Abstract
Luteinizing hormone-releasing hormone (LHRH) agonist therapy to induce medical castration has become the most common form of hormonal therapy for advanced and metastatic prostate cancer. When treatment is started, LHRH agonists initially stimulate the release of LH, causing a surge in serum testosterone that can precipitate a “flare” phenomenon or worsening of disease, particularly in patients with bone metastatic disease. Gonadotropin-releasing hormone (GnRH) receptor antagonism represents a newer approach to medical castration. Abarelix is a pure GnRH receptor antagonist that is devoid of any LHRH agonist activity. Results from 1 phase II and 3 phase III clinical trials demonstrate that abarelix produces medical castration more quickly and without causing testosterone surge, as compared with LHRH agonists with or without a nonsteroidal antagonist. The safety profile in terms of adverse events is comparable between the 2 types of treatment, but the lack of testosterone surge with abarelix might confer a safety advantage by abolishing the risk of a disease flare.
Key words: Prostate cancer, Testosterone surge, Luteinizing hormone-releasing hormone agonists, Abarelix, Leuprolide, Goserelin, Bicalutamide
Prostate cancer is the most common malignancy affecting men in many Western countries.1,2 When prostate cancer is diagnosed at an advanced stage or recurs after definitive therapy, hormonal therapy in the form of androgen ablation is usually the treatment of choice.3
Because prostate tumors are, at least initially, predominantly made of androgen-dependent cells, the removal of endogenous androgen represents a rational approach to prostate cancer therapy. Huggins and colleagues4,5 first demonstrated the effectiveness of this strategy more than 60 years ago in their landmark study of surgical castration and medical castration with estrogen therapy.
Since that time, luteinizing hormone-releasing hormone (LHRH) agonists have become the most common form of hormonal therapy for advanced and metastatic prostate cancer. LHRH agonists act by releasing LH, which controls the production of testosterone, from the anterior pituitary. When treatment is started, LHRH agonists initially stimulate the release of LH, causing a surge in serum testosterone that can precipitate a “flare” phenomenon or worsening of disease, particularly in patients with bone metastatic disease.6,7 With continued therapy, however, these drugs ultimately downregulate the gonadotropin-releasing hormone (GnRH) receptors in the pituitary that regulate LH secretion and, in turn, suppress production of testosterone and its active intracellular metabolite dihydrotestosterone (DHT), resulting in chemical castration.8 Antiandrogens are often used in combination with LHRH agonists to lessen the clinical sequelae of the initial testosterone surge,9 but they might cause adverse events on their own.8
GnRH receptor antagonism represents a newer approach to medical castration.10,11 Abarelix is a pure GnRH receptor antagonist that is devoid of any LHRH agonist activity.12 After initial pharmacologic and pharmacokinetic studies,13–15 abarelix was tested in prostate cancer patients who were candidates for initial hormonal therapy. The first results were described after phase II studies,13,16 followed by phase III studies.17,18 After this initial clinical research, further evaluation of abarelix in the management of advanced metastatic prostate cancer was conducted in an 81-patient study.19 The aim of this article is to provide an overview of the current available data on the GnRH antagonist abarelix in the management of patients with advanced, metastatic prostate cancer who qualify for hormonal treatment.
Phase II Studies
Tomera and colleagues16 reported the initial phase II data. In their study, they compared the endocrinologic and biochemical efficacy of abarelix depot with that of LHRH agonists administered with or without antiandrogen to a prospective concurrent control cohort. A total of 242 prostate cancer patients requiring initial hormonal treatment were included in this open-label study: 209 patients received abarelix depot and 33 an LHRH agonist with or without an antiandrogen. Abarelix depot (total 100 mg) was delivered intramuscularly (IM) every 28 days, with an additional injection on day 15. LHRH agonists with or without antiandrogen were administered according to the depot formulation used. Endocrine efficacy was measured by the absence of testosterone surge and rapidity of castration onset. The rate of prostate-specific antigen (PSA) decrease was also assessed.
