Abstract
A new transdermal preparation, Testim® 1% testosterone gel, has recently become available for normalization of serum testosterone in hypogonadal men. In short-term studies, it has been shown to reverse the clinical signs and symptoms of low testosterone and to be well tolerated with less application-site irritation than with testosterone patches. In 2 long-term studies with Testim, the predose early morning serum testosterone (T), dihydrotestosterone (DHT), and free testosterone (FT) levels were assessed. Serum T, DHT, and FT were all maintained in the normal range for up to 12 months. In these studies, involving a total of 371 hypogonadal men, evaluation by means of dual-energy x-ray absorptiometry revealed a significant increase from baseline in bone mineral density. A significant improvement was also observed in body composition (increased lean body mass, decreased fat mass, and decreased percent fat). In addition, significant improvements in mood and sexual function were maintained for up to 12 months of treatment. The parameters measured included sexual performance, sexual motivation, sexual desire, and occurrence of spontaneous erections. These data from the 2 long-term studies support the results of the 90-day studies with Testim showing that the gel significantly improves the signs and symptoms associated with low testosterone compared with placebo. The data also support the conclusion that these improvements are maintained for up to 1 year of additional treatment.
Key words: Testosterone gel, Hypogonadism, Mood, Sexual function, Bone mineral density, Body composition
It has been demonstrated that as men age, serum testosterone (T) concentration falls approximately 1% each year.1–4 This gradual decline results in 1 in 5 men having a serum T level below the normal range after the age of 50 years.2 Abnormally low serum T has been associated with a clinical syndrome that includes decreased sexual function, loss of muscle mass, increased fat mass (FM), depressed mood, and decreased cognitive function, as well as loss of bone mineral density (BMD).4–6
In several studies, low testosterone has been identified as a risk factor for osteoporotic fractures. Approximately 50% of men who present with hip fractures and 20% of men with vertebral fractures have been shown to be hypogonadal.7–11 Osteoporosis in men is a significant health problem as hip fractures are associated with a high mortality.12
Testim®, a new testosterone gel for normalization of serum T, has recently become available. In short-term studies up to 90 days, Testim has proved to be well tolerated and to produce significant improvement in sexual function and mood as well as increased BMD and improved lean body mass (LBM), FM, and percentage of fat (%F).13,14 Here we report the results observed with long-term treatment with Testim 1% testosterone gel in an aging hypogonadal population.
Patients and Methods
Patients
Hypogonadal men who had previously entered and completed 2 short-term double-blind studies with Testim were enrolled in 2 open-label, multicenter, long-term studies (39 centers in the United States and 10 centers in Denmark, Sweden, and The Netherlands). Male patients between the ages of 21 and 81 years were included. These patients had a baseline original morning serum T level (before double-blind therapy) of 300 ng/dL or less plus 1 or more symptoms of low testosterone. Except for hypogonadism, the patients were in generally good health, as evidenced by medical history and based on the results of the final physical examination and clinical laboratory and urine analysis assessments performed at the conclusion of the antecedent short-term study.
Patients were excluded from the study if they had any generalized skin irritation or disease that might have interfered with androgen absorption or metabolism.
Study Drug
Testim 1% testosterone gel was supplied by Auxilium Pharmaceuticals, Inc. (Norristown, PA). Testim 1% testosterone gel is supplied in foil-lined tubes containing 50 mg testosterone. A dosage of 100 mg requires 2 tubes of Testim to be applied to the skin.
All Testim 1% testosterone gel treatments were applied in the morning, with repeated applications occurring at the same time of day for the duration of the study. Each day patients applied the contents of 1 or 2 tubes, depending on the dose, to the shoulders and upper arms.
Study Design
Hypogonadal men who had previously entered and completed 2 short-term, double-blind studies and had been randomized to receive Testim 50 mg or 100 mg, testosterone patch, or placebo for up to 90 days were treated with Testim in 2 open-label, multi-center, extension studies.
