Abstract
Serum testosterone levels decline progressively as men age, with resulting pathophysiological changes. Because the onset of andropause is gradual and many of its symptoms mirror those associated with medications or disease states common in the elderly, a clinical diagnosis can be difficult to make. Additionally, because of a lack of established normal testosterone levels for different age groups, as well as confusion regarding what subset of testosterone to measure, simply testing testosterone levels is inadequate. Although clinical studies have shown testosterone supplementation to be safe, no long-term placebo-controlled trials have been performed. Among the possible side effects of testosterone replacement therapy is an increased risk of prostate cancer. While there is no evidence that supplemental testosterone will initiate prostate cancer or cause clinically significant progression of an established occult malignancy, the initial evaluation of patients prior to administration of testosterone requires screening for prostatic carcinoma. A number of formulations of testosterone are currently available; transdermal gels, although expensive, are the preferred modality because of their ease of administration.
Key words: Testosterone replacement, Andropause, Prostate-specific antigen, Treatment option
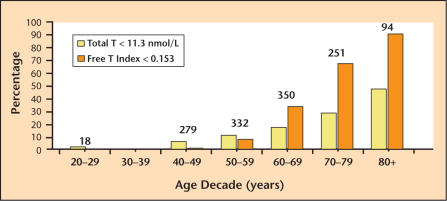
Hormone replacement therapy in women has long been the standard of care to obviate menopausal symptoms resulting from estrogen deficiency. It is increasingly widely recognized that similar, although nonclimacteric, decreases in serum testosterone with resulting symptoms occur in aging men. This testosterone reduction and associated symptom complex has been variously referred to as andropause, ADAM (androgen deficiency in the aging male), and PADAM (partial androgen deficiency in the aging male). It has been established that testosterone decreases by approximately 1% per year after age 30.1–3 As illustrated in Figure 1, which shows the prevalence of hypogonadism in men at different ages, approximately 20% of men in their 60s have biochemical evidence of androgen deficiency, increasing to 50% of men in the eighth decade of life.2
Figure 1.
The prevalence of hypogonadism in the aging male. Each bar indicates the percentage of men in each decade with total testosterone (T) < 11.3 nmol/L (325 ng/dL) (yellow bars) or free testosterone index (total T/sex hormone- binding globulin) < 0.153 (orange bars), both < 2.5th percentile. Numbers over each pair of bars represent the number of men studied in each decade. Adapted with permission from Harman SM et al.2
Unlike the female menopause, the male counterpart is a slowly progressive condition. In many ways the resulting symptoms are different from those experienced in the young hypogonadal male, because of the slow nature of testosterone decline in the elderly. This may render the clinical diagnosis of andropause difficult. In this overview I will describe the andropause syndrome and discuss the rationale for treatment, explore the risk/benefit ratio of therapy, and discuss the various treatment options currently available.
Biochemical and Clinical Characteristics of Andropause
Andropause has been perhaps best described by Morales and Lunenfeld4 as a “biochemical syndrome associated with advancing age and characterized by a deficiency in serum androgen levels with or without a decreased genomic sensitivity to androgens. It may result in significant alterations in the quality of life and adversely affect the function of multiple organ-systems.”5
The progressive decrease in serum testosterone with age has been documented in several investigations.1–3 Reduction in testosterone results in a number of physiologic changes. These include alteration in body composition (decrease in lean body mass, increase in fat mass),6,7 decrease in energy and muscle strength, decreased libido and erections,4,5 increased osteoporosis,8 mood changes, and reduction in cognitive function.9 Despite these observations, specific studies demonstrating the connection between reduction in testosterone and these physiologic events have found only a weak correlation. This is perhaps explained by the myriad of other disease states occurring in the aging male that can also give rise to these symptoms.
One of the limitations in the study of andropause is the lack of established normal testosterone levels for different age groups. It is readily apparent that, given the wide range of testosterone in so-called “normal” men, a single value may be appropriate for broad-based populations but irrelevant in the individual patient. This is particularly problematic given the fact that the level of serum testosterone required for well-being may be variable. If a man is accustomed to a serum testosterone level in the high-normal range, reduction to a lower level, even though still within the established normal range, may no longer be adequate for maintenance of well-being. Unfortunately, because we rarely measure testosterone in men prior to the point at which they are experiencing symptoms, clinicians are obliged to use established normal ranges. Another confounding factor is the fact that many of the symptoms of andropause can be caused by other comorbid disease processes or side effects from commonly utilized medications in this population.
