Seattle group noted 0.7% lung migration in patients treated with stranded seeds, whereas 11% of those treated with loose seeds experienced such migration. An update of 1000 patients treated at the Seattle Prostate Institute demonstrated that 24% of patients implanted with loose seeds experienced seed migration to the lung versus 2% of patients treated with seeds stranded in Vicryl suture (P = .002).58 Moreover, patients treated with stranded seeds had a significantly lower incidence of migration of seeds out of the prostate and into the pelvis: 40% versus 20% (P = .02).58 Although there are no data demonstrating any side effects of seed migration to the lung, the goal of permanent seed implantation is to place seeds according to plan throughout the prostate and for the seeds to remain in position throughout an effective half-life of the seed.
Figure 2.
Free or loose seeds and connected seeds in Vicryl suture (RAPID Strand). Image courtesy of Oncura, Inc.
Stranded seeds and dosimetry. Multiple studies have demonstrated improved dosimetry with seeds stranded in Vicryl suture versus loose seeds. Lee and colleagues59 compared 20 loose seed implants with their first 20 connected seed implants (RAPID Strand) and found significantly improved postoperative dosimetry on dose-volume histogram (DVH) analysis: V100 of 86.5% with loose seed implants and V100 of 96.1% with stranded seed implants. Fagundes60 also showed significant improvement in DVH dosimetry when he switched from using loose seeds and the Mick applicator to afterloading the Mick applicator needles with seeds stranded in Vicryl suture.60 The Seattle Prostate Institute noted improved dose homogeneity and lower V150 and slightly better (not statistically significant) V100s on DVH analysis with stranded seeds versus loose seeds.61
In summary, the advantages of using radioactive seeds that are stranded in absorbable material, eg, Vicryl suture, include; less seed migration to the lungs and pelvis, seed placement outside the capsule, and improved dosimetry. To date, there have been no studies demonstrating an improved biochemical outcome with stranded seeds. However, the demonstrated advantages and high-quality implants using stranded radioactive seeds with absorbable material may become more important for reducing side effects.
Imaging. Ultrasound imaging has essentially remained unchanged over the past 10 years. However, anecdotal evidence indicates that the use of specialized condoms designed for brachytherapy has improved the consistency and clarity of images.62 Increasingly, the use of sagittal imaging is used to better visualize needle depth/penetration and better seed placement and coverage of the base of the prostate. The Seattle team has adopted the extensive use of sagittal imaging for determining the base of the prostate and positioning of the needle tip. The reported advantage of this technique is that the prostate movement cephalad during needle placement can be seen and needle placement adjusted, maximizing seed distribution at the base.
Needle placement. Almost all centers use ultrasound imaging to identify needle placement. The use of fluoroscopy to determine needle placement is controversial and not recommended as the sole modality for needle placement. The two primary methods of seed placement are the preloaded needle and the afterloading technique. Many centers have reported on the differences in these technique18,30,63,64; both techniques have strong advocates. With the preloaded technique, the seeds are placed into the needle and the needle is inserted into the gland; with the afterloading technique, a needle and stylet are first positioned into the gland, the stylet is removed, and the seeds are inserted into the needle. With afterloading, several options, such as the Mick applicator two-stage system, or the cartridge described by Fagundes, are available.60 In several studies, the use of seeds stranded in Vicryl suture and the afterloading technique resulted in a significant reduction in seed migration and improved dosimetry compared with loose seeds.60,62 The most significant factor was the use of the stranded seeds and not the technique, however. Whether the preloaded or afterloading technique will prove superior for clinical control of the cancer is unclear.
Advances in EBRT. Recent advances that have reduced the incidence of rectal bleeding and radiation proctitis in EBRT patients can now be applied to intermediate- and high-risk patients receiving combination EBRT and seed implantation. IMRT has reduced the incidence of rectal bleeding.65
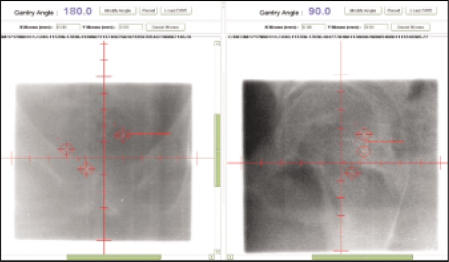
Even more accurate methods of localizing the prostate immediately before treatment each day have been developed recently. One exciting technique involves the placement of three gold nonradioactive marker seeds into the prostate by the urologist a few days before the treatment-planning computed tomography (CT) scan. The IMRT plan is then developed, and each day, the gold seeds are identified with the patient in treatment position under the linear accelerator just prior to treatment (Figure 3). The treatment table (couch) is then moved a few millimeters in/out, up/down, or right/left, depending on how much the prostate moved compared with the original treatment plan.66 This method allows for a much more accurate treatment and thus a reduction in the amount of the rectum and the bulb of the penis that is in the external beam field. It is expected that this method will decrease the risk of radiation proctitis and impotency in patients receiving either IMRT alone or IMRT plus brachytherapy.
Figure 3.
Gold seeds marking prostate location.
Toxicity
Major acute operative symptoms and complications are extremely rare. Surgical events such as bleeding that require transfusion, admission to the intensive care unit for any postoperative acute events, and death have not been noted in the literature.5,67 At the Seattle Prostate Institute where physicians have performed more than 7000 implantation procedures, no deaths or serious intraoperative or postoperative morbidity have been observed.
