Abstract
Although there is renewed interest in conservative therapies for stress urinary incontinence, such as pelvic floor exercises, electrical stimulation, and duloxetine therapy, surgery remains the primary choice in managing this condition. Surgical options include paravaginal defect repair, the Marshall-Marchetti-Krantz procedure, open and laparoscopic Burch urethropexy, and pubovaginal sling procedures. There is a growing trend in the United States toward use of the pubovaginal sling procedure as the primary operation for urinary incontinence due to less invasive techniques. Studies comparing the pubovaginal sling with open urethropexy have shown similar short-term cure rates. More large prospective, randomized studies are needed to assess long-term rates.
Key words: Stress urinary incontinence, Pelvic floor exercises, Electrical stimulation, Duloxetine, Marshall-Marchetti-Krantz procedure, Burch urethropexy, Transvaginal tape procedure
There are numerous treatment options available for patients with stress urinary incontinence (SUI). These include conservative therapies, such as pelvic floor exercises, electrical stimulation, and pharmacotherapy, and surgical therapies, such as the Marshall-Marchetti-Krantz procedure, Burch urethropexy, and pubovaginal slings.
Conservative Treatment of SUI
The conservative treatment of incontinence has experienced a resurgence of interest in the past several years. The methods of performing and investigating conservative therapies have improved, allowing for a better understanding of the benefit or lack of benefit associated with various interventions.
Pelvic Floor Exercises
Pelvic floor exercises were performed as early as the mid-19th century, although Kegel, reporting in 1948, was the first to investigate the long-term effects using somewhat objective methods with the aid of a perineometer.1 The results of past studies are variable, with poor follow-up, infrequent urodynamic testing, and lack of standardized procedures and descriptive terms. More recently, studies utilizing objective methods have been published. It appears that pelvic floor exercises, when properly performed, are a useful adjunct to treatment in patients with mild to moderate incontinence. A Cochrane systematic review that included studies of stress, urge, and mixed incontinence found pelvic floor muscle training to be more effective than no treatment or placebo.2
Mouritsen and colleagues3 studied the long-term effect of pelvic floor exercises on female incontinence. Seventy-six women underwent a 3-month exercise program and were followed for 1 year. At the 12-month evaluation, 30% of subjects were considered cured and 17% were improved. Subjects with severe incontinence did not benefit from the therapy, whereas 72% of patients with mild incontinence experienced a cure. Patients in this study were instructed in self-digital biofeedback.
Some studies have suggested that formal biofeedback increases the success rate of pelvic floor exercises. Burgio and colleagues4 compared the results of digital biofeedback (n = 11) with bladder-sphincter biofeedback (n = 13) in a group of women with SUI. The subjects who received bladder-sphincter biofeedback had a 76% decrease in incontinence, compared with a 51% decrease in those who received digital biofeedback. However, a recent Cochrane Review that included women with SUI concluded that there appears to be no advantage to combining pelvic floor muscle training with biofeedback over the use of well-done pelvic floor muscle training alone.5
Bladder training may be as efficacious as pelvic floor muscle training.6 However, pelvic floor muscle training with concomitant bladder retraining appears to be better than pelvic floor training alone.7 Bladder training combined with drug therapy does not demonstrate significant improvement rates over bladder training alone.8
Functional Electrical Stimulation
Electrical stimulation has been used throughout the past 3 decades for the treatment of SUI. This technique has been employed to either aid in the treatment of bladder instability or increase sphincteric/outlet resistance. Devices have included implanted electrodes, electronic pessaries, anal electrodes, and intravaginal electrodes of various types.
The goal of electrical stimulation for the treatment of SUI is to increase the strength of the pelvic and periurethral muscles. Animal studies have shown that increases in urethral pressure with intravaginal probes are secondary to direct stimulation of efferent motor axons. Electrical stimulation of the pelvic floor muscles results in reflexive contraction of paraurethral musculature and reflexive detrusor inhibition. Patients with SUI may benefit from the feedback of electrically induced muscle contractions, thus learning how to contract the appropriate muscles when performing pelvic floor exercises.
