Abstract
Finasteride selectively inhibits the Type 2 isoenzyme of 5α-reductase (5AR) (the enzyme responsible for converting testosterone to dihydrotestosterone [DHT]) whereas dutasteride inhibits both Type 1 and Type 2 5AR. General conclusions regarding the differences and similarities of these 2 agents, in terms of pharmacologic effect, safety, and efficacy, can be drawn from evaluation of short-term comparative trials and similar but non-comparative long-term trials. Dutasteride therapy reduces serum DHT significantly more than does finasteride. In men with benign prostatic hyperplasia (BPH), treatment with either agent results in similar prostate gland volume reduction, flow rate and symptom improvement, and similar reductions in long-term risk of BPH development in terms of symptom progression and acute urinary retention (AUR) and BPH-related surgery. There does not appear to be any clinically significant difference between the adverse event profiles of dutasteride and finasteride. Although weak evidence suggests a difference in the onset of clinical benefit, the available non-comparative trial data do not confirm this finding. Patients with symptomatic BPH who receive dutasteride or finasteride, either as monotherapy or combination therapy with α-blockers, can expect to experience significant prostate gland size reduction, improved symptoms, and reduced risk of progression in terms of long-term adverse outcomes.
Key words: Dutasteride, Finasteride, 5α-reductase, Benign prostatic hyperplasia, Medical therapy, Dihydrotestosterone
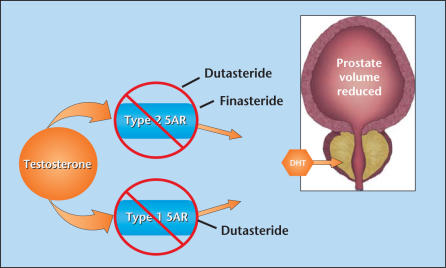
Dutasteride and finasteride are 4-aza steroid competitive inhibitors of 5α-reductase (5AR), the enzyme responsible for converting testosterone to dihydrotestosterone (DHT). Finasteride selectively inhibits the Type 2 isoenzyme whereas dutasteride inhibits both Type 1 and Type 2 5AR (Figure 1). Head-to-head clinical studies comparing dutasteride with finasteride are available to evaluate pharmacologic parameters, time to onset of clinical effect, and short-term clinical efficacy and safety; however, non-comparative trials must be evaluated as well in order to compare the long-term efficacy and safety of these 2 agents. Because such clinical studies have not been performed previously, this review will describe the differences and similarities between these 2 agents in the way they affect prostate size, flow rate, symptoms, risk of progression (including acute urinary retention [AUR] in surgery), and safety by comparing available comparative and non-comparative clinical trial data.
Figure 1.
The 2 5α-reductase inhibitors (5ARIs), dutasteride and finasteride, suppress dihydrotestosterone (DHT) by inhibiting the conversion of testosterone to DHT. Finasteride inhibits only the Type 2 5AR isoenzyme, whereas dutasteride, the only dual 5ARI, selectively inhibits both Type 1 and Type 2 5AR isoenzymes. Data from Bartsch G et al.1
Pharmacology Comparisons
Two isoenzymes of 5AR exist: Type 1 and Type 2.1 Type 1 is most abundant in the liver and skin, but is found, to a lesser degree, in the prostate as well. It is also the dominant isoform in sebaceous glands. Type 2 is the dominant isoenzyme in the prostate, and is minimally present in the liver and skin. Their primary function is to convert testosterone to DHT. Finasteride has proven to selectively inhibit the Type 2 isoenzyme whereas dutasteride competitively inhibits both forms of the enzyme. This is significant because DHT is a primary androgen and is believed to be important in the development and progression of benign prostatic hyperplasia (BPH).2
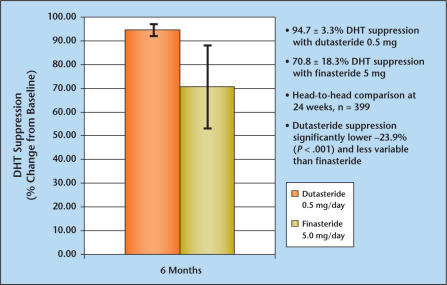
Results of a phase II, double-blind, placebo-controlled, comparative dose-ranging trial of dutasteride and finasteride clearly demonstrated that serum DHT suppression was significantly greater with dutasteride (0.5 mg daily) than with finasteride (5 mg daily).3 The mean reduction in baseline DHT concentration in patients receiving 0.5 mg dutasteride daily was 94.7 ± 3.3% and for patients receiving finasteride 5 mg daily was 70.8 ± 18.3%, respectively (P < .001) (Figure 2). In non-comparative clinical trials, chronic therapy with dutasteride (0.5 mg daily) for up to 2 years in patients with BPH resulted in median reductions in serum DHT of 93%.4 In contrast, therapy with finasteride (5 mg daily) suppressed serum DHT concentrations by approximately 70% for up to 4 years in patients with BPH.5 These observations raise the question whether the pharmacologic differences in DHT suppression between a selective versus a dual inhibitor of 5AR results in clinically significant differences in the treatment of BPH.