Confusion also exists because of the subset of testosterone that is measured. The majority (up to 98%) of circulating testosterone is bound to serum proteins, primarily sex hormone- binding globulin and albumin, rendering only 1% to 2% of testosterone free in the circulation.10 All conventional total testosterone assays measure both free and bound testosterone. Measurement of the free testosterone level is difficult and generally requires a specialty laboratory. A significant factor for clinicians to recognize when measuring serum testosterone levels is that there is a circadian variation to these levels, with peak concentrations in the early morning.
The decline in testosterone with aging is related to decreases in both hypothalamic and testicular function. There is an age-related reduction in gonadotropin-releasing hormone, resulting in decreased production of luteinizing hormone (LH) by the pituitary gland. The number of testicular Leydig cells also decreases with age,11 resulting in reduction in testosterone production. Associated with the decline in serum testosterone is an increase in serum gonadotropin, follicle-stimulating hormone, and LH.1–3 This results from negative feedback on the hypothalamus. The action of androgens is by binding to the androgen receptor, which leads to activation and/or repression of tissue-specific factors. This results in changes in the transcription genes encoding a number of androgen-regulated proteins.12
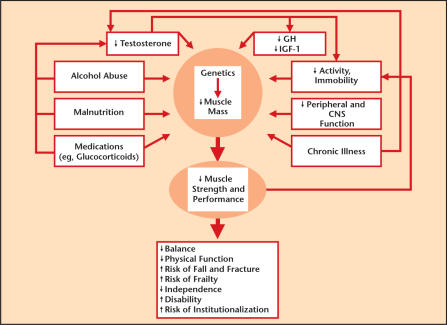
As noted, there are a number of physiologic changes that can occur with androgen deficiency in the aging male (Figure 2). These include decreased lean body mass and increased fat, reduction in muscle mass and resulting strength, decrease in bone mineral density, decreased body hair and skin thickness, diminished libido and sexual activity, reduced energy and sense of well being, irritability and depression, impaired quality of sleep, and deterioration in cognitive function.11
Figure 2.
Physiological changes from androgen deficiency in the aging male. GH, growth hormone; IGF-1, insulin-like growth factor-1; CNS, central nervous system. Adapted with permission from Matsumoto.11
With respect to sexual dysfunction the clinical manifestation of andropause may be confused with other causes of erectile dysfunction, such as those of a vascular or neurogenic origin. Absence of nocturnal erections in a patient without other markers of vascular disease may suggest andropause as a cause. Moreover, there can be confusing overlap with other conditions associated with sexual dysfunction, most notably depression. It should be noted that erectile dysfunction is not a prerequisite for the diagnosis of andropause, but certainly in the urologic practice it is the most likely cause to institute investigation.
The psychological and cognitive hints of andropause including loss of cognitive function, depression, and “loss of drive” may be identified with a careful history. Physical examination may demonstrate a decrease in lean body mass and changes in hair, skin, and fat distribution. Fractures, one complication of osteopenia and osteoporosis, may suggest andropause as well.
Indications for Treatment
The goal of testosterone therapy can be divided into 2 major areas: improvement in symptoms and restoration of alterations in normal physiology. Symptom improvement might include improved psychosexual function, physical activity, quality of life, and overall mood. Restoration of organ systems that have suffered deleterious effects because of a relative hypogonadal state include improvement of bone mineral density, body composition, strength, and cognitive function.
The degree of increase in testosterone level necessary for efficacy has not been established in older men. Due to individual variability with respect to sensitivity of the androgen receptor, as well as different target tissue sensitivity, there will likely be heterogeneity in the level that needs to be achieved in each individual patient. Tenover13 has suggested that symptoms involving mood and psychosexual function may require a lesser increase in testosterone than those involving bone density or muscle mass. Problematic in this regard is the fact that we monitor testosterone replacement by measuring serum levels of testosterone and other sex steroids and have no ability to determine the more valid measurement, androgen levels in the target tissue.
The patient who is considered a candidate for treatment of andropause because of biochemical evidence of low testosterone and some or all of the clinical symptoms noted above, must undergo careful evaluation and be informed of the potential risks and benefits before treatment. The absence of long-term placebo-controlled trials renders definitive evidence of the safety of supplemental androgens unknown. The potential risk factors noted below should be described to the patient.