All patients initially received Testim 50 mg testosterone gel daily. At subsequent visits beginning at 2 weeks after enrollment, titration of Testim was done based on serum T levels and clinical symptoms. The patient’s dose could be adjusted from 50 mg to 100 mg or from 100 mg to 50 mg at subsequent visits.
Patients were to remain in the extension studies for 12 months and were assessed at 2 weeks, monthly for the first 3 months, and every 3 months thereafter until completion. Each study visit consisted of a predose morning serum T level measurement, skin irritation examination, and clinical laboratory analyses. In addition, at month 6 and month 12, a body composition and BMD test of the lumbar spine (L1–L4) using dual-energy x-ray absorptiometry (DEXA) was performed. All body composition and BMD measurements were centrally monitored and analyzed by Synarc, Inc., San Francisco, CA. At months 3, 6, and 12, prostate-specific antigen levels (PSA) were measured. Clinical laboratory assessments and serum T, dihydrotestosterone (DHT), and free testosterone (FT) measurements were done at a central laboratory. Serum T and DHT levels were measured using validated radioimmunoassay kits (T and FT kits from Diagnostic Products, Los Angeles, CA, and DHT kits from Diagnostic Systems Laboratories, Inc., Webster, TX). Adverse events were recorded throughout the study.
Sexual function and mood were assessed via a validated questionnaire. The sexual function parameters elicited by the questionnaire were performance, motivation, spontaneous erections (day and/or night), desire, enjoyment (with and without a partner), satisfaction with erection duration, and percentage of full erection experienced. The men also provided ratings on positive mood items (alert, full of energy, friendly, well/good) and negative mood items (angry, irritable, sad or depressed, tired, nervous). In the United States, patients were to complete questionnaires using a telephone interactive voice response system (IVRS), concerning mood and sexual function for 7 days before each visit. Elsewhere, patients manually completed the daily questionnaires but did not use IVRS.
Methods
Descriptive statistics were used to summarize predose morning serum T, DHT, and FT levels; BMD; body composition; sexual function parameters; and mood parameters at baseline (before double-blind therapy) and at each extension study visit.
Repeated-measure analysis of variance with a factor for visit was used to compare whether the percent change from baseline for BMD was different from zero and whether the baseline values for T, DHT, FT, sexual function parameters, and body composition were different from each of the extension study visits. For each comparison, a significance level of α = 0.05 (2-sided) was considered significant. SAS version 9.1 was used for all statistical analyses. All data in figures are means ± SE.
Results
Patients
A total of 371 patients were recruited; of these patients, 76 received Testim for less than 6 months, 38 received Testim for between 6 and 9 months, and 257 patients received Testim for more than 9 months. Baseline demographics are presented in Table 1. The average age was 58.5 years, the average screening morning serum T level was 234 ng/dL, 96% were Caucasian, and 90% were hypogonadal because of aging or were normogonadotropic at baseline.
Table 1.
Study Population Demographics
| Demographics | (N = 371) |
|---|---|
| Age (y) | 58.5 ± 10.0 |
| Testosterone (ng/dL)* | 234 ± 59.2 |
| Race | |
| Caucasian | 96.0% |
| Black | 1.4% |
| Hispanic | 2.2% |
| Other | 0.5% |
| Cause of Hypogonadism† | |
| Primary (n = 22) | 5.9% |
| Secondary (n = 348) | 93.8% |
| Aging | 65.8%‡ |
| Normogonadotrophic | 24.3%‡ |
8 A.M. serum testosterone concentration at screening exam.
One patient had missing cause of hypogonadism.
Distribution bu cause is shown only if it occured in 4% of patients.
Data presented as mean ± SD.