The prespecified endocrine endpoint was the medical castration (defined as a testosterone level ≤ 50 ng/dL) success rate over 12 weeks. The castration rate on day 8 was the primary endpoint and was a measure of the rapidity of action of abarelix. A secondary endpoint was testosterone surge (defined as an increase in testosterone by 10% above baseline values) on any of days 2, 4, or 8. Testosterone, LH, follicle-stimulating hormone (FSH), DHT, and PSA were measured on days 1, 13, 27, 57, and every 28 days thereafter.
During week 1, no patient treated with abarelix depot had testosterone surge. In contrast, two thirds of those receiving LHRH agonists (22 of 33) experienced surge, and concomitant administration of antiandrogen had no effect on surge. During the first week of drug administration, medical castration was achieved in 75% of patients treated with abarelix depot, compared with none of those treated with LHRH agonists. Decrease of PSA occurred more quickly, with no flare or surge, in patients treated with abarelix depot. A careful safety evaluation of abarelix depot administration was conducted throughout the study, and abarelix depot was well tolerated in all patients. This phase II study16 demonstrated that abarelix depot represented a new class of hormonal therapy, the GnRH antagonist, which produced medical castration while avoiding the testosterone surge characteristic of LHRH agonists.
These phase II results led to the initiation of 2 phase III studies in the United States and 1 phase III study in Europe.
Phase III Studies
US Phase III Studies
The first phase III study was an open-label, randomized study of abarelix versus leuprolide acetate in men with prostate cancer.17 Levels of testosterone and other hormones were evaluated in 269 patients randomized 2 to 1 to receive open-label abarelix (100 mg) or leuprolide acetate (7.5 mg IM). Baseline patient demographics and disease characteristics are shown in Table 1.
Table 1.
Baseline Demographic Data and Disease Characteristics from a Phase III Study of Abarelix Depot Versus Leuprolide Depot for the Treatment of Prostate Cancer
| Leuprolide Depot | Abarelix Depot | |
|---|---|---|
| (n = 89) | (n = 180) | |
| Race (%) | ||
| Caucasian | 82 | 88 |
| African American | 9 | 6 |
| Hispanic | 7 | 3 |
| Asian | 2 | 3 |
| Median age (y) | 74 | 73 |
| Median weight (lb) | 184 | 190 |
| Body mass index (kg/m2) | 27.4 | 27.5 |
| Reason for treatment (%) | ||
| D1/D2 stage | 8 | 8 |
| Rising PSA | 33 | 37 |
| NHT | 36 | 37 |
| IHT | 24 | 17 |
| None of the above | 0 | 1 |
| Baseline PSA (ng/mL) (%) | ||
| 0–<4 | 17 | 17 |
| 4–<10 | 36 | 33 |
| 10–20 | 22 | 21 |
| >20 | 24 | 26 |
| Unknown | 1 | 4 |
PSA, prostate-specific antigen; NHT, neoadjuvant hormone therapy; IHT, intermittent hormone therapy.
McLeod and colleagues17 reported the results of the first 84 days of the study. The primary efficacy endpoints included avoidance of testosterone surge, castration on day 8, and achievement and maintenance of castration from days 29 through 85. The secondary endpoints included castration on days 2, 4, and 15, a reduction in PSA level, and measurements of other hormones (DHT, LH, and FSH). Patients were monitored for clinical adverse events and laboratory abnormalities.