A study conducted by Bent and colleagues9 that included patients with stress, urge, and mixed incontinence showed significant subjective patient improvement (mixed, 52%; urge, 70%; pure SUI, 71%) with electrical stimulation, whereas objective parameters of improvement remained unchanged. If a patient is able to perform Kegel exercises, functional electrical stimulation may not be required. Bo and Talseth10 demonstrated that voluntary muscle contraction increased urethral pressure more significantly than did functional electrical stimulation. In addition, electrical stimulation may not cause a typical pelvic floor contraction, or “Kegel contraction,” in the majority of women.11
Pharmacologic Management
Duloxetine, a selective serotonin and norepinephrine reuptake inhibitor, increases rhabdosphincter contractility via stimulation of pudendal motor neuron receptors. A controlled trial of duloxetine for the treatment of SUI revealed a significant dose response in the median decrease in incontinence frequency compared with placebo.12 Dmochowski and colleagues13 reported a double-blind, placebo-controlled study of 683 women with SUI; the case definition required subjects to have a pretreatment weekly incontinence episode frequency of 7 or more and a bladder capacity of 400 mL or greater. Duloxetine significantly decreased incontinence episode frequency compared with placebo (50% vs 27%; P < .001) over the spectrum of incontinence severity. Quality-of-life scores also improved significantly with duloxetine compared with placebo (11.0 vs 6.8; P < .001).
Surgical Treatment of SUI
A number of features of urethral function distinguish symptom-free women from patients with genuine SUI. These include maximum urethral pressure at rest or closure pressure, intra-abdominal pressure variations, sustained response to stress (drop in urethral pressure from sustained coughing), and pressure-transmission ratios.
The aims of incontinence surgery have been variously defined as tightening of the pubocervical fascia, elevation of the bladder neck, increase in urethral resistance, and increase in urethral functional length. Postoperative urodynamic studies have not shown consistent increases in urethral pressure or functional length in patients cured of incontinence. In fact, most studies have shown that there is no increase in urethral resting or closure pressure. Surgical success does appear to be urodynamically associated with increased pressure transmission to the urethra; this may also be suggestive of the longevity of surgical success.14
The pressure-transmission ratio appears to have an all-or-none effect in the determination of SUI. The exact cause of the impairment in a given woman may or may not be completely apparent. The ability to maintain positive pressure in the urethra during stress may be the result of multiple factors acting in concert, including a functional rhabdosphincter, anatomic support of the urethrovesical junction, a healthy estrogenized urethral lumen, and proper function of the levator musculature.
Surgery for urinary incontinence results in support to the urethrovesical junction, arrest of downward descent with straining, and increased pressure transmission to the urethra. The classic pubovaginal sling causes obstruction to the urethra coincident with coughing and increased pressure transmission to the urethra. Recent modifications result in less obstruction.
The distribution of pressure transmission along the urethra following successful colposuspension is quite different from that seen in healthy women.15 In healthy women, the maximum transmission ratios are at or just distal to the resting profile peak, which is at the mid-distal urethra. In women made continent by colposuspension, maximum transmission is achieved within the proximal half of the functional urethra. Thus, most incontinence operations do not restore a woman to a fully physiologic state.
The Paravaginal Defect Repair
The paravaginal defect repair (PVDR), or vagino-levator shelf procedure, by virtue of its close reapproximation to normal anatomy, may restore the extrinsic continence mechanism to a degree somewhat comparable to that of the healthy female. Support of the urethrovesical junction is re-established, and attachment to the levator musculature may again allow movement and increased function. In the majority of cases, patients are able to void almost immediately following surgery.
Shull and Baden16 reported a 6-year experience with the PVDR for SUI. Of 149 patients who underwent the procedure, 97% were reported as having excellent results, with no further subjective stress incontinence. In contrast, Colombo and colleagues17 performed a prospective comparison of Burch urethropexy and the PVDR and reported cure rates of 100% and 61%, respectively, at 6 months. However, the study was severely limited by its small number of subjects; only 36 patients were enrolled, with 29 patients completing the study. To show a difference in success of 12.5% with an α level of 0.05 and a power of 0.80 would have required 80 patients in the 2 arms of the surgical study.18
PVDR failure may be secondary to persistent or recurrent urethrovesical junction descent from relaxation of the anterior vaginal wall in the midline, despite the restoration of paravaginal anatomy. This may be evidenced by the fact that, in Colombo’s study, all of the postoperative Burch patients, but only 33% of the PVDR patients, had a negative urethral angle on cotton swab testing. Patients who continued to have midline urethral descent despite paravaginal support demonstrated high rates of failure. Thus, patient selection by documenting lack of urethral descent during instrumental support of the anterior lateral sulci may help to increase the cure rate in PVDR patients.
Concomitant PVDR at the time of urethrolysis for the management of voiding dysfunction secondary to urethropexy is a consideration. Webster and Kreder19 reported on 15 women with voiding dysfunction following cystourethropexy who underwent takedown and substitution with a PVDR. All 13 women who had symptoms of bladder instability experienced resolution of their symptoms. In addition, of 7 patients who required self-intermittent catheterization preoperatively, only 1 required catheterization postoperatively. A successful outcome was achieved in 14 of the 15 patients. This article underscores the anatomic correction of the PVDR technique, which does not result in elevation of the urethrovesical junction beyond its normal anatomic position.
