Abstract
Bone pain associated with advanced prostate and other cancers is a frequent and significant complication, and the effective management of metastatic bone disease and accompanying symptoms continues to be one of the major problems facing patients and their physicians. Treatment is in part dependent on prior treatments; usually a combination of systemic and local modalities is used because no single treatment regimen is effective for an extended period of time. The 3 radionuclides currently approved for treatment of bone pain (phosphorus-32, strontium-89, and samarium-153) are discussed in this review as viable treatment options, with emphasis on the third-generation agent in this category, samarium Sm 153 lexidronam. Clinical trial data are described that support the use of this agent in patients with hormone-refractory prostate cancer with painful metastatic bone disease, and the efficacy of and role for combination therapies are also discussed.
Key words: Prostate cancer, Samarium Sm 153 lexidronam, Metastatic bone disease, Bone scan, Radionuclides
Aclinical hallmark of prostate cancer is a predisposition to metastasize to the skeleton. Up to 80% of patients with prostate cancer have evidence of metastatic bone disease at autopsy,1 and bone is the only site of metastasis in 65% of patients presenting with metastatic prostate carcinoma.2 The propensity of cancer to metastasize to the skeleton, as shown in Table 1,3 has been recognized for over a century, and a number of theories have been developed to account for this observation. In 1889, Paget4 proposed the “seed-and-soil” theory that emphasized the importance of compatibility between the cancer (“seed”) that metastasized and the host milieu (“soil”) where local factors allowed or even enhanced proliferation of the metastasis.
Table 1.
Incidence and Prognosis of Bone Metastases
| Incidence of | Median Survival | ||
|---|---|---|---|
| Cancer Type | Advanced Disease, % | (Months) | 5-Year Survival, % |
| Myeloma | 95–100 | 20 | 10 |
| Breast | 65–75 | 24 | 20 |
| Prostate | 65–75 | 40 | 25 |
| Lung | 30–40 | <6 | <5 |
| Kidney | 20–25 | 6 | 10 |
| Thyroid | 60 | 48 | 40 |
| Melanoma | 14–45 | <6 | <5 |
Reprinted from Coleman RE3, with permission of Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc.
Copyright © 1997 American Cancer Society.
Recently it has been shown that the preferential localization of prostate cancer in bone is influenced by a variety of cytokines and bone-derived growth factors. These include transforming growth factor beta (TGF-β), bone-morphogenic proteins (BMPs), and epidermal growth factor (EGF), which promote the growth and differentiation of prostate cancer cells. TGF-β, which is expressed on prostate cancer cells, plays a role in the maturation of osteoclasts and facilitates cellular adhesion to the bone matrix—processes that are involved in the expression of androgen-independent cellular growth.5–7 In a similar manner, EGF facilitates the development of bone metastases by promoting migration of prostate cancer cells to bone tissue.8 In addition, prostate cancer cells themselves produce a variety of growth factors that interact either directly with osteoblasts and osteoclasts to promote growth and differentiation or indirectly by influencing a series of complex cellular and stromal interactions involved in normal skeletal homeostasis. Taken together, tumor-bone interactions in prostate cancer involve a series of soluble factors, insoluble matrix factors, and direct cell-to-cell interactions among the tumor, bone stroma, osteoclasts, and osteoblasts. The result is a disease that is markedly more osteoblastic in nature than skeletal lesions secondary to most other types of tumors.9
Metastatic bone disease contributes significantly to the morbidity and mortality associated with prostate cancer. Patients with bone metastasis complain of bone pain as well as of symptoms arising from bone marrow failure, nerve entrapment, and spinal cord compression. There is a direct relationship between the extent of osseous involvement and patient survival.10,11 Several characteristics related to bone metastases secondary to prostate cancer are unique in comparison with most other solid tumors. As a result of many of the factors discussed above, the frequency of clinically significant metastases to bone is very high while the incidence of clinically significant soft-tissue lesions is low. In addition, patients with bone metastases secondary to prostate cancer have a prolonged survival compared with patients at a similar stage with other malignancies such as lung cancer. This results in a higher prevalence of bone metastases among patients with prostate cancer in comparison with other malignancies. Thus, the effective management of metastatic bone disease and accompanying symptoms is one of the most common and difficult processes to address in patients with advanced prostate cancer.
The most reliable method of detecting bone metastases in a prostate cancer patient is a radionuclide bone scan. However, even in a patient with prostate cancer, not all areas of enhanced uptake on bone scan are associated with metastatic disease, particularly in the case of solitary lesions or uptake in joints.12 Confirmation of metastases by additional imaging modalities such as plain radiographs or MRI scans is often needed prior to making a diagnosis. Bone metastases may or may not be associated with painful symptoms when initially detected. In patients with stage D2 prostate cancer with bone metastases treated with hormonal therapy, increases in serum prostate-specific antigen (PSA) levels occur approximately 6 months before changes are detected with bone scans, which in turn occur approximately 4 months before the patient reports pain.13 The timing of this sequence of events in patients treated with hormonal therapy prior to the onset of bone metastases has not been well studied, but the interval between a rise in PSA values and bone scan positivity is suspected to be much longer (clearly more than 2 years).
