Abstract
Hormonal therapy has been the standard of care for advanced prostate cancer for over 6 decades. Treatments to suppress testosterone have expanded beyond surgical castration and estrogens to include steroidal and nonsteroidal antiandrogens, luteinizing hormone-releasing hormone agonists, and, most recently, gonadotropin-releasing hormone antagonists. Yet, despite this extensive therapeutic armamentarium, long-term survival of patients with advanced prostate cancer remains poor. Many issues regarding hormonal treatment of prostate cancer continue to be controversial, including the benefits of combined androgen blockade versus monotherapy, the optimal timing of treatment, and the value of new therapeutic approaches and strategies, such as intermittent androgen deprivation and adjuvant chemotherapy.
Key words: Hormone therapy, Combined androgen blockade, Intermittent androgen deprivation
In 1941, the pioneering work of Huggins and Hodges provided clear evidence that patients with symptomatic metastatic disease benefit from some form of testosterone suppression.1 Yet, despite over 6 decades of research and clinical experience, few areas of medicine generate as much uncertainty and debate as the hormonal manipulation of advanced prostate cancer. A key area of controversy is the relative utility of surgical and/or medical therapies. Bilateral surgical orchiectomy, estrogens, and luteinizing hormone-releasing hormone (LHRH) agonists all provide patients with castrate levels of testosterone. However, in many patients, the small amounts of androgens released by the adrenal glands continue to support tumor growth.1,2 High-risk surgical removal of the adrenal glands has been replaced by steroidal and nonsteroidal antiandrogens, which block the activity of adrenal androgens. Combining an antiandrogen with medical or surgical castration is considered combined androgen blockade (CAB) and results in total androgen suppression. However, the debate continues regarding the safety, efficacy, and long-term utility of this approach. This review examines the evolution of hormonal manipulation in advanced prostate cancer and many of the unresolved issues pertaining to the optimal use of hormonal treatment in prostate cancer, including the benefits of CAB versus monotherapy and the optimal timing of treatment. Newer treatment approaches, such as gonadotropin-releasing hormone (GnRH) antagonists, intermittent androgen deprivation (IAD), and adjuvant chemotherapy are also discussed.
Early History of Hormonal Therapy for Prostate Cancer
The use of androgen deprivation as therapy for advanced prostate cancer began in 1941, when Huggins and Hodges first treated men with prostate cancer with either orchiectomy or estrogen.1 They monitored changes in prostate size and observed that improvements in acid and alkaline phosphatases were associated with cancer-related symptom relief. Largely due to the absence of other therapies, hormonal manipulation became a mainstay of treatment for symptomatic metastatic disease. Although it was originally hoped that suppression of testicular androgens would be curative, this proved not to be the case.
Although the testes are the primary source of testosterone, the adrenal glands also produce androgens. As a result, many patients with castrate levels of testosterone continue to have measurable levels of dihydrotestosterone (DHT) in the prostate, thereby allowing continued stimulation of prostate cancer cells.2 The importance of adrenal androgens in prostate cancer was observed by Huggins and Hodges in their pioneering study, as many patients continued to have measurable levels of serum acid phosphatase, a surrogate marker of the disease, following medical or surgical castration. The authors considered this a clear indication that androgen production by the adrenal glands was ongoing.1
In 1945, Huggins and colleagues attempted to eliminate the contribution of adrenal androgens by performing bilateral adrenalectomy in patients with progressing prostate cancer. However, patients undergoing this procedure experienced high morbidity in the early postoperative period from mineralocorticoid deficiency, and the investigators thus deemed bilateral adrenalectomy an impractical method of treatment.3 The availability of exogenous cortisone replacement in the 1950s rekindled interest in adrenalectomy as a treatment for advanced prostate cancer. The “immediate and persistent relief of crippling bone pain” was the principal effect reported by Huggins and Bergenstal in their 1952 study.4 Ultimately, however, adrenalectomy was abandoned for prostate cancer due to the poor objective response rates and the tremendous morbidity associated with the surgery and cortisone replacement.
In 1954, encouraged by the work of Huggins and his colleagues, Miller and Hinman attempted to induce “medical adrenalectomy” using large doses of cortisone.5 Marked subjective improvement was noted in 8 of 10 patients and objective improvement in 6 of 10. However, the duration of beneficial response was short-lived, averaging 82 days.5 Medical blockade of adrenocortical function attempted 30 years later, using aminoglutethimide, had similar results: 61% of patients experienced subjective pain relief, but again, objective responses were rare and short-lived. Side effects were common and included lethargy, ankle edema, weakness, and nausea.6 As a result, interest in combining treatments to completely block androgen production waned until improved methods of medical castration became available.