Pharmacokinetics
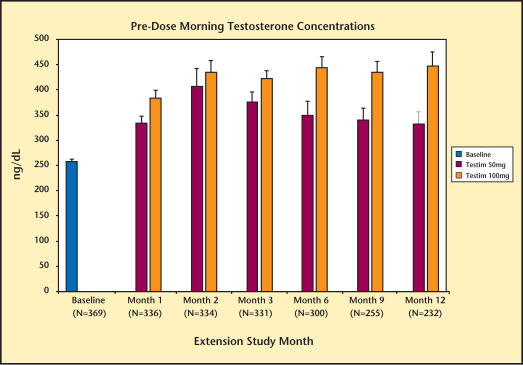
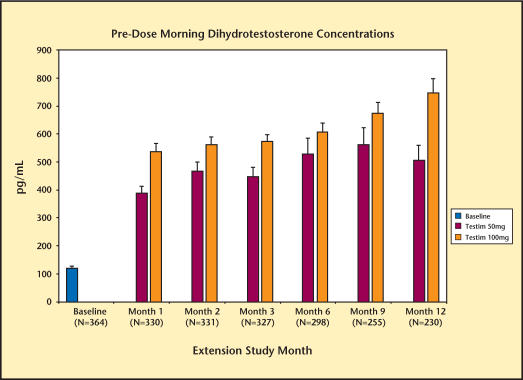
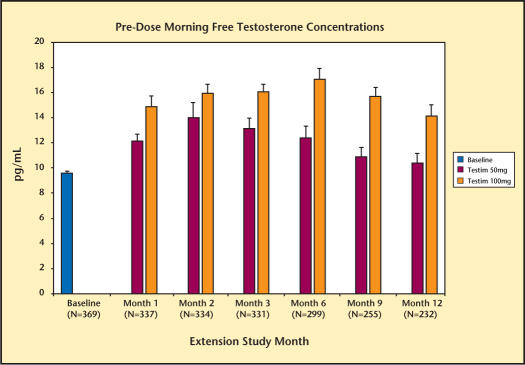
Serum T, DHT, and FT levels are summarized in Figures 1–3. By the end of the first month, the mean morning predose serum T levels were in the normal range, regardless of the treatment received by the patient in the previous short-term, 90-day double-blind study. Serum T levels were maintained in the normal range for up to 12 months of treatment. Consistent with the changes in total testosterone level, Testim increased serum levels of DHT and FT. These increases were also maintained for up to 12 months.
Figure 1.
Levels of morning total testosterone in study participants over 12 months.
Figure 3.
Levels of morning dihydrotestosterone in study patients over 12 months.
BMD
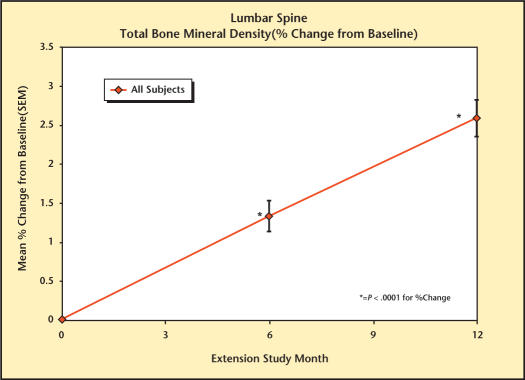
At baseline, mean BMD was 1.1747 g/cm.2 Long-term treatment with Testim resulted in a significant (P < 0.001) percent change increase from baseline in BMD of the lumbar spine as measured by DEXA scan, reaching 2.58% at month 12 (Figure 4). Month 12 data showed a greater increase than that found at month 6 (1.33%), indicating an ongoing repletion of BMD with continuing treatment with Testim.
Figure 4.
Percent change in total bone mineral density from baseline in study participants.