To provide adequate treatment to patients undergoing a Burch or Marshall-Marchetti-Krantz (MMK) procedure who have a concomitant paravaginal defect, a PVDR may be accomplished simultaneously. This is often referred to as a paravaginalplus or MMK-plus procedure. In this setting, the Burch procedure is viewed as the primary incontinence operation and the PVDR as correcting a defect and further supporting the anterior vaginal wall.
MMK Procedure
The MMK procedure was first accomplished in a female patient on June 8, 1944.20 Modifications of the procedure must involve suturing of the periurethral tissues to the midline cartilage or periosteum of the symphysis pubis in order to maintain the MMK designation.
Considering the limitation of mobility following this procedure and the high position of the anterior wall, it is unlikely that the continence mechanism is commensurate with that of the healthy female. The MMK procedure may work through a combination of slight obstruction, creating positive pressure transmission by an unknown mechanism, and additional features that act at the urethrovesical junction subsequent to its elevation.
In a review of 56 articles published through 1988, Mainprize and Drutz20 reported the overall success rate of the MMK procedure to be 86.1% in 2712 cases. The authors noted that, even in repeat procedures, the cure rate was high at 84.5%. Lee and colleagues21 reported data from a series of 549 patients who underwent an MMK procedure and were followed for 2 to 16 years: results demonstrated subjective cure rates of 91% in the 227 patients who underwent the surgery as a primary procedure and 90% in the 322 patients who underwent the surgery as a repeat procedure.
Colombo and colleagues22 reported results of a randomized comparison of the MMK and Burch procedures. A full urodynamic investigation was performed 6 months postsurgery. The cure rate with the MMK procedure was 65% on urodynamic testing, and a subjective cure rate of 85% was seen at mean 3.5 years. The Burch procedure resulted in an objective cure rate of 80%.
The MMK procedure is effective in patients with low urethral pressure and hypermobility of the urethra. Quadri and colleagues23 conducted a prospective, randomized comparison of MMK urethropexy and Burch colposuspension in patients with low urethral pressures who demonstrated urethral descent. Only 15 patients were studied in each treatment group. At 1 year postsurgery, stress tests were negative in 93% of the women who had undergone the MMK procedure and 53% of those who had undergone the Burch procedure.
In their review, Mainprize and Drutz20 reported the overall complication rate of the MMK procedure to be 21.1%, with a 5% wound complication rate, 3.8% urinary tract infection rate, and 2.5% incidence of osteitis pubis. Kammerer-Doak and colleagues24 reported 15 cases of osteitis pubis diagnosed after 2030 MMK procedures performed at the Mayo Clinic.
Burch Urethropexy
The majority of Burch procedures performed today are similar to the modification described by Emil A. Tanagho, MD, in 1976.25 Tanagho placed his sutures in a far-lateral position, used 2 sutures bilaterally (No. 1 Dexon), and emphasized avoidance of undue tension on the anterior vaginal wall. He commented that 2 fingers could be placed between the symphysis and urethra, thus stressing that the vagina does not have to be contiguous with Cooper’s ligament.
The concept of not overelevating the vaginal wall at the time of retropubic urethropexy was an important one, and it presaged the hammock hypothesis of John O. L. DeLancey, MD.26 Patients could experience cure with less risk of urinary retention and bladder overactivity secondary to obstruction. It is consistent with the concepts of the hammock hypothesis that Burch procedures should stabilize and not elevate the anterior vaginal wall. Tanagho’s modification, by its lateral placement of sutures, reduced compression and overelevation of the urethra. It is important when reviewing articles describing the results of Burch procedures to note the technique used and to what degree the anterior vaginal wall was elevated. Articles that describe apposition of the wall to Cooper’s ligament or significant elevation will report a higher incidence of prolonged voiding dysfunction and enterocele formation.
Several studies have reported long-term results after Burch urethropexy. Herbertsson and Iosif27 studied 72 women who underwent Burch colposuspension with preoperative and postoperative urodynamics. Objective follow-up was performed a mean 9.4 years postsurgery. The objective surgical cure rate was 90.3%, with cure being defined as a negative stress test with at least 300 mL within the bladder. The enterocele formation rate was 4%.