Even in patients with a positive bone scan who report painful symptoms, a comprehensive examination may be indicated to establish the etiology of the pain and evaluate any possible complicating factors such as spinal cord compression, neuropathic conditions, and pathologic fractures.14,15 Patients with bone metastases may also have nonmalignant sources of bone pain, the causes of which need to be evaluated on a case-by-case basis.
The approach to palliation of pain from bone metastases varies depending on many factors, including degree of symptoms, extent of disease, comorbidities, and prior treatments. Analgesics, antitumor agents, hormones, chemotherapy, steroids, local surgery, bisphosphonates (zoledronic acid), anesthesia, and radiation therapy (local and systemic) are all appropriate treatments under selected circumstances. In general, a combination of systemic and local modalities is required and no single treatment regimen is efficacious for an extended period of time.16 Although a variety of treatments are commonly employed in treating metastatic bone pain, this review focuses on the use of systemically administered bone-targeted radionuclides in general and the third-generation agent in that category, samarium Sm 153 lexidronam, in specific.
Bone-Targeted Radionuclide Therapy
For many patients with multiple symptomatic osteoblastic bone metastases who have relapsed following an initial course of hormonal or chemotherapy, bone-targeted systemic radionuclides have emerged as a viable treatment option. These agents are also helpful for patients previously treated to their maximal normal tissue tolerance with focal external beam radiation who have progressive or recurrent symptoms at the treated sites.17 The indications and contraindications for the use of bone-targeted radionuclides are presented in Table 2. Baseline complete blood counts are necessary to establish adequate pretreatment levels of platelets (PLTs) and neutrophils because these agents result in some suppression of bone marrow function. Severe renal dysfunction is a contraindication to the use of bone-targeted radionuclides, as currently available agents are predominantly excreted by the kidney.
Table 2.
Use of Bone-Targeted Radionuclides for Treatment of Metastatic Bone Pain: Indications and Contraindications
| Indications |
|
| Relative Contraindications |
|
| Absolute Contraindications |
|
Three radionuclides are currently approved for the treatment of bone pain: first-generation phosphorus-32 (32P), second-generation strontium-89 (89Sr), and third-generation samarium- 153 (153Sm). These radionuclides all localize to regions of enhanced bone turnover and deliver high local doses of radiation through the emission of beta particles. The mechanism of bone targeting varies for each of them. 32P is targeted to bone through inorganic phosphate pathways and, in a similar manner, 89Sr is taken up as a calcium analog. 153Sm, however, is the only agent in its class targeted to bone via chelation to the aminotetraphosphonate EDTMP (ethylenediaminetetra- methylenephosphonic acid). The relevant nuclear decay properties of these radionuclides are shown in Table 3.
Table 3.
Nuclear Decay Properties of Radionuclides Approved for Treatment of Metastatic Bone Pain
| Half-life | β-Emission | Penetration | γ-Emission | |
|---|---|---|---|---|
| Radionuclide | (Days) | (Average, MeV) | (mm) | (keV, %) |
| Phosphorus-32 | 14.3 | 0.7 | 2.7 | None |
| Strontium-89 | 50.5 | 0.58 | 2.4 | None |
| Samarium-153 | 1.9 | 0.22 | 0.55 | 103, 29 |
MeV, million electron volt; keV, kiloelectron volt.
Decay properties such as half-life and particle energy (and resultant range) play significant roles in clinical characteristics of these agents, such as onset and duration of palliative effects and degree of and time to recovery from myelosuppression. The particle emissions from 32P and 89Sr and the corresponding ranges in bone and soft tissue are much greater than those of 153Sm. Higher-energy particles are associated with greater marrow toxicity as result of the larger volumes of marrow exposed to radiation. The shorter physical half-life of 153Sm (1.9 days) results in a more rapid delivery of radiation than either 32P (14.3 days) or 89Sr (50.5 days). For example, delivery of 90% of the total dose of radiation requires approximately 3.5 half-lives of decay, a time interval of approximately 1 week for 153Sm, 7 weeks for 32P, and 25 weeks for 89Sr.
Samarium Sm 153 Lexidronam
Samarium-153 is produced by the neutron bombardment of isotopically enriched 152Sm2O3 in a nuclear reactor. Soluble ionic 153Sm3+ when administered intravenously has very little propensity for bone, but when chelated by a variety of aminocarboxylate and aminophosphonate ligands it can be quite effectively targeted to the skeleton. Goeckeler and colleagues18 demonstrated that the complex formed with EDTMP had a combination of biologic properties necessary for a bone-targeted radiotherapeutic agent. These include rapid clearance from the vascular compartment following intravenous injection, high uptake and retention in the skeleton, and rapid renal clearance and urinary excretion of the portion not bound to bone. In addition, it was found that localization of the complex to newly formed bone, such as that laid down by osteoblastic bone metastases, was 10- to 20-fold higher per gram than that found in normal bone.