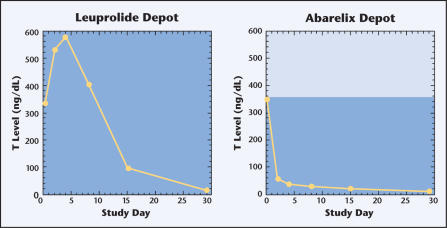
As in the phase II study discussed above, significant differences were seen in the occurrence of testosterone surge: 0% of patients in the abarelix group, compared with 82% of men in the leuprolide acetate group (P < .001) (Figure 1). Rapid medical castration was seen with abarelix in 25% of men 1 day after treatment and 78% after 7 days. Conversely, none of the leuprolide acetate-treated men demonstrated medical castration on either day. A comparable percentage of men achieved and maintained castration between days 29 and 85 in each group (Table 2). In abarelix-treated patients, PSA levels showed a statistically significant decrease for the first month. DHT, LH, and FSH showed similar rapid reductions without an initial increase. Overall, adverse events occurred equally across treatment groups, and most were sequelae of comorbid disorders.
Figure 1.
Testosterone (T) levels in a phase III study comparing leuprolide depot with abarelix depot for the treatment of prostate cancer.
Table 2.
Median Testosterone Levels (ng/dL) in a Phase III Study of Abarelix Depot Versus Leuprolide Depot for the Treatment of Prostate Cancer
| Leuprolide Depot | Abarelix Depot | ||
|---|---|---|---|
| (n = 89) | (n = 180) | P Value* | |
| Baseline | 338 | 350 | .90 |
| Day 2 | 529 | 59 | <.001 |
| Day 4 | 578 | 37 | <.001 |
| Day 8 | 406 | 29 | <.001 |
| Day 15 | 94 | 20 | <.001 |
| Day 29 | 15 | 11 | † |
| Day 57 | 10 | 12 | † |
| Day 85 | 9 | 15 | † |
| Day 113 | 8 | 11 | † |
| Day 141 | 8 | 13 | † |
| Day 169 | 9 | 15 | † |
Wilcoxon rank sum test.
Not determined per the statistical analysis plan.
This phase III study demonstrated that, compared with leuprolide acetate, treatment with abarelix was more successful at avoiding testosterone surge. Abarelix also caused testosterone suppression more rapidly, with a higher rate of medical castration 1 day after treatment and greater reductions in testosterone LH, FSH, and DHT during the first 2 weeks of treatment. The achievement and maintenance of castration was comparable between the 2 groups. Apart from the consequences of androgen deprivation and comorbidity, toxicity of treatment was minimal.
In the second phase III study, reported by Trachtenberg and colleagues,18 a similar comparison was performed, whereby the endocrine-logic and biochemical efficacy of abarelix depot was compared this time with the combination of an LHRH and a nonsteroidal antiandrogen. In this study, 255 patients were randomized 2 to 1 to receive open-label abarelix depot (100 mg) or leuprolide acetate (7.5 mg IM) on days 1, 29, 57, 85, 113, and 141 for 24 weeks. The patients in the abarelix group received an additional injection on day 15 and those in the leuprolide acetate group received bicalutamide (50 mg) daily. Baseline demographic data and disease characteristics are shown in Table 3. Patients could continue treatment with study drug for an additional 28 weeks. Comparative rates of avoidance of testosterone surge (>10% increase) within 7 days of the first injection and the rapidity of achieving castrate levels (≤ 50 ng/dL or less) of serum testosterone on day 8 were the efficacy endpoints. Patients were monitored for adverse events and laboratory abnormalities.
Table 3.
Baseline Demographic Data and Disease Characteristics from a Phase III Study of Abarelix Depot Versus Leuprolide Depot Plus Bicalutamide for the Treatment of Prostate Cancer
| Leuprolide Depot | ||
|---|---|---|
| Plus Bicalutamide | Abarelix Depot | |
| (n = 83) | (n = 168) | |
| Race (%) | ||
| Caucasian | 83 | 80 |
| African American | 12 | 13 |
| Hispanic | 2 | 5 |
| Asian | 2 | 2 |
| Other | 0 | 1 |
| Median age (y) | 74 | 73 |
| Median weight (lb) | 179 | 183 |
| Body mass index (kg/m2) | 26.3 | 26.5 |
| Reason for treatment (%) | ||
| D1/D2 stage | 5 | 7 |
| Rising PSA | 43 | 36 |
| NHT | 40 | 40 |
| IHT | 12 | 17 |
| None of the above | 0 | 1 |
| Baseline PSA (ng/mL) (%) | ||
| 0–<4 | 22 | 27 |
| 4–<10 | 46 | 30 |
| 10–20 | 20 | 18 |
| >20 | 11 | 23 |
| Unknown | 1 | 1 |
PSA, prostate-specific antigen; NHT, neoadjuvant hormone therapy; IHT, intermittent hormone therapy.