Urinary symptoms. Moderate postoperative side effects, however, are common and are primarily urinary irritative and obstructive symptoms such as increased urinary frequency, urgency, discomfort on urination, and weakening of the urinary stream.20,29,68,69 The symptoms are at their worst between 2 and 6 weeks after the operation, but they may be bothersome for 2 to 6 months or longer. The need for a temporary catheter occurs in approximately 10% of patients.64,68–72 In one study at the Seattle Prostate Institute, the average duration of catheterization was 13 days, and 2% of patients required Foley, suprapubic, or intermittent self-catheterization for more than 6 months. No patient has required a permanent catheter.37 In the small percentage of patients who require a catheter more than a few weeks, self-catheterization is either taught or a suprapubic catheter is placed until the swelling and retention resolve.
Bowel symptoms. Increased bowel frequency and urgency is uncommon, and when it occurs, the symptoms respond to diet and medications such as loperamide. Blood in the urine is to be expected for a few days, and occasionally a few weeks, after implant. We recommend that patients use stool softeners or fiber supplements to keep their stools soft for at least the first half-life of the seeds. This can decrease distention of the rectum and the pressure of the anterior rectal wall against the prostate, thus lowering the dose of radiation to the anterior rectal wall.
Sexual issues. Ten percent to 20% of sexually active patients will experience some level of discomfort (usually mild) with orgasm; a problem that generally resolves itself gradually.73 Although the volume of the ejaculate will decrease dramatically following an implant, sperm may still be present. Occasionally, blood may be seen in the ejaculate, but it is not harmful or dangerous. Whether the sperm is significantly damaged by radiation exposure is unknown, but to be safe, birth control measures are recommended for couples who are still fertile. Ejaculation of a seed is rarely reported. The Seattle team is aware of fewer than 5 patients who have noted this event over the past 15 years.74
Quality assurance. As quantitative parameters for quality implantation have become available, brachytherapy teams have the ability to evaluate the quality of each implant. The assessment of implant quality begins in the operating room as a qualitative evaluation, with the use of ultrasound and possibly fluoroscopy to visualize the needles and seeds as they are being placed. Although there have been numerous attempts and claims at intraoperative dosimetry, no current system can intraoperatively determine seed placement and calculate dosimetry accurately.
The quantitative evaluation of implant quality is performed postoperatively using CT scans.75 The CT scan allows identification of the position of each seed and allows the brachytherapy team to calculate the dose delivered by the seeds to the gland. CT dosimetry shows the radioactive seeds in cross-sectional images as they lie within the prostate. With the aid of treatment-planning software, the dose is calculated and compared with the preplan dosimetry. CT dosimetry has allowed brachytherapists to substantially improve both planning and introperative technique.13,76,77 Some centers are also adopting the use of magnetic resonance imaging (MRI) to outline the anatomy more accurately.78 Because swelling of the prostate may sometimes make it difficult to accurately define the gland and to perform the required calculations, the postoperative CT and/or MRI study is usually performed 4 weeks post operation; however, day 0 or day 1 is acceptable.
Postoperative CT dosimetry provides important, immediate feedback on each implant. If there is an area or areas with significant underdosing, the deficiency can be addressed in a timely manner with supplemental EBRT, HDR, or a second, corrective implantation. Currently, the ABS recommends the use of CT-based, postoperative dosimetry on all patients and, in addition, the inclusion of such findings in published reports of clinical research on implantation.19
Quality Assurance and Quality of Life
With evidence that the various treatments for prostate cancer are likely to be equally successful in terms of long-term cancer control, emphasis is now being placed on the quality of life (QOL) after treatment. QOL can be difficult to measure because men may perceive problems after treatment very differently. Previous attempts to define QOL have been marred by the fact that patient reports of problems and physician reports have differed substantially and treatment planning and techniques are constantly changing. Therefore, studies are now incorporating patient self-reported and validated QOL-questionnaires that can help decrease, but not totally eliminate, bias.
Postimplantation surgery. A TURP after implantation increases the risk of urinary incontinence.79 Because most patients with brachytherapy-related urinary obstruction will eventually spontaneously void, a TURP should be approached with extreme caution and only after substantial time, eg, 9 months to 1 year, has elapsed.80 Generally, if obstructive symptoms are persistent beyond 12 months, a TUIP is recommended rather than a TURP.
Short-term effects. Prophylactic α-blockers (eg, tamsulosin) significantly improve the immediate obstructive effects of the implant.81 The use of steroids (eg, methylprednisolone dose packs) has been reported to be of benefit in reducing postoperative obstruction in some patients with high preoperative obstructive scores. Smokers and patients receiving hormone therapy have been demonstrated to have slightly higher obstructive problems. Urethral morbidity is associated with dose and can be reduced by minimizing the high dose to the urethra to 100% to 140% of the minimum peripheral dose.
QOL comparisons. Several recent QOL studies compared current implantation techniques with EBRT and radical prostatectomy. In one study, the analysis showed a decrease in QOL with radical prostatectomy and implantation at 1 month after the operation, but the overall QOL for both treatments returned to near baseline by the 1-year mark.82 In another study, the patients treated with radical prostatectomy reported significantly worse QOL in terms of urinary function and sexual function and bother. The patients treated with EBRT reported significantly worse QOL with regard to bowel function and fear of cancer recurrence.82 More studies in this important area will not only help compare QOL for each of the treatments but will also allow comparison of QOL for each of the several brachytherapy techniques currently in use.