Preclinical escalating single (0.5–2.0 mCi/kg) and multiple (1.0 mCi/kg) dose studies in dogs showed that the only apparent toxicity related to the administration of samarium Sm 153 lexidronam was a decrease in circulating levels of white blood cells (WBCs) and PLTs.19 Dose-related decreases in WBCs and PLTs were observed following administration, reaching a nadir at 2 to 4 weeks, recovering to pretreatment levels by 5 to 6 weeks. In a separate study,20 very high (up to 30 mCi/kg) single doses were administered to dogs with the intent of ablating bone marrow. Unexpectedly, spontaneous recovery of marrow function was observed in all animals. Pathologic analyses showed that marrow function was retained in the mid-shaft of the long bones presumably as a result of the inability of the beta particle emissions from the 153Sm deposited in these areas of predominantly cortical bone to uniformly penetrate the marrow space.
Phase I and II Clinical Studies
Initial human clinical studies21 of samarium Sm 153 lexidronam over the dose range from 0.1 mCi/kg to 1.0 mCi/kg showed that both clearance from blood and soft tissue and uptake in bone and bone lesions were independent of administered dose over the range studied. Clearance from blood is biexponential with half-lives of 5.5 minutes and 65 minutes.22 Radioactivity not localized to the skeleton is rapidly cleared via the urine with excretion complete by 6 hours. Total skeletal uptake is highly correlated with the number of skeletal lesions. Through imaging of its gamma ray emissions (103 keV, 29% abundance) a number of studies have evaluated biolocalization and estimated dosimetry.23–25 Imaging studies have also evaluated uptake in bone lesions compared with that in adjacent normal bone. Across a number of studies, uptake in bone lesions has been found to be both qualitatively and quantitatively indistinguishable from that obtained using technetium-99m (Tc-99m)-based diagnostic bone scanning agents. Accordingly, diagnostic bone scans are useful for identifying patients eligible for treatment.
Turner and Claringbold26 treated 35 patients with symptomatic bone metastases with single and repeat doses ranging from 0.28 mCi/kg to 0.84 mCi/kg. Pain relief was observed in 22 of 34 (65%) evaluable patients for periods of 4 to 35 weeks. Collins and colleagues27 carried out a dose escalation study in patients with hormone-refractory prostate cancer and symptomatic bone metastases. Five groups of 4 patients each were sequentially treated with single 1.0, 1.5, 2.0, 2.5, and 3.0 mCi/kg doses. At the 3.0 mCi/kg dose level (which is 3 times the current approved dose), 3 of 4 patients experienced grade III neutropenia, and as a result, 2.5 mCi/kg was established as the maximum tolerated dose in this patient population. Subsequently, 16 additional patients were treated at both the 1.0 and 2.5 mCi/kg dose levels. Overall, 70% to 80% of patients, independent of dose level, experienced relief of pain, generally within 1 week of dosing.
Controlled Clinical Studies
On the basis of the responses observed in these phase I and II studies, a number of prospective, randomized, controlled studies have been performed to evaluate the efficacy of samarium Sm 153 lexidronam in relieving the pain of bone metastases from a variety of primary tumors. These studies have been carried out in large populations of patients in North America, Europe, and Asia. In studies having a placebo arm, the placebo was a formulation identical to the active drug with the exception that the samarium used was non-radioactive Sm-152.
Resche and colleagues28 carried out a multicenter, randomized, single-blind, dose-controlled study in patients with painful bone metastases from a variety of primary tumors. One hundred fourteen patients received single doses of either 0.5 mCi/kg (n = 55) or 1.0 mCi/kg (n = 59). The majority of the patients had either primary prostate (n = 67) or breast (n = 36) cancer. Efficacy was evaluated by blinded patient evaluations of pain using a visual analog scale (VAS) and recording of daily opioid analgesic use. In addition, quality of life was also evaluated by means of patient ratings of daytime discomfort and sleep patterns. VAS scores decreased from baseline for both treatment groups at each of the first 4 weeks following administration, with decreases larger at each week for the higher dose group. Decreases from baseline in VAS scores were statistically significant (P < .005) for the 1.0 mCi/kg group at both weeks 3 and 4. Changes from baseline for the lower dose group were not statistically significant at any week. The difference between groups in VAS scores was statistically significant at week 4 (P < .0476). An unblinded physician’s global assessment (PGA) judged that 70% of the 1.0 mCi/kg patients and 55% of those receiving 0.5 mCi/kg were improved at week 4. Patients receiving the higher dose also exhibited significant improvements over baseline in both daytime discomfort and sleep at week 4. Long-term follow-up revealed a significantly longer survival among breast cancer patients receiving the high dose than among those treated with the low dose. No such difference was observed for the prostate cancer patients.
