Abstract
Although testosterone replacement therapy (TRT) is indicated for the management of symptomatic hypogonadism, there is still controversy over whether TRT should be administered to middle-aged men for the clinical manifestations of andropause, regardless of whether the serum testosterone levels are depressed or not. Side effects of TRT may include fluid retention, gynecomastia, polycythemia, and exacerbation of existing prostate cancer. As a result, patients on TRT require meticulous surveillance including regular digital rectal examination and serum prostate-specific antigen (PSA) testing. Herein, we present the case of a middle-aged man with andropause and a rising PSA on TRT.
Key words: Testosterone, Sildenafil citrate, Prostate-specific antigen, Benign prostatic hyperplasia
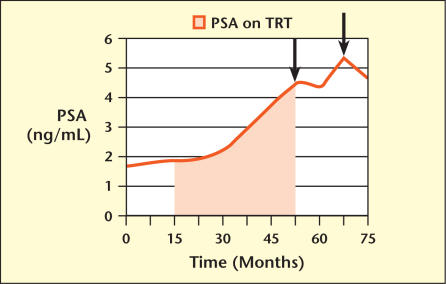
A 53-year-old man presented for evaluation of erectile dysfunction (ED) and a lack of libido. Laboratory results from his primary care physician included a normal serum prostate-specific antigen (PSA), which was 1.8 ng/mL, as well as a normal free PSA fraction (34%) and a normal total serum testosterone (594 ng/dL). He was empirically started on sildenafil citrate for on-demand treatment of his ED, and a repeat serum testosterone months later was again within the normal range (422 ng/dL). He continued to complain of ED and decreased libido, and was empirically started on exogenous androgen therapy with biweekly intramuscular injections of testosterone decanoate. Over the next 3 years his PSA progressively increased to 3.8 ng/mL, an average PSA velocity of 0.68 ng/mL/y (Figure 1). His sexual complaints remained unresolved, and he was referred to a urologist.
Figure 1.
Change in prostate-specific antigen (PSA) over time. Shaded area shows time period during which patient was on TRT. Arrows indicate time point at which prostate biopsy was performed. TRT, testosterone replacement therapy.
On evaluation, the patient admitted that testosterone replacement therapy (TRT) failed to ameliorate his sexual complaints, and that sildenafil citrate did not improve his ED. He also noted nocturia up to 2 times nightly, slight hesitancy and intermittency, a sensation of incomplete emptying, and mild postvoid dribbling. His American Urological Association (AUA) symptom index was 14. His past medical history was otherwise significant for hypertension and hypercholesterolemia. On physical examination, he was a moderately obese male with no gynecomastia, skin, or hair alterations. His genital examination revealed normal testes that measured approximately 20 cc each. Rectal examination demonstrated a moderate-sized, 40-g prostate with no nodules or asymmetry. Repeat serum PSA was 4.0 ng/mL with a total serum testosterone of 561 ng/dL (normal, 200–800 ng/dL).
As a result of the elevated PSA, the patient elected to stop androgen therapy and he underwent an extended 12-core biopsy of the prostate. By transrectal ultrasonography his prostate measured 57 cc. Pathology revealed benign prostatic hyperplasia (BPH). Follow-up serum PSA 6 months later remained elevated at 4.4 ng/mL, and his serum testosterone off TRT was 561 ng/dL. Three months later, his serum PSA spiked to 5.5 ng/mL with a free PSA fraction of 16%, even though he remained off androgen therapy. Because of the increased PSA and decreased free PSA fraction, a repeat 12-core biopsy was performed. By transrectal ultrasonography his prostate now measured 68 cc. Pathology again revealed BPH.
Currently, the patient is doing well off testosterone replacement. His erectile dysfunction responded to intracorporeal alprostadil injections and his last PSA measurement was 4.7 ng/mL. His urinary complaints improved on oral tamsulosin, and his last AUA symptom index was 9.
Discussion
This case illustrates several important concepts. First, it is evident from recent data that the use of TRT is increasing, often in patients who have no discrete indication other than a sexual complaint. Under the umbrella of age-related hormonal changes in men, or andropause, patients with various complaints are being offered an opportunity to try androgen therapy. Sexual symptoms often prompt evaluation and treatment. Although a recent consensus panel made recommendations for the initiation of TRT, suggesting limitation of its use to men with a serum testosterone level below 200 ng/dL and symptoms of hypogonadism, evidence-based data to support this recommendation are certainly lacking.1 A recent report from the Institute of Medicine addressed this issue, admonishing the use of testosterone for nonspecific symptoms and emphasizing the need to further clarify populations that clearly benefit from TRT.2 As our patient’s case attests, use of TRT in eugonadal men may provide no recognizable clinical benefit and may even unnecessarily expose patients to certain risks and side effects of exogenous androgens.