Estrogens
Until the late 1960s and 1970s, synthetic estrogens were the primary means of medical castration. The most commonly used agent, diethylstilbestrol (DES), inhibits LHRH through negative feedback on the hypothalamic-pituitary axis. However, although DES was highly effective in achieving castrate levels of testosterone, the Veterans Administration Cooperative Urological Research Group (VACURG) studies raised serious concerns regarding cardiac events in patients receiving DES, particularly the 5 mg daily dose. The VACURG studies demonstrated that although DES had therapeutic efficacy comparable to orchiectomy, the 5 mg/d dose was associated with excess mortality from cardiovascular causes.7,8
Antiandrogens
Investigations into antiandrogenic compounds in the late 1960s and early 1970s led to the availability of several agents that inhibited or blocked the testosterone receptor with few or minor side effects.9,10 Antiandrogens can be classified as steroidal or nonsteroidal, the distinctions being the molecular chemical structure and the physiologic progestational effects of steroidal compounds, principally impotence and loss of libido. Nonsteroidal antiandrogens act only at the androgenic receptors and have no progestational side effects. This local effect results in increased hormone and serum testosterone, which preserves libido and potency in most patients. The nonsteroidal antiandrogens currently available include flutamide, nilutamide, and bicalutamide. The steroidal antiandrogens include cyproterone acetate (CPA), megestrol acetate, and medroxyprogesterone acetate (Table 1).
Table 1.
Steroidal and Nonsteroidal Antiandrogens
| Steroidal | Nonsteroidal |
|---|---|
| Megestrol acetate | Flutamide |
| Cyproterone acetate* | Nilutamide |
| Medroxyprogesterone | Bicalutamide |
Not available for prescription in the United States.
Luteinizing Hormone-Releasing Hormone Agonists
The availability of long-acting synthetic LHRH agonists in the 1980s revolutionized the hormonal treatment of prostate cancer, enabling many men to avoid the emotional and psychological effects of surgical castration. The administration of an LHRH agonist results in release and depletion of pituitary hormone, followed by a downregulation of LHRH receptors. As a result, the pituitary becomes refractory to further stimulation by LHRH, resulting in castrate levels of testosterone. However, LHRH agonists usually cause an early increase or surge in serum testosterone over the first 2 weeks of therapy, with castrate levels attained by 4 weeks.11 Several LHRH agonists are currently available and include goserelin, leuprolide, buserelin, and triptorelin. These compounds are available in various formulations but are commonly given as intramuscular or subcutaneous depots that last between 1 and 4 months, with longer acting implants/depots available or being developed. Luteinizing hormone-releasing hormone agonist therapy offers the advantages of tolerance and reversibility; however, these compounds have high repeating costs and result in a loss of libido, impotence, and hot flashes. Also of concern is the testosterone surge that occurs after initial LHRH administration.12 This surge in testosterone may cause tumor flare, resulting in an increase in pain and serious side effects such as ureteric obstruction and paralysis in patients with extensive spinal column metastases.13 The surge in testosterone may in most cases be prevented by the short-term administration of an antiandrogen.12
Gonadotropin-Releasing Hormone Antagonists
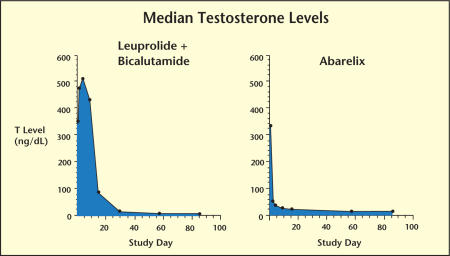
The GnRH antagonists represent the newest class of agents introduced for the hormonal treatment of prostate cancer. The advantages of GnRH antagonists are that they do not cause the initial surge in testosterone associated with LHRH agonists and they rapidly achieve castrate androgen levels.14 Abarelix (Plenaxis®; Praecis Pharmaceuticals Inc., Waltham, MA) is a GnRH antagonist available in depot injectable formulation. In clinical trials, monotherapy with abarelix was shown to achieve medical castration more rapidly than leuprolide alone or in combination with bicalutamide and without an early surge in testosterone (Figure 1).14–16 These studies also demonstrated that, unlike LHRH agonists, abarelix also suppresses follicle-stimulating hormone, which may contribute to the growth of prostate cancer cells.14–16
Figure 1.
Abarelix as monotherapy achieves medical castration significantly more rapidly than combination therapy and without the testosterone surge associated with luteinizing hormone-releasing hormone agonist therapy.16 T, testosterone.