Body Composition
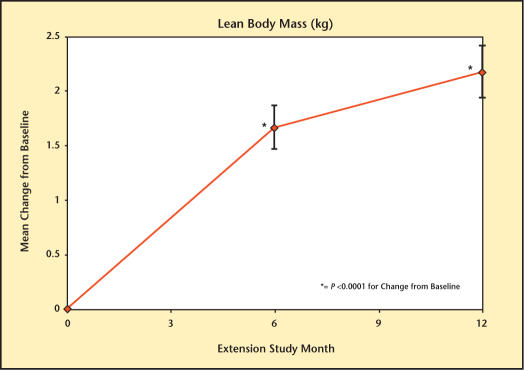
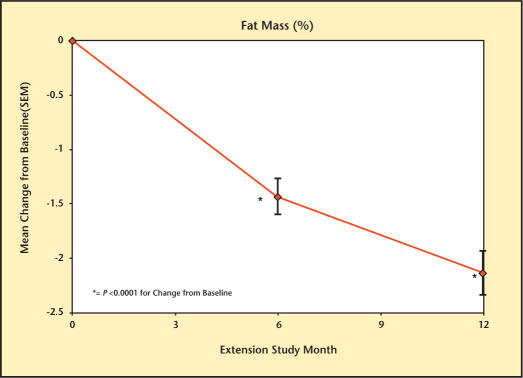
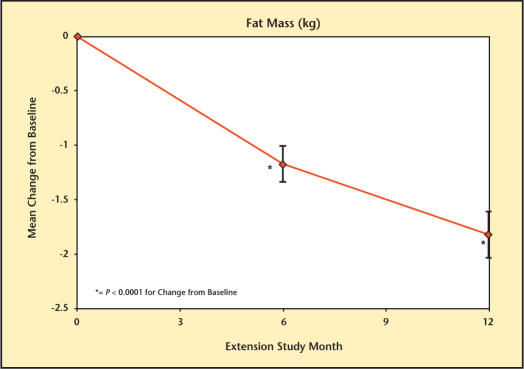
At baseline, LBM was 61.9 kg, FM was 27.8 kg, and %F was 29.5%. Testim treatment resulted in significant changes in body composition at months 6 and 12 (Figures 5–7). These changes included significant increases in LBM (1.7 kg [month 6] and 2.2 kg [month 12]), significant decreases in FM (1.2 kg [month 6] and 1.8 kg [month 12]), and %F (1.4% [month 6], and 2.1% [month 12]) but with no significant change in total body mass (data not shown).
Figure 5.
Change in lean body mass from baseline in study participants.
Figure 7.
Percentage change in fat mass from baseline in study participants.
Sexual Function
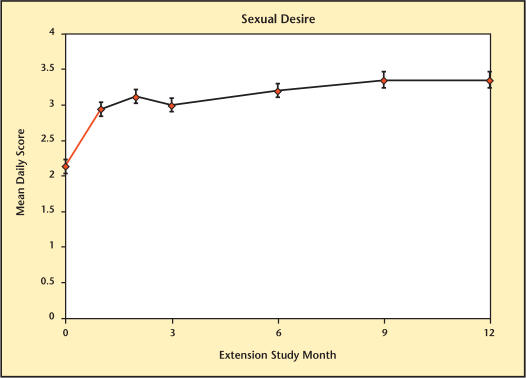
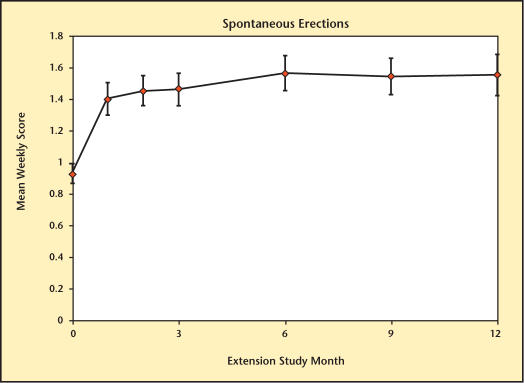
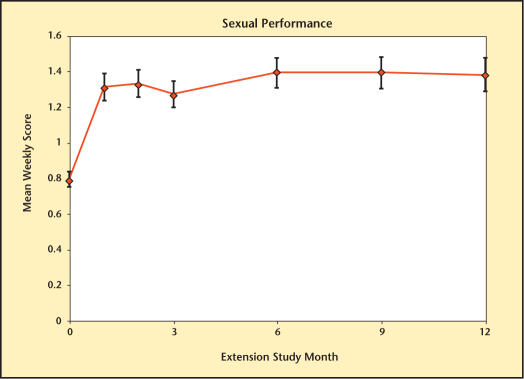
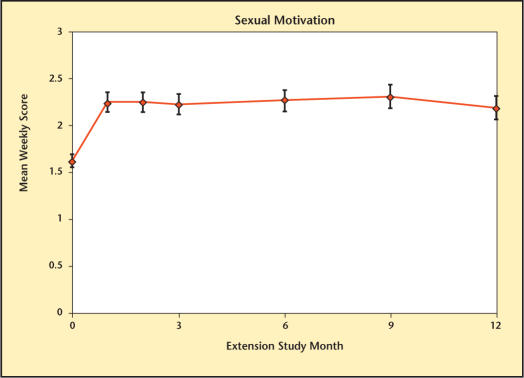
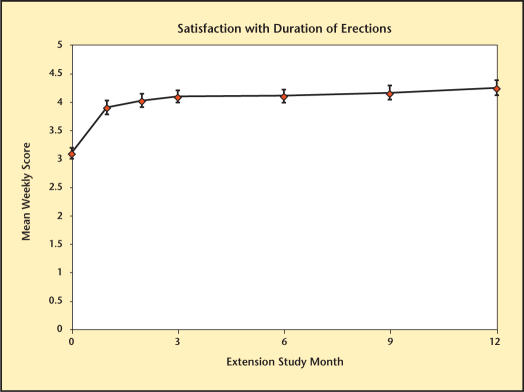
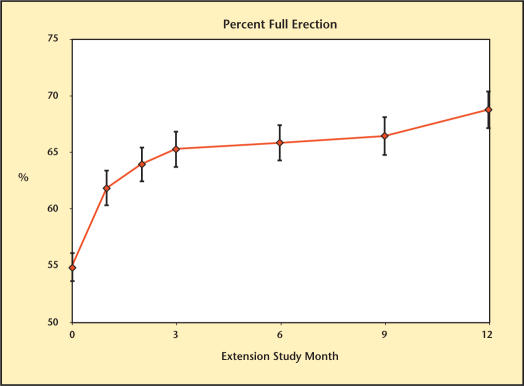
The different domains of sexual function are summarized in Figures 8–13. Desire, performance, motivation, satisfaction with duration of erection, percent of full erection, and spontaneous erections showed significant improvement (P < .001) from baseline at all