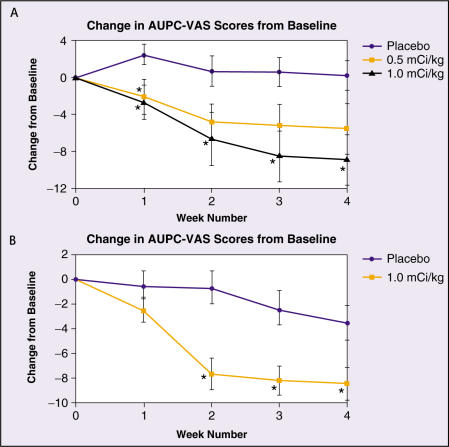
Serafini and associates29,30 reported the results of the first prospective, multicenter, randomized, double-blind, placebo-controlled study (Study BA-106/110) in patients with bone metastases from a variety of primary tumors. One hundred eighteen patients were randomized equally to receive single doses of placebo (n = 39) or active drug at doses of 0.5 mCi/kg (n = 40) or 1.0 mCi/kg (n = 39) and were followed-up for up to 16 weeks. The majority (86%) of the patients had either primary prostate (n = 80) or breast (n = 21) cancer. Efficacy was evaluated by blinded patient evaluations of pain using a VAS, recording of daily opioid analgesic use, and a blinded PGA. Patients who received the 1.0 mCi/kg dose of active drug exhibited significant improvements (compared with the placebo group) in both VAS scores and in the PGA at each of the first 4 weeks following administration. In contrast, patients receiving the 0.5 mCi/kg dose had a significant improvement in VAS scores for week 1 only, and in the PGA at week 4 only. Change from baseline in VAS scores for each of the treatment groups is shown in Figure 1A.
Figure 1.
Change from baseline in the area under the pain curve (AUPC) derived from daily VAS scores for studies BA-106/110 (A) and 424Sm10/11 (B). AUPC is a weekly integral measure of VAS scores obtained by summing daily scores over weekly time intervals. *Indicates a statistically significant improvement over placebo. VAS, visual analog scale. Adapted with permission from Serafini AN et al.29
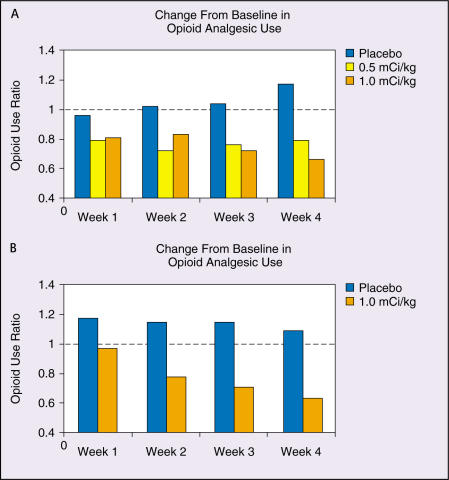
In the blinded PGA, 62% to 72% of those in the 1.0 mCi/kg group and 40% to 70% of those in the 0.5 mCi/kg group were judged to have relief of pain during the first 4 weeks following administration of the drug. Of the 0.5 mCi/kg patients judged to be responders at week 4, less than half were still responders at week 12. For the patients receiving the 1.0 mCi/kg dose, two-thirds of those responding at week 4 were also judged to be responding at week 16. A significant correlation (P = .01) was observed between reductions in daily opioid analgesic use and VAS scores for patients receiving 1.0 mCi/kg but not in any other group. Change in opioid analgesic use relative to baseline is shown in Figure 2A.
Figure 2.
Ratio of mean daily opioid analgesic use relative to baseline for the first 4 weeks post-administration in studies BA-106/110 (A) and 424Sm10/11 (B). Figure 2A adapted with permission from Serafini AN et al.29Figure 2B from data on file, Cytogen Corporation.
Sartor and colleagues31 reported the results of a prospective, multicenter, randomized, double-blind, placebo-controlled study (Study 424Sm10/11) in patients with bone metastases from hormone-refractory prostate cancer. A total of 152 patients were randomized in a 1:2 ratio, placebo (n = 51) or 1.0 mCi/kg active drug (n = 101), and were followed for up to 16 weeks. Pain intensity was measured twice daily (by patients) using validated linear and non-linear scales. A 100-mm VAS, with 0 mm representing the “least possible pain” and 100 mm the “worst possible pain,” was used, as was a pain descriptor scale (PDS), which consisted of 8 words or phrases (no pain, just noticeable, weak, mild, moderate, strong, severe, and excruciating) randomly arranged.32 Daily opioid analgesic use was also recorded.
Patients who received the active drug exhibited significant improvements (compared with the placebo group) in VAS scores for weeks 2 through 4 (P < .0232) and in PDS scores at each of the first 4 weeks following administration (P < .003).
Change from baseline in VAS scores for both treatment groups is shown in Figure 1B. A statistically significant correlation (r = 0.78, P < .0001) was found between VAS and PDS scores. In addition, patients who received active drug had significant decreases in opioid analgesic use at weeks 3 and 4 (P < .0284). Changes in VAS scores were correlated with reductions in opioid analgesic use in the active treatment group (r=0.349, P=.0004), but no such correlation occurred in the placebo group (r = 0.059, P = 0.685). Change in opioid analgesic use relative to baseline is shown in Figure 2B.
