Abstract
Recent studies have discussed the benefits of medical therapy for benign prostatic hyperplasia (BPH), but have not provided physicians with the necessary tools needed to translate the information into individualized, evidence-based recommendations for clinically important questions. Nomograms can help physicians individualize their treatment decisions and predict a likely outcome by assessing the key risk factors for BPH progression.
Key words: Nomogram, Benign prostatic hyperplasia, Progression, 5α-reductase inhibitor
In the 1980s, advances in our understanding of the pathophysiology of benign prostatic hyperplasia (BPH), including the discovery and characterization of adrenergic receptors and of androgen action in the prostate, ushered in the era of medical therapy for BPH, using inhibitors of 5α-reductase (5AR) and of α-receptors in the prostate. Through results of myriad randomized, placebo-controlled trials, 5AR inhibitors (5ARIs) and α-blockers have been shown to be both safe and effective for the treatment of BPH. Because these 2 classes of agents appear to work through unrelated mechanisms, the Medical Therapy of Prostatic Symptoms (MTOPS) trial1 was initiated to determine if 5ARIs and selective α-blockers would act synergistically to delay or prevent the progression of BPH. In the MTOPS trial, the combination of finasteride and doxazosin was found to be more effective than either therapy alone in preventing the progression of BPH; the risk of clinical progression was reduced by 39%, 34%, and 67% in the doxazosin, finasteride, and combination therapy groups, respectively, compared with placebo.1 Only the 5ARI finasteride, alone or in combination with doxazosin, significantly reduced the risk of acute urinary retention (AUR) and BPH-related invasive therapy. Doxazosin did not reduce the long-term incidence of either AUR or BPH-related invasive therapy, thus clearly establishing that only 5ARIs affect the natural history of prostate growth and prevent the progression of BPH.
Data from the MTOPS trial demonstrated that baseline prostate-specific antigen (PSA) level and prostate volume predicted AUR, which had been shown in other studies such as the Proscar Long-term Efficacy and Safety Study2 (PLESS) and the dutasteride phase III pivotal trials.3 Along with these parameters, age, flow rate, symptom severity, and post-void residual urine were also significant predictors of overall BPH and symptom progression. The MTOPS trial emphasized key points regarding the natural history of BPH and the expectations associated with medical therapy for this disease. In addition to reinforcing previous notions regarding the progressive nature of BPH, the relationship between PSA level and prostate size and growth, and progression as measured by AUR and BPH surgery rates, the MTOPS trial added to our understanding of the importance of multiple disease parameters in predicting symptom and overall disease progression. Furthermore, the MTOPS trial increased our appreciation of the long-term natural history of BPH, which until recently, had been evaluated primarily in short-term 1- or 2-year studies.
Within this context of renewed scientific and clinical support for the unique role of finasteride in the long-term management of BPH, the dual 5ARI dutasteride was approved by the US Food and Drug Administration (FDA) on November 20, 2001 for the treatment of symptomatic BPH and was launched in February 2003 as the first 5ARI to inhibit both isoenzymes of 5AR. In addition, it was approved for the reduction in risk of AUR and surgery in patients with BPH.
Approval by the FDA was supported by data from three 2-year multicenter, placebo-controlled, double-blind studies, each with 2-year open-label extensions evaluating dutasteride 0.5 mg/day (n = 2167) or placebo (n = 2158) in male subjects with BPH.3 Subjects were at least 50 years of age with a serum PSA level > 1.5 ng/mL and < 10 ng/mL, and had been diagnosed with BPH through medical history and physical examination, which revealed an enlarged prostate (30 cc) and BPH symptoms that were moderate to severe according to the American Urological Association-Symptom Index (AUA-SI). Most of the 4325 subjects randomly assigned to receive either dutasteride or placebo completed 2 years of treatment (70% and 67%, respectively).
Effect on Symptom Scores
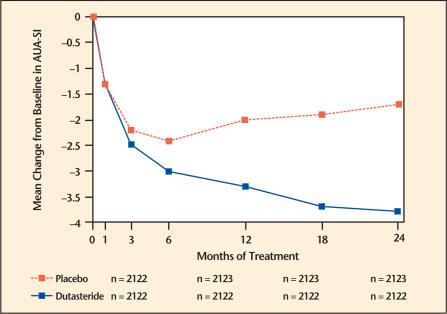
The baseline AUA-SI score across the 3 studies was approximately 17 units in both treatment groups. Subjects receiving dutasteride achieved statistically significant improvement in symptoms versus placebo by month 3 in 1 study, and by month 12 in the other 2 pivotal studies. At month 12, the mean decrease from baseline in AUA-SI symptom scores across the 3 studies pooled was −3.3 units for dutasteride and −2.0 units for placebo with a mean difference between the 2 treatment groups of −1.3 (range −1.1 to −1.5 units in each of the 3 studies, P < .001) and was consistent across the 3 studies. At month 24, the mean decrease from baseline was −3.8 units for dutasteride and −1.7 units for placebo with a mean difference of −2.1 (range −1.9 to −2.2 units in each of the 3 studies, P < .001) (Figure 1).