visits during the extension study. Satisfaction with sexual activity, with or without a partner, also showed a significant improvement from baseline. The data support the conclusion that the significant improvements in sexual function observed with Testim in the short-term studies, including those versus placebo, were maintained for up to an additional year of treatment.
Figure 8.
Sexual desire over time in study participants.
Figure 13.
Spontaneous erections over time in study participants.
Mood
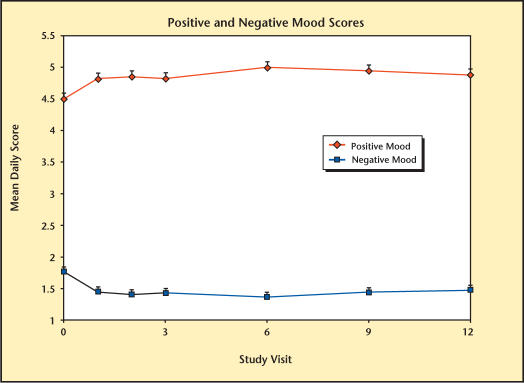
Treatment with Testim was shown to improve mood at month 1 compared with baseline (Figure 14). Scores were increased for positive mood parameters and decreased for negative mood parameters. These improvements were maintained through month 12. The results observed by month 1 of the extension studies were of the same magnitude as seen at the end of the short-term studies.
Figure 14.
Mood scores over time during therapy with Testim®.
Safety
Adverse Events
Forty patients experienced an adverse event that resulted in study drug discontinuation. The most frequent adverse events leading to discontinuation were increased PSA level (2.4%), increased hematology parameters (2.4%), and application site reactions (1.1%).