The results of the 3 controlled studies described above, all of which were sponsored by the US manufacturer (Cytogen Corporation, Princeton, NJ), have been supplemented by additional large multicenter studies performed independently in China and by the International Atomic Energy Agency (IAEA). In the multicenter trial conducted in China,33 patients with painful bone metastases from a variety of primary tumors were treated with single doses of either 0.5 mCi/kg (n = 70) or 1.0 mCi/kg (n =35). Pain was assessed using a composite of pain scores and analgesic consumption. Positive responses were observed for 58 of 70 (83%) patients in the 0.5 mCi/kg group and 30 of 35 (86%) in the 1.0 mCi/kg group. In the IAEA multicenter study,34 417 patients were divided into 3 groups, receiving single doses of 0.5 mCi/kg, 1.0 mCi/kg, or 1.5 mCi/kg, respectively. Overall, 73% of patients, independent of dose level, experienced effective pain palliation. Analgesic use was reduced significantly or completely in 82% of those responding.
Safety
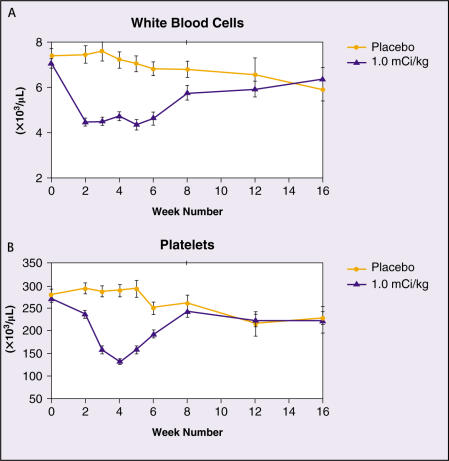
In all clinical studies of samarium Sm 153 lexidronam, the only clinically significant toxicity reported has been mild and transient myelosuppression. WBC and PLT counts decrease beginning at 1 to 2 weeks to a nadir of approximately 50% of the baseline level at 3 to 5 weeks post administration. Recovery to pretreatment levels is typically observed by week 8. No differences have been seen in hemoglobin levels between patients receiving 1.0 mCi/kg active drug and those administered a placebo. Pooled WBC and PLT levels as a function of time for 1.0 mCi/kg and placebo patients in the 2 placebo-controlled studies are shown in Figure 3.
Figure 3.
White blood cell and platelet counts in placebo-controlled studies.
Table 4 summarizes the hematologic toxicity grades in the double-blind portions of the 2 placebo-controlled studies. Grade 2 or less WBC and PLT toxicities were observed in 92% and 97%, respectively, of patients administered 1.0 mCi/kg samarium Sm 153 lexidronam in the placebo-controlled studies. Additionally, no grade 4 WBC or PLT toxicities occurred among the 128 patients treated at this dose level.
Table 4.
Hematologic Toxicity Grades in Placebo-Controlled Studies
| Parameter and | Placebo (n = 83) | 1.0 mCi/kg (n = 128) |
|---|---|---|
| Toxicity Grade | n (%) | n (%) |
| Hemoglobin* | ||
| 0–2 | 76 (92) | 115 (90) |
| 3 | 6 (7) | 12 (9) |
| 4 | 1 (1) | 1 (1) |
| Platelets† | ||
| 0–2 | 83 (100) | 124 (97) |
| 3 | 0 (0) | 4 (3) |
| 4 | 0 (0) | 0 (0) |
| WBCs‡ | ||
| 0–2 | 83 (100) | 117 (92) |
| 3 | 0 (0) | 10 (8) |
| 4 | 0 (0) | 0 (0) |
Hemoglobin: grade 0–2, ≥ 8.0 g/dL; grade 3, 6.5–7.9 g/dL, grade 4, < 6.5 g/dL.
Platelets: grade 0–2, ≥ 50,000/μL; grade 3, 25,000–49,900/μL, grade 4, < 25,000/μL.
WBCs: grade 0–2,≥ 2000/μL; grade 3, 1000–1900/μL, grade 4, < 1000/μL.
WBCs, white blood cells.
Nonhematologic adverse events (Table 5) occurred at similar rates in patients who received placebo and 1.0 mCi/kg of active drug. The most commonly observed nonhematologic adverse events, nausea and/or vomiting and constipation, which are known to be associated with the use of opioid-containing analgesics, had a somewhat lower incidence in patients receiving 1.0 mCi/kg than that found among the patients in the placebo group. This may be related to the reductions in opioid analgesic consumption discussed above for patients receiving active treatment. In controlled clinical studies, spinal cord compression occurred at the same frequency (6%) in patients receiving 1.0 mCi/kg as for those receiving placebo, despite the fact that the active- treatment patients on average remained on study nearly twice as long as those receiving a placebo. Painful flare reactions, an adverse event that has been observed with all skeletal-targeted radionuclides, occurred at similar rates for those receiving 1.0 mCi/kg (7%) as with those administered the placebo (6%). In the blinded placebo-controlled study of patients with hormone- refractory prostate cancer,31 the response rate for the active-treatment patients who experienced a painful flare reaction was similar to that of the remainder of the group.