Safety
A number of studies have been conducted in which testosterone has been administered to older men with andropause syndrome. Several different testosterone formulations have been utilized and the resulting serum testosterone levels achieved have generally been in the low-normal level. Most studies demonstrated beneficial effects of testosterone replacement in this population, including increase in lean body mass and associated decrease in fat mass, increased bone mineral density, increase in muscle strength and sexual function, improved generalized feelings of well-being, and improved cognitive function. Unfortunately, long-term trials have not been carried out. Long-term trials are needed to answer some important questions. For example, will testosterone therapy produce a reduction in pathologic fracture occurrences? In all studies reported, the administration of supplemental testosterone in these patients has been safe. In some men, primarily those living in high altitudes, there was an increase in erythrocytosis, but this was of little clinical consequence.
A number of side effects have been reported in connection with testosterone therapy, among them an increase in body weight related to fluid retention (Table 1). There is some circumstantial evidence that testosterone administration may have a negative effect on cardiovascular health due to changes in the lipid profile; however, this has not been established in prospective trials. To the contrary, there is some evidence to support improvement in cardiovascular health with phenomena such as improvement in the direct arterial vaso-dilatory effect, and prolongation of time until ischemia with exercise.14–16 Tenover demonstrated no deleterious change in cholesterol profiles and indeed improvement in total cholesterol in older men treated with testosterone.17
Table 1.
Potential Hormone-Related and Non-Hormone-Related Testosterone Adverse Effects of Therapy
| Hormone-Related | |
| Promotion of fluid retention | |
| Increase in cardiovascular disease risk | |
| Precipitation or worsening of sleep apnea | |
| Gynecomastia | |
| Polycythemia | |
| Fluctuations in mood | |
| Increased rate of development of benign or malignant prostate disease | |
| Non-Hormone-Related | |
| Preparation | Effect |
| Testosterone undecanoate | Gastrointestinal bloating and irritation |
| Injectable esters | Pain at sight of injection |
| Implantable pellets | Pain or infection at site; extravasation of pellet |
| Scrotal patch | Local site irritation |
| Nonscrotal patch | Local site skin irritation, sometimes significant |
| Transdermal gels | Occasional mild skin irritation |
Reprinted with permission from Tenover 13
Some investigators have demonstrated worsening of sleep apnea;18 however, this has been recently refuted.19 Breast tenderness or enlargement is a rare outcome of testosterone administration owing to conversion of testosterone to estradiol by the aromatase enzyme in fat. Increased hemoglobin and hematocrit levels have been widely reported17 in men treated with supplemental testosterone, but not to the level necessitating phlebotomy.
Prostate Cancer Risk
Of greatest concern when supplementing testosterone is an increase in prostatic disease, namely benign prostatic hyperplasia or the development of, or increased occurrence of, prostatic carcinoma. While there has been a slight increase in prostate-specific antigen (PSA) levels demonstrated in some studies,11,20 this has not been associated with a clear increase in prostate cancer occurrence. Even though there may be a small number of men in whom an increase in PSA will facilitate the diagnosis of an existing carcinoma, there is no evidence that supplemental testosterone will initiate prostatic carcinoma. Moreover, in the properly evaluated and monitored patient, I do not believe that treatment with supplemental testosterone will result in clinically significant progression of an established occult malignancy. The subject of prostatic disease in men treated with androgen supplementation has been recently reviewed in a Reviews in Urology supplement.21
Even though it is highly doubtful that the administration of testosterone will result in any promotional effect of an occult malignancy, initial evaluation of patients prior to administration of testosterone requires screening for prostatic carcinoma. A standard urologic evaluation including careful digital rectal examination (DRE) and serum PSA measurement should be conducted. The threshold to indicate biopsy for PSA in this cohort probably should be lower than the widely utilized cut-off of 4.0 ng/mL (which is, even for all men, considered by many experts to be too high). I recommend biopsy for a PSA greater than 3.0 ng/mL prior to administration of testosterone. If the patient has a negative biopsy, or has a normal PSA and DRE, I believe there is no risk of a trial of testosterone supplementation in a man with symptoms of andropause. However, careful monitoring for change in PSA and DRE is warranted. An additional prostate assessment 3 months after initiation of therapy and every 6 months thereafter is recommended. If the PSA level rises above 3.0 ng/mL, a biopsy is warranted.
Treatment Options
A number of testosterone preparations are suitable for men with andropause symptoms. These include oral agents, injectable formulations, transdermal patches, transdermal gels, and buccal tablets. Theoretically, each of these can restore the physiologic levels of testosterone. A number of factors go into the selection of therapy including the pattern of serum testosterone levels produced, side effects, convenience and ease of administration, cosmetic issues, and cost. While many of the potential adverse effects of testosterone are universal with all preparations, different formulations provide individual benefits and side effects.