Six patients reported 8 serious adverse events that were possibly related to the study drug; there was no pattern among the adverse events. Specific adverse events that were possibly or probably related to the study drug and reported by more than 1% of Testim patients are presented in Table 2. The most frequently reported related adverse events were PSA level increase (5.7%), hematocrit increase (4.9%), hemoglobin increase (4.3%), and application site erythema (4.0%).
Table 2.
Adverse Events Judged Related to Study Medication
| Event | Total (N = 371) |
|---|---|
| Application site erythema | 4.0% |
| Application site rash | 1.9% |
| Increase in PSA level | 5.7% |
| Increase in hematocrit | 4.9% |
| Increase in hemoglobin level | 4.3% |
| Increase in red blood cell count | 1.6% |
| Benign prostatic hypertrophy | 1.6% |
| Acne | 1.6% |
| Hypertension aggravated | 1.6% |
| Decrease in libido | l.1%. |
PSA, prostate-specific antigen.
Hematology
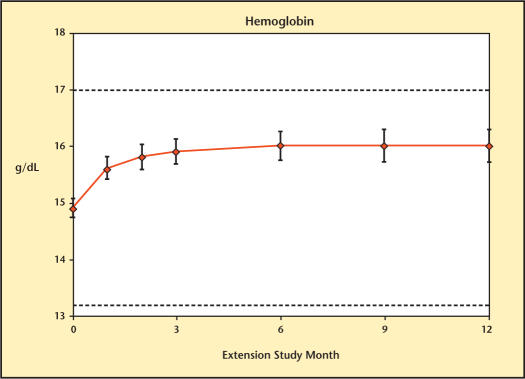
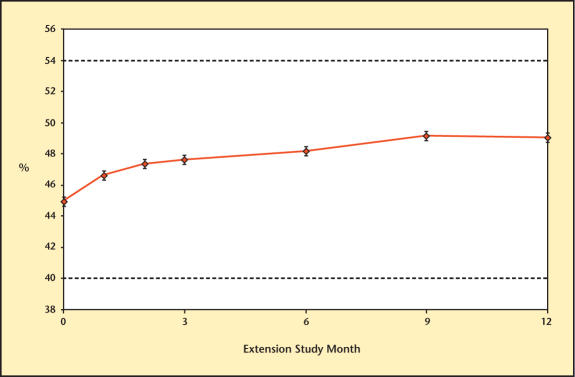
Mean hemoglobin level and hematocrit are presented in Figures 15 and 16. Mean values remained within normal limits for the entire extension study.
Figure 15.
Hemoglobin levels over time in study participants during 12-month treatment.
Figure 16.
Hematocrit levels over time in study participants receiving therapy with Testim®.
Prostate
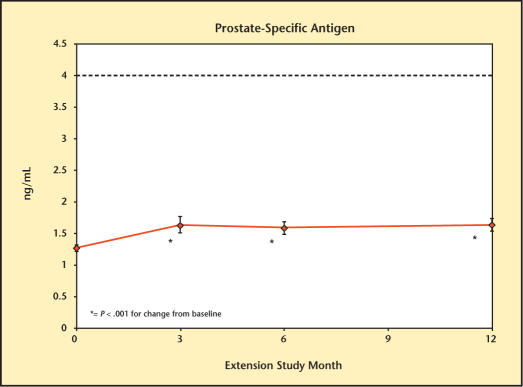
Mean PSA values are presented in Figure 17. Baseline PSA concentrations were 1.26 ± 0.05 ng/mL. The changes from baseline were significant at each of the visits (0.39 ng/mL [month 3], 0.37 ng/mL [month 6], and 0.45 ng/mL [month 12]). Three patients received a diagnosis of prostate cancer. All 3 had positive prostate biopsies; 2 biopsies were positive for adenocarcinoma. One patient underwent a radical prostatectomy. Further analysis of the patients with a diagnosis of prostate cancer reveals that 1 patient, aged 53 years had an entrance PSA level of 1.3 ng/mL and an exit PSA level of 2.9 ng/mL. This patient discontinued treatment at day 37 for an elevated hematocrit. The diagnosis of prostate cancer was made over 60 days post-treatment by biopsy performed because of asymmetrical prostate.