Figure 2.
A comparative phase II evaluation of dutasteride and finasteride in a double-blind placebo-controlled trial clearly demonstrates that serum dihydrotestosterone (DHT) suppression is significantly greater with dutasteride (0.5 mg daily) than with finasteride (5 mg daily). Data from Clark RV et al.3
Short-Term Clinical Efficacy
The theoretical value of DHT reduction in BPH was confirmed in 2, 1-year placebo-controlled, double-blind trials evaluating finasteride for patients with symptoms of BPH and enlarged prostate glands determined by digital rectal exam.6 These trials (which led to the approval of finasteride by the US Food and Drug Administration for the treatment of BPH in 1992) demonstrated that 5AR inhibition resulted in reduction in baseline prostate volume, improvement of urinary flow rate, and reduction in symptoms compared with placebo in patients with BPH. One randomized, double-blind, active-controlled trial (Enlarged Prostate International Comparative Study), which compared 12 months of dutasteride and finasteride, was conducted in Europe and Canada to fulfill European registration requirements.7 Although the primary objective of the study was the change in baseline prostate volume at year 1, other efficacy, safety, and tolerability data were obtained. For 12 months, 1630 BPH patients were randomized to either dutasteride 0.5 mg daily (n = 813) or finasteride 5 mg daily (n = 817). In total, 1454 patients completed the 12-month double-blind phase (719 dutasteride, 88% and 735 finasteride, 90%).8 The data from this comparative trial have not been presented at international meetings.
For the intent to treat population, prostate volume was reduced from baseline in both the dutasteride and finasteride groups at month 12. The differences between the 2 agents were not statistically significant. Dutasteride produced numerically but not statistically significant greater improvements in symptoms and urinary flow rates compared with finasteride. The most frequent drug-related adverse events, as expected, were sexual in nature and are listed in Table 1. Although fewer drug-related sexual adverse events occurred in patients who received dutasteride than finasteride (17% of the dutasteride group compared with 20% of the finasteridetreated patients), there were no substantial differences between the 2 drugs.8 As is acknowledged from longer trials with dutasteride and finasteride, most adverse events occurred in the first year and few led to patient withdrawal from the study (5% of dutasteride-treated patients and 4% of finasteride-treated patients). Overall, dutasteride and finasteride appeared to have a similar safety profile. These results are especially important as this study represents the only 1-year data comparing finasteride to dutasteride in a head-to-head comparative trial.
Table 1.