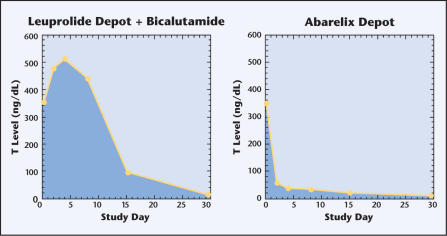
The study showed that, compared with combination LHRH-antiandrogen therapy, abarelix caused less testosterone surge (P < .001) and produced a more rapid reduction of testosterone to castrate levels on day 8 (P < .001) (Figure 2, Table 4). No significant difference was seen between the groups in the initial rate of decline of serum PSA or the ability to achieve and maintain castrate levels of testosterone. No unusual or unexpected adverse events were reported.
Figure 2.
Testosterone (T) levels in a phase III study comparing leuprolide depot plus bicalutamide with abarelix depot for the treatment of prostate cancer.
Table 4.
Median Testosterone Levels (ng/dl) in a Phase III Study of Abarelix Depot Versus Leuprolide Depot Plus Bicalutamide for the Treatment of Prostate Cancer
| Leuprolide Depot | |||
|---|---|---|---|
| Plus Bicalutamide | Abarelix Depot | ||
| (n = 83) | (n = 168) | P Value* | |
| Baseline | 353 | 340 | .79 |
| Day 2 | 480 | 58 | <.001 |
| Day 4 | 512 | 40 | <.001 |
| Day 8 | 435 | 35 | <.001 |
| Day 15 | 90 | 22 | <.001 |
| Day 29 | 16 | 10 | † |
| Day 57 | 10 | 9 | † |
| Day 85 | 10 | 14 | † |
| Day 113 | 8 | 9 | † |
| Day 141 | 11 | 12 | † |
| Day 169 | 10 | 11 | † |
Wilcoxon rank sum test.
Not determined per the statistical analysis plan.
From this study it could be concluded that abarelix monotherapy achieves medical castration significantly more rapidly than combination therapy and avoids the testosterone surge characteristic of agonist therapy.
European Phase III Study
The European study, reported by Debruyne and colleagues,20 also aimed at comparing the efficacy and safety of abarelix with those of goserelin depot (LH hormone agonist) combined with bicalutamide, a non-steroidal androgen (a combination treatment used commonly in Europe in patients with advanced prostate cancer). A total of 177 men were randomly assigned to receive abarelix depot (100 mg) on days 1, 15, and 29 and then every 28 days thereafter or goserelin depot (3.6 mg) every 28 days plus bicalutamide (50 mg) once daily for 48 weeks. The primary efficacy endpoint was the time to induction of medical castration (serum testosterone < 50 ng/dL) during the first 12 weeks of therapy.
As in the US phase III study comparing abarelix with LHRH-antiandrogen combination therapy, this study demonstrated that abarelix induced castration significantly earlier than goserelin plus bicalutamide (median 7 vs 21 days, P < .001). In addition, a significantly higher percentage of patients receiving abarelix were at castrate serum testosterone levels on day 3 (35% vs 0, P < .001). Testosterone surge was not seen with abarelix but occurred in 96% of patients in the goserelin-plus-bicalutamide group (P < .001). Serum LH levels were significantly lower in the abarelix group on days 1, 7, 14, and 21 (P < .001), and PSA levels were significantly lower on day 7 (P = .047). Escape from castration after 12 weeks of therapy (22% vs 8%, P = .007) and early withdrawals due to lack of endocrine efficacy (9% vs 0, P = .003) occurred more commonly in the abarelix group. Disease progression occurred in 9% of both treatment groups, and the rates were similar between treatments for patients entering with rising PSA levels and for those enrolling with stage D1/D2 disease. The incidence of adverse events and discontinuations due to these events occurred at similar rates in the 2 groups. The most common treatment-related events were asthenia (abarelix group) and gynecomastia (goserelin-plus- bicalutamide group). In addition, symptomatic increases in liver function test results were observed in both groups with similar frequency.
