Abstract
In the treatment of prostatic carcinoma, neoadjuvant androgen deprivation therapy has been shown to confer several clinical and quality-of-life benefits. Compared with the luteinizing hormone-releasing hormone agonists, gonadotropin-releasing hormone antagonists can achieve castrate levels of testosterone much faster, without the risks associated with testosterone flare. They can provide equal or superior efficacy in apoptosis, radiation sensitization, and prostate volume reduction. Urologists are increasingly employing neoadjuvant androgen deprivation in patients undergoing prostatectomy, radiation, brachytherapy, and cryotherapy.
Key words: Prostatic carcinoma, Neoadjuvant androgen deprivation, Luteinizing hormone-releasing hormone agonist, Gonadotropin-releasing hormone antagonist
Neoadjuvant androgen deprivation has several advantages in the definitive treatment of prostate cancer. These include apoptosis of cancer cells and benign prostatic epithelium,1,2 decreased surgical margins and extracapsular extension in prostatectomy specimens, radiation sensitization, and decreased lower urinary tract symptoms in men undergoing brachytherapy. This article will review these aspects of androgen deprivation, particularly in regard to new modalities in prostate downsizing such as gonadotropin-releasing hormone (GnRH) antagonists.
Although GnRH antagonists have a similar castrating effect on serum testosterone levels as traditional luteinizing hormone-releasing hormone (LHRH) agonists, the effect of GnRH antagonists is immediate, with 72% of men reaching castrate levels of testosterone 8 days after injection and 94% of men achieving castrate levels by 28 days. A comparable decrease in testosterone level in men receiving LHRH agonists can require 3 months to achieve.3 This immediate nadir with the GnRH antagonists can potentially enhance radiosensitivity, apoptotic effect, and downsizing, potentially translating to increased efficacy and/or decreased time required for androgen deprivation before definitive treatment. Furthermore, because GnRH antagonists do not increase serum testosterone levels on initial administration (“testosterone flare”), the addition of an anti-androgen agent is unnecessary during this period. Not only can avoidance of this phenomenon potentially result in cost savings, it can also decrease patients’ exposure to the morbidities of anti-androgen medications, including teratogenesis and hypospadias in neonates and rare death or hospitalization secondary to severe liver injury.
Data from the National Cancer Institute suggest that urologists throughout the country are increasingly using neoadjuvant androgen deprivation therapy when developing a treatment plan for prostate cancer. A recent report4 shows that the number of urologists using neoadjuvant androgen deprivation before prostatectomy has increased, from 2.9% in 1989 to 7.8% in 2001. Similarly, neoadjuvant androgen deprivation with brachytherapy treatment increased from 7.4% to 24.6% over the same time period. Although cryotherapy was not widely practiced in 1989, current rates of neoadjuvant androgen deprivation likely approximate those with brachytherapy—since both focalized therapies technically benefit from prostate downsizing. Appropriately, the use of neoadjuvant androgen deprivation with external beam radiation has dramatically increased: from 9.8% in 1989 to 74.6% in 2001.4 Justifications for these trends will be examined in detail, beginning with the effect of neoadjuvant androgen deprivation on the patient undergoing radical retropubic prostatectomy.
Many quality studies have evaluated the effect of neoadjuvant androgen deprivation on surgical outcome. It is clear that patients treated with neoadjuvant androgen deprivation have fewer positive surgical margins and smaller prostate volumes, and the percentage with extracapsular extension may be decreased.5–9 No study has shown an increased survival rate despite these positive findings, with the exception of a small subgroup analysis of men with prostate-specific antigen (PSA) levels greater than 20 ng/mL in a study performed by the Canadian Urologic Oncology Group.10
It is possible that further dedicated evaluation of high-risk men with PSA values greater than 20 ng/mL or men with metastatic disease treated non-conventionally with adjuvant androgen deprivation as well as focal therapy to the prostate may have improved survival rates and/or an improved quality of life with neoadjuvant androgen deprivation (E. D. Crawford, personal communication). It is also possible that previously studied neoadjuvant androgen deprivation regimens of 3 months of therapy may not be as effective as extended deprivation periods (although at least 1 study has shown equivalence between a 3-month and an 8-month schema11). Three months of androgen deprivation with a GnRH antagonist may confer survival benefits, given the longer exposure to castrate testosterone levels and lack of testosterone flare. Although anti-androgen treatment protects men from the clinical effects of a serum testosterone surge, this rise in testosterone level is also accompanied by a surge in dihydrotestosterone and estrogen, both of which may play a role in prostate cancer growth.