Most Frequent Drug-Related Adverse Events Noted in EPICS
| Dutasteride | Finasteride | |
|---|---|---|
| 0.5 mg | 5 mg | |
| n = 813 | n = 817 | |
| Any adverse event | 17% | 20% |
| Any sexual event | 11% | 14% |
| Impotence | 7% | 8% |
| Decreased libido | 5% | 6% |
| Ejaculatory disorder | 1% | l% |
| Gynaecomastia | l% | l% |
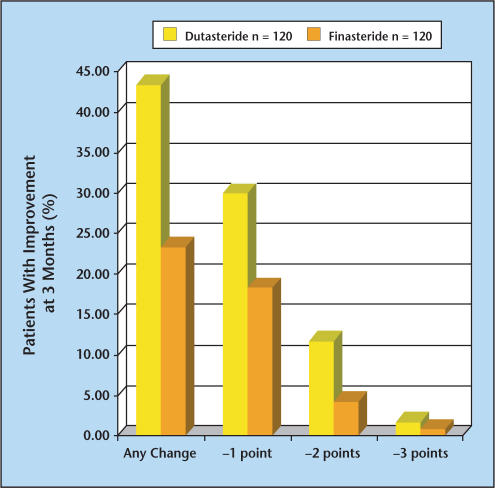
The results of a 3-month prospective and consecutive study conducted to evaluate the onset of symptom relief in men treated with dutasteride versus finasteride were reported at the 2004 American Urological Association (AUA) meeting.9 One hundred and twenty men with symptomatic BPH were treated with dutasteride followed by an additional 120 men treated consecutively with finasteride for 3 months. Among patients who received dutasteride, there were significantly greater reductions in AUA symptom scores compared with those treated with finasteride. Forty-three percent (n = 52) of patients experienced improvement over the 3-month period with dutasteride compared with 23% (n = 28) of patients treated with finasteride. One unit improvements in the AUA-Symptom Index (SI) were noted in 30% and 18% of patients treated with dutasteride and finasteride, respectively, whereas 2 unit improvements were noted in 12% and 4% of the dutasteride- and finasteridetreated patients, respectively (Figure 3). Unfortunately, firm conclusions cannot be drawn from this study because it was not a randomized or controlled study and it only examined the first 3 months of what should be very long-term therapy.
Figure 3.
A 3-month prospective study was conducted to evaluate the onset of symptom relief in patients treated with dutasteride (n = 120) vs those treated with finasteride (n = 120). One hundred and twenty men with symptomatic benign prostatic hyperplasia (BPH) were treated with dutasteride for 3 months followed by an additional 120 men treated consecutively with finasteride for 3 months. Data from Hagerty JA et al.9
Long-Term Efficacy and Safety
Many studies have confirmed that BPH is a gradually progressive disease.10 Therefore, it is difficult to comment on potential long-term clinical differences in outcomes between dutasteride and finasteride treatment by examining only data from short-term studies (and in terms of BPH, 1 year is short-term). It is necessary to evaluate data from non-comparative, long-term, placebo-controlled trials of finasteride and dutasteride.
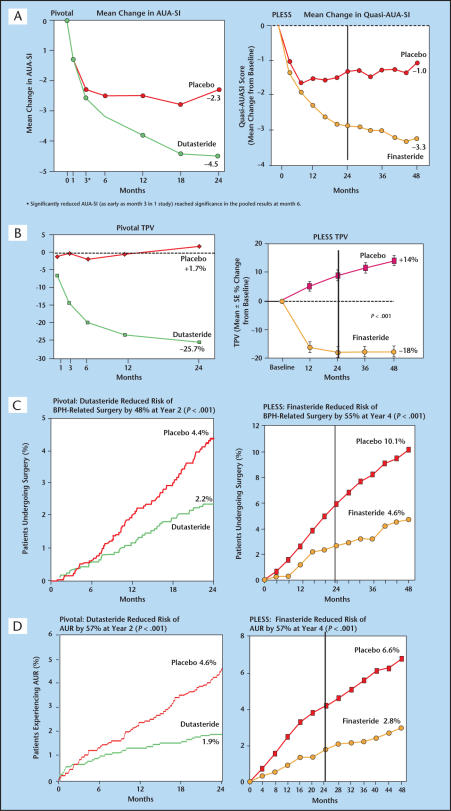
The Proscar Long-Term Efficacy and Safety Study (PLESS)5 was the first long-term placebo-controlled evaluation of 5AR inhibition in BPH. In PLESS, 3040 men with symptomatic BPH and large prostates were randomized to receive finasteride (n = 1524) or placebo (n = 1516) for 4 years. Finasteride treatment resulted in significant improvement in symptom scores (−3.3 in the finasteride group compared to −1.0 in the placebo group) (Figure 4A), and main prostate volume decreased in the finasteride group (−18%) in the first year. Prostate volume in the placebo group continued to have a gradual increase in average size over the 4 years compared to baseline (+14%) (Figure 4B). At 4 years, the risk of undergoing BPH-related surgery was reduced by 55% (Figure 4C) in the finasteride group compared with placebo, whereas the risk reduction for experiencing AUR was reduced by 57% (P < .001) (Figure 4D).
Figure 4.