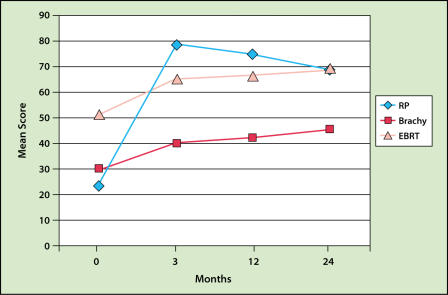
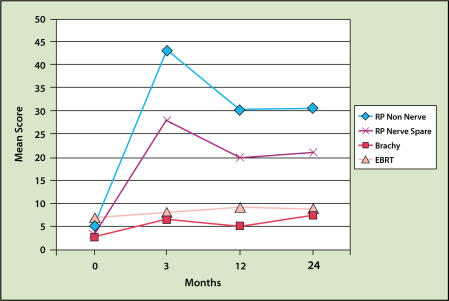
The most recent QOL study was reported by Talcott and colleagues.83 This study showed that radical prostatectomy resulted in higher incontinence and impotency rates (even though the patients were younger) and lower urinary obstruction complaints than brachytherapy (Figures 4 and 5). Rectal bleeding occurs in approximately 2% to 5% of patients receiving only an implant and 6% to 10% of those treated with both EBRT and implantation. The bleeding is usually minor and not apparent until 1 to 2 years after the implantation. Rectal bleeding rarely occurs after 3 years.31,40,68,71,84,85 One study demonstrated that careful planning of the dose to the rectum substantially reduces the risk of rectal bleeding.86 A severe rectal ulcer or fistula is rare in patients not undergoing electrocautery.67,87 Biopsy or electrocautery to stop bleeding is to be avoided in all patients with rectal bleeding after implantation because it may increase the risk of a non-healing ulcer or fistula.
Figure 4.
Incidence of sexual dysfunction as a result of modern brachytherapy (Brachy), radical prostatectomy (RP), and conformal external beam radiotherapy (EBRT). Adapted with permission from Talcott J, et al.83
Figure 5.
Incidence of incontinence as a result of modern brachytherapy (Brachy), radical prostatectomy (RP), and conformal external beam radiotherapy (EBRT). Adapted with permission from Talcott J, et al.83
Most published reports note that long-term urinary morbidity and/or incontinence following implantation is rare. In patients without severe obstructive urinary symptoms or significant benign prostatic hypertrophy, and without a prior TURP, the risk of chronic urinary irritation or incontinence following implantation is less than 3%.67,87
Sexual functioning and impotency are more challenging to evaluate because of differences in patient perception, the definition of potency, age differences, baseline functioning, comorbid diseases, and the sexual functioning and interest of the patient’s partner. The Seattle team has reported the results of a patient self-reported questionnaire. Patients who noted full, normal erection-ability prior to implantation maintained the ability to obtain an erection “adequate for intercourse” at 2 years in 80% of the seed monotherapy cases and 69% of the EBRT plus implantation cases.88–90 In other studies, 75% of implant patients maintained erection function at 1 year after implantation. At 3 years, 81% of the implant patients reported the ability to maintain an erection.31,85 Of interest is that several studies may have identified the cause of impotence in some men. The dose to the bulb of the penis may correlate to erectile dysfunction. In a small series of retrospectively reviewed patients, Dr Merrick noted that 19 of 23 patients who lost erectile function received a dose to the bulb of the penis of > 40% of the minimal peripheral dose, whereas 19 of 23 who maintained erectile function received a dose (to the bulb of the penis) of < 40% of the minimal peripheral dose.91 These findings have changed the Seattle Prostate Institute’s future planning of dose to this area, as noted in Table 1.
Summary
During the past 15 years, brachytherapy has advanced significantly in all areas, including patient selection, treatment planning, technique, and seed technology. Continuing efforts are focused on improving QOL for implant patients. Long-term biochemical disease-free survival continues to match published radical prostatectomy results (Table 3).15,38,92,93
Table 3.
Biochemical Relapse-Free Survival
| RP | 3D-CRT | I-125 | EBRT+Seeds | |||
|---|---|---|---|---|---|---|
| D’Amico38 | Zelefsky92 | Grimm15 | Sylvester93 | |||
| (HUP)(B&W) | (MSKCC) | (Seattle) | (Seattle) | |||
| Risk | 5-yr BRFS | 10-yr BRFS | 10-yr BRFS | 10-yr BRFS | ||
| Group | (DRG) | (DRG) | (SRG) | (SRG) | (SRG) | (DRG) |
| Low | 85% | 83% | 83% | 87% | 85% | 84 |
| Intermediate | 65% | 50% | 50% | 76% | 77% | 90% |
| High | 32% | 28% | 42% | — | 47% | 46% |
RP, radical prostatectomy; HUP, Hospital of the University of Pennsylvania; B & W Brigham and Women’s Hospital; BRFS biochemical relapse-free survival; DRG, D’Amico risk grouping; 3D-CRT, three-dimensional conformal radiotherapy; MSKCC, Memorial Sloan-Kettering Cancer Center; SRG, standard MSKCC risk grouping; I-125, iodine-125; EBRT, external beam radiotherapy.
Main Points.
Significant advances in patient selection, treatment planning, technique, and technology (eg, seeds stranded in Vicryl suture [RAPID Strand]) have made transperineal seed implantation an accurate, practical treatment option for patients with low-, intermediate-, and high-risk prostate cancer.