Cryotherapy is an emerging area of treatment in prostate cancer. Although formal randomized studies have not been performed to prove the efficacy of neoadjuvant androgen deprivation for primary or salvage treatment of prostate cancer, one of the largest experiences comes from Columbia University. All patients undergoing prostate cryotherapy at Columbia undergo a staging lymph node dissection before treatment as well as 3 months of neoadjuvant androgen deprivation. Using this protocol, the reported 2-year survival rate with salvage cryotherapy is 74%.12 Follow-up studies from this group show that quality-of-life scores are high in men undergoing neoadjuvant androgen deprivation.13
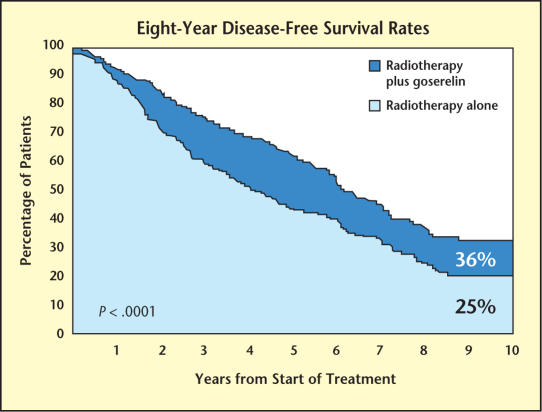
Neoadjuvant androgen deprivation acts synergistically with radiation and has been shown to improve disease-free and overall survival in various groups of men who undergo radiation therapy for prostate cancer. The prospective Radiation Therapy Oncology Group (RTOG) Trial 85-31 showed that disease-free survival rates were higher in men with early and continuous androgen deprivation treated definitively with external beam radiation versus men treated with radiation alone (60% vs 44% at 5 years; P < .001) (see Figure 1 from follow-up study by Lawton et al.14). This patient population included 977 men with T3 disease or nodal metastases. Overall survival rates were higher in men treated with adjuvant androgen deprivation who had Gleason score 8–10 prostate cancers as well (66% vs 55%; P = .03).15
Figure 1.
The prospective Radiation Therapy Oncology Group Trial 85-31 showed that disease-free survival rates were higher in men with early and continuous androgen deprivation treated definitively with external beam radiation versus those treated with radiation alone. Adapted from Lawton CA et al.14
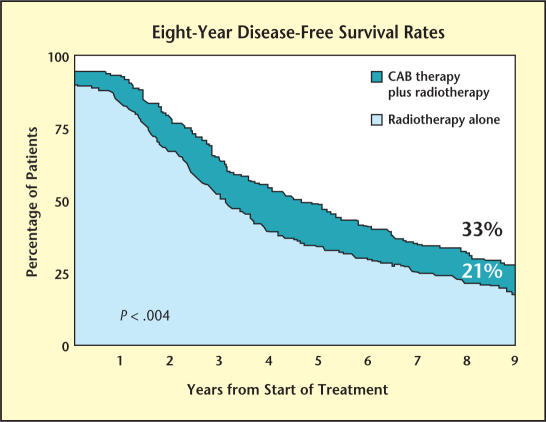
Another trial coordinated by the RTOG, 86-10, showed that disease-free survival was also improved in patients who underwent brief neoadjuvant androgen deprivation followed by external beam radiation compared with radiation alone (33% vs 21% at 8 years; P < .004) (see Figure 2). This patient population included 233 men with bulky T2 and T3 prostate cancer, and treatment included 2 months of neoadjuvant and 2 months of adjuvant combined androgen blockade during radiation.16
Figure 2.