Study in the Indicated Patient Population
Effects of Abarelix in Advanced Symptomatic Prostate Cancer Patients
An open label, multicenter, uncontrolled study (N=81) of abarelix19 was conducted in 72 evaluable men (9 men were not included in the efficacy evaluation owing to regulatory noncompliance at their site) with advanced symptomatic prostate cancer who were at risk for clinical exacerbation (“clinical flare”) if treated with an LHRH agonist. This study defined the patient population approved in the United States for the use of abarelix. Eligible patients had at least 1 of the 4 following conditions: bone pain from skeletal metastases, retroperitoneal adenopathy causing ureteral obstruction, impending neurological compromise, or the presence of an enlarged prostate gland or pelvic mass causing bladder neck outlet obstruction. The study objective was to demonstrate that such patients could avoid surgical castration through at least 12 weeks of treatment.
Results
Efficacy. All patients were able to avoid surgical castration. Although the study was not designed to assess specific clinical outcomes, outcomes consistent with the expected benefits of castration and avoidance of adverse consequences of clinical flare were observed and included relief of bladder outlet and ureteral obstruction; decreased bone pain and need for narcotic analgesics; avoidance of neurologic compromise, including spinal cord compression in those with vertebral or epidural metastases.
Sixty-five patients (90%) experienced improvement in at least 1 baseline sign or symptom of prostate cancer (other than PSA) between Day 29 and Day 169. None of the patients with impending neurological compromise at study entry developed spinal cord compression during the study. At treatment Day 85, 60% of patients had improvement in bladder neck obstruction; 76% of patients with urinary catheters at baseline no longer required a catheter by Day 85; 43% of patients with hydronephrosis at baseline had no evidence of this on Day 85.
Safety. At least 1 adverse event, generally comorbid disorder, underlying malignancy, or effect of medical castration, was experienced by 95% of 81 patients; 41% experienced at least 1 potentially study-related event. Nine events in 6 patients were reported as severe and treatment related; none was considered life-threatening. Three of 81 patients experienced an immediate-onset systemic allergic reaction within minutes of receiving abarelix. The reactions were urticaria (Day 15), urticaria and pruritis (Day29), and hypotension and syncope (Day 141).25 All recovered uneventfully. Slight decreases in mean and median hemoglobin and hematocrit were observed. Three out of 78 patients had elevations of aspartate aminotransferase; two of these also had elevations of alanine aminotransferase although both values returned to normal or near normal with continued dosing in 2 of the 3 patients (3rd lost to followup).
Discussion
The early and rapid achievement of medical castration is essential for the successful treatment of prostate cancer, increasing the chances for cancer remission, palliation, and survival. The results of the above-mentioned studies demonstrate that abarelix reduces serum testosterone to castrate levels more rapidly than LHRH agonists with or without an antiandrogen. Reductions in testosterone were evident after 1 day of treatment, and castration was achieved after a median of 7 days in the abarelix group. Importantly, medical castration with abarelix was not associated with a testosterone surge, as seen in most patients treated with LHRH agonists.