The development of the transperineal, ultrasound-guided approach provided a theoretical means to more accurately place seeds and improve dose coverage.
Virtually all studies agree that the keys to successful outcomes are appropriate patient selection and a high-quality implant.
The three key considerations involved in selecting patients for ultrasound-guided implantation are stage of cancer, technical suitability, and toxicity issues.
Treatment planning is a three-step process consisting of a volume study, an outline of an implant volume, and a computerized ideal seed placement plan.
Compared with loose seeds, seeds stranded in Vicryl suture have been shown to substantially lower the incidence of seed migration to the lung. Other studies demonstrated improved dosimetry with RAPID Strand versus loose seeds.
With evidence that the various treatments for prostate cancer are likely to be equally successful in terms of long-term cancer control, emphasis is now being placed on quality of life after treatment.
References
- 1.Sharkey J. Trends in prostate brachytherapy. Presented at: Sixth Annual Advanced Prostate Brachytherapy Conference; November 10–11, 2003; Seattle, WA. [Google Scholar]
- 2.Scardino P, Carlton C. Combined interstitial and external irradiation for prostatic cancer. In: Javadpour N, editor. Principles and Management of Urologic Cancer. Baltimore, MD: Williams & Wilkins; 1983. pp. 392–408. [Google Scholar]
- 3.Sylvester J, Blasko JC, Grimm P, Ragde H. Interstitial implantation techniques in prostate cancer. J Surg Oncol. 1997;66:65–75. doi: 10.1002/(sici)1096-9098(199709)66:1<65::aid-jso13>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 4.Martinez A, Edmundson GK, Cox RS, et al. Combination of external beam irradiation and multiple-site perineal applicator (MUPIT) for treatment of locally advanced or recurrent prostatic, anorectal, and gynecologic malignancies. Int J Radiat Oncol Biol Phys. 1985;11:391–398. doi: 10.1016/0360-3016(85)90163-4. [DOI] [PubMed] [Google Scholar]
- 5.Puthawala A, Syed A, Tansey L. Temporary iridium implant in the management of carcinoma of the prostate. Endocurie Hyper Oncol. 1985;1:25–33. [Google Scholar]
- 6.Whitmore WF, Jr, Hilaris B, Grabstald H. Retropubic implantation to iodine 125 in the treatment of prostatic cancer. J Urol. 1972;108:918–920. doi: 10.1016/s0022-5347(17)60906-6. [DOI] [PubMed] [Google Scholar]
- 7.DeLaney TF, Shipley WU, O’Leary MP, et al. Preoperative irradiation, lymphadenectomy, and 125iodine implantation for patients with localized carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 1986;12:1779–1785. doi: 10.1016/0360-3016(86)90319-6. [DOI] [PubMed] [Google Scholar]
- 8.Fuks Z, Leibel SA, Wallner KE, et al. The effect of local control on metastatic dissemination in carcinoma of the prostate: long-term results in patients treated with I125 implantation. Int J Radiat Oncol Biol Phys. 1991;21:537–547. doi: 10.1016/0360-3016(91)90668-t. [DOI] [PubMed] [Google Scholar]
- 9.Kovacs G, Galalae R, Loch T, et al. Prostate preservation by combined external beam and HDR brachytherapy in nodal negative prostate cancer. Strahlenther Onkol. 1999;175(suppl 2):87–88. doi: 10.1007/BF03038899. [DOI] [PubMed] [Google Scholar]
- 10.Hilaris B, Fuks Z, Nori D, et al. Interstitial irradiation in prostatic cancer: report of 10-year results. In: Sauer R, et al., editors. Interventional Radiation Therapy Techniques/Brachytherapy. Berlin, Germany: Springer-Verlag; 1991. p. 235. [Google Scholar]
- 11.Holm HH, Juul N, Pedersen JF, et al. Transperineal 125iodine seed implantation in prostatic cancer guided by transrectal ultrasonography. J Urol. 1983;130:283–286. doi: 10.1016/s0022-5347(17)51108-8. [DOI] [PubMed] [Google Scholar]
- 12.Blasko JC, Radge H, Schumacher D. Transperineal percutaneous iodine-125 implantation for prostatic carcinoma using transrectal ultrasound and template guidance. Endocurietherapy Hyperthermia Oncol. 1987;3:131–139. [Google Scholar]
- 13.Stock RG, Stone NN, Tabert A, et al. A doseresponse study for I-125 prostate implants. Int J Radiat Oncol Biol Phys. 1998;41:101–108. doi: 10.1016/s0360-3016(98)00006-6. [DOI] [PubMed] [Google Scholar]
- 14.Potters L, Cao Y, Calugaru E, et al. A comprehensive review of CT-based dosimetry parameters and biochemical control in patients treated with permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2001;50:605–614. doi: 10.1016/s0360-3016(01)01473-0. [DOI] [PubMed] [Google Scholar]
- 15.Grimm P, Blasko J, Sylvester JE, et al. 10-year biochemical (prostate-specific antigen) control of prostate cancer with (125)I brachytherapy. Int J Radiat Oncol Biol Phys. 2001;51:31–40. doi: 10.1016/s0360-3016(01)01601-7. [DOI] [PubMed] [Google Scholar]