Table 5.
Incidence of Most Common Nonhematologic Adverse Events in Controlled Clinical Studies
| Adverse Event | Placebo (%) | 1.0 mCi/kg (%) |
|---|---|---|
| Nausea and/or vomiting | 41 | 33 |
| Constipation | 13 | 8 |
| Asthenia | 11 | 10 |
| Fever | 10 | 6 |
| Anorexia | 7 | 8 |
| Spinal cord compression | 6 | 6 |
| Pain flare | 6 | 7 |
| Dyspnea | 6 | 5 |
| Urinary tract infection | 4 | 6 |
From data on file, Cytogen Corporation.
Repeat Dosing
Retreatment with samarium Sm 153 lexidronam has been reported primarily in a setting of an initial favorable palliative response followed by recurrence of symptoms. Sartor and colleagues35 reported on 54 multiple administrations to 18 patients (range 2–11 doses/patient) with prostate (n = 15) and breast (n = 3) cancer. Percentage decreases from baseline in WBC and PLT counts did not increase as a function of increasing number of administrations, and there was likewise no significant increase in the percentage of patients experiencing grade 3 or 4 hematologic toxicities. Menda and associates36 reported treatment of a patient with bone metastases from hormone refractory prostate cancer with 11 doses of 1.0 mCi/kg each over a period of 28 months. None of the WBC and PLT nadir counts following any of the repeat doses were lower than those following the initial dose.
Combination Treatments
Recently considerable interest has emerged in the use of skeletal-targeted radionuclides in combination with other agents such as bisphosphonates and taxane-based chemotherapeutics. Marcus and colleagues37 studied the skeletal uptake of samarium Sm 153 lexidronam prior to and 1 and 4 days following administration of pamidronate and again at 1, 2, 3, and 4 weeks following administration of the bisphosphonate. There was no difference in uptake of samarium Sm 153 lexidronam at any time following administration, of the pamidronate.
Preliminary data are available regarding the combination of samarium Sm 153 lexidronam and docetaxel in patients with hormone-refractory prostate cancer. In a phase I study examining the biodistribution and preliminary efficacy, 6 patients were treated with weekly docetaxel at a dose of 30 mg/m2, in combination with 1.0 mCi/kg given on week 4, 24 hours before treatment with docetaxel.38 Optimal uptake by tumor sites was seen 8 to 24 hours after injection. Five of 6 patients had a decrease in PSA values of >50% and 4 of 6 patients had a decrease of >80% that persisted for more than 6 months. Toxicity was not dose limiting, with 1 episode of neutropenic fever reported. This study clearly deserves additional follow-up and expanded patient numbers.
In another preliminary study,39 6 patients with metastatic prostate cancer were treated with paclitaxel 200 mg/m2 every 3 weeks combined with estramustine and 1.0 mCi/kg of samarium Sm 153 lexidronam. Subsequent groups of 6 patients were treated with paclitaxel 90 mg/m2 every 3 weeks. Samarium Sm 153 lexidronam was administered with chemotherapy, starting at a dose of 1 mCi/kg and escalating by 0.5 mCi/kg increments. Grade 3 leucopenia was seen in 1 of 6 patients at the 1.5 mCi/kg level, and maximally tolerated dose had not been reached at the time of the report.
Summary
Bone metastases with pain are a common and significant problem for patients with advanced prostate cancer. The treatments for bone-metastatic prostate cancer are dependent in part on prior treatments. Extensive data from prospective randomized clinical trials now support the use of samarium Sm 153 lexidronam in patients with hormone-refractory disease and painful bone metastases. Pain relief and decreases in analgesic consumption can be expected in the majority of patients treated. Side effects are limited to transient and relatively mild platelet and neutrophil suppression. Repeated doses can be used in patients whose marrow reserve is adequate at the time of administration. Combination therapies that incorporate cytotoxic or bone-targeted agents into samarium Sm 153 lexidronam-based regimens are now being actively explored in clinical trials.
Main Points.
Metastatic bone disease contributes significantly to morbidity and mortality of patients with advanced cancer, with a direct relationship evident between extent of bone involvement and survival.
The radionuclide bone scan is the most reliable method of detecting bone metastasis in patients with prostate cancer.
Bone-targeted systemic radionuclides have become viable treatment options for patients with hormone-refractory prostate cancer and painful multiple bone metastases.
Currently, there are 3 radionuclides approved for treatment of bone pain, phosphorus-32, strontium-89, and samarium-153, each with differing bone targeting mechanisms.
Beta particle emissions are of higher energy in phosphorus-32 and strontium-89 than samarium-153 and result in greater bone marrow toxicity. The half-life of samarium-153 is 1.9 days, much shorter than that for the other 2 agents, and results in a more rapid delivery of radiation.
Samarium Sm 153 lexidronam has been found to be effective at palliating pain and reducing opioid analgesic use, and associated with mild and transient adverse events in prostate cancer patients with hormone-refractory disease and metastatic bone disease.