Figure 1.
American Urological Association-Symptom Index (AUA-SI) score* change from baseline (pivotal studies pooled).
Effect on AUR and the Need for Surgery
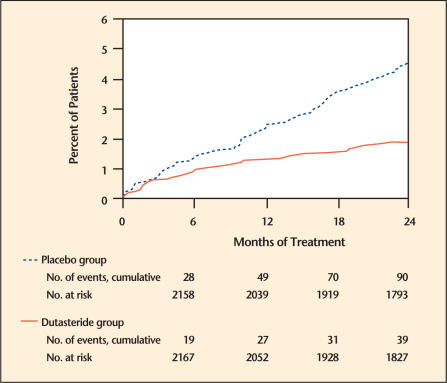
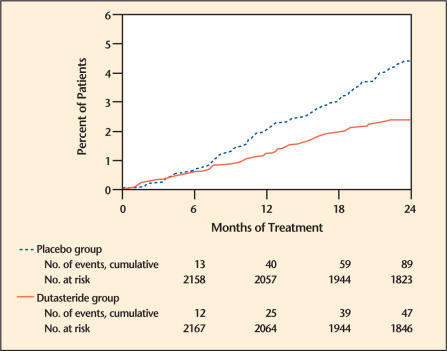
Efficacy was also assessed after 2 years of treatment by the incidence of AUR requiring catheterization and BPH-related urological surgical intervention. Compared with placebo, dutasteride was associated with a statistically significant lower incidence of AUR (1.8% for dutasteride vs 4.2% for placebo, P < .001; 57% reduction in risk, 95% CI: [38%–71%]) and with a statistically significant lower incidence of surgery (2.2% for dutasteride vs 4.1% for placebo, P < .001; 48% reduction in risk, 95% CI: [26%–63%]) (Figure 2 and Figure 3).
Figure 2.
Percent of subjects developing acute urinary retention over a 24-month period (pivotal studies pooled). Adapted from Roehrborn.3
Figure 3.
Percent of subjects having surgery for benign prostatic hyperplasia over a 24-month period (pivotal studies pooled). Adapted from Roehrborn.3
Summary of Clinical Studies
Thus, data from 3 large, well-controlled efficacy studies demonstrated that treatment with dutasteride (0.5 mg once daily) reduced the risk of both AUR and BPH-related surgical intervention relative to placebo, improved BPH-related symptoms, decreased prostate volume, and increased maximum urinary flow rates. These compelling data served as the basis to support the clinical use of dutasteride for the treatment of men with BPH.
Despite the increasing number of published studies that have enhanced general knowledge regarding the best candidates for α-blockers and 5ARIs, physicians and patients still lack tools to help translate this body of general knowledge into individualized, evidence-based recommendations for clinically important questions such as:
Do I need to perform a prostate biopsy on this patient?
What are the chances that the biopsy will identify cancer?
For patients with BPH only, what is the long-term risk of developing prostate cancer?
Who needs a repeat biopsy if an initial biopsy fails to detect prostate cancer?
What is the long-term risk of experiencing BPH progression in this patient?
Will this patient experience a significant reduction in BPH symptoms if medical therapy is initiated?
What would the reduction in risk of developing either prostate cancer or BPH progression be if I start the patient on a 5ARI?
Unfortunately, physicians are not currently equipped to provide the best answers tailored for individual patients. Until now, physicians have been encouraged to make clinically important decisions based on only 1 or just a few of the important parameters that impact course of treatment for their patients. An example is the considerable confusion over the appropriate cut-points to use in decisions regarding prostate cancer and BPH therapy. For BPH, physicians often agree that a 5ARI is appropriate for patients with a “large prostate,” but often disagree as to whether this includes patients with prostate volumes > 30 cc, > 40 cc, or larger. Similarly, urologists agree that higher PSA levels are associated with a greater risk of finding prostate cancer on prostate biopsy, but disagree as to whether the correct cut-point for prostate biopsy should be PSA level < 4, < 2.5, or whether age-specific cut-points should be applied. Nomograms allow physicians to individualize these decisions, rather than applying a “one-size fits all” approach to medical decision-making.