Figure 17.
Serum prostate-specific antigen level during 12-month extension study treatment.
The second patient with a prostate cancer diagnosis was 63 years old. He had an entrance PSA level of 2.4 ng/mL and an exit PSA level of 3.1 ng/mL at the 1-year completion of study. He had a biopsy because of rising PSA values. The third patient was a 73-year-old who underwent biopsy on day 273 of the study because his PSA level rose from 1.4 ng/mL on entrance into the study to 4.3 ng/mL at 6 months. However, the last PSA value obtained at completion of the study was 1.9 ng/mL.
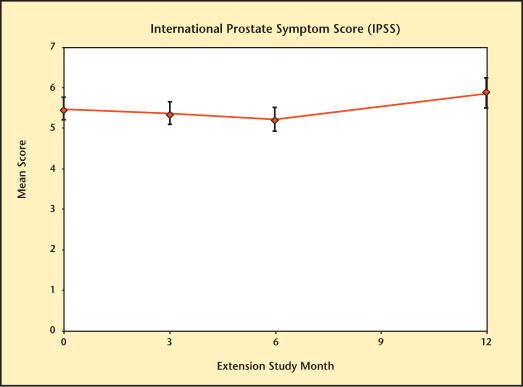
International Prostate Symptom Score (IPSS)
Mean IPSSs are presented in Figure 18. Values exhibited no clinically significant rise from baseline through the entire extension study.
Figure 18.
International Prostate Symptom Score over time in subjects receiving testosterone therapy with Testim®.
Discussion
In these 2 long-term studies, treatment of hypogonadal men with Testim resulted in normalization of mean serum T levels by the end of the first month of treatment, with T levels remaining in the normal range throughout the 12 months of treatment. Consistent with the changes in total T, Testim increased serum levels of DHT and FT, with these increases also maintained throughout the 12 months of treatment.
Ongoing repletion of BMD was observed over time with prolonged treatment with Testim. After 12 months of treatment, a significant percentage change increase from baseline in BMD was seen, greater than that observed at 6 months. In addition, significant improvements in body composition parameters, including LBM, FM, and %F, were noted at months 6 and 12 of treatment.
The significant improvements in mood and sexual function parameters observed with short-term treatment, including sexual performance, sexual motivation, sexual desire, and occurrence of spontaneous erections, were maintained for up to 12 months with Testim treatment.
The safety results from the 2 long-term studies were consistent with those reported from short-term studies with Testim and are comparable to changes observed in other studies with T treatment. In addition, the laboratory monitoring confirmed the known class effects of T on erythropoiesis and PSA values. Of note, only 1 of the 3 patients with prostate cancer had a PSA value above 4.3 ng/mL. The occurrence of 3 prostate cancers in this study population of aging males (< 1%) is not unexpected.
The beneficial effects of testosterone with regard to sexual function, mood, muscle strength and body composition are maintained through 12 months of treatment, suggesting that tolerance to testosterone effects does not occur.
It can be concluded from these data that Testim can be given safely to patients with age-related and other types of hypogonadism for prolonged periods of time, producing serum T levels that remain in the normal range. It has also been shown that the beneficial effects on sexual function and mood are maintained over time and that improvement in BMD and body composition increases with continued administration of Testim.
Main Points.
Abnormally low serum testosterone is associated with decreased sexual function, loss of muscle mass, increased fat mass, depressed mood, decreased cognitive function, and loss of bone mineral density (BMD).
The study included hypogonadal men who had previously been involved in 2 short-term, double-blind studies comparing Testim® gel, testosterone patch, or placebo for up to 90 days; participants received Testim in 2 open-label, multi-center, extension studies.
Testim resulted in increases in total testosterone, serum levels of dihydrotestosterone, and free testosterone, as well as in BMD and lean body mass.