Feyereisl and colleagues28 reported urodynamic outcomes in 87 patients, 5 to 10 years after Burch urethropexy. Patients with greater than grade I prolapse or cystocele were excluded from the study. Inclusion criteria included objective stress leakage in the absence of detrusor instability and documented hypermobility of the urethra. Stress incontinence was objectively cured in 81.6% of patients, with cure being defined as a dry, symptom-free patient without objective urine loss during coughing in the standing position at a bladder volume of 400 mL.
Alcalay and colleagues29 reported data from a 10- to 20-year follow-up in patients who had undergone Burch colposuspension. Follow-up in this longitudinal retrospective study included symptom review, uroflowmetry, and an extended pad test. An objective cure was defined as the inability to demonstrate stress incontinence during clinical examination and provocative urodynamics. The investigators reported the incontinence cure rate to be time dependent, with a decline for 10 to 12 years, when a plateau of 69% is reached.
Langer and colleagues30 reported long-term (10- to 15-year) follow-up data after Burch colposuspension in 127 patients; 109 patients underwent an additional urodynamic examination performed at least 10 years after surgery. The cure rate was 93.7%, with cure defined as subjective and objective dryness. Following surgery, there was an improvement in symptoms of frequency (P < .001), urgency (P < .01), and urge incontinence (P < .001).
Prospective, Randomized Studies Comparing Burch Urethropexy and Anterior Colporrhaphy
Anterior colporrhaphy, or the Kelly procedure, has been used in the management of female urinary incontinence for decades. This technique became popular after the concepts of the PVDR were lost to common awareness and before the creation of other retropubic urethropexies. There remain proponents of anterior colporrhaphy for incontinence correction to date, despite a preponderance of objective evidence supporting retropubic urethropexy for this indication. There are now several prospective, randomized studies comparing Burch urethropexy with anterior colporrhaphy.
In 1995, Bergman and Elia31 reported data from a 5-year objective follow-up comparing 3 surgical procedures for the treatment of urinary incontinence. The series consisted of 127 patients without a history of previous incontinence surgery. Multichannel urodynamics were performed preoperatively and at 3 months, 12 months, and 5 years postoperatively. Patients were randomized to anterior colporrhaphy with Kelly plication, the Pereyra procedure, or Burch urethropexy. Ninety-three subjects were available for the 5-year objective follow-up. The success rates were 37% with anterior colporrhaphy, 43% with the Pereyra procedure, and 82% with Burch urethropexy. Seventy percent of the colporrhaphy patients had a urethrovesical junction descent at 5 years, compared with 7% of the Burch patients. Many patients who had cure at 1 year but demonstrated a positive cotton swab test were noted to be failures at 5 years.
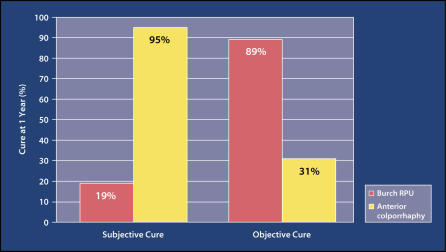
Kammerer-Doak and colleagues32 conducted a randomized trial comparing Burch urethropexy with the modified anterior colporrhaphy. Thirty-five patients were randomized; subjects underwent preoperative and postoperative urodynamic testing. At 1 year postsurgery, 16 (89%) of 18 Burch patients were objectively cured, compared with 5 (31%) of 16 colporrhaphy patients (Figure 1). Subjective and objective ratings of incontinence severity as determined by questionnaires and pad testing were significantly lower for the Burch patients compared with the colporrhaphy patients. Mobility of the urethrovesical junction was lower for the Burch patients.
Figure 1.
In a study by Kammerer-Doak and colleagues, 16 (89%) of 18 patients who underwent Burch retropubic urethropexy (RPU) were objectively cured at 1 year postsurgery, compared with 5 (31%) of 16 colporrhaphy patients. Subjective and objective ratings of incontinence severity as determined by questionnaires and pad testing were significantly lower for the Burch patients compared with the colporrhaphy patients. Data from Kammerer-Doak DM et al. Obstet Gynecol. 1999;93:75–78.32
Liapis and colleagues33 reported results of a randomized study of 3 operations for stress incontinence: the MMK procedure, Burch urethropexy, and anterior colporrhaphy. Subjects were clinically and urodynamically examined preoperatively and at 60 months postsurgery. At 5 years, results achieved with the 3 procedures differed significantly: the cure rate was 89% with the Burch procedure, compared with 56% and 67% with anterior colporrhaphy and the MMK procedure, respectively (P < .001).