Combined Androgen Blockade
Geller and colleagues confirmed significant androgenic stimulation of prostate cancer cells by adrenal androgens in men who had undergone medical or surgical castration.2 These investigators noted that in castrated men, the concentration of DHT in prostate tissue remained at up to 40% of the level detected in untreated men. They also noted that extremely small levels of DHT resulted in an increase in prostatic protein synthesis. Recognition of the role of adrenal androgens in prostate cancer led to the development of the therapeutic strategy known as CAB. This approach combines either a steroidal or a nonsteroidal antiandrogen with an LHRH agonist or orchiectomy to block androgens of both adrenal and testicular origin. Interest in CAB increased following a highly positive study by Labrie and colleagues.17 In this investigation, 37 patients with stage T3 and M+ prostate cancer received buserelin and nilutamide. None of the patients had received prior hormonal therapy. A positive objective response was reported in 97% of the patients. The authors thus suggested that first-line CAB produced response rates 25% to 30% higher than conventional testicular androgen ablation.17
Trials Supporting Combined Androgen Blockade
The excellent results reported by Labrie and colleagues were met with considerable skepticism, as the study had neither a large patient population nor a randomized design. Many controlled studies have since been initiated to test the hypothesis that CAB is superior to conventional monotherapy. The study designs have varied, and different LHRH agonists or orchiectomy have been used for castration in combination with different antiandrogens. Three independent randomized studies have shown significant benefits for CAB over castration alone in either time to progression or survival.18–20 They are summarized briefly below.
Study INT 0036
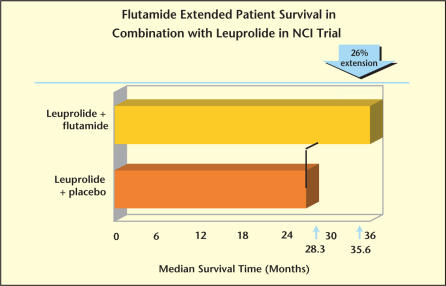
Study INT 0036, conducted by the Southwest Oncology Group (SWOG), randomized 603 patients with previously untreated stage D2 carcinoma of the prostate to leuprolide plus placebo versus leuprolide plus flutamide.18 The National Prostatic Cancer Project criteria were used to evaluate response. Patients who received CAB experienced significantly longer progression-free survival (16.5 vs 13.9 months, P = .039) and overall survival (35.6 vs 28.3 months, P = .035; Figure 2). Patients with minimal disease and good performance status should significantly benefit. However, this subgroup consisted of only 41 patients in each treatment arm. The conclusion of the INT 0036 study was that leuprolide combined with flutamide was superior to leuprolide alone and, thus, the extrapolation that CAB was superior to monotherapy (Figure 2).18
Figure 2.
In Study INT 0036, conducted by the Southwest Oncology Group, patients who received combination therapy with leuprolide and flutamide experienced a 26% increase in survival compared with patients receiving leuprolide plus placebo (35.6 months vs 28.3 months, respectively).18 NCI, National Cancer Institute.
EORTC 30853
The European Organization of Research and Treatment of Cancer (EORTC) conducted a phase III randomized study (EORTC 30853) that compared CAB (goserelin acetate, 3.6 mg every 4 weeks subcutaneously; plus flutamide, 250 mg tid orally) with bilateral orchiectomy in 310 patients.19 In the final analysis of this trial, with a median duration of follow-up of 7.2 years, the median duration of survival was 27 months on orchiectomy and 34 months on CAB.19 This statistically significant difference closely approximates that found in the INT 0036 study.18 Additionally, CAB was most beneficial to patients with minimal disease. The most frequent side effects reported in both treatment groups were hot flashes and gynecomastia, both of which were more common in patients who received the combination therapy. Hot flashes were reported for 59% of patients who underwent orchiectomy and 70% of patients treated with CAB. The corresponding incidence of gynecomastia was 8% and 22%, for orchiectomy and CAB, respectively.19
The International Anandron Study Group
The International Anandron Study Group compared bilateral orchiectomy alone versus bilateral orchiectomy plus 300 mg/d of nilutamide in 457 patients.20 After 8.5 years of follow-up, a 7-month increase was found in the CAB treatment group in both time to progression (21.2 months vs 14.7 months) and survival rate (37 months vs 29.8 months). The percentage of patients with a normal prostate-specific antigen (PSA) at 3 months was significantly greater (P < .001) in the nilutamide plus orchiectomy group (59%) than with orchiectomy alone (28%). Early normalization of PSA was predictive of improved long-term response to hormonal therapy in terms of time to disease progression and death.20
Trials Refuting Combined Androgen Blockade
Although these studies support the superiority of CAB over monotherapy, others refute these findings or showed trends that did not achieve statistical significance.21,22