These figures compare the 4-year PLESS finasteride results with the 2-year pivotal dutasteride results. The vertical line in the PLESS data graphs represents the 2-year time point for comparison purposes. (A) symptoms, (B) prostate volume, (C) surgery, (D) acute urinary retention. PLESS, Proscar Long-Term Efficacy and Safety Study; AUA-SI, American Urological Association-Symptom Index; BPH, benign prostatic hyperplasia; TPV, total prostate volume; AUR, acute urinary retention. Reprinted from Roehrborn CG et al.4 with permission form Elsevier and McConnell JD et al.5 Copyright © 1998 Massachusetts Medical Society. All rights reserved.
The pivotal data from the evaluation of the efficacy and safety of dutasteride consisted of a pooled analysis of 3 essentially identical randomized, double-blind, placebo-controlled, parallel-group clinical trials of 2 years duration4 followed by a 2-year open-label extension. The trials were generally similar to those of the PLESS trial with finasteride (Table 2). Differences in trial design included a larger number of patients in dutasteride trials (n = 4325) and the fact that the patients received double-blind therapy for only 2 years, as compared to 4 years in PLESS. Patients were excluded from the dutasteride trials if their serum prostate-specific antigen (PSA) values were < 1.5 mg/mL and/or their prostate volume was < 30 cc. Symptom scores improved by 3 months in 1 of the 3 studies, and by 6 months in the other 2 studies. At the end of 2 years of follow-up, the symptom score decreased by −4.5 in the dutasteride group compared with −2.3 in the placebo group (Figure 4A). Prostate volume in the dutasteride group was reduced by −25.7% compared to baseline versus an increase in prostate volume of +1.7% in the placebo group (Figure 4B). Significant reduction in prostate volume was observed at 1 month and continued throughout the 24-month period (prostate volume change was not measured at 1 month in the PLESS study). At 2 years, dutasteride therapy reduced the risk of BPH-related surgery by 48% (Figure 4C) and AUR by 57% compared with placebo (P < .001) (Figure 4D). Table 2 describes the similarities in the baseline characteristics of the patients enrolled in the pivotal study compared to those enrolled in the PLESS study.
Table 2.
Comparing the Baseline Entry Characteristics
| Pivotal | Pivotal | PLESS | PLESS | |
|---|---|---|---|---|
| 2-Year | 2-Year | 4-Year | 4-Year | |
| Double-Blind | Double-Blind | Double-Blind | Double-Blind | |
| Placebo | Dutasteride | Placebo | Finasteride | |
| Baseline Data | n = 2158 | n = 2167 | n = 1516 | n = 1524 |
| Age (years) | 66.1 ± 7.4 | 66.5 ± 7.6 | 64 ± 7 | 64 ± 6 |
| AUA-SI | 17.1 ± 6.1 | 17.0 ± 6.0 | 15 ± 6* | 15 ± 6* |
| Qmax (mL/sec) | 10.4 ± 3.6 | 10.1 ± 3.5 | 11 ± 4 | 11 ± 4 |
| PSA (ng/mL) | 4.0 ± 2.1 | 4.0 ± 2.1 | 2.8 ± 2.1 | 2.8 ± 2.1 |
| Prostate volume (cc) | 54.0 ± 21.9 | 54.9 ± 23.9 | 55 ± 26 | 54 ± 25 |
| Transition zone (cc) | 26.8 ± 17.4 | 26.8 ± 17.1 | N/A | N/A |
Pivotal — ARIA 3001 (USA), ARIA 3002 (USA), ARIA 3003 (19 countries); PLESS (USA).
PLESS used a quasi-AUA symptom score, adapted from the AUA-SI and described in the trial’s method section.
PLESS, Proscar Long-Term Efficacy and Safety Study; AUA-SI, American Urological Association-Symptom Index; Qmax, maximum flow rate; PSA, prostate-specific antigen; N/A, not available.
In both the finasteride and dutasteride trials, drug-related sexual adverse events, gynaecomastia, and rash occurred more frequently in the 5α-reductase inhibitor (5ARI) groups than in the placebo groups. Table 3 describes the adverse events reported in these trials; however, the trials were not comparative, and therefore, there may be significant differences in reported adverse event rates, which may reflect differences in patient populations, trial design, or even methods of collecting and coding adverse events. Nonetheless, it was apparent that for both agents, the most frequently reported drug-related adverse events were related to sexual function. These included impotence, decreased libido, decreased volume of ejaculation, and ejaculatory disorders as well as breast tenderness and/or enlargement or gynaecomastia. For both agents, the onset of drug-related sexual adverse events appeared in the first year and there was no evidence of increased adverse events compared to placebo after the first year of therapy. Both agents appeared to be reasonably safe and well tolerated.