Laparoscopic Burch Urethropexy
Vancaillie and Schuessler34 reported the first laparoscopic colposuspension (MMK) case series in 1991. The literature reflects a lack of standardization and precise outcome measurements. Prospective, randomized comparisons of laparoscopic and open techniques include studies by Summitt and colleagues35 and Fatthy and colleagues,36 both of which report comparable rates of SUI cure between the 2 patient groups. Prospective, randomized studies that demonstrate an increased cure rate with an open technique include those by Burton37 and Su and colleagues.38 Su and colleagues examined success at 1 year and demonstrated cure rates of 84% with the laparoscopic approach and 95.6% with the open technique. The randomized study by Burton,37 which included a 3-year follow-up, showed a failure rate of 40% in the laparoscopic group, compared with 15% in the open-technique group.
Several authors have reported an increased complication rate with the laparoscopic approach. Speight and colleagues39 reported on frequency of lower urinary tract injury with laparoscopic Burch and PVDR: there were no ureteral injuries, and 4 of 171 patients had cystotomies.
Walter and colleagues40 compared morbidity and costs of laparoscopic versus open Burch when performed with concomitant vaginal prolapse repairs. The investigators performed a retrospective review of 76 laparoscopic and 143 open Burch procedures with at least 1 concomitant vaginal repair for symptomatic prolapse. The patients who underwent open urethropexy were older and had a greater degree of prolapse, fewer concurrent hysterectomies, and a greater number of vaginal procedures than those who underwent the laparoscopic Burch procedure. There were minimal differences in complications and no differences between the 2 groups in estimated blood loss, operative time, hemoglobin change, hospitalization, or hospital charges. Therefore, because a significant percentage of incontinent patients require some type of concomitant prolapse repair, the benefits of laparoscopy in this setting are less evident. Kholi and colleagues41 showed that, despite a shorter hospital stay, the direct costs of laparoscopic Burch were higher than those of the open technique.
Persson and Wolner-Hanssen42 demonstrated the benefit of laparoscopic Burch colposuspension using 2 sutures on each side of the urethra compared with 1 suture: the objective cure rate was 83% in the women who received 2 sutures, compared with 58% in those who received 1 suture.
The current literature indicates that additional large prospective, randomized studies of laparoscopic Burch urethropexy that are adequately powered are needed.
Retropubic Urethropexy Versus Pubovaginal Sling
The past several years have seen an increasing trend toward selection of the pubovaginal sling as a primary incontinence procedure in the United States. Two phenomena accelerated this trend: the first was the accumulation of data on the poor outcomes of needle-suspension procedures; the second was the development of a less invasive pubovaginal sling in the form of the transvaginal tape procedure (TVT).43 In addition, the misconception of intrinsic sphincteric deficiency as an entity with a possible laboratory diagnosis further resulted in increased numbers of patients receiving slings. Practice trends may reach the point at which the majority of patients are simply relegated to a TVT procedure, with little clinical evaluation or attention to paravaginal anatomy.
Pubovaginal Sling Procedures
In the Cochrane Review of pubovaginal slings, 12 trials were identified, in which 543 women received suburethral slings.44 Nine trials compared the sling procedure with retropubic suspension. Overall rates of short-term cure were similar with the pubovaginal sling procedure and open urethropexy. Long-term data are too few to reliably estimate outcomes.
The TVT procedure and similar products have resulted in minimized dissection and cure rates commensurate with classic pubovaginal sling procedures. Nilsson and colleagues45 reported 5-year data on 90 consecutive patients who underwent a TVT procedure for SUI: the cure rate was 84.7%. Seven-year data show similar cure rates. TVT surgery is not appropriate for patients with intrinsic sphincteric deficiency who lack urethrovesical junction descent. In a series by Rezapour and colleagues,46 patients who lacked urethrovesical junction descent failed TVT surgery for incontinence. Ward and Hilton47 compared the TVT procedure with colposuspension for the treatment of SUI and found the 2 procedures to have similar cure rates.
Main Points.
Conservative therapies for stress urinary incontinence (SUI), including pelvic floor exercises, functional electrical stimulation, and pharmacologic management with duloxetine, have experienced a resurgence of interest over the past several years.
Surgery for urinary incontinence results in support to the urethrovesical junction, arrest of downward descent with straining, and increased pressure transmission to the urethra.
The paravaginal defect repair, by virtue of its close reapproximation to normal anatomy, may restore the extrinsic continence mechanism to a degree somewhat comparable to that of the normal female.
The Marshall-Marchetti-Krantz procedure is effective in patients with low urethral pressure and hypermobility of the urethra.