A trial by the Radiation Therapy Oncology Group 86-10 showed that diseasefree survival was improved in patients who underwent brief neoadjuvant androgen deprivation followed by external beam radiation compared with radiation alone. Adapted from Pilepich MV et al.17
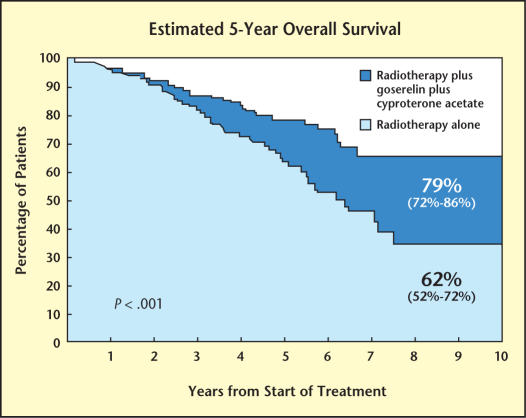
A prospective multi-institutional study from Europe confirmed these findings in 415 men with Gleason 8–10 or T3–4 or node-positive prostate cancer. These men were randomized to receive treatment with combined androgen blockade beginning 1 week before radiation therapy and continuing for 3 years after treatment or radiation therapy alone. The disease-free survival rate at 5 years was 74% in the androgen-deprivation group versus 40% in the radiation-alone group (P = .0001). In addition to an improvement in disease-free survival, a significant overall survival improvement in the patients treated with neoadjuvant androgen deprivation was seen as well (79% vs 62% at 5 years; P = .0002) (see Figure 3).17
Figure 3.
In a prospective multi-institutional study from Europe, men with prostate cancer were randomized to receive treatment with combined androgen blockade beginning 1 week before radiation therapy and continuing for 3 years after treatment or radiation therapy alone. In addition to an improvement in disease-free survival, a significant overall survival improvement in the patients treated with neoadjuvant androgen deprivation was seen. Adapted from Bolla M et al.18
A different look at neoadjuvant androgen deprivation retrospectively evaluated 105 men treated with 73–87 Gy of external beam radiation therapy, 67 of whom had received neoadjuvant androgen deprivation for an average of 4 months before radiation. Patients who had received neoadjuvant androgen deprivation had a more rapid time to PSA nadir (6 vs 12 months) and a lower average nadir value (0.25 vs 1.35 ng/mL).18 Importantly, a PSA nadir value less than 1 ng/mL after external beam radiation has been shown to be an independent predictor of a significantly improved disease-free survival (63% vs 22% at 3 years; P < .001).19
The benefits of androgen deprivation in a salvage radiation setting are less clear but have been evaluated in a recent retrospective study from Memorial Sloan-Kettering Cancer Center. In this study, it was concluded that neoadjuvant androgen deprivation improved the 4-year disease-free survival rate in patients given treatment when initial prostatectomy pathology revealed negative margins, absence of extracapsular extension, or invasion of cancer into the seminal vesicle.20 Furthermore, a subset analysis of 139 men in the RTOG 85-31 trial showed a 5-year disease-free survival improvement in men treated with androgen deprivation during salvage radiation in whom initial surgical pathology revealed extracapsular extension or seminal vesicle invasion (65% vs 42%; P = .002).21
Patients undergoing brachytherapy as the sole treatment for prostate cancer may not benefit from enhanced cancer control with neoadjuvant androgen deprivation. This may be because of the low-risk nature of prostate cancer generally treated by this modality or the theorized decreased significance of radiation sensitization when high doses of radiation are delivered to the prostate. Neoadjuvant androgen deprivation, however, is extremely useful in decreasing the size of prostates greater than 50 to 60 cc in volume. It has been shown that implantation of larger prostates is technically more demanding, has less optimal dosimetry secondary to pubic arch interference, and requires more seeds. On computed tomography and magnetic resonance imaging scans obtained in patients undergoing androgen deprivation with traditional LHRH agonists, a prostate volume reduction of 33% is seen after a mean of 3.7 months of treatment.22
Volume reduction has been studied using a GnRH antagonist as well and is, as expected, more expedient. A similar 30% to 35% prostate volume reduction is seen after a mean of 57 days of treatment with a GnRH antagonist.3,23 An interesting analysis in a small cohort of patients in European Organization for Research and Treatment of Cancer (EORTC) study 22863 evaluated neoadjuvant androgen deprivation and prostate size and showed that prostate volume continued to drop long after castration levels of testosterone had been achieved (a reduction of 46% at 12 months, when castration was reached at 2–3 months).24 Treatment with a GnRH antagonist may have less downsizing lag time because of earlier castrate testosterone levels.