Nomograms: Enabling Technology for Data Convergence in Predictive Medicine
Nomograms that incorporate novel diagnostic and clinical information can provide personalized, evidence-based answers to clinically important questions. Practically, a nomogram is a device or model that uses an algorithm or mathematical formula to predict the probability of an outcome, optimized for predictive accuracy.4,5 Nomograms allow continuous variables to remain continuous, maximizing their predictive power. They allow for the convergent use of all important data parameters, so that the most accurate prediction model can be built. Furthermore, nomograms can be constantly updated by building on prior knowledge rather than replacing it. Thus, novel markers such as PSA level, proteomics, and genomics are evaluated by their ability to improve the overall accuracy of prediction models and are added to nomogram models when they provide significant improvement in the accuracy of predictions.
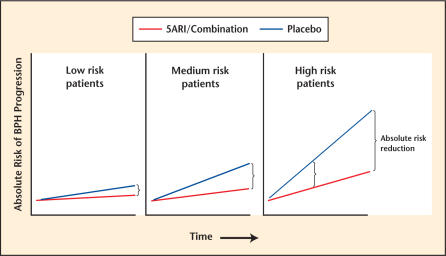
In order to generate a nomogram to predict BPH progression, one first needs to assemble the key risk factors. For BPH, these risk factors have been suggested through numerous analyses of population-based6,7 and clinical trials databases.8,9 These risk factors have included: 1) higher age, 2) more severe (obstructive) symptoms, 3) lower maximal urinary flow, 4) greater prostate volume, 5) large endovesical lobe, and 6) higher serum PSA level. Individually, each of these is a risk factor for BPH progression, but for individual patients, the increasing number and severity of these risk factors increases the absolute risk of BPH progression. Identifying patients at highest risk for BPH progression, while improving patient care decision-making at the individual patient level, also helps to select patients who would benefit the most from 5ARI/combination therapy (Figure 4).
Figure 4.
Impact of overall risk of benign prostatic hyperplasia (BPH) progression on absolute risk reduction with therapy. 5ARI, 5α-reductase inhibitor.
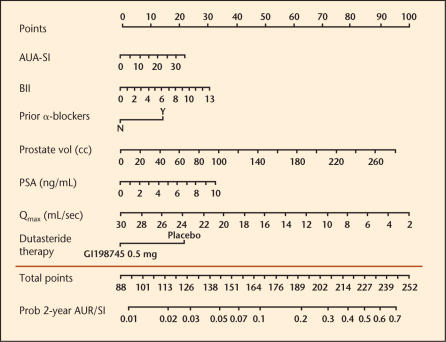
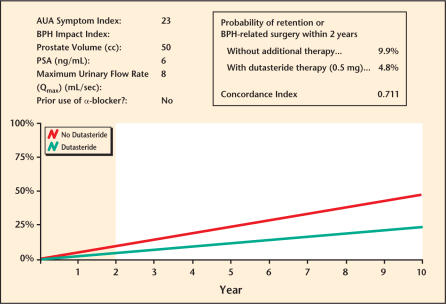
With these benefits in mind, a nomogram to predict the risk of BPH progression was constructed using phase III pivotal trial data (ARIA 3001, 3002, 3003), which was used to establish the safety and efficacy of dutasteride prior to FDA approval and was presented at the 98th Annual Meeting of the American Urological Association in Chicago, Illinois, in May 2003 (Figure 5). For each individual predictive parameter, points are awarded by drawing a perpendicular line to the point scale along the top of the nomogram. After all points are added, a perpendicular line is drawn from the bottom, total points scale to the line below, indicating the 2-year probability of a patient developing retention or requiring BPH-related surgery within 2 years. Note that the use of dutasteride versus placebo leads to a total point score reduced by approximately 20 to 25 points, which translates to a 50% relative risk reduction across the entire range of total points for any patient. This nomogram was shown to have an accuracy of approximately 71%, better than the flip of a coin (50%), but less than 100% perfect predictive accuracy. This research nomogram demonstrated that although the median risk of progression to a combined endpoint of AUR/surgery was only 6.8%, the maximum risk of progression in the most severely affected patients was 27% at 2 years, an absolute increase in the risk of progression of > 20%. Thus, a 50% relative risk reduction over 2 years translates into approximately 13% to 14% absolute risk reduction over this very short time frame, perhaps resulting in a greater benefit over time (Figure 4, last panel). In order to make nomograms more convenient, electronic versions have been created that are PDA- or Web-accessible, and that provide graphic reports of the risk of BPH progression for patients either treated or not treated with dutasteride (Figure 6).