INT 0105/SWOG 8894
The most significant of the studies to refute the benefits of CAB was INT 0105/SWOG 8894, a large randomized study that enrolled almost 1400 patients and compared bilateral orchiectomy alone and orchiectomy plus flutamide.22 This study failed to demonstrate the superiority of CAB versus monotherapy in terms of time to progression (20.4 months vs 18.6 months, respectively) and overall survival (33.5 months vs 29.9 months, respectively). It also failed to demonstrate an advantage of CAB in patients with minimal disease with respect to overall survival and progression-free survival. It did show a statistically significant difference in serum PSA response (74% vs 61.5%) in favor of the combination arm. However, the decrease in PSA levels did not correlate to a survival advantage, and this finding calls into question the validity of PSA level as a prognostic marker in advanced disease.22
Quality of Life and Combined Androgen Blockade
INT 0105/SWOG 8894 has been the only study to also enroll patients in a quality-of-life (QOL) protocol.23 Patients completed a comprehensive battery of QOL questions at assignment and then at 1, 3, and 6 months after initiating treatment. Most patients showed improvement, but those in the flutamide arm showed more statistically significant episodes of diarrhea and worse overall emotional functioning. Those receiving flutamide also more often discontinued the treatment and showed less overall improvement in most QOL dimensions, suggesting that the addition of flutamide might actually detract from the palliative benefits of bilateral orchiectomy.23
Meta-analysis of Controlled Studies
Most meta-analyses have reported slightly in favor of CAB for reasons ranging from slightly decreased rates of progression or slightly increased survival. The Prostate Cancer Trialists Collaborative Group (PCTCG) updated an overview of 27 randomized trials with 5932 deaths (72%) in 8275 patients.24 The 5-year survival rate was 23.6% and 25.4% in the monotherapy and combined therapy groups, respectively. This study subcategorized the results to evaluate different types of antiandrogens and castration. It appears that CAB using CPA is no more effective than castration alone and is slightly unfavorable in terms of benefit, while studies including only nilutamide and flutamide appear to be slightly favorable.24 In another meta-analysis of 20 randomized trials using nonsteroidal antiandrogens, Schmitt and colleagues estimated this improvement to be as high as 5%.25 The analysis from the PCTCG concluded that the addition of an antiandrogen was likely to confer approximately 2% to 3% survival advantage at 5 years.24
Currently, no clear consensus exists about the advantage of CAB as first-line therapy for metastatic prostate cancer over castration alone. A review of the current literature indicates that the original observations made by Labrie and colleagues concerning the significant advantage of CAB have not been confirmed to the extent hoped. Also, most studies found a higher incidence of adverse events in the CAB groups, which can be attributed to the addition of the antiandrogens.
Antiandrogen Monotherapy
The sole use of antiandrogens for initial hormonal therapy has a potential role in the treatment of advanced or metastatic prostate cancer as a means of satisfactorily controlling disease and maintaining quality of life. Direct inhibition of the testosterone receptor by antiandrogens allows for some hormonal effects of circulating testosterone, namely, the maintenance of sexual interest and function. However, initial comparisons of low-dose bicalutamide (50 mg) versus castration postponed enthusiasm for this approach, as response and survival parameters proved the superiority of medical and surgical castration despite improved QOL in antiandrogen monotherapy groups.9,10 Largescale randomized trials with higher doses of bicalutamide have provided mixed results in patients with metastatic and locally advanced T3/4 disease.26 However, bicalutamide monotherapy may still be an option in well-counseled, younger patients with metastatic disease who wish to maintain their potency.26
Initial Versus Delayed Hormone Therapy
Since the introduction of androgen withdrawal therapy, controversy has existed over its optimal timing. Many have advocated beginning treatment at the time of diagnosis in hopes of delaying disease progression and possibly prolonging survival. Others have argued that survival is not prolonged and the treatment may be deferred until symptoms develop.
Early studies by Nesbit and Baum provide evidence to support the immediate treatment of advanced prostate cancer.27 They compared patients who were treated with orchiectomy, DES, or both and then compared them with untreated historical controls. The treated group showed a 5-year survival rate of 34% versus 10% in the untreated group.27 These and other similar studies provided the basis for the early treatment of advanced prostate cancer.