The only testosterone preparation available for oral delivery is testosterone undecanoate (Andriol®; Organon, The Netherlands). This is not commercially available in the United States. It achieves low-normal levels of testosterone but delivery is poor and absorption is highly dependant on food intake. The considerable variability of testosterone levels obtained with this oral preparation renders it not useful in the opinion of most clinicians.
Until recently the primary formulation in the United States was the injectable esters. The propionate ester is not widely used because it requires dosing every 2 to 3 days. The cypionate or enanthate esters have essentially equivalent pharmacokinetics22 and provide for dosing every 1 to 2 weeks by intramuscular injection. Unfortunately, as has been demonstrated by Snyder23 and Dobs and colleagues,24 there is considerable variability in the testosterone levels achieved with these preparations, and super-physiologic levels often occur soon after injection. In some men this may create a sense of euphoria in the period immediately following injection, followed by a rapid return of andropause symptoms as the testosterone level falls before the next dose injection. The advantages of the injectable forms of testosterone include ease of adjustment of dose as well as cost.
In an effort to provide a more constant level of serum testosterone without the need for frequent injections, a variety of transdermal approaches have been developed. Initially the so-called “testosterone patches” (Testoderm®; Alza Corp., Mountain View, CA) required application to the scrotum. In general, low-normal levels of testosterone were achieved with Testoderm.25 Patches are applied daily, allowing establishment of the normal circadian rhythm of testosterone.26,27 The high level of 5-alpha reductase in scrotal skin results in super-physiologic levels of dihydrotestosterone.26 A number of problems exist with this approach, including high variability in the level of testosterone achieved, problems with patch adherence, and dermal complications.
Because of the problems with scrotal administration, a nonscrotal patch has been developed (Androderm®, Watson Pharma, Inc., Morristown, NJ) that provides easier application with fewer complications. The Androderm patch achieves mid-range normal testosterone levels with fairly good reliability. Like the scrotal patch, 2 dose formulations are available to afford adjustment in serum level of testosterone achieved. This patch may also produce skin irritation at the application site.
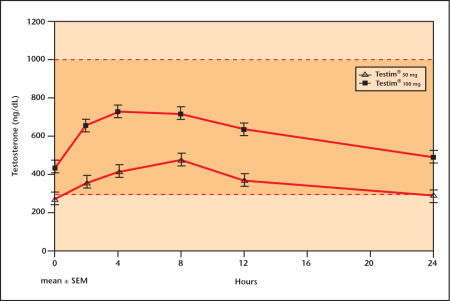
The major advancement in administration of testosterone was the development of the so-called “transdermal” gels (AndroGel®, Solvay Pharmaceuticals, King of Prussia, PA, and Testim®, Auxilium Pharmaceuticals, Inc., Norristown, PA). Daily administration of the gels affords mid-range normal testosterone levels.28 Steidle and colleagues29 have reported the largest placebo-controlled clinical trial ever conducted in testosterone replacement. In this investigation, Testim achieved excellent serum testosterone levels and improvement in lean body mass and sexual function. A number of other reports support Testim’s efficacy and safety.30–33 Like the dermal patches, the transdermal gels result in some variability in testosterone levels achieved. As shown in Figure 3, 24-hour serum testosterone levels with daily administration of Testim demonstrate an excellent dose-response curve.
Figure 3.
24-hour serum testosterone level dose response with daily administration of Testim®.
The decision as to the formulation of testosterone supplementation to be utilized requires careful discussion with the patient. The easiest to administer are the transdermal gels and, except for their cost, these are the preferred modality of therapy today.
Main Points.
Andropause, the progressive decline of sex hormones, particularly testosterone, in men as they age, can cause an array of unwanted physiological changes. Testosterone replacement therapy can alleviate and in some cases reverse many of these symptoms.
Among the difficulties in establishing a diagnosis of andropause is a lack of agreement as to what constitutes a “normal” level of serum testosterone. There is tremendous interpatient variability as well as changes within a patient at different times of the day. In addition, many of the symptoms of andropause can be caused by comorbid disease states or commonly used medications.