References
- 1.Galasko CSB. The anatomy and pathways of skeletal metastases. In: Weiss L, Gilbert HA, editors. Bone Metastasis. Vol. 6. Boston, Mass: GK Hall; 1981. pp. 49–63. [Google Scholar]
- 2.McRea LE, Karafin L. Carcinoma of the prostate: metastases, therapy, and survival. A statistical analysis of 500 cases. Int Coll Surg J. 1958;29:723–728. [PubMed] [Google Scholar]
- 3.Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80(suppl 8):1588–1594. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 4.Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98–101. [PubMed] [Google Scholar]
- 5.Fong CJ, Sherwood ER, Braun EJ, et al. Regulation of prostatic carcinoma cell proliferation and secretory activity by extracellular matrix and stromal secretions. Prostate. 1992;21:121–131. doi: 10.1002/pros.2990210205. [DOI] [PubMed] [Google Scholar]
- 6.Muir GH, Butta A, Shearer RJ, et al. Induction of transforming growth factor beta in hormonally treated human prostate cancer. Br J Cancer. 1994;69:130–134. doi: 10.1038/bjc.1994.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kostenuik PJ, Singh G, Orr FW. Transforming growth factor beta upregulates the integrin-mediated adhesion of human prostatic carcinoma cells to type I collagen. Clin Exp Metastasis. 1997;15:41–52. doi: 10.1023/a:1018484323210. [DOI] [PubMed] [Google Scholar]
- 8.Rajan R, Vanderslice R, Kapur S, et al. Epidermal growth factor (EGF) promotes chemomigration of a human prostate tumor cell line, and EGF immunoreactive proteins are present at sites of metastasis in the stroma of lymph nodes and medullary bone. Prostate. 1996;28:1–9. doi: 10.1002/(SICI)1097-0045(199601)28:1<1::AID-PROS1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 9.Gandhok N, Sartor O. Bone-targeted therapy for prostate cancer. In: Klein EA, editor. Current Clinical Urology: Management of Prostate Cancer. 2nd edition. Totowa, NJ: Humana Press; 2004. pp. 589–606. [Google Scholar]
- 10.Soloway MS, Hardeman SW, Hickey D, et al. Stratification of patients with metastatic prostate cancer based on extent of disease on initial bone scan. Cancer. 1988;61:195–202. doi: 10.1002/1097-0142(19880101)61:1<195::aid-cncr2820610133>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 11.Sabbatini P, Larson SM, Kremer A, et al. Prognostic significance of extent of disease in bone in patients with androgen-independent prostate cancer. J Clin Oncol. 1999;17:948–957. doi: 10.1200/JCO.1999.17.3.948. [DOI] [PubMed] [Google Scholar]
- 12.Jacobson AF, Cronin EB, Stomper PC, Kaplan WD. Bone scans with one or two new abnormalities in cancer patients with no known metastases: frequency and serial scintigraphic behavior of benign and malignant lesions. Radiology. 1990;175:229–232. doi: 10.1148/radiology.175.1.2315486. [DOI] [PubMed] [Google Scholar]
- 13.Newling DW, Denis L, Vermeylen K. Orchiectomy versus goserelin and flutamide in the treatment of newly diagnosed metastatic prostate cancer: analysis of the criteria of evaluation used in the European Organization for Research and Treatment of Cancer-Genitourinary Group Study 30853. Cancer. 1993;72(12 suppl):3793–3798. doi: 10.1002/1097-0142(19931215)72:12+<3793::aid-cncr2820721706>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 14.Garrett IR. Bone destruction in cancer. Semin Oncol. 1993;20:4–9. [PubMed] [Google Scholar]
- 15.Ventafridda V, Sbonatto A, DeConno F. Pain in prostate cancer. Palliat Med. 1990;4:173–184. [Google Scholar]
- 16.Serafini AN. Therapy of metastatic bone pain. J Nucl Med. 2001;42:895–906. [PubMed] [Google Scholar]
- 17.Sartor O. Radioisotopic treatment of bone pain from metastatic prostate cancer. Curr Oncol Rep. 2003;5:258–262. doi: 10.1007/s11912-003-0119-2. [DOI] [PubMed] [Google Scholar]
- 18.Goeckeler WF, Edwards B, Volkert WA, et al. Skeletal localization of samarium-153 chelates: potential therapeutic bone agents. J Nucl Med. 1987;28:495–504. [PubMed] [Google Scholar]
- 19.Lattimer JC, Corwin LA, Jr, Stapleton J, et al. Clinical and clinicopathologic effects of Samarium- 153-EDTMP administered intravenously to normal beagle dogs. J Nucl Med. 1990;31:586–593. [PubMed] [Google Scholar]
- 20.Applebaum FR, Sandmaier B, Brown PA, et al. Myelosuppression and mechanism of recovery following administration of samarium-153 EDTMP. Antibod Immunoconj Radiopharm. 1988;1:263–270. [Google Scholar]