Table 3.
Drug-Related Sexual Adverse Events
| Pivotal | PLESS | |
|---|---|---|
| % Dutasteride Patients | % Finasteride Patients | |
| (% Placebo Patients) | (% Placebo Patients) | |
| Impotence | 6.0 (3.0) | 8.1 (3.7) |
| Decreased libido | 3.7 (1.9) | 6.4 (3.4) |
| Ejaculatory disorder | 1.8 (0.7) | 3.7 (0.8) |
| Gynaecomastia* | 1.3 (0.5) | 0.9 (0.2) |
Percentage of patients who withdrew due to sexual adverse events or gynaecomastia over 4 years of dutasteride treatment is low.
Long-Term Extension Studies
Both the 4-year PLESS study and the 2-year pivotal dutasteride study incorporated a 2-year open extension. The evaluation of the 2-year open extension of the PLESS study11 demonstrated that patients who were originally randomized to finasteride continued to experience stable prostate size reduction, durable symptom improvement, and progressive risk reduction (AUR and surgery). The 2-year open-label extension in the dutasteride trial12 clearly demonstrated that patients who remained on dutasteride for 4 years continued to have sustained and durable benefits in terms of prostate size, clinical symptoms, and AUR and surgery risk reduction when compared with the finasteride group in the 4-year PLESS study.
The placebo group in the extension studies for both finasteride and dutasteride experienced similar (but delayed when compared with the treatment group) clinical benefits comparable to the initial 2-year results experienced by patients who were initially randomized to either finasteride or dutasteride. This finding provides some evidence that patients may experience more risk reduction in terms of AUR and surgery when treated earlier compared to delayed treatment. These open-label extension studies confirm the long-term safety and tolerability of both dutasteride and finasteride.
Combination Therapy: 5AR Inhibition Combined With α-Blocker Therapy
Short 1-year combination therapy trials comparing finasteride, α-blocker, α-blocker/finasteride combination, and placebo13,14 failed to show any benefit for combining these 2 independent modalities of therapy. As discussed previously, evaluation of short-term studies in a chronic, slowly progressive disease can lead to erroneous conclusions. The Medical Treatment of Prostate Symptoms Study (MTOPS),15 the longest and largest trial of medical management of BPH, was developed to be a prospective, double-blind, placebo-controlled, multi-center, randomized clinical trial to evaluate the effect of medical therapy on overall BPH progression for over 4 years. Patients were treated with doxazosin, finasteride, the combination of finasteride and doxazosin, and placebo. This trial, which randomized 3047 men with BPH, confirmed significant prostate gland volume reduction with finasteride (−16%), which was not different from the size reduction seen with combination therapy (−13%). As expected, both the doxazosin and placebo groups had a gradual increase in prostate volume over the 4 years (+18%). However, combination therapy was shown to be clearly superior in improving AUA symptom scores at year 4. The combination group experienced a decrease in symptom score of 7.0 points compared to finasteride (5.0 points), doxazosin (6.0 points), and placebo (4.0 points). Similarly, combination therapy clearly showed a significantly better risk reduction in terms of BPH progression (primarily driven by symptom progression) compared with finasteride, doxazosin, or placebo. Interestingly, however, patients being treated with combination therapy experienced similar reduction in risk of developing AUR or requiring BPH-related surgery to those patients treated with finasteride alone (as opposed to those with a higher risk of AUR and BPHrelated surgery in the doxazosin and placebo group). In the long term, combination therapy was clearly more effective than monotherapy for symptom amelioration and prevention of BPH progression.
The Combination Avodart Tamsulosin trial is an international, multi-center, randomized, double-blind, parallel-group study that is currently investigating whether combination therapy with dutasteride and tamsulosin is more effective than monotherapy with either drug alone in the improvement of symptoms and long-term clinical outcome of AUR and BPH-related surgery. This study is similar in design to the MTOPS study except that a comparative placebo arm has not been incorporated in the study design. The study will answer the question of whether combination therapy with an α-blocker and dutasteride will achieve similar results as finasteride and an α-blocker as demonstrated in the MTOPS study.