- 16.Wallner K, Chiu-Tsao ST, Roy J, et al. An improved method for computerized tomography- planned transperineal 125iodine prostate implants. J Urol. 1991;146:90–95. doi: 10.1016/s0022-5347(17)37721-2. [DOI] [PubMed] [Google Scholar]
- 17.Beyer DC, Priestley JB., Jr Biochemical diseasefree survival following 125I prostate implantation. Int J Radiat Oncol Biol Phys. 1997;37:559–563. doi: 10.1016/s0360-3016(96)00609-8. [DOI] [PubMed] [Google Scholar]
- 18.Grimm PD, Blasko JC, Ragde H. Ultrasoundguided transperineal implantation of iodine-125 and palladium-103 for the treatment of earlystage prostate cancer: technical concepts in planning, operative technique, and evaluation. In: Schellhammer PF, editor. New Techniques in Prostate Surgery. Vol. 2. Philadelphia, PA: WB Saunders Company; 1994. pp. 113–126. [Google Scholar]
- 19.Nag S, Beyer D, Friedland J, et al. American Brachytherapy Society (ABS) recommendations for transperineal permanent brachytherapy of prostate cancer. Int J Radiat Oncol Biol Phys. 1999;44:789–799. doi: 10.1016/s0360-3016(99)00069-3. [DOI] [PubMed] [Google Scholar]
- 20.Blasko JC, Ragde H, Luse RW, et al. Should brachytherapy be considered a therapeutic option in localized prostate cancer? Urol Clin North Am. 1996;23:633–649. doi: 10.1016/s0094-0143(05)70342-6. [DOI] [PubMed] [Google Scholar]
- 21.Wallner K, Blasko J, Dattoli M. Prostate Brachytherapy Made Complicated. Seattle, WA: Smart Medicine Press; 1997. [Google Scholar]
- 22.Partin AW, Yoo J, Carter HB, et al. The use of prostate specific antigen, clinical stage and Gleason score to predict pathological stage in men with localized prostate cancer [see comments] J Urol. 1993;150:110–114. doi: 10.1016/s0022-5347(17)35410-1. [DOI] [PubMed] [Google Scholar]
- 23.Partin AW, Kattan MW, Subong EN, et al. Combination of prostate-specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer. A multi-institutional update [see comments] JAMA. 1997;277:1445–1451. [erratum in JAMA. 1997;278:118] [PubMed] [Google Scholar]
- 24.Partin AW, Mangold LA, Lamm DM, et al. Contemporary update of prostate cancer staging nomograms (Partin Tables) for the new millennium. Urology. 2001;58:843–848. doi: 10.1016/s0090-4295(01)01441-8. [DOI] [PubMed] [Google Scholar]
- 25.Davis BJ, Pisansky TM, Wilson TM, et al. The radial distance of extraprostatic extension of prostate carcinoma: implications for prostate brachytherapy. Cancer. 1999;85:2630–2637. [PubMed] [Google Scholar]
- 26.Sohayda C, Kupelian PA, Ciezki J, et al. Extent of extracapsular extension: implications for planning for conformal radiotherapy and brachytherapy [abstract 16] Int J Radiat Oncol Phys. 1998;42(suppl):132. [Google Scholar]
- 27.Epstein JI, Carmichael MJ, Pizov G, Walsh PC. Influence of capsular penetration on progression following radical prostatectomy: a study of 196 cases with long-term followup. J Urol. 1993;150:135–141. doi: 10.1016/s0022-5347(17)35415-0. [DOI] [PubMed] [Google Scholar]
- 28.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 29.Blasko JC, Grimm PD, Ragde H. External beam irradiation with palladium-103 implantation for prostate carcinoma. Int J Radiat Oncol Biol Phys. 1994;30(suppl):219. [Google Scholar]
- 30.Wallner K. I-125 brachytherapy for early stage prostate cancer: new techniques may achieve better results. Oncology. 1991;5:115–126. [PubMed] [Google Scholar]
- 31.Wallner K, Roy J, Harrison L. Tumor control and morbidity following transperineal iodine 125 implantation for stage T1/T2 prostatic carcinoma. J Clin Oncol. 1996;14:449–453. doi: 10.1200/JCO.1996.14.2.449. [DOI] [PubMed] [Google Scholar]
- 32.Schellhammer PF, Ladaga LE, El-Mahdi A. Histological characteristics of prostatic biopsies after 125iodine implantation. J Urol. 1980;123:700–705. doi: 10.1016/s0022-5347(17)56096-6. [DOI] [PubMed] [Google Scholar]
- 33.Prestidge BR, Hoak DC, Grimm PD, et al. Posttreatment biopsy results following interstitial brachytherapy in early-stage prostate cancer. Int J Radiat Oncol Biol Phys. 1997;37:31–39. doi: 10.1016/s0360-3016(96)00390-2. [DOI] [PubMed] [Google Scholar]
- 34.Merrick GS, Butler WM, Galbreath RW, Lief JH. Five-year biochemical outcome following permanent interstitial brachytherapy for clinical T1–T3 prostate cancer. Int J Radiat Oncol Biol Phys. 2001;51:41–48. doi: 10.1016/s0360-3016(01)01594-2. [DOI] [PubMed] [Google Scholar]
- 35.Roy JN, Ling CC, Wallner KE, Anderson LL. Determining source strength and source distribution for a transperineal prostate implant. Endocurie/Hyperthermia Oncology. 1996;12:35–41. [Google Scholar]