Although antiandrogens can be used to ameliorate the clinical sequelae of these surges, these drugs might cause adverse events, most commonly diarrhea, liver function abnormalities, pulmonary fibrosis, and visual disturbances, and they do not actually address the biochemical surges in LH and testosterone.8 The lack of testosterone surge with abarelix treatment allows the potential risks associated with the flare phenomenon to be avoided and circumvents the need for adding more drugs to the treatment regimen.6,7
The differing profile of abarelix as compared with goserelin plus bicalutamide reflects the different mechanisms of the 2 regimens. Goserelin is an agonist analogue of GnRH (LHRH), and as such it initially stimulates GnRH receptors to increase LH release before desensitizing the receptor to further activation.21 In contrast, abarelix is a pure GnRH receptor antagonist, thus blocking LH production from the start of therapy.12 Consequently, hormone levels can begin to decline on the first day of treatment, and as a result, castrate levels of serum testosterone and lower levels of DHT, LH, and FSH are achieved more quickly. All studies demonstrated this effect, regardless of whether abarelix was compared with leuprolide acetate monotherapy or the combination with a nonsteroidal antiandrogen. In all these studies, abarelix did not cause a testosterone surge in any patient. The immediate reduction in hormone levels with abarelix likely accounts for an earlier reduction in PSA levels. PSA levels were significantly lower in the abarelix group on day 7.
In the European study,20 escape from castration was found to be higher in the abarelix group, and the time to escape was significantly shorter. Most escapes, however, occurred after at least 24 weeks of treatment. Serial PSA measurements might be an appropriate surrogate for determining the clinical relevance of testosterone fluctuations during escape, particularly because clinicians often consider changes in PSA when making treatment decisions. In the European study, the clinical relevance of escape from castration was evaluated in a blinded manner to determine whether the changes in PSA, taking into consideration the normal variations in PSA over time, would have necessitated a change in therapy. For most patients, especially those with a rising PSA value or stage D1 disease, the escapes from castration were of little clinical relevance.
During follow-up in the European study, the rate of disease progression was also evaluated. The rate of disease progression observed in the abarelix-treated patients was similar to that seen in the goserelin-plus-bicalutamide group and consistent with rates of progression in patients treated with standard hormonal therapies through 1 year of treatment.22 Indeed, in stage D2 patients receiving hormonal therapy, disease progression occurs commonly within 1 year.
FSH might be a possible source of stimulation for androgen-independent prostate cancer. FSH receptors are expressed on androgen-independent prostate cancer cells, as well as in malignant prostate tissue, and FSH is capable of stimulating proliferation of these cells.23 In all the studies discussed here, abarelix produced an immediate and sustained reduction in FSH, whereas FSH levels increased initially with LHRH agonist therapy, fell to a nadir by 3 weeks, and then trended upward for the remainder of the study. Garnick and colleagues13 also observed this when they evaluated the differential effects of abarelix on FSH. However, it remains to be demonstrated that the greater effect on FSH induced by abarelix translates into clinical efficacy in patients with androgen-independent prostate cancer. In a recent phase II study, abarelix was administered to 20 men with prostate cancer who had progressed during LHRH agonist therapy or with bilateral orchiectomy.24 Serum FSH levels were reduced by more than 50% from baseline and remained suppressed after 20 weeks of treatment. However, only 2 patients remained stable during abarelix therapy without rising PSA levels or other evidence of disease progression. Further investigation is needed to determine whether patients with androgen-independent disease have tumors that are receptive to FSH levels and FSH-receptive expression.
The safety of abarelix was comparable to that of LHRH agonists with or without antiandrogen. Increased liver enzymes and aggravated malignant neoplasm were the most common causes of treatment discontinuation in the European study, and they occurred at similar rates in the 2 groups.
In summary, it can be concluded that abarelix produces medical castration more quickly and without causing testosterone surge, as compared with LHRH agonists with or without a non-steroidal antiandrogen. Although the safety profile in terms of adverse events was comparable between the 2 study groups in all studies, the lack of testosterone surge with abarelix might confer a safety advantage by abolishing the risk of a disease flare, which is particularly relevant in patients with stage D2 disease.
Main Points.
The early and rapid achievement of medical castration is essential for the successful treatment of prostate cancer; luteinizing hormone-releasing hormone (LHRH) agonist therapy to induce medical castration has become the most common form of hormonal therapy for advanced and metastatic prostate cancer.