Figure 5.
Nomogram prediction of benign prostatic hyperplasia (BPH) progression. AUA-SI, American Urological Association-Symptom Index; BII, BPH impact index; PSA, prostate-specific antigen; Qmax, maximal urinary flow; AUR, acute urinary retention; SI, BPH-related surgery.
Figure 6.
Benign prostatic hyperplasia (BPH) progression nomogram. AUA, American Urological Association; PSA, prostate-specific antigen.
Conclusions
Patients and physicians desire accurate knowledge regarding the risks and benefits of therapy when contemplating any new course of treatment for any disease. Nomograms provide the most accurate predictions for individual patients in this setting. Therefore, we developed an accurate nomogram to predict the risk of BPH progression after 2 years for patients facing the choice of beginning therapy with dutasteride for symptomatic BPH.
Main Points.
Due to the findings of the Medical Therapy of Prostatic Symptoms trial, which showed that only 5α-reductase inhibitors (5ARIs) affect the natural history of benign prostatic hyperplasia (BPH), the dual inhibitor dutasteride was developed.
Dutasteride has caused significant improvement in patients’ American Urological Association symptom scores and has resulted in lower incidences of acute urinary retention and surgery.
The numerous studies on 5ARI therapy have not helped physicians translate the information into individualized, evidence-based treatments. The use of a nomogram, a model that uses an algorithm or mathematical formula to predict the probability of an outcome, has proven to be useful in this regard.
To generate a nomogram to predict BPH progression, one must examine key risk factors. In the individual, the increasing number and severity of these risk factors increases the absolute risk of BPH progression.
The use of a nomogram to predict BPH progression has been shown to be accurate approximately 71% of the time.
Footnotes
AUA-SI score ranges from 1 to 35. Adapted from Roehrborn.3
References
- 1.McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. MTOPS Research Group. N Engl J Med. 2003;349:2387–2398. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 2.McConnell JD, Bruskewitz R, Walsh P, et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. Finasteride Long-Term Efficacy and Safety Study Group. N Engl J Med. 1998;338:557–563. doi: 10.1056/NEJM199802263380901. [DOI] [PubMed] [Google Scholar]
- 3.Roehrborn CG, Boyle P, Nickel JC, et al. Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urology. 2002;60:434–441. doi: 10.1016/s0090-4295(02)01905-2. [DOI] [PubMed] [Google Scholar]
- 4.Eastham JA, Kattan MW, Scardino PT. Nomograms as predictive models. Semin Urol Oncol. 2002;20:108–115. doi: 10.1053/suro.2002.32936. [DOI] [PubMed] [Google Scholar]
- 5.Kattan MW. Comparison of Cox regression with other methods for determining prediction models and nomograms. J Urol. 2003;170:S6–S9. doi: 10.1097/01.ju.0000094764.56269.2d. discussion S10. [DOI] [PubMed] [Google Scholar]
- 6.Jacobsen SJ, Jacobson DJ, Girman CJ, et al. Natural history of prostatism: risk factors for acute urinary retention. J Urol. 1997;158:481–487. doi: 10.1016/s0022-5347(01)64508-7. [DOI] [PubMed] [Google Scholar]
- 7.Jacobsen SJ, Girman CJ, Guess HA, et al. Natural history of prostatism: longitudinal changes in voiding symptoms in community dwelling men. J Urol. 1996;155:595–600. doi: 10.1016/s0022-5347(01)66461-9. [DOI] [PubMed] [Google Scholar]
- 8.Roehrborn CG, Boyle P, Bergner D, et al. Serum prostate-specific antigen and prostate volume predict long-term changes in symptoms and flow rate: results of a four-year, randomized trial comparing finasteride versus placebo. PLESS Study Group. Urology. 1999;54:662–669. doi: 10.1016/s0090-4295(99)00232-0. [DOI] [PubMed] [Google Scholar]
- 9.Roehrborn CG, McConnell JD, Lieber M, et al. Serum prostate-specific antigen concentration is a powerful predictor of acute urinary retention and need for surgery in men with clinical benign prostatic hyperplasia. PLESS Study Group. Urology. 1999;53:473–480. doi: 10.1016/s0090-4295(98)00654-2. [DOI] [PubMed] [Google Scholar]