- 36.Lederman GS, Cavanagh W, Albert PS, et al. Retrospective stratification of a consecutive cohort of prostate cancer patients treated with a combined regimen of external-beam radiotherapy and brachytherapy. Int J Radiat Oncol Biol Phys. 2001;49:1297–3003. doi: 10.1016/s0360-3016(00)01442-5. [DOI] [PubMed] [Google Scholar]
- 37.Sylvester J. Modern permanent prostate brachytherapy. Paper presented at: American Society for Therapeutic Radiology and Oncology 43rd Annual Meeting; November 4–8, 2001; San Francisco. [Google Scholar]
- 38.D’Amico AV, Whittington R, Malkowicz SB, et al. Clinical utility of the percentage of positive prostate biopsies in defining biochemical outcome after radical prostatectomy for patients with clinically localized prostate cancer [see comments] J Clin Oncol. 2000;18:1164–1172. doi: 10.1200/JCO.2000.18.6.1164. [DOI] [PubMed] [Google Scholar]
- 39.Merrick GS, Butler WM, Wallner KE, et al. Prognostic significance of percent positive biopsies in clinically organ-confined prostate cancer treated with permanent prostate brachytherapy with or without supplemental external-beam radiation. Cancer J. 2004;10:54–60. doi: 10.1097/00130404-200401000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Blasko JC, Ragde H, Grimm PD. Transperineal ultrasound-guided implantation of the prostate: morbidity and complications. Scand J Urol Nephrol. 1991;137(suppl):113–118. [PubMed] [Google Scholar]
- 41.Talcott JA, Clark JA, Stark PC, Mitchell SP. Long-term treatment related complications of brachytherapy for early prostate cancer: a survey of patients previously treated. J Urol. 2001;166:494–499. [PubMed] [Google Scholar]
- 42.Wallner K, Lee H, Wasserman S, Dattoli M. Low risk of urinary incontinence following prostate brachytherapy in patients with a prior transurethral prostate resection. Int J Radiat Oncol Biol Phys. 1997;37:565–569. doi: 10.1016/s0360-3016(96)00570-6. [DOI] [PubMed] [Google Scholar]
- 43.Stone NN, Ratnow ER, Stock RG. Prior transurethral resection does not increase morbidity following real-time ultrasound-guided prostate seed implantation. Tech Urol. 2000;6:123–127. [PubMed] [Google Scholar]
- 44.Wallner KE, Blasko J, Dattoli MJ. Prostate Brachytherapy Made Complicated. Seattle, WA: Smart Medicine Press; 1997. [Google Scholar]
- 45.Crook J. SPIRIT Trial. Presented at: Sixth Annual Advanced Prostate Brachytherapy Conference; November 10–11, 2003; Seattle, WA. [Google Scholar]
- 46.Nath R, Anderson LL, Luxton G, et al. Dosimetry of interstitial brachyhtherapy source: recommendations of the AAPM Radiation Therapy Committee Task Group 43. Med Phy. 1995;22:209–243. doi: 10.1118/1.597458. [DOI] [PubMed] [Google Scholar]
- 47.Ling CC. Permanent implants using Au-198, Pd- 103 and I-125: radiobiological considerations based on the linear quadratic model. Int J Radiat Oncol Biol Phys. 1992;23:81–87. doi: 10.1016/0360-3016(92)90546-t. [DOI] [PubMed] [Google Scholar]
- 48.Ling CC, Li WX, Anderson LL. The relative biological effectiveness of I-125 and Pd-103. Int J Radiat Oncol Biol Phys. 1995;32:373–378. doi: 10.1016/0360-3016(95)00530-C. [DOI] [PubMed] [Google Scholar]
- 49.Merrick GS, Butler WM, Wallner KE, et al. The importance of radiation dose to the penile bulb vs crura in the development of postbrachytherapy erectile dysfunction. Int J Radiat Oncol Biol Phys. 2002;54:1055–1062. doi: 10.1016/s0360-3016(02)03031-6. [DOI] [PubMed] [Google Scholar]
- 50.Blasko JC, Grimm PD, Sylvester JE, et al. Palladium-103 brachytherapy for prostate carcinoma. Int J Radiat Oncol Biol Phys. 2000;46:839–850. doi: 10.1016/s0360-3016(99)00499-x. [DOI] [PubMed] [Google Scholar]
- 51.Cha C, Potters L, Ashley R, et al. Isotope selection for patients undergoing prostate brachytherapy [abstract 19] Int J Radiat Oncol Phys. 1998;42(suppl):134. doi: 10.1016/s0360-3016(99)00187-x. [DOI] [PubMed] [Google Scholar]
- 52.Tapen EM, Blasko JC, Grimm PD, et al. Reduction of radioactive seed embolization to the lung following prostate brachytherapy. Int J Radiat Oncol Biol Phys. 1998;42:1063–1067. doi: 10.1016/s0360-3016(98)00353-8. [DOI] [PubMed] [Google Scholar]
- 53.Merrick GS, Butler WM, Dorsey AT. Seed fixity in the periprostatic region following brachytherapy. Int J Radiat Oncol Biol Phys. 2000;46:215–220. doi: 10.1016/s0360-3016(99)00405-8. [DOI] [PubMed] [Google Scholar]
- 54.Sommerkamp H, Rupprecht M, Wannemacher M. Seed loss in interstitial radiotherapy of prostate carcinoma with I-125. Int J Radiat Oncol Biol Phys. 1988;14:389–392. doi: 10.1016/0360-3016(88)90448-8. [DOI] [PubMed] [Google Scholar]