VACURG Studies
The results of the VACURG studies suggested that delaying hormonal therapy did not compromise overall survival and that many of the patients died of causes other than prostate cancer.7,8 As a result, the pendulum shifted toward advocating delayed treatment for those with advanced prostate cancer. However, a stringent reanalysis of the VACURG data showed that younger patients with high-grade tumors and those with stage M+ disease derive a survival benefit from the early initiation of androgen withdrawal therapy.28
Medical Research Council Study
Two recent studies provide convincing clinical evidence supporting the early treatment of advanced prostate cancer: the randomized trial reported by the Medical Research Council (MRC)29 and the Eastern Cooperative Oncology Group (ECOG)/SWOG Stage D1(N+) study.30 The MRC study randomized 934 patients with locally advanced prostate cancer or asymptomatic metastasis to either immediate treatment (orchiectomy or LHRH agonist) or to the same treatment deferred until an indication occurred. This study showed that there was a more rapid local and distant disease progression in the deferred treatment group, as evidenced by an earlier onset of pain and an increased need for transurethral resection of the prostate. There was also a 2-fold increase in serious complications, such as pathologic fractures, spinal cord compression, and extraskeletal metastasis, in the deferred treatment group compared with those who received immediate treatment (Table 2).29
Table 2.
Summary of the Results from the Medical Research Council Prostate Cancer Working Party Investigators Group Trial
| Immediate Treatment | Deferred Treatment | |
|---|---|---|
| Group | Group | |
| Complication | (n = 469) | (n = 465) |
| Pain from metastasis | 121 | 211 |
| Need for transurethral | ||
| resection of the prostate | 65 | 141 |
| Pathologic fracture | 11 | 21 |
| Cord compression | 9 | 23 |
| Ureteric obstruction | 33 | 55 |
| Extraskeletal metastasis | 37 | 55 |
| Death from prostate cancer | 203 | 257 |
Source: Data from The Medical Research Council Prostate Cancer Working Party Investigators Group. Immediate versus deferred treatment for advanced prostatic cancer: initial results of the Medical Research Council Trial.29
The Eastern Cooperative Oncology Group (ECOG/SWOG) Trial
The ECOG trial randomized 98 patients with pathologically positive lymph nodes after radical prostatectomy with lymphadenectomy to immediate or delayed hormonal therapy with goserelin or orchiectomy.30 Survival in the delayed therapy and immediate therapy arms was 65% and 85%, respectively, at a median of over 7 years of follow-up. Patients receiving immediate therapy were 75% less likely to progress and die of prostate cancer.30
The appropriate time to initiate hormonal therapy for prostate cancer remains a matter of debate. Certainly, a patient with painful metastasis can expect symptomatic relief from castration. D1(N+) (lymph node positive) patients after prostatectomy and patients with advanced disease undergoing external beam radiation therapy have solid evidence that hormonal therapy delays progression and extends survival.30,31 Treatment of M0 and locally advanced cancer becomes less clear; however, the most recent findings of the MRC trial suggest that these patients will benefit from early initiation of androgen deprivation.29
Prostate-specific Antigen-only Recurrence
Patients with biochemical or PSA-only recurrence after local therapy present another group with debatable treatment options. Historically, these patients have been started on hormonal therapy if further local therapy is not indicated, despite a lack of direct data supporting this broad approach. Based on recent results by Moul and colleagues,32 starting hormonal therapy early, before PSA rises to 5 ng/mL (10 ng/mL in postprostatectomy patients with Gleason scores > 7 or PSA doubling time < 12 months), will delay time to clinical metastasis.
A recent investigation by Barqawi and colleagues suggests that the combination of the α-reductase inhibitor, finasteride, with low-dose flutamide may be an effective option for lowering PSA levels in patients with PSA-only recurrence and especially in older patients who have previously achieved a PSA nadir of 0.1 ng/mL. Theoretically, finasteride enhances the efficacy of antiandrogens by inhibiting α-reductase, thus limiting the intracellular concentration of DHT.33
Intermittent Hormone Therapy
Unfortunately, most prostate cancers eventually develop hormone-independent growth characteristics during continuous androgen deprivation as the cells lose the ability to undergo apoptosis (programmed cell death). Experimental animal models demonstrate that IAD may allow recovery of apoptosis and slow the progression to the androgen-independent state.34,35 Using this approach, patients receive hormonal therapy until PSA reaches a nadir. Subsequent androgen deprivation therapy is withheld until PSA begins to rise to a predetermined level. During the period when therapy is withheld, serum testosterone increases to normal levels. The side effects of testosterone deprivation, such as impotence and hot flashes, may resolve during this period.