Finally, in addition to improving dosimetry and technical feasibility of brachytherapy, downsizing prostate glands before brachytherapy has been shown to decrease morbidity following treatment. In a study by Gelblum and coworkers,25 Grade 2 urinary toxicity (men requiring treatment with an α-blocker after implantation) was significantly decreased in men with prostate volumes less than 35 cc (P = .001). Although not yet extensively studied, these results are probably similar when other focal treatments for prostate cancer are used.
In summary, it is clear why doctors who treat prostate cancer are utilizing neoadjuvant androgen deprivation more frequently. First, neoadjuvant treatment has been shown to make radical prostatectomy, brachytherapy, and cryotherapy technically more feasible and therefore theoretically more effective in men with large glands. Second, neoadjuvant androgen deprivation has been shown to improve survival rates in men with intermediate- and high-risk prostate cancer who undergo treatment with external beam radiation. Similarly, neoadjuvant androgen deprivation appears to improve disease-free survival in men undergoing salvage radiation therapy when surgical pathology shows seminal vesicle invasion, absence of positive margins, or absence of extracapsular extension. Finally, neoadjuvant androgen deprivation is well tolerated and can improve urinary obstructive symptoms in men with large glands undergoing brachytherapy.
GnRH antagonists are a new class of drugs available to treat prostate cancer that do not require treatment with anti-androgen medications and provide almost immediate castrate levels of serum testosterone. GnRH antagonists clearly may provide equal if not superior efficacy in apoptosis, radiation sensitization, and downsizing, secondary to a quicker achievement of castrate levels of serum testosterone and avoidance of testosterone (as well as estrogen and dihydrotestosterone) flare. Further advantages include avoidance of the cost and morbidity associated with anti-androgen treatment and potential decreased treatment durations secondary to a superior onset of action. Clearly, further studies should be pursued to better define the efficacy and cost-benefit ratios of GnRH antagonist neoadjuvant prostate cancer treatment.
Main Points.
Although gonadotropin-releasing hormone (GnRH) antagonists have a similar castrating effect on serum testosterone levels as traditional luteinizing hormone-releasing hormone (LHRH) agonists, the effect of GnRH antagonists is almost immediate, compared with the 3 months needed to achieve this effect with LHRH agonists. This advantage can potentially enhance radiosensitivity, apoptotic effect, and prostate downsizing, leading to increased efficacy and/or decreased time required for androgen deprivation.
GnRH antagonists do not cause a testosterone flare and its associated morbidities; thus, concomitant anti-androgen therapy is not necessary with these agents.
As the benefits of prostate downsizing have become clear, more urologists are employing androgen deprivation therapy before surgical and radiation procedures for prostate cancer.
Neoadjuvant androgen deprivation has many benefits: it has been shown to make radical prostatectomy, brachytherapy, and cryotherapy more feasible and theoretically more effective in men with large glands; it has been shown to improve survival rates in men who are treated with external beam radiation and when used in a salvage setting results in a durable disease-free survival. It is well tolerated and can also improve urinary obstructive symptoms in men with large glands undergoing brachytherapy.