A number of clinical studies have demonstrated beneficial effects of testosterone replacement; in all studies reported, the administration of supplemental testosterone has been safe. Although no clear evidence exists that androgen supplementation produces an increase in cancer or promotes the effect of an established occult malignancy, initial evaluation of patients prior to administration of testosterone should include screening for prostatic carcinoma, and regular digital rectal examinations and monitoring of prostate-specific antigen levels is warranted.
There are a number of testosterone preparations available to treat andropause symptoms. These include oral agents, injectable formulations, and transdermal patches and gels. While many of the potential adverse effects of testosterone are universal with all preparations, different formulations provide individual benefits and side effects. Easiest to administer are the transdermal gels and, except for their cost, these are the preferred modality of therapy today.
References
- 1.Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589–598. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 2.Harman SM, Metter EJ, Tobin JD, et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 3.Morley JE, Kaiser FE, Perry HM, 3rd, et al. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism. 1997;46:410–413. doi: 10.1016/s0026-0495(97)90057-3. [DOI] [PubMed] [Google Scholar]
- 4.Morales A, Lunenfeld B. Investigation, treatment and monitoring of late-onset hypogonadism in males. Official recommendations of ISSAM. International Society for the Study of the Aging Male. Aging Male. 2002;5:74–86. [PubMed] [Google Scholar]
- 5.Morales A, Heaton JPW. Hormonal erectile dysfunction: evaluation and management. Urol Clin North Am. 2001;28:279–288. doi: 10.1016/s0094-0143(05)70138-5. [DOI] [PubMed] [Google Scholar]
- 6.Urban RJ, Bodenberg YH, Gilkison C, et al. Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am J Physiol. 1995;269:E820–E826. doi: 10.1152/ajpendo.1995.269.5.E820. [DOI] [PubMed] [Google Scholar]
- 7.Tenover JS. Androgen administration to aging men. Endocrinol Metab Clin North Am. 1994;23:877–892. [PubMed] [Google Scholar]
- 8.Behre HM, Kliesch S, Leifke E, et al. Long-term effect of testosterone therapy on bone mineral density in hypogonadal men. J Clin Endocrinol Metab. 1997;82:2386–2390. doi: 10.1210/jcem.82.8.4163. [DOI] [PubMed] [Google Scholar]
- 9.Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- 10.Dunn JF, Nisula BC, Rodbard D. Transport of steroid hormones: binding of 21 endogenous steroids to both testosterone-binding globulin and corticosteroid-binding globulin in human plasma. J Clin Endocrinol Metab. 1981;53:58–68. doi: 10.1210/jcem-53-1-58. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto AM. Andropause: clinical implications of the decline in serum testosterone levels with aging in men. J Gerontol A Biol Sci Med Sci. 2002;57:M76–M99. doi: 10.1093/gerona/57.2.m76. [DOI] [PubMed] [Google Scholar]
- 12.Lamb DJ, Weigel NL, Marcelli M. Androgen receptors and their biology. Vitam Horm. 2001;62:199–230. doi: 10.1016/s0083-6729(01)62005-3. [DOI] [PubMed] [Google Scholar]
- 13.Tenover L. The androgen-deficient aging male: current treatment options. Rev Urol. 2003;5(suppl 1):S22–S28. [PMC free article] [PubMed] [Google Scholar]
- 14.Barrett-Conner E, Khaw RT, Yen SS. Testosterone and risk factors for cardiovascular disease in men. Diabetes Metab. 1995;21:156–161. [PubMed] [Google Scholar]
- 15.Webb CM, McNeill JG, Hayward CS, et al. Effects of testosterone on coronary vasomotor regulation in men with coronary artery disease. Circulation. 1999;100:1690–1696. doi: 10.1161/01.cir.100.16.1690. [DOI] [PubMed] [Google Scholar]
- 16.Webb CM, Adamson DL, deZeigler D, Collins P. Effect of acute testosterone on myocardial ischemia in men with coronary artery disease. Am J Cardiol. 1999;83:437–439. doi: 10.1016/s0002-9149(98)00880-7. [DOI] [PubMed] [Google Scholar]