The long-term clinical benefits of 5AR inhibition are secondary to prostate gland reduction, a slow process that takes months to achieve. Smooth muscle relaxation achieved by α-blockade is quick in patients who then experience rapid amelioration of their symptoms. It has been suggested that following a period of combination therapy, the long-term benefits in terms of risk reduction and symptom amelioration of 5AR inhibition can be maintained, even after stopping α-blocker therapy.
The first study that suggested this outcome was a prospective, nonrandomized, uncontrolled study of 240 men with a favorable response to finasteride (5 mg/day) and various doses of doxazosin (2, 4, and 8 mg/day) who discontinued doxazosin at 3, 6, 9, or 12 months while continuing finasteride.16 Patients were evaluated 1 month after discontinuation of doxazosin to determine whether any deterioration had resulted. In patients discontinuing doxazosin at 3 months, success (defined as no increase in symptom score and no desire to resume doxazosin) was reported in 13% to 20%; in patients discontinuing doxazosin at 6 months, success was reported in 40% to 48%; in patients discontinuing doxazosin at 9 months, success was reported in 73% to 84%; and in patients discontinuing doxazosin at 12 months, success was reported by 84% to 87%. The authors concluded that the patients receiving combination therapy using finasteride and an α-blocker were likely to experience no significant symptom deterioration after discontinuing the α-blocker after 9 to 12 months of combination therapy (regardless of the dose of α-blocker chosen).
The Symptom Management After Reducing Therapy17 trial was a better designed and powered study to examine withdrawal of α-blocker therapy after treatment with combination therapy. In this study, 327 BPH patients were randomized either to dutasteride and tamsulosin for 36 weeks (DT 36) or dutasteride and tamsulosin for 24 weeks followed by dutasteride and tamsulosin-matched placebo for the remaining 12 weeks (DT 24 + D 12). Seventy-seven percent of DT 24 to D 12 patients felt the same or better at week 30 compared with week 24 (changes in International Prostate Symptom Score [IPSS] were consistent with this finding). In subjects with an IPSS < 20 who changed to dutasteride monotherapy at week 24, 84% switched without a noticeable deterioration of their symptoms. In 27% of men with severe baseline symptoms (IPSS ≥ 20) who withdrew from tamsulosin therapy at week 24, 42.5% reported worsening of symptoms compared with 14% in the DT 36 group. The authors concluded that dutasteride could be used in a 24-week combination with tamsulosin to achieve a rapid onset of symptom relief in patients at risk of underlying disease progression. The study demonstrated that this effect was maintained in the majority of patients after the α-blocker was removed from the combination. However, patients with severe symptoms appeared to benefit from long-term combination therapy.
Although these combinations with α-blocker discontinuation studies were investigating the same clinical question, different study designs preclude making any direct comparisons. It appears, however, that there is no major reason to dismiss a trial of α-blocker discontinuation after 6 to 12 months of successful combination therapy with either dutasteride or finasteride.
Conclusion
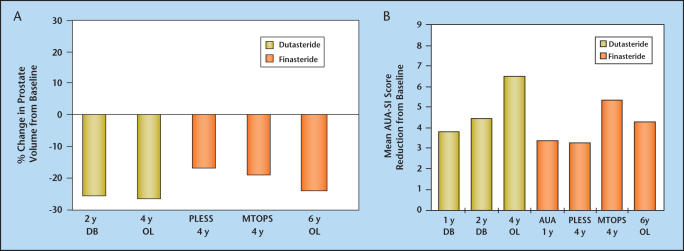
Without evidence from long-term clinical trials comparing the safety and efficacy of dutasteride and finasteride, firm conclusions regarding the relative efficacy and safety of one agent over the other cannot be made. However, indirect comparisons of long-term changes in prostate volume and symptoms (Figure 5A and B) and general conclusions drawn from evaluation of short-term comparative trials and similar but non-comparative long-term trials can be made (Table 4). One such conclusion is that dutasteride therapy reduces serum DHT significantly more than does finasteride. In addition, treatment with either agent results in similar prostate gland volume reduction, flow rate, and symptom improvement, and similar reductions in long-term risk of BPH development in terms of symptom progression and AUR and BPH-related surgery. There does not appear to be any clinically significant difference between the adverse event profile of dutasteride and finasteride. Although weak evidence suggests a difference in the onset of clinical benefit, the available non-comparative trial data do not confirm this finding. Patients with symptomatic BPH who receive dutasteride or finasteride, either as monotherapy or combination therapy with α-blockers, can expect to experience significant prostate gland size reduction, improved symptoms, and reduced risk of progression in terms of long-term adverse outcomes.