Results of the longer-term study of Testim showed benefits in sexual function, mood, and body composition, which were maintained for 12 months, with a safety profile similar to that seen with short-term therapy and no rise in prostate symptom score.
Figure 2.
Levels of morning free testosterone over 12 months during Testim® extension study.
Figure 6.
Change in fat mass from baseline in study participants.
Figure 9.
Sexual performance over time in study participants.
Figure 10.
Sexual motivation over time in study participants.
Figure 11.
Satisfaction with duration of erections over time in study participants.
Figure 12.
Percentage of full erection over time in study participants.
References
- 1.Gray A, Berlin JA, McKinlay JB, Longcope C. An examination of research design effects on the association of testosterone and male aging: results of a meta-analysis. J Clin Epidemiol. 1991;44:671–684. doi: 10.1016/0895-4356(91)90028-8. [DOI] [PubMed] [Google Scholar]
- 2.Harman SM, Metter EJ, Tobin JD, et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 3.Morley JE, Kaiser FE, Perry HM, 3rd, et al. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism. 1997;46:410–413. doi: 10.1016/s0026-0495(97)90057-3. [DOI] [PubMed] [Google Scholar]
- 4.Tenover JL. Male hormone replacement therapy including “andropause.”. Endocrinol Metab Clin North Am. 1998;27:969–987. doi: 10.1016/s0889-8529(05)70050-5. [DOI] [PubMed] [Google Scholar]
- 5.Petak SM. American association of clinical endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hypogonadism in adult male patients-2002 update. Endocr Pract. 2002;6:439–456. [PubMed] [Google Scholar]
- 6.Matsumoto AM. Andropause: clinical implications of the decline in serum testosterone levels with aging in men. J Gerontol A Biol Sci Med Sci. 2002;57:M76–M99. doi: 10.1093/gerona/57.2.m76. [DOI] [PubMed] [Google Scholar]
- 7.Francis RM. Pathogenesis and management of osteoporosis in men. J of Endocrinology Ltd. 1996:23–29. [Google Scholar]
- 8.Francis RM. The effects of testosterone on osteoporosis in men. Clin Endocrinol (Oxf) 1999;50:411–414. doi: 10.1046/j.1365-2265.1999.00730.x. [DOI] [PubMed] [Google Scholar]
- 9.Jackson JA, Riggs MW, Spiekerman AM. Testosterone deficiency as a risk factor for hip fractures in men: a case-control study. Am J Med Sci. 1992;304:4–8. doi: 10.1097/00000441-199207000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Jackson JA, Waxman J, Spiekerman AM. Prostatic complications of testosterone replacement therapy. Arch Intern Med. 1989;149:2365–2366. [PubMed] [Google Scholar]
- 11.Szulc P, Claustrat B, Marchand F, Delmas PD. Increased risk of falls and increased bone resorption in elderly men with partial androgen deficiency: the MINOS Study. J Clin Endrocrinol Metab. 2003;88:5240–5247. doi: 10.1210/jc.2003-030200. [DOI] [PubMed] [Google Scholar]
- 12.Diamond TH, Thornley SW, Sekel R, Smerdely P. Hip fracture in elderly men: prognostic factors and outcomes. Med J Aust. 1997;167:412–415. doi: 10.5694/j.1326-5377.1997.tb126646.x. [DOI] [PubMed] [Google Scholar]
- 13.McNicholas TA, Dean JD, Mulder HA, et al. A novel testosterone gel formulation normalizes androgen levels in hypogonadal men, with improvements in body composition and sexual function. BJU Int. 2003;91:69–74. doi: 10.1046/j.1464-410x.2003.04016.x. [DOI] [PubMed] [Google Scholar]
- 14.Steidle C, Schwartz S, Jacoby K, et al. AA2500 testosterone gel normalizes androgen levels in aging males with improvements in body composition and sexual function. J Clin Endocrinol Metab. 2003;88:2673–2681. doi: 10.1210/jc.2002-021058. [DOI] [PubMed] [Google Scholar]