Several small clinical trials with IAD have been conducted.35–38 In these studies, the response of serum PSA to treatment was predictive of a patient’s long-term prognosis, and failure of serum PSA to decrease to normal levels during induction usually signaled early progression to androgen independence and a poor prognosis. The evidence also suggests that 32 weeks of treatment is needed to bring the serum PSA level into the normal range.36
One randomized trial of IAD has been published to date.38 de Leval and colleagues examined 68 patients with advanced or relapsing prostate cancer and randomized these patients to CAD or IAD with the primary outcome as time to androgen independence.38 The 3-year progression rate was 39% in the CAD group and 7% in the IAD group. Although promising, IAD should be considered investigational. A large multicenter NCI/SWOG trial is underway in patients with metastatic disease that should definitively answer this question in this population. Other randomized trials are ongoing in Europe. It is hoped that several issues will be addressed in future IAD protocols: 1) appropriate populations; 2) type of therapy (monotherapy or CAB); 3) optimal point of cessation and reinitiation of therapy; and 4) cost/QOL analyses.
Combination Chemotherapy and Hormonal Therapy for Advanced Prostate Cancer
Alternative and additional therapies in patients with hormone refractory, metastatic, and locally advanced disease have been investigated in an effort to improve survival. Despite poor outcomes in the past, the availability of new chemotherapeutic agents has resulted in renewed interest in chemotherapy for advanced prostate cancer. A recent study by Wang and colleagues examined the role of mitozantrone as an adjuvant to hormonal treatment in men with locally advanced prostate cancer.39 Ninety-six patients were entered into a stratified, randomized single institution study of hormonal therapy with an LHRH agonist and flutamide with or without 4 cycles of adjuvant mitozantrone. The results show that patients with localized prostate cancer receiving chemotherapy had a higher initial objective response rate (95% vs 53%, P = .008) and longer median survival (60 vs 36 months, P = .04) than patients treated with hormonal therapy alone. The study demonstrated no advantages to chemotherapy in patients with metastatic disease. There were insignificant advantages to chemotherapy in overall response rates (55% vs 39%, P = .3) and PSA responses (82% vs 64%, P = .11). There was no difference between the patient groups in time to treatment failure.
The Future
Despite our increased understanding of the hormonal influences on prostate cancer and the increasing array of therapeutic options, no significant improvement in survival benefit has been demonstrated for any of the currently available treatments. Novel therapeutic strategies are thus urgently needed to improve long-term survival in patients with advanced prostate cancer.
Main Points.
Despite over 6 decades of research and clinical experience, few areas of medicine generate as much uncertainty and debate as the hormonal manipulation of advanced prostate cancer.
The availability of long-acting synthetic luteinizing hormone-releasing hormone (LHRH) agonists in the 1980s revolutionized the hormonal treatment of prostate cancer, enabling many men to avoid the emotional and psychological effects of surgical castration.
Gonadotropin-releasing hormone (GnRH) antagonists represent the newest class of agents introduced for the hormonal treatment of prostate cancer. The advantages of GnRH antagonists are that they do not cause the initial surge in testosterone associated with LHRH agonists and they rapidly achieve castrate androgen levels.
Recognition of the role of adrenal androgens in prostate cancer led to the development of the therapeutic strategy known as combined androgen blockade. This approach combines either a steroidal or a nonsteroidal antiandrogen with an LHRH agonist or orchiectomy to block androgens of both adrenal and testicular origin.
Alternative and additional therapies in patients with hormone refractory, metastatic, and locally advanced disease have been investigated in an effort to improve survival. Despite poor outcomes in the past, the availability of new chemotherapeutic agents has resulted in renewed interest in chemotherapy for advanced prostate cancer.