Figure 5.
Long-term prostate volume changes (A) and symptom improvement (B) between dutasteride and finasteride can be determined by evaluating changes from baseline in long-term non-comparative trials. As these are not comparative data, patient populations from the various studies may differ. Dutasteride data for 1, 2, and 4 years are shown in yellow. Continuing improvement in symptoms over the 4 years is seen. Finasteride data for 1, 4, and 5 years are shown in orange, including those from PLESS and MTOPS. DB, double-blind; OL, open-label; PLESS, Proscar Long-Term Efficacy and Safety Study; MTOPS, Medical Treatment of Prostate Symptoms Study; AUA, American Urological Association; SI, AUA Symptom Index. Data from Roehrborn et al4,11,12 and McConnell JD et al.5,15
Table 4.
Summary of Dutasteride Versus Finasteride
| Dutasteride (0.5 mg) | Finasteride (5 mg) | |
|---|---|---|
| 5AR inhibition | Type 1 and 2 | Type 2 only |
| % DHT inhibition (serum) | 93% | 70% |
| Half-life | 5 weeks | 6 to 8 hours |
| Prostate size reduction | −25.7% (2 y) | −18% (4 y, PLESS) |
| (from baseline) | −27.3% (4 y) | −16% (4.5 y, MTOPS) |
| Urinary flow | 2.2 mL/sec (2 y) | 1.7 mL/sec (4 y, PLESS) |
| improvement (Qmax) | 2.7 mL/sec (4 y) | 2.2 mL/sec (4.5 y, MTOPS) |
| Improvement in symptoms | −4.4 (2 y) | −3.3 (4 y, PLESS) |
| (AUA-SI) | −6.5 (4 y) | −5.0 (4.5 y, MTOPS) |
| % Reduction in AUR | 57% (2 y) | 57% (4 y, PLESS) |
| 79% (4.5 y, MTOPS) | ||
| % Reduction in surgery | 48% (2 y) | 55% (4 y, PLESS) |
| 69% (4.5 y, MTOPS) |
5AR, 5α-reductase; DHT, dihydrotestosterone; PLESS, Proscar Long-Term Efficacy and Safety Study; MTOPS, Medical Treatment of Prostate Symptoms Study; Qmax, maximum flow rate; AUA-SI, American Urological Association-Symptom Index; AUR, acute urinary retention.
Main Points.
To fully determine the efficacy and safety of dutasteride and finasteride in the treatment of benign prostatic hyperplasia (BPH), both comparative and non-comparative studies must be examined.
5α-reductase, the enzyme responsible for converting testosterone to dihydrotestosterone, exists in 2 forms: Type 1 and Type 2. Finasteride selectively inhibits Type 2 whereas dutasteride inhibits both forms. This is significant because dihydrotestosterone is thought to be important in the development of BPH; its suppression by these 2 agents may prove to be beneficial for patients with BPH.
Both short- and long-term treatment with finasteride and dutasteride result in prostate volume reduction, urinary flow rate and symptom improvement, and a risk reduction for acute urinary retention and BPH-related surgery.
Dutasteride and finasteride appear to have a similar safety profile.
For both agents, the onset of drug-related sexual adverse events appeared in the first year and there was no evidence of increased adverse events compared to placebo after the first year of therapy.
In the long term, combination therapy with α-blockers can be more effective than monotherapy for symptom amelioration and prevention of BPH progression. These benefits can be maintained even after dropping the α-blocker from the combination.