References
- 1.Denmeade SR, Lin XS, Isaacs JT. Role of programmed (apoptotic) cell death during the progression and therapy for prostate cancer. Prostate. 1996;28:251–265. doi: 10.1002/(SICI)1097-0045(199604)28:4<251::AID-PROS6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 2.Buttyan R, Ghafar MA, Shabsigh A. The effects of androgen deprivation on the prostate gland: cell death mediated by vascular regression. Curr Opin Urology. 2000;10:415–420. doi: 10.1097/00042307-200009000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Wong SL, Lau DTW, Baughman SA, et al. Phamacokinetics and pharmacodynamics of abarelix, a gonadotropin-releasing hormone antagonist, after subcutaneous continuous infusion in patients with prostate cancer. Clin Pharmacol Ther. 2003;73:304–311. doi: 10.1016/s0009-9236(02)17637-5. [DOI] [PubMed] [Google Scholar]
- 4.Cooperberg MR, Grossfeld GD, Lubeck DP, et al. National practice patterns and time trends in androgen ablation for localized prostate cancer. J Natl Cancer Inst. 2003;95:981–989. doi: 10.1093/jnci/95.13.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soloway MS, Pareek K, Sharifi R, et al. Neoadjuvant androgen ablation before radical prostatectomy in cT2bNxM0 prostate cancer: 5-year results. J Urol. 2002;167:112–116. [PubMed] [Google Scholar]
- 6.Aus G, Abrahamsson PA, Ahlgren G, et al. Hormonal treatment before radical prostatectomy: a 3-year follow-up. J Urol. 1998;159:2013–2016. doi: 10.1016/S0022-5347(01)63230-0. [DOI] [PubMed] [Google Scholar]
- 7.Wildschutz T, Louis L, Hourriez L, et al. Neoadjuvant hormonal treatment prior to radical prostatectomy: follow-up of a prospective randomized study. Eur Urol. 1996;30:210. Abstract 774. [Google Scholar]
- 8.Meyer F, Bairate I, Bedard C, et al. Duration of neoadjuvant androgen deprivation therapy before radical prostatectomy and disease-free survival in men with prostate cancer. Urology. 2001;58:71–77. doi: 10.1016/s0090-4295(01)01245-6. [DOI] [PubMed] [Google Scholar]
- 9.Schulman CC, Debruyne FM, Forster G, et al. 4-Year follow-up results of a European Prospective Randomized Study on neoadjuvant hormonal therapy prior to radical prostatectomy in T2-3N0M0 prostate cancer. European Study Group on Neoadjuvant Treatment of Prostate Cancer. Eur Urol. 2000;38:706–713. doi: 10.1159/000020366. [DOI] [PubMed] [Google Scholar]
- 10.Klotz LH, Goldenberg SL, Jewett MA, et al. Long-term follow-up of a randomized trial of 0 versus 3 months of neoadjuvant androgen ablation before radical prostatectomy. J Urol. 2003;170:791–794. doi: 10.1097/01.ju.0000081404.98273.fd. [DOI] [PubMed] [Google Scholar]
- 11.Gleave ME, Goldenberg SL, Chin JL, et al. Randomized comparative study of 3 versus 8-month neoadjuvant hormonal therapy before radical prostatectomy: biochemical and pathological effects. J Urol. 2001;166:500–506. [PubMed] [Google Scholar]
- 12.Ghafar MA, Johnson CW, De La Taille A, et al. Salvage cryotherapy using an argon based system for locally recurrent prostate cancer after radiation therapy: the Columbia experience. J Urol. 2001;166:1333–1338. [PubMed] [Google Scholar]
- 13.Anastasiadis AG, Sachdev R, Salomon L, et al. Comparison of health-related quality of life and prostate-associated symptoms after primary and salvage cryotherapy for prostate cancer. J Cancer Res Clin Oncol. 2003;129:676–682. doi: 10.1007/s00432-003-0472-4. [DOI] [PubMed] [Google Scholar]