When treatment is started, LHRH agonists initially stimulate the release of LH, causing a surge in serum testosterone that can precipitate a “flare” phenomenon or worsening of disease, particularly in patients with bone metastatic disease.
Gonadotropin-releasing hormone (GnRH) receptor antagonism represents a newer approach to medical castration; abarelix is a pure GnRH receptor antagonist that is devoid of any LHRH agonist activity.
Results of 3 phase III studies demonstrate that abarelix reduces serum testosterone to castrate levels more rapidly than LHRH agonists with or without an antiandrogen (reductions in testosterone were evident after 1 day of treatment, and castration was achieved after a median of 7 days in the abarelix groups); importantly, medical castration with abarelix was not associated with a testosterone surge, as seen in most patients treated with LHRH agonists.
The safety of abarelix is comparable to that of LHRH agonists with or without antiandrogen.
References
- 1.Boyle P, Severi G, Giles GG. The epidemiology of prostate cancer. Urol Clin N Am. 2003;30:209–217. doi: 10.1016/s0094-0143(02)00181-7. [DOI] [PubMed] [Google Scholar]
- 2.Moul JW, Anderson J, Penson DF, et al. Early prostate cancer: prevention, treatment modalities, and quality of life issues. Eur Urol. 2003;44:283–293. doi: 10.1016/s0302-2838(03)00296-3. [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network, authors. [Accessed October 23, 2003];NCCN Practice Guidelines in Oncology: Prostate Cancer. 2002 Available at: http://www.nccn.org.
- 4.Huggins C, Stevens RE, Jr, Hodges CV. Studies on prostatic cancer: II. The effects of castration on advanced carcinoma of the prostate gland. Arch Surg. 1941;43:209–223. [Google Scholar]
- 5.Huggins C, Hodges CV. Studies on prostate cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293–297. Reprinted in J Urol. 2002;167: 948–951. [Google Scholar]
- 6.Thompson IM, Zeidman EJ, Rodriguez FR. Sudden death due to disease flare with luteinizing hormone-releasing hormone agonist therapy for carcinoma of the prostate. J Urol. 1990;144:1479–1480. doi: 10.1016/s0022-5347(17)39774-4. [DOI] [PubMed] [Google Scholar]
- 7.Bubley GJ. Is the flare phenomenon clinically significant? Urology. 2001;58(suppl 2A):5–9. doi: 10.1016/s0090-4295(01)01235-3. [DOI] [PubMed] [Google Scholar]
- 8.Denis LJ, Griffiths K. Endocrine treatment in prostate cancer. Semin Surg Oncol. 2000;18:52–74. doi: 10.1002/(sici)1098-2388(200001/02)18:1<52::aid-ssu8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 9.Kuhn J-M, Billebaud T, Navratil H, et al. Prevention of the transient adverse effects of a gonadotropin-releasing hormone analogue (buserelin) in metastatic prostatic carcinoma by administration of an antiandrogen (nilutamide) N Engl J Med. 1989;321:413–418. doi: 10.1056/NEJM198908173210701. [DOI] [PubMed] [Google Scholar]
- 10.Cook T, Sheridan WP. Development of GnRH antagonists for prostate cancer: new approaches to treatment. The Oncologist. 2000;5:162–168. doi: 10.1634/theoncologist.5-2-162. [DOI] [PubMed] [Google Scholar]
- 11.Stricker HJ. Luteinizing hormone-releasing hormone antagonists in prostate cancer. Urology. 2001;58(suppl 2A):24–27. doi: 10.1016/s0090-4295(01)01238-9. [DOI] [PubMed] [Google Scholar]
- 12.Doehn C, Jocham D. Technology evaluation: abarelix, Praecis Pharmaceuticals. Curr Opin Mol Ther. 2000;2:579–585. [PubMed] [Google Scholar]