References
- 1.Bartsch G, Rittmaster RS, Klocker H. Dihydrotestosterone and the concept of 5α-reductase inhibition in human benign prostatic hyperplasia. Eur Urol. 2000;37:367–380. doi: 10.1159/000020181. [DOI] [PubMed] [Google Scholar]
- 2.Steers WD. 5α-reductase activity in the prostate. Urol. 2001;58(suppl 6A):17–24. doi: 10.1016/s0090-4295(01)01299-7. [DOI] [PubMed] [Google Scholar]
- 3.Clark RV, Hermann DJ, Cunningham GR, et al. Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5α-Reductase inhibitor. J Clin Endocrin Metab. 2004;89:2179–2184. doi: 10.1210/jc.2003-030330. [DOI] [PubMed] [Google Scholar]
- 4.Roehrborn CG, Boyle P, Nickel JC, et al. Efficacy and safety of a dual inhibitor of 5α-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urol. 2002;60:434–441. doi: 10.1016/s0090-4295(02)01905-2. [DOI] [PubMed] [Google Scholar]
- 5.McConnell JD, Bruskewitz R, Walsh P, et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. N Engl J Med. 1998;338:557–563. doi: 10.1056/NEJM199802263380901. [DOI] [PubMed] [Google Scholar]
- 6.Gormley GJ, Stoner E, Bruskewitz RC, et al. The effect of finasteride in men with benign prostatic hyperplasia. The Finasteride Study Group. N Engl J Med. 1992;327:1185–1191. doi: 10.1056/NEJM199210223271701. [DOI] [PubMed] [Google Scholar]
- 7. GlaxoSmithKline Document BP2001/00017/00. Data on File. Clinical Trial Report, Study ARI 40001.
- 8.Andriole GL, Kirby R. Safety and tolerability of the dual 5α-reductase inhibitor dutasteride in the treatment of benign prostatic hyperplasia. Eur Urol. 2003;44:82–88. doi: 10.1016/s0302-2838(03)00198-2. [DOI] [PubMed] [Google Scholar]
- 9.Hagerty JA, Ginsberg PC, Metro MJ, Harkaway RC. A prospective, comparative study of the onset of symptomatic benefit of dutasteride versus finasteride in men with benign prostatic hyperplasia in clinical practice. J Urol. 2004;171:356. [Google Scholar]
- 10.Emberton M, Andriole GL, de la Rosette J, et al. BPH: a progressive disease of the ageing male. Urol. 2004;61:267–273. doi: 10.1016/s0090-4295(02)02371-3. [DOI] [PubMed] [Google Scholar]
- 11.Roehrborn CG, Bruskewitz R, Nickel JC, et al. Sustained decrease in incidence of acute urinary retention and surgery with finasteride for 6 years in men with benign prostatic hyperplasia. J Urol. 2004;171:1194–1198. doi: 10.1097/01.ju.0000112918.74410.94. [DOI] [PubMed] [Google Scholar]
- 12.Roehrborn C, Marks L, Fenter T, et al. Efficacy and safety of dutasteride in the four-year treatment of men with benign prostatic hyperplasia. Urol. 2004;63:709–715. doi: 10.1016/j.urology.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Lepor H, Williford WO, Barry MJ, et al. The efficacy of terazosin, finasteride or both in benign prostatic hyperplasia. Veterans’ Affairs Cooperative Studies Benign Prostatic Hyperplasia Study Group. N Engl J Med. 1996;335:533–539. doi: 10.1056/NEJM199608223350801. [DOI] [PubMed] [Google Scholar]
- 14.Kirby RS, Roehrborn C, Boyle P, et al. Efficacy and tolerability of doxazosin and finasteride, alone or in combination, in treatment of symptomatic benign prostatic hyperplasia: the prospective European doxazosin and combination therapy (PREDICT) trial. Urol. 2003;61:119–126. doi: 10.1016/s0090-4295(02)02114-3. [DOI] [PubMed] [Google Scholar]
- 15.McConnell JD, Roehrborn CG, Oliver OM, et al. The long term effect of doxazosin, finasteride and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349:2385–2396. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 16.Baldwin KC, Ginsberg PC, Roehrborn CG, Harkaway RC. Discontinuation of alpha-blockade after initial treatment with finasteride and doxazosin in men with lower urinary tract symptoms and clinical evidence of benign prostatic hyperplasia. Urol. 2001;58:203–208. doi: 10.1016/s0090-4295(01)01201-8. [DOI] [PubMed] [Google Scholar]
- 17.Barkin J, Guimaraes M, Jacobi G, et al. Alpha-blocker therapy can be withdrawn in the majority of men following initial combination therapy with the dual 5α-reductase inhibitor dutasteride. Eur Urol. 2003;44:461–466. doi: 10.1016/s0302-2838(03)00367-1. [DOI] [PubMed] [Google Scholar]