- 14.Lawton CA, Winter K, Murray K, et al. Updated results of the Phase III Radiation Therapy Oncology Group (RTOG) Trial 85-31 evaluating the potential benefit of androgen support following standard radiation therapy for unfavorable prognosis carcinoma of the prostate. Radiat Oncol Biol Phys. 2001;49:937–946. doi: 10.1016/s0360-3016(00)01516-9. [DOI] [PubMed] [Google Scholar]
- 15.Pilepich MV, Caplan R, Byhardt RW, et al. Phase III trial of androgen suppression using goserelin in unfavorable-prognosis carcinoma of the prostate treated with definitive radiotherapy: report of the Radiation Therapy Oncology Group Protocol 85-31. J Clin Oncol. 1997;15:1013–1021. doi: 10.1200/JCO.1997.15.3.1013. [DOI] [PubMed] [Google Scholar]
- 16.Pilepich MV, Winter K, Roach M, et al. RTOG Trial 86-10 of androgen deprivation before and during radiotherapy in locally advanced carcinoma of the prostate. Am J Clin Oncol. 1998:308a. doi: 10.1016/s0360-3016(01)01579-6. [DOI] [PubMed] [Google Scholar]
- 17.Pilepich MV, Winter K, John MJ, et al. Phase III radiation therapy oncology group (RTOG) trial 86-10 of androgen deprivation adjuvant to definitive radiotherapy in locally advanced carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 2001;50:1243–1252. doi: 10.1016/s0360-3016(01)01579-6. [DOI] [PubMed] [Google Scholar]
- 18.Bolla M, Gonzales D, Warde P, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. Lancet. 2002;360:103–106. doi: 10.1056/NEJM199707313370502. [DOI] [PubMed] [Google Scholar]
- 19.Velasco J, Tekyi-Mensah S, Bolton S, et al. Postneoadjuvant hormone PSA levels and prognosis in locally advanced prostate cancer. Urology. 1999;54:325–328. doi: 10.1016/s0090-4295(99)00123-5. [DOI] [PubMed] [Google Scholar]
- 20.Katz MS, Zelefsky MJ, Venkatraman ES, et al. Predictors of biochemical outcome with salvage conformal radiotherapy after radical prostatectomy for prostate cancer. J Clin Onc. 2003;21:483–489. doi: 10.1200/JCO.2003.12.043. [DOI] [PubMed] [Google Scholar]
- 21.Corn BW, Winter K, Pilepich MV. Does androgen suppression enhance the efficacy of post-operative irradiation? A secondary analysis of RTOG 85-31. Urology. 1999;54:495–502. doi: 10.1016/s0090-4295(99)00186-7. [DOI] [PubMed] [Google Scholar]
- 22.Kucway R, Vicini F, Huang R, et al. Prostate volume reduction with androgen deprivation therapy before interstitial brachytherapy. J Urol. 2002;167:2443–2447. [PubMed] [Google Scholar]
- 23.Phase 2 multi-center, open-labeled study of PPI-149, administered as a subcutaneous, continuous infusion for 57 to 85 days (8 to 12 weeks) in patients undergoing radiation therapy final study report, study no 149-97-03. Waltham, Mass: Praecis Pharmaceuticals Incorporated; 2000. May 10, Data on file (unpublished) [Google Scholar]
- 24.Lilleby W, Fossa SD, Knutsen BH, et al. Computed tomography/magnetic resonance based volume changes of the primary tumor in patients with prostate cancer with or without androgen deprivation. Radiother Oncol. 2000;57:195–200. doi: 10.1016/s0167-8140(00)00219-x. [DOI] [PubMed] [Google Scholar]
- 25.Gelblum DY, Potters L, Ashley R, et al. Urinary morbidity following ultrasound-guided transperineal prostate seed implantation. Int J Radiat Oncol Biol Phys. 1999;45:59–67. doi: 10.1016/s0360-3016(99)00176-5. [DOI] [PubMed] [Google Scholar]