References
- 1.Huggins C, Hodges CU. Studies on prostate cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293. [Google Scholar]
- 2.Geller J, Albert J, Vik A. Advantages of total androgen blockade in the treatment of advanced prostate cancer. Semin Oncol. 1988;15(suppl 1):53. [PubMed] [Google Scholar]
- 3.Huggins C, Scott WW. Bilateral adrenalectomy in prostatic cancer. Ann Surg. 1945;122:1031. [PubMed] [Google Scholar]
- 4.Huggins C, Bergenstal DM. Effect of bilateral adrenalectomy on certain human tumors. Proc Natl Acad Sci USA. 1952;38:73. doi: 10.1073/pnas.38.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller GM, Hinman F., Jr Cortisone therapy in advanced carcinoma of the prostate. J Urol. 1954;72:485–496. doi: 10.1016/S0022-5347(17)67614-6. [DOI] [PubMed] [Google Scholar]
- 6.Crawford DE, Ahmann FR, Davis MA, et al. Aminoglutethimide in metastatic adenocarcinoma of the prostate. Prog Clin Biol Res. 1985;243:283–289. [PubMed] [Google Scholar]
- 7.Veterans Administration Cooperative Urological Research Group, authors. Treatment and survival of patients with cancer of the prostate. Surg Gynecol Obstet. 1967;124:1011. [PubMed] [Google Scholar]
- 8.Byar DP, Corle DK. Hormone therapy for prostate cancer: results of the Veterans Administration Cooperative Urological Research Group studies. Natl Cancer Inst Monogr. 1988;7:165. [PubMed] [Google Scholar]
- 9.Kaisary AV, Tyrrell CJ, Beacock C, et al. A randomised comparison of monotherapy with Casodex 50 mg daily and castration in the treatment of metastatic prostate carcinoma. Casodex Study Group. Eur Urol. 1995;28:215–222. doi: 10.1159/000475054. [DOI] [PubMed] [Google Scholar]
- 10.Iversen P, Tveter K, Varenhorst E. Randomised study of Casodex 50 MG monotherapy vs orchidectomy in the treatment of metastatic prostate cancer. The Scandinavian Casodex Cooperative Group. Scand J Urol Nephrol. 1996;30:93–98. doi: 10.3109/00365599609180896. [DOI] [PubMed] [Google Scholar]
- 11.The Leuprolide Study Group, authors. Leuprolide versus diethylstilbesterol for metastatic prostate cancer. N Engl J Med. 1984;311:1281–1286. doi: 10.1056/NEJM198411153112004. [DOI] [PubMed] [Google Scholar]
- 12.Labrie F, Dupont A, Belanger A, Lachance R. Flutamide eliminates the risk of disease flare in prostatic cancer patients treated with luteinizing hormone-releasing hormone agonist. J Urol. 1987;138:804–806. doi: 10.1016/s0022-5347(17)43380-5. [DOI] [PubMed] [Google Scholar]
- 13.el-Rayes BF, Hussain MH. Hormonal therapy for prostate cancer: past, present and future. Expert Rev Anticancer Ther. 2002;2:37–47. doi: 10.1586/14737140.2.1.37. [DOI] [PubMed] [Google Scholar]
- 14.Beer TM, Garzotto M, Eilers KM, et al. Targeting FSH in androgen-independent prostate cancer progressing after orchiectomy. Urology. 2004;63:342–347. doi: 10.1016/j.urology.2003.09.045. [DOI] [PubMed] [Google Scholar]
- 15.McLeod DG, Zinner N, Tomera K, et al. A phase 3, multicenter, open-label randomized study of abarelix versus leuprolide acetate in men with prostate cancer. Urology. 2003;61(suppl 1):3–7. doi: 10.1016/s0090-4295(01)01342-5. [DOI] [PubMed] [Google Scholar]
- 16.Trachtenberg J, Gittleman M, Steidle C, et al. A phase 3, multicenter, open-label randomized study of abarelix versus leuprolide plus daily antiandrogen in men with prostate cancer. J Urol. 2002;167:1670–1674. doi: 10.1097/00005392-200204000-00021. [DOI] [PubMed] [Google Scholar]
- 17.Labrie F, Dupont A, Belanger A, et al. New approach in the treatment of prostate cancer: complete instead of partial withdrawal of androgens. Prostate. 1983;4:579–594. doi: 10.1002/pros.2990040605. [DOI] [PubMed] [Google Scholar]
- 18.Crawford ED, Eisenberger MA, McLeod DG, et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med. 1989;321:419–424. doi: 10.1056/NEJM198908173210702. [DOI] [PubMed] [Google Scholar]
- 19.Denis LJ, Keuppens F, Smith PH, et al. Maximal androgen blockade: final analysis of EORTC phase III trial 30853. Eur Urol. 1998;33:144–151. doi: 10.1159/000019546. [DOI] [PubMed] [Google Scholar]
- 20.Dijkman GA, Janknegt RA, De Reijke TM, Debruyne FM. Long-term efficacy and safety of nilutamide plus castration in advanced prostate cancer, and the significance of early prostate specific antigen normalization. International Anandron Study Group. J Urol. 1997;158:160–163. doi: 10.1097/00005392-199707000-00051. [DOI] [PubMed] [Google Scholar]