- 13.Garnick MB, Campion M. Abarelix Depot Study Group. Abarelix depot, a GnRH antagonist, v LHRH superagonists in prostate cancer: differential effects on follicle-stimulating hormone. Mol Urol. 2000;4:275–277. [PubMed] [Google Scholar]
- 14.Wong SL, Lau D, Baughman SA, et al. Pharmacokinetics and pharmacodynamics of abarelix, a gonadotropin-releasing hormone antagonist, after subcutaneous continuous infusion in patients with prostate cancer. J Clin Pharmacol. 2003;73:304–311. doi: 10.1016/s0009-9236(02)17637-5. [DOI] [PubMed] [Google Scholar]
- 15.Wong SL, Lau D, Baughman SA, et al. Pharmacokinetics and pharmacodynamics of a novel depot formulation of abarelix, a gonadotropin-releasing hormone (GnRH) antagonist, in healthy men ages 50 to 75. J Clin Pharmacol. 2004;44:495–502. doi: 10.1177/0091270004264920. [DOI] [PubMed] [Google Scholar]
- 16.Tomera K, Gleason D, Gittelman M, et al. The gonadotropin-releasing hormone antagonist abarelix depot versus luteinizing hormone releasing hormone agonists leuprolide or goserelin: initial results of endocrinological and biochemical efficacies in patients with prostate cancer. J Urol. 2001;165:1585–1589. [PubMed] [Google Scholar]
- 17.McLeod D, Zinner N, Tomera K, et al. A phase 3, multicenter, open-label, randomized study of abarelix versus leuprolide acetate in men with prostate cancer. Urology. 2001;58:756–761. doi: 10.1016/s0090-4295(01)01342-5. [DOI] [PubMed] [Google Scholar]
- 18.Trachtenberg J, Gittleman M, Steidle C, et al. A phase 3, multicenter, open label, randomized study of abarelix versus leuprolide plus daily antiandrogen in men with prostate cancer. J Urol. 2002;167:1670–1674. doi: 10.1097/00005392-200204000-00021. [DOI] [PubMed] [Google Scholar]
- 19.Koch M, Steidle C, Brosman S, et al. An open-label study of abarelix in men with symptomatic prostate cancer at risk of treatment with LHRH agonists. Urology. 2003;62:877–882. doi: 10.1016/s0090-4295(03)00656-3. [DOI] [PubMed] [Google Scholar]
- 20.Debruyne F, Teillac P, Campion M, Garnick MB. A 1-year, open-label, randomized, multicenter, phase III study of abarelix compared with goserelin plus bicalutamide in patients with advanced or metastatic prostate cancer. Eur Urol. 2004 in press. [Google Scholar]
- 21.Chrisp P, Goa KL. Goserelin. A review of its pharmacodynamic and pharmacokinetic properties, and clinical use in sex hormone-related conditions. Drugs. 1991;41:254–288. doi: 10.2165/00003495-199141020-00008. [DOI] [PubMed] [Google Scholar]
- 22.Crawford ED, Eisenberger MA, McLeod DG, et al. A controlled trial of leuprolide with or without flutamide in prostatic carcinoma. N Engl J Med. 1989;321:419–424. doi: 10.1056/NEJM198908173210702. [DOI] [PubMed] [Google Scholar]
- 23.Ben-Josef E, Yang S-Y, Ji TH, et al. Hormone-refractory prostate cancer cells express functional follicle-stimulating hormone receptor (FSHR) J Urol. 1999;161:970–976. [PubMed] [Google Scholar]
- 24.Beer TM, Garzatto M, Eilers KM, Lemmon D. Phase II study of abarelix depot for androgen-independent prostate cancer progression during gonadotropin-releasing hormone agonist therapy. J Urol. 2003;169:1738–1741. doi: 10.1097/01.ju.0000059584.47272.9d. [DOI] [PubMed] [Google Scholar]
- 25.Plenaxis®, [package insert] Waltham, MA: Praecis Pharmaceuticals Incorporated; 2003. [Google Scholar]