Second, and as this case clearly demonstrates, a rising PSA does not necessarily imply that an undetected prostate cancer is present. Although the hormonal responsiveness of prostate cancer is well established, it is generally accepted that TRT does not induce the development of prostate cancer.3 Furthermore, a recent report demonstrated no increased risk of prostate cancer in men with prostatic intraepithelial neoplasia, a precursor of prostate cancer, on TRT, although appropriate controls were not included in the study.4
It is not surprising to see PSA changes in men receiving androgen therapy. Hypogonadal men have depressed PSA levels compared to normal age-matched men, and patients on finasteride treatment experience a 2.2-fold reduction in serum PSA.5,6 Averaging several investigations of the effect of TRT on PSA, men receiving testosterone will have an associated increase of 0.30 ng/mL/y in serum PSA, with older men experiencing a greater increase of 0.43 ng/mL/y.5 Changes in PSA are expected in patients on TRT, but this expectation should not downplay the need for rigorous follow up in this patient population.
Finally, patients on TRT should be monitored for obstructive voiding symptoms as testosterone, through its conversion to dihydrotestosterone, can exacerbate manifestations of BPH. Similar to the trend in serum PSA levels, prostate volumes are lower in hypogonadal men. With TRT, these volumes normalize to age-matched controls. Evaluation of hypogonadal men on exogenous androgen therapy has shown no significant increase in AUA symptom index; however, patients with pre-existing BPH symptomatology can experience rapid progression of their obstructive symptoms.5 As such, the presence of significant BPH symptoms may be considered a relative contraindication to the use of TRT.
This case illustrates a patient that has become a common referral to our practice. Despite published consensus recommendations on the use of TRT, middle-aged men with sexual symptoms receive exogenous androgens in the setting of a normal hormonal evaluation. The rising PSA in this setting is a cause for concern and commonly initiates urologic referral. It is our recommendation that clinicians perform a baseline digital rectal examination and PSA before starting TRT, and that the PSA should be checked 6–12 weeks after initiation of androgen therapy, regardless of the route of administration. The PSA should then be monitored semi-annually as long as the patient remains on TRT, in addition to having an annual digital rectal examination. A PSA velocity greater than 0.75 ng/mL/y, regardless of the baseline PSA, or a nodule on digital rectal examination while on TRT should prompt further investigation with a prostate biopsy.
Main Points.
Sexual complaint, in particular erectile dysfunction, is the main reason older patients are placed on exogenous testosterone; however, patients treated with testosterone replacement therapy for sexual symptoms may not benefit.
A rising prostate-specific antigen (PSA) in the setting of androgen therapy does not necessarily mean the patient has prostate cancer.
PSA levels need to be monitored at regular intervals in patients being treated with testosterone replacement therapy.
Testosterone replacement therapy can exacerbate obstructive symptoms related to benign prostatic hyperplasia.
References
- 1.ASRM Practice Committee, authors. Treatment of androgen deficiency in the aging male. Fertil Steril. 2004;81:1437–1440. doi: 10.1016/j.fertnstert.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 2.Liverman CT, Blazer DG National Research Council (U.S.), authors . Testosterone and Aging: Clinical Research Directions. Washington, DC: National Academies Press; 2004. Committee on Assessing the Need for Clinical Trials of Testosterone Replacement Therapy. [PubMed] [Google Scholar]
- 3.Rhoden EL, Morgentaler A. Risks of testosterone-replacement therapy and recommendations for monitoring. N Engl J Med. 2004;350:482–492. doi: 10.1056/NEJMra022251. [DOI] [PubMed] [Google Scholar]
- 4.Rhoden EL, Morgentaler A. Testosterone replacement therapy in hypogonadal men at high risk for prostate cancer: results of 1 year of treatment in men with prostatic intraepithelial neoplasia. J Urol. 2003;170:2348–2351. doi: 10.1097/01.ju.0000091104.71869.8e. [DOI] [PubMed] [Google Scholar]
- 5.Bhasin S, Singh AB, Mac RP, et al. Managing the risks of prostate disease during testosterone replacement therapy in older men: recommendations for a standardized monitoring plan. J Androl. 2003;24:299–311. doi: 10.1002/j.1939-4640.2003.tb02676.x. [DOI] [PubMed] [Google Scholar]
- 6.Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215–224. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]