- 21.Iversen P, Rasmussen F, Klarskov P, Christensen IJ. Long-term results of Danish Prostatic Cancer Group trial 86. Goserelin acetate plus flutamide versus orchiectomy in advanced prostate cancer. Cancer. 1993;72(suppl 12):3851. doi: 10.1002/1097-0142(19931215)72:12+<3851::aid-cncr2820721717>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 22.Eisenberger MA, Blumenstein BA, Crawford ED, et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med. 1998;339:1036–1042. doi: 10.1056/NEJM199810083391504. [DOI] [PubMed] [Google Scholar]
- 23.Moinpour CM, Savage MJ, Troxel A, et al. Quality of life in advanced prostate cancer: results of a randomized therapeutic trial. J Natl Cancer Inst. 1998;90:1537–1544. doi: 10.1093/jnci/90.20.1537. [DOI] [PubMed] [Google Scholar]
- 24.Prostate Cancer Trialists’ Collaborative Group, authors. Maximum androgen blockade in advanced prostate cancer: an overview of the randomized trials. Lancet. 2000;355:1491–1498. [PubMed] [Google Scholar]
- 25.Schmitt B, Wilt TJ, Schellhammer PF, et al. Combined androgen blockade with nonsteroidal antiandrogens for advanced prostate cancer: a systematic review. Urology. 2001;57:727–732. doi: 10.1016/s0090-4295(00)01086-4. [DOI] [PubMed] [Google Scholar]
- 26.Iversen P, Tyrrell CJ, Kaisary AV, et al. Bicalutamide monotherapy compared with castration in patients with nonmetastatic locally advanced prostate cancer: 6.3 years of followup. J Urol. 2000;164:1579–1582. [PubMed] [Google Scholar]
- 27.Nesbit RM, Baum WC. Endocrine control of prostatic carcinoma: clinical and statistical survey of 1818 cases. J Am Med Assoc. 1950;143:1317. doi: 10.1001/jama.1950.02910500019005. [DOI] [PubMed] [Google Scholar]
- 28.Christensen MM, Aagaard J, Madsen PO. Reasons for delay of endocrine treatment in cancer of the prostate (until symptomatic metastases occur) Prog Clin Biol Res. 1990;359:7–14. [PubMed] [Google Scholar]
- 29.The Medical Research Council Prostate Cancer Working Party Investigators Group, authors. Immediate versus deferred treatment for advanced prostatic cancer: initial results of the Medical Research Council Trial. Br J Urol. 1997;79:235–246. doi: 10.1046/j.1464-410x.1997.d01-6840.x. [DOI] [PubMed] [Google Scholar]
- 30.Messing EM, Manola J, Sarosdy M, et al. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with nodepositive prostate cancer. N Engl J Med. 1999;341:1781–1788. doi: 10.1056/NEJM199912093412401. [DOI] [PubMed] [Google Scholar]
- 31.Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360:103–106. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 32.Moul JW, Wu H, Sun L, et al. Early versus delayed hormonal therapy for prostate specific antigen only recurrence of prostate cancer after radical prostatectomy. J Urol. 2004;171:1141–1147. doi: 10.1097/01.ju.0000113794.34810.d0. [DOI] [PubMed] [Google Scholar]
- 33.Barqawi AB, Moul JW, Ziada A, et al. Combination of low-dose flutamide and finasteride for PSA-only local recurrent prostate cancer after primary therapy. Urology. 2003;62:872–876. doi: 10.1016/s0090-4295(03)00667-8. [DOI] [PubMed] [Google Scholar]
- 34.Bruchovsky N, Rennie PS, Coldman AJ, et al. Effects of androgen withdrawal on the stem cell composition of the Shionogi carcinoma. Cancer Res. 1990;50:2275–2282. [PubMed] [Google Scholar]
- 35.Gleave M, Bruchovsky N, Bowden M, et al. Intermittent androgen suppression prolongs time to androgen-independent progression in the LnCAP prostate tumor model [abstract] J Urol. 1994;151:457A. [Google Scholar]
- 36.Gleave M, Bruchovsky N, Goldenberg SL, et al. Intermittent androgen suppression for prostate cancer: rationale and clinical experience. Eur Urol. 1998;34(suppl 3):37. doi: 10.1159/000052297. [DOI] [PubMed] [Google Scholar]
- 37.Grossfeld GD, Chaudhary UB, Reese DM, et al. Intermittent androgen deprivation: update of cycling characteristics in patients without clinically apparent metastatic prostate cancer. Urology. 2001;58:240–245. doi: 10.1016/s0090-4295(01)01114-1. [DOI] [PubMed] [Google Scholar]
- 38.de Leval J, Boca P, Yousef E, et al. Intermittent versus continuous total androgen blockade in the treatment of patients with advanced hormone- naive prostate cancer: results of a prospective randomized multicenter trial. Clin Prostate Cancer. 2002;1:163–171. doi: 10.3816/cgc.2002.n.018. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Halford S, Rigg A, et al. Adjuvant mitozantrone chemotherapy in advanced prostate cancer. BJU Int. 2000;86:675–680. doi: 10.1046/j.1464-410x.2000.00894.x. [DOI] [PubMed] [Google Scholar]