- 55.Davis BJ, Bresnahan JF, Stafford SL, et al. Prostate brachytherapy seed migration to a coronary artery found during angiography. J Urol. 2002;168:1103. doi: 10.1016/S0022-5347(05)64589-2. [DOI] [PubMed] [Google Scholar]
- 56.Shanahan T, Mueller P, Roszhart D, et al. Image guided I-125 prostate brachytherapy with hybrid interactive Mick technique in the community setting: how does it compare? Technol Cancer Res Treat. 2004;3 doi: 10.1177/153303460400300214. In press. [DOI] [PubMed] [Google Scholar]
- 57.Eshleman JS, Davis B, Pisansky TM, et al. Radioactive seed migration to the chest following transperineal interstitial prostate brachytherapy: extraprostatic seed placement correlates with migration. Int J Radiat Oncol Biol Phys. 2004 doi: 10.1016/j.ijrobp.2003.10.050. in press. [DOI] [PubMed] [Google Scholar]
- 58.Grimm P, Sylvester J, Blasko J. See migration strands vs loose seeds. Presented at: Sixth Annual Advanced Prostate Brachytherapy Conference; November 10–11, 2003; Seattle, WA. [Google Scholar]
- 59.Lee WR, deGuzman AF, Tomlinson SK, McCullough DL. Radioactive sources embedded in suture are associated with improved postimplant dosimetry in men treated with prostate brachytherapy. Radiother Oncol. 2002;65:123–127. doi: 10.1016/s0167-8140(02)00305-5. [DOI] [PubMed] [Google Scholar]
- 60.Fagundes U. Dosimetric analysis comparing free seeds vs a novel technique using Rapid Strand prostate brachytherapy. Presented at: Sixth Annual Advanced Prostate Brachytherapy Conference; November 10–11, 2003; Seattle, WA. [Google Scholar]
- 61.Meier R. Loose seeds vs. stranded seeds. Presented at: Sixth Annual Advanced Prostate Brachytherapy Conference; November 10–11, 2003; Seattle, WA. [Google Scholar]
- 62.Grimm P. Quality assurance for brachytherapy. Presented at: Seventh Annual Advanced Prostate Brachytherapy Conference; April 2–3, 2003; Seattle, WA. [Google Scholar]
- 63.Stock RG, Stone NN, Wesson MF, DeWyngaert JK. A modified technique allowing interactive ultrasound-guided three-dimensional transperineal prostate implantation. Int J Radiat Oncol Biol Phys. 1995;32:219–225. doi: 10.1016/0360-3016(95)00521-Y. [DOI] [PubMed] [Google Scholar]
- 64.Beyer DC, Priestley JB. Biochemical disease-free survival following I-125 prostate implantation [abstract 1062] Int J Radiat Oncol Biol Phys. 1995;32(suppl):254. doi: 10.1016/s0360-3016(96)00609-8. [DOI] [PubMed] [Google Scholar]
- 65.Zelefsky MJ, Fuks Z, Hunt M. High dose radiation delivered by intensity modulated conformal radiotherapy improves the outcome of localized prostate cancer. J Urol. 2001;166:876–881. [erratum in J Urol. 2001;166:1839] [PubMed] [Google Scholar]
- 66.Jin-Song Y, Sylvester J, Grimm P, et al. Advances in EBRT. To be presented at the 46th Annual Meeting of the American Society of Therapeutic Radiology Oncology; October 3–7, 2004; Atlanta, GA. [Google Scholar]
- 67.Blasko J. Complications of implantation. Presented at: Transperineal Brachytherapy: Into the Mainstream Conference; 1995; Pacific NW Cancer Foundation; Seattle, WA. [Google Scholar]
- 68.Arterbery VE, Wallner K, Roy J, Fuks Z. Shortterm morbidity from CT-planned transperineal I-125 prostate implants. Int J Radiat Oncol Biol Phys. 1993;25:661–667. doi: 10.1016/0360-3016(93)90013-l. [DOI] [PubMed] [Google Scholar]
- 69.Grier D. Complications of permanent seed implantation. J Brachytherapy Int. 2001;17:205–210. [Google Scholar]
- 70.D’Amico AV, Coleman CN. Role of interstitial radiotherapy in the management of clinically organ-confined prostate cancer: the jury is still out [see comments] J Clin Oncol. 1996;14:304–315. doi: 10.1200/JCO.1996.14.1.304. [DOI] [PubMed] [Google Scholar]
- 71.Dattoli MJ, Wasserman SG, Koval JM, et al. Conformal brachytherapy boost to external beam irradiation for localized high risk prostate cancer [abstract 1056] Int J Radiat Oncol Biol Phys. 1995;32(suppl):251. [Google Scholar]
- 72.Sylvester JE, Grimm P, Blasko J, et al. Transperineal permanent brachytherapy for local recurrence following external beam radiation for early-stage prostate cancer. J Brachytherapy Int. 2001;17:181–188. [Google Scholar]
- 73.Merrick G, Butler W, Wallner K, et al. Short-term sexual function after prostate brachytherapy. Int J Radiat Oncol Invest. 2001;910:313–319. doi: 10.1002/ijc.1028. [DOI] [PubMed] [Google Scholar]
- 74.Stock RG, Stone NN, DeWyngaert JK. PSA findings and biopsy results following interactive ultrasound guided transperineal brachytherapy for early stage prostate cancer. Proceedings of the American Radium Society 78th Annual Meeting; 1995; Paris. p. 58. [DOI] [PubMed] [Google Scholar]