Central to the Burch procedure is the concept of not overelevating the vaginal wall at the time of retropubic urethropexy. Several prospective, randomized studies comparing Burch urethropexy with anterior colporrhaphy have shown cure rates to be consistently higher with the Burch procedure.
There is a growing trend in the United States toward use of the pubovaginal sling as the primary incontinence procedure. Studies comparing the pubovaginal sling with open urethropexy have shown similar short-term cure rates; long-term data are too few to reliably estimate outcomes.
References
- 1.Kegel AH. Progressive resistance exercise in the functional restoration of the perineal muscle. Am J Obstet Gynecol. 1948;56:238–249. doi: 10.1016/0002-9378(48)90266-x. [DOI] [PubMed] [Google Scholar]
- 2.Burgio KL, Goode PS, Locher JL, et al. Behavioral training with and without biofeedback in the treatment of urge incontinence in older women: a randomized controlled trial. JAMA. 2002;288:2293–2299. doi: 10.1001/jama.288.18.2293. [DOI] [PubMed] [Google Scholar]
- 3.Mouritsen L, Frimodt-Moller C, Moller M. Long-term effect of pelvic floor exercises on female urinary incontinence. Br J Urol. 1991;144:99–101. doi: 10.1111/j.1464-410x.1991.tb15252.x. [DOI] [PubMed] [Google Scholar]
- 4.Burgio KL, Robinson JC, Engel BT. The role of biofeedback in Kegel exercise training for stress urinary incontinence. Am J Obstet Gynecol. 1986;154:58–64. doi: 10.1016/0002-9378(86)90393-5. [DOI] [PubMed] [Google Scholar]
- 5.Morkved S, Bo K, Fjoroft T. Effect of adding biofeedback to pelvic floor muscle training to treat urodynamic stress incontinence. Obstet Gynecol. 2002;100:730–739. doi: 10.1016/s0029-7844(02)02160-9. [DOI] [PubMed] [Google Scholar]
- 6.Wyman JF, Fantl JA, McClish DK, Bump RC. Comparative efficacy of behavioral interventions in the management of female urinary incontinence. Am J Obstet Gynecol. 1998;179:999–1007. doi: 10.1016/s0002-9378(98)70206-6. [DOI] [PubMed] [Google Scholar]
- 7.Hay-Smith EJ, Bo K, Berghmans LC, et al. The Cochrane Library. 1. Chichester, UK: John Wiley & Sons, Ltd; 2003. Pelvic floor muscle training for urinary incontinence in women (Cochrane Review) [Google Scholar]
- 8.Roe B, Williams K, Palmer M. The Cochrane Library. 1. Chichester, UK: John Wiley & Sons, Ltd; 2003. Bladder training for urinary incontinence in adults (Cochrane Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bent AE, Sand PK, Ostergard DR, Brubaker LT. Transvaginal electrical stimulation in the treatment of genuine stress incontinence in detrusor instability. Int J Urogynecol. 1993;4:9–13. [Google Scholar]
- 10.Bo K, Talseth T. Change in urethral pressure during voluntary pelvic floor muscle contraction and vaginal electrical stimulation. Int Urogynecol J Pelvic Floor Dysfunct. 1997;8:3–7. doi: 10.1007/BF01920286. [DOI] [PubMed] [Google Scholar]
- 11.Bo K, Maanum M. Does vaginal electrical stimulation cause pelvic floor muscle contraction? A pilot study. Scan J Urol Nephrol. 1996;179:39–45. [PubMed] [Google Scholar]
- 12.Norton PA, Zinner NR, Yalcin I, Bump RC. Duloxetine vs placebo in the treatment of stress urinary incontinence. Am J Obstet Gynecol. 2002;187:40–48. doi: 10.1067/mob.2002.124840. [DOI] [PubMed] [Google Scholar]
- 13.Dmochowski RR, Miklos JR, Norton PA, et al. Duloxetine versus placebo for the treatment of North American women with stress urinary incontinence. J Urol. 2003;170:1259–1263. doi: 10.1097/01.ju.0000080708.87092.cc. [DOI] [PubMed] [Google Scholar]
- 14.Rosenzweig BA, Bhatia NN, Nelson AL. Dynamic urethral pressure profilometry, pressure transmission ratio: What do the numbers really mean? Obstet Gynecol. 1991;77:586–590. [PubMed] [Google Scholar]
- 15.Hilton P, Stanton SL. Urethral pressure measurement by microtransducer: the results in symptom-free women and in those with genuine stress incontinence. Br J Obstet Gynaecol. 1983;90:919–933. doi: 10.1111/j.1471-0528.1983.tb06764.x. [DOI] [PubMed] [Google Scholar]