- 17.Tenover JL. Effects of androgen supplementation in the aging male. In: Oddens BJ, Vermeulen A, editors. Androgens and the Aging Male. New York, NY: Parthenon Publishing Group; 1996. pp. 191–204. [Google Scholar]
- 18.Matsumoto AM, Sandblom RE, Schoene RB, et al. Testosterone replacement in hypogonadal men: effects on obstructive sleep apnea, respiratory drives, and sleep. Clin Endocrinol (Oxf) 1985;22:713–721. doi: 10.1111/j.1365-2265.1985.tb00161.x. [DOI] [PubMed] [Google Scholar]
- 19.Snyder PJ, Preachy H, Hannoush P, et al. Effect of testosterone treatment on bone mineral density in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:1966–1972. doi: 10.1210/jcem.84.6.5741. [DOI] [PubMed] [Google Scholar]
- 20.Gruenewald DA, Matsumoto AM. Testosterone supplementation therapy for older men: potential benefits and risks. J Am Geriatr Soc. 2003;51:101–115. doi: 10.1034/j.1601-5215.2002.51018.x. [DOI] [PubMed] [Google Scholar]
- 21.Brawer MK. Androgen supplementation and prostate cancer risk: strategies for pretherapy assessment and monitoring. Rev Urol. 2003;5(suppl 1):S29–S33. [PMC free article] [PubMed] [Google Scholar]
- 22.Schulte-Beerbuhl M, Nieschlag E. Comparison of testosterone, dihydrotestosterone, luteinizing hormone, and follicle-stimulating hormone in serum after injection of testosterone cypionate. Fertil Steril. 1980;33:201–203. doi: 10.1016/s0015-0282(16)44543-7. [DOI] [PubMed] [Google Scholar]
- 23.Snyder PJ. Clinical use of androgens. Annu Rev Med. 1984;35:207–217. doi: 10.1146/annurev.me.35.020184.001231. [DOI] [PubMed] [Google Scholar]
- 24.Dobs AS, Meikle AW, Arver S, et al. Pharmacokinetics, efficacy, and safety of a permeation-enhanced testosterone transdermal system in comparison with bi-weekly injections of testosterone enanthate for the treatment of hypogonadal men. J Clin Endocrinol Metab. 1999;84:3469–3478. doi: 10.1210/jcem.84.10.6078. [DOI] [PubMed] [Google Scholar]
- 25.Place VA, Atkinson L, Prather DA, et al. Transdermal testosterone replacement through genital skin. In: Nieschlag E, Behre HM, et al., editors. Testosterone: Action, Deficiency, Substitution. Berlin: Springer-Verlag; 1990. pp. 165–181. [Google Scholar]
- 26.Findlay JC, Place V, Snyder PJ. Treatment of primary hypogonadism in men by the transdermal administration of testosterone. J Clin Endocrinol Metab. 1989;68:369–373. doi: 10.1210/jcem-68-2-369. [DOI] [PubMed] [Google Scholar]
- 27.Bremner WJ, Vitiello MV, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J Clin Endocrinol Metab. 1983;56:1278–1281. doi: 10.1210/jcem-56-6-1278. [DOI] [PubMed] [Google Scholar]
- 28.Swerdloff RS, Wang C, Cunningham G, et al. Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. J Clin Endocrinol Metab. 2000;85:4500–4510. doi: 10.1210/jcem.85.12.7045. [DOI] [PubMed] [Google Scholar]
- 29.Steidle C, Schwartz S, Jacoby K, et al. AA2500 testosterone gel normalizes androgen levels in aging males with improvements in body composition and sexual function. J Clin Endocrinology Metab. 2003;88:2673–2681. doi: 10.1210/jc.2002-021058. [DOI] [PubMed] [Google Scholar]
- 30.Bachand R, Jones N, Secrest A, et al. Long-term treatment with Testim® 1% testosterone gel significantly improves sexual function, mood, body composition, and bone mineral density [Abstract]. The Aging Male IV-International Society for the Study of the Aging Male Annual Meeting; February 2004; Prague, Czech Republic. [Google Scholar]
- 31.Jones N, Smith T, Rodsvilla J. Evaluation of the pharmacokinetic profiles of a new testosterone gel (Testim®) compared to Androgel® [Abstract]. 3rd World Congress of Men’s Health; October 2003; Vienna, Austria. [Google Scholar]
- 32.Mack R, Rodzvilla J, Secrest A, Smith T. Testosterone supplementation improves sexual function in the most severely impaired patients [Abstract]. American Society of Andrology Annual Meeting; April 2004; Baltimore, MD. [Google Scholar]
- 33.Marbury T, Hamill E, Bachand R, et al. Evaluation of the pharmacokinetic profiles of the new testosterone topical gel formulation, Testim®, compared to Androgel®. Biopharm Drug Dispos. 2003;24:115–120. doi: 10.1002/bdd.345. [DOI] [PubMed] [Google Scholar]