- 75.Willins J, Wallner K. CT-based dosimetry for transperineal I-125 prostate brachytherapy. Int J Radiat Oncol Biol Phys. 1997;39:347–353. doi: 10.1016/s0360-3016(97)00322-2. [DOI] [PubMed] [Google Scholar]
- 76.Roy JN, Wallner KE, Chiu-Tsao ST, et al. CT-based optimized planning for transperineal prostate implant with customized template. Int J Radiat Oncol Biol Phys. 1991;21:483–489. doi: 10.1016/0360-3016(91)90800-j. [DOI] [PubMed] [Google Scholar]
- 77.Stock RG, Stone NN, DeWyngaert JK, et al. Prostate specific antigen findings and biopsy results following interactive ultrasound guided transperineal brachytherapy for early stage prostate carcinoma. Cancer. 1996;77:2386–2392. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2386::AID-CNCR30>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 78.Crook J. Quality assessment with respect to toxicity. Presented at: Sixth Annual Advanced Prostate Brachytherapy Conference; November 10–11, 2003; Seattle, WA. [Google Scholar]
- 79.Merrick G, Butler W, Wallner K, et al. Effect of transurethral resection of urinary quality of life after permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2004;58:81–88. doi: 10.1016/s0360-3016(03)00776-4. [DOI] [PubMed] [Google Scholar]
- 80.Wehle M, Lisson SW, Buskirk SJ, et al. Prediction of genitourinary tract morbidity after brachytherapy for prostate adenocarcinoma. Mayo Clin Proc. 2004;79:314–317. doi: 10.4065/79.3.314. [DOI] [PubMed] [Google Scholar]
- 81.Merrick G, Butler W, Wallner K, et al. Long-term urinary quality of life after permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2003;56:454–461. doi: 10.1016/s0360-3016(02)04600-x. [DOI] [PubMed] [Google Scholar]
- 82.Lee WR, Hall MC, McQuellon RP, et al. A prospective quality-of-life study in men with clinically localized prostate carcinoma treated with radical prostatectomy, external beam radiotherapy, or interstitial brachytherapy. Int J Radiat Oncol Biol Phys. 2001;5:614–623. doi: 10.1016/s0360-3016(01)01707-2. [DOI] [PubMed] [Google Scholar]
- 83.Talcott JA, Manola J, Clark JA, et al. Time course and predictors of symptoms after primary prostate cancer therapy. J Clin Oncol. 2003;21:3979–3986. doi: 10.1200/JCO.2003.01.199. [DOI] [PubMed] [Google Scholar]
- 84.Wallner K, Roy J, Zelefsky M, et al. Short-term freedom from disease progression after I-125 prostate implantation. Int J Radiat Oncol Biol Phys. 1994;30:405–409. doi: 10.1016/0360-3016(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 85.Kaye KW, Olson DJ, Payne JT. Detailed preliminary analysis of 125iodine implantation for localized prostate cancer using percutaneous approach. J Urol. 1995;153:1020–1025. [PubMed] [Google Scholar]
- 86.Snyder K, Stock R, Hong S, et al. Defining the risk of developing grade 2 proctitis following 125I prostate brachytherapy using a rectal dosevolume histogram analysis. Int J Radiat Oncol Biol Phys. 2001;50:335–341. doi: 10.1016/s0360-3016(01)01442-0. [DOI] [PubMed] [Google Scholar]
- 87.Blasko JC, Grimm PD, Ragde H. Brachytherapy and organ preservation in the management of carcinoma of the prostate. Semin Radiat Oncol. 1993;3:240–249. doi: 10.1053/SRAO00300240. [DOI] [PubMed] [Google Scholar]
- 88.Grimm P. Clinical results of prostate brachytherapy. Presented at: 84th Annual Radiological Society of North America Meeting; 1998; Chicago, IL. [Google Scholar]
- 89.Grimm P. Patient selection. Presented at: the First Advanced Prostate Brachytherapy Workshop, Seattle Prostate Institute; May 15–16, 1998; Seattle, WA. [Google Scholar]
- 90.Ragde H, Blasko J, Grimm P. Transperineal Brachytherapy: Into the Mainstream. Seattle, WA: Pacific NW Cancer Foundation; 1995. Complications of permanent seed implantation. [Google Scholar]
- 91.Merrick GS, Wallner K, Butler WM, et al. A comparison of radiation dose to the bulb of the penis in men with and without prostate brachytherapy-induced erectile dysfunction. Int J Radiat Oncol Biol Phys. 2001;50:597–604. doi: 10.1016/s0360-3016(01)01475-4. [DOI] [PubMed] [Google Scholar]
- 92.Zelefsky M, Fuks Z, Chan H, et al. Ten-year results of dose escalation with 3-dimensional conformal radiotherapy for patients with clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2003;57(suppl 2):S149–S150. [Google Scholar]
- 93.Sylvester JE, Blasko JC, Grimm PD, et al. Ten-year biochemical relapse-free survival after external beam radiation and brachytherapy for localized prostate cancer: the Seattle experience. Int J Radiat Oncol Biol Phys. 2003;57:944–952. doi: 10.1016/s0360-3016(03)00739-9. [DOI] [PubMed] [Google Scholar]