- 16.Shull R, Baden W. A six-year experience with paravaginal defect repair for stress urinary incontinence. Am J Obstet Gynecol. 1989;160:1432–1440. doi: 10.1016/0002-9378(89)90867-3. [DOI] [PubMed] [Google Scholar]
- 17.Colombo M, Milani R, Vitobello D, Maggioni A. A randomized comparison of Burch colposuspension and abdominal paravaginal defect repair for female stress urinary incontinence. Am J Obset Gynecol. 1996;175:78–84. doi: 10.1016/s0002-9378(96)70254-5. [DOI] [PubMed] [Google Scholar]
- 18.Miklos J, Saye W. A randomized comparision of Burch colposuspension and abdominal paravaginal defect repair [letter] Am J Obstet Gynecol. 1997;176:255–256. doi: 10.1016/s0002-9378(97)80047-6. [DOI] [PubMed] [Google Scholar]
- 19.Webster GD, Kreder KJ. Voiding dysfunction following cystourethropexy. J Urol. 1990;144:670–673. doi: 10.1016/s0022-5347(17)39550-2. [DOI] [PubMed] [Google Scholar]
- 20.Mainprize TC, Drutz HP. The Marshall-Marchetti-Krantz procedure: a critical review. Obstet Gynecol Surv. 1988;43:724–729. doi: 10.1097/00006254-198812000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Lee RA, Symmonds RE, Goldstein RA. Surgical complications and results of modified Marshall-Marchetti-Krantz procedure for urinary incontinence. Am J Obstet Gynecol. 1979;53:447–450. [PubMed] [Google Scholar]
- 22.Colombo M, Scalambrino S, Maggioni A, Miliani R. Burch colposuspension versus modified Marshall-Marchetti-Krantz urethropexy for primary genuine stress urinary incontinence: a prospective, randomized trial. Am J Obstet Gynecol. 1994;171:1573–1579. doi: 10.1016/0002-9378(94)90404-9. [DOI] [PubMed] [Google Scholar]
- 23.Quadri G, Magatti F, Belloni C, et al. Marshall-Marchetti-Krantz urethropexy and Burch colposuspension for stress urinary incontinence in women with low pressure and hypermobility of the urethra: early results of a prospective randomized clinical trial. Am J Obstet Gynecol. 1999;181:12–18. doi: 10.1016/s0002-9378(99)70428-x. [DOI] [PubMed] [Google Scholar]
- 24.Kammerer-Doak DN, Cornella JL, Magrina JF, et al. Osteitis pubis after Marshall-Marchetti-Krantz urethropexy: a pubic osteomyelitis. Am J Obstet Gynecol. 1998;179:586–590. doi: 10.1016/s0002-9378(98)70049-3. [DOI] [PubMed] [Google Scholar]
- 25.Tanagho EA. Colpocystourethropexy: the way we do it. J Urol. 1976;116:751–753. doi: 10.1016/s0022-5347(17)58997-1. [DOI] [PubMed] [Google Scholar]
- 26.DeLancey JOL. Structural support of the urethra as it relates to stress urinary incontinence: the hammock hypothesis. Am J Obstet Gynecol. 1994;170:1713–1720. doi: 10.1016/s0002-9378(94)70346-9. [DOI] [PubMed] [Google Scholar]
- 27.Herbertsson G, Iosif GS. Surgical results and urodynamic studies ten years after retropubic urethropexy. Acta Obstet Gynecol Scand. 1993;72:299–301. doi: 10.3109/00016349309068041. [DOI] [PubMed] [Google Scholar]
- 28.Feyereisl J, Dreher E, Haenggi W, et al. Long-term results after Burch colposuspension. Am J Obstet Gynecol. 1994;1771:647–652. doi: 10.1016/0002-9378(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 29.Alcalay M, Monga A, Stanton SL. Burch colposuspension: a 10–20 year follow up. Br J Obstet Gynaecol. 1995;102:740–745. doi: 10.1111/j.1471-0528.1995.tb11434.x. [DOI] [PubMed] [Google Scholar]
- 30.Langer R, Lipshitz Y, Halperin R, et al. Long-term (10–15 years) follow-up after Burch colposuspension for urinary stress incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12:323–327. doi: 10.1007/s001920170034. [DOI] [PubMed] [Google Scholar]