- 21.Singh A, Holmes RA, Farhangi M, et al. Human pharmacokinetics of samarium 153 EDTMP in metastatic cancer. J Nucl Med. 1989;30:1814–1818. [PubMed] [Google Scholar]
- 22.Bayouth JE, Macey DJ, Kasi LP, Fossella FV. Dosimetry and toxicity of samarium-153-EDTMP administered for bone pain due to skeletal metastases. J Nucl Med. 1994;35:63–69. [PubMed] [Google Scholar]
- 23.Eary JF, Collins C, Stabin M, et al. Samarium- 153-EDTMP biodistribution and dosimetry estimation. J Nucl Med. 1993;34:1031–1036. [PubMed] [Google Scholar]
- 24.Heggie JCP. Radiation absorbed dose calculations for samarium-153-EDTMP localized in bone. J Nucl Med. 1991;32:840–844. [PubMed] [Google Scholar]
- 25.Podoloff DA, Kasi LP, Kim EE, et al. Evaluation of Sm-153-EDTMP as a bone imaging agent during a therapeutic trial [abstract] J Nucl Med. 1991;32(suppl) [Google Scholar]
- 26.Turner JH, Claringbold PG. A phase II study of treatment of painful multifocal skeletal metastases with single and repeated dose of samarium-153 ethylenediaminetetramethylene-phosphonate. Eur J Cancer. 1991;27:1084–1086. doi: 10.1016/0277-5379(91)90297-q. [DOI] [PubMed] [Google Scholar]
- 27.Collins C, Eary JF, Donaldson G, et al. Samarium-153 EDTMP in bone metastases of hormone refractory prostate cancer: a phase I/II trial. J Nucl Med. 1993;34:1839–1844. [PubMed] [Google Scholar]
- 28.Resche I, Chatal JF, Pecking A, et al. A dose controlled study of 153Sm-ethylenediaminetetra-methylephosphonate (EDTMP) in patients with painful bone metastases. Eur J Cancer. 1997;33:1583–1591. doi: 10.1016/s0959-8049(97)00155-x. [DOI] [PubMed] [Google Scholar]
- 29.Serafini AN, Houston SJ, Resche I, et al. Palliation of pain associated with metastatic bone cancer using samarium-153 lexidronam: a double-blind placebo-controlled clinical trial. J Clin Oncol. 1998;16:1574–1581. doi: 10.1200/JCO.1998.16.4.1574. [DOI] [PubMed] [Google Scholar]
- 30.Serafini AN. Samarium Sm-153 lexidronam for the palliation of bone pain associated with metastases. Cancer. 2000;88(suppl):2034–2039. doi: 10.1002/1097-0142(20000615)88:12+<2934::aid-cncr9>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 31.Sartor O, Reid RH, Hoskin PJ, et al. Samarium- 153-lexidronam complex for treatment of painful bone metastases in hormone-refractory prostate cancer. Urology. 2004;63:940–945. doi: 10.1016/j.urology.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 32.Fishman B, Pasternak S, Wallenstein SL, et al. The Memorial pain assessment card: a valid instrument for the evaluation of cancer pain. Cancer. 1980;60:1151–1158. doi: 10.1002/1097-0142(19870901)60:5<1151::aid-cncr2820600538>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 33.Tian JH, Zhang JM, Hou QT, et al. Multicentre trial on the efficacy and toxicity of single dose samarium-153-ethylene diamine tetra-methylene phosphonate as a palliative treatment for painful skeletal metastases in China. Eur J Nucl Med. 1999;26:2–7. doi: 10.1007/s002590050351. [DOI] [PubMed] [Google Scholar]
- 34.Olea E, Riccabona G, Tian J, et al. Efficacy and toxicity of Sm-153-EDTMP in the palliative treatment of skeletal metastases: results of an IAEA international multicenter study [abstract] J Nucl Med. 2000;41(suppl) [Google Scholar]
- 35.Sartor O, Bushnell D, Reid R, et al. Repeated administration of samarium-153 lexidronam in the treatment of painful bone metastases [abstract] Proc Am Soc Clin Oncol. 2001;20:1036. [Google Scholar]
- 36.Menda Y, Bushnell DL, Williams RD. Efficacy and safety of repeated samarium-153 lexidronam treatment in a patient with prostate cancer and metastatic bone pain. Clin Nucl Med. 2000;25:698–700. doi: 10.1097/00003072-200009000-00009. [DOI] [PubMed] [Google Scholar]
- 37.Marcus CS, Saeed S, Mlikotic A, et al. Lack of effect of a bisphosphonate (pamidronate disodium) infusion on subsequent skeletal uptake of Sm-153-EDTMP. Clin Nucl Med. 2002;27:427–430. doi: 10.1097/00003072-200206000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Widmark A, Linne T, Modig H, Johansson L. Optimizing the time of co-administration of docetaxel and samarium-153 for advanced androgen independent carcinoma of the prostate [abstract] Proc Am Soc Clin Oncol. 2003;22:433. [Google Scholar]
- 39.Arnsmeier SI, Spies S, Shervin D, et al. Phase I/II study of taxane and estramustine with samarium in patients with hormone refractory prostate cancer [abstract] Proc Am Soc Clin Oncol. 2004;23:438. [Google Scholar]