- 31.Bergman A, Elia G. Three surgical procedures for genuine stress incontinence: five-year follow-up of a prospective randomized study. Am J Obstet Gynecol. 1995;173:66–71. doi: 10.1016/0002-9378(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 32.Kammerer-Doak DN, Dorin MH, Rogers RG, Cousin MO. A randomized trial of Burch urethropexy and anterior colporrhaphy for stress urinary incontinence. Obstet Gynecol. 1999;93:75–78. doi: 10.1016/s0029-7844(98)00360-3. [DOI] [PubMed] [Google Scholar]
- 33.Liapis AE, Asimiadis V, Loghis CD, et al. A randomized prospective study of three operative methods for genuine stress incontinence. J Gynecol Surg. 1996;12:7–13. [Google Scholar]
- 34.Vancaillie TG, Schuessler W. Laparoscopic bladderneck suspension. J Laparoendosc Surg. 1991;1:169–173. doi: 10.1089/lps.1991.1.169. [DOI] [PubMed] [Google Scholar]
- 35.Summitt RL, Lucente V, Karram MM, et al. Randomized comparison of laparoscopic and transabdominal Burch urethropexy for the treatment of genuine stress incontinence. Obstet Gynecol. 2000;95(suppl 1):S2. [Google Scholar]
- 36.Fatthy H, El Hoa M, Samaha I, Abdallah K. Modified Burch colposuspension: laparoscopy versus laparotomy. J Am Assoc Gynecol Laparosc. 2001;8:99–106. doi: 10.1016/s1074-3804(05)60557-9. [DOI] [PubMed] [Google Scholar]
- 37.Burton G. A three-year randomized urodynamic study comparing open and laparoscopic colposuspension. Neurourol Urodyn. 1993;16:353–354. [Google Scholar]
- 38.Su TH, Wang KG, Hsu CY, et al. Prospective comparison of laparoscopic and traditional colposuspension in the treatment of genuine stress incontinence. Acta Obstet Gynecol. 1997;76:576–582. doi: 10.3109/00016349709024588. [DOI] [PubMed] [Google Scholar]
- 39.Speight SE, Moore RD, Miklos JR. Frequency of lower urinary tract injury at laparoscopic Burch and paravaginal repair. J Am Assoc Gynecol Laparosc. 2000;7:515–518. doi: 10.1016/s1074-3804(05)60365-9. [DOI] [PubMed] [Google Scholar]
- 40.Walter AJ, Morse AN, Hammer RH, et al. Laparoscopic versus open Burch retropubic urethropexy: comparison of morbidity and costs when performed with concurrent vaginal prolapse repairs. Am J Obstet Gynecol. 2002;186:723–728. doi: 10.1067/mob.2002.121893. [DOI] [PubMed] [Google Scholar]
- 41.Kholi N, Jacobs PA, Sze EHM, et al. Open compared with laparoscopic approach to Burch colposuspension: a cost analysis. Obstet Gynecol. 1997;90:411–415. doi: 10.1016/s0029-7844(97)00267-6. [DOI] [PubMed] [Google Scholar]
- 42.Persson J, Wolner-Hanssen P. Laparoscopic Burch colposuspension for stress urinary incontinence: a randomized comparison of one to two sutures on each side of the urethra. Obstet Gynecol. 2000;95:151–155. doi: 10.1016/s0029-7844(99)00529-3. [DOI] [PubMed] [Google Scholar]
- 43.Ulmsten U, Henriksson L, Johnson P, Varhos G. An ambulatory surgical procedure under local anesthesia for treatment of female urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1996;7:81–86. doi: 10.1007/BF01902378. [DOI] [PubMed] [Google Scholar]
- 44.Bezerra CA, Bruschini H, Cody DJ. The Cochrane Library. 1. Chichester, UK: John Wiley & Sons, Ltd; 2004. Suburethral sling operations for urinary incontinence in women (Cochrane Review) [Google Scholar]
- 45.Nilsson CG, Kuuva N, Falconer C, et al. Long-term results of the tension-free vaginal tape (TVT) procedure for surgical treatment of female stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12(suppl 2):S5–S8. doi: 10.1007/s001920170003. [DOI] [PubMed] [Google Scholar]
- 46.Rezapour M, Falconer C, Ulmsten U. Tension-free vaginal tape (TVT) in stress incontinent women with intrinsic sphincter deficiency (ISD)—a long-term follow-up. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12(suppl 2):S12–S14. doi: 10.1007/s001920170005. [DOI] [PubMed] [Google Scholar]
- 47.Ward KL, Hilton P. A prospective multicenter randomized trial of tension-free vaginal tape and colposuspension for primary urodynamic stress incontinence: two-year follow-up. Am J Obstet Gynecol. 2004;190:324–331. doi: 10.1016/j.ajog.2003.07.029. [DOI] [PubMed] [Google Scholar]