Abstract
Efforts to evaluate and discover diagnostic and therapeutic markers for prostate cancer continue. One of these, prostate-specific membrane antigen (PSMA), a transmembrane protein expressed in all types of prostatic tissue, remains a useful diagnostic and possibly therapeutic target. The radio-immunoconjugate form of the anti-PSMA monoclonal antibody 7E11 is used in the commercially available and US Food and Drug Administration-approved diagnostic tool, the ProstaScint® (Cytogen Corporation, Princeton, NJ) scan. Recent studies have demonstrated other possible useful roles for PSMA as a target, not only in prostate cancer, but in other malignancies.
Key words: Prostate-specific membrane antigen, Prostate cancer, Monoclonal antibody, Angiogenesis
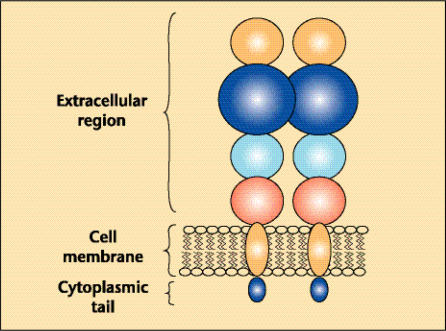
Prostate-specific membrane antigen (PSMA) is a type II membrane protein originally characterized by the murine monoclonal antibody (mAb) 7E11-C5.3 and is expressed in all forms of prostate tissue, including carcinoma.1–6 The PSMA protein has a unique 3-part structure: a 19-amino-acid internal portion, a 24-amino-acid transmembrane portion, and a 707-amino-acid external portion (Figure 1).7,8 The PSMA gene is located on the short arm of chromosome 11 in a region that is not commonly deleted in prostate cancer.9
Figure 1.
Schematic of prostate-specific membrane antigen.
PSMA has known enzymatic activities and acts as a glutamate-preferring carboxypeptidase.10–12 The impact of these enzymatic functions on human prostate tissue and perhaps elsewhere, however, remains unclear, as does the question regarding the existence of a natural ligand for PSMA. What has been demonstrated recently is that PSMA does have an internalization signal that allows internalization of the protein on the cell surface into an endosomal compartment.13 This recently recognized characteristic might prove useful in future diagnostic and therapeutic maneuvers in which PSMA is used as an antigenic target.
Anti-PSMA Antibodies
Originally developed with a type of prostate cancer cell line known as LNCaP cells, the mAb 7E11 was the first anti-PSMA antibody. It recognizes and binds a PSMA intracellular or cytoplasmic epitope.2,6,14 New mAbs, however, continue to be discovered and developed.15–17 A key difference of these newer antibodies is where the binding interaction take s place, although this distinction may be less relevant for radionuclide-based imaging and therapeutic applications. The more recently developed anti-PSMA mAbs bind the extracellular portion of PSMA and, in fact, can be internalized by PSMA-expressing cells.18 Recent anti-PSMA antibodies have identified dimer-specific epitopes on PSMA-expressive tumor cells.19 In addition, several of these next-generation antibodies are now either fully human or humanized as opposed to murine antibodies, thus making them even more likely to be diagnostically and therapeutically effective without possible antimouse reactions, although the incidence of such antimouse reactions with ProstaScint (or capromab pendetide) have been extremely low.
Clinical Evaluation of PSMA
Tissue Expression
Studies have consistently demonstrated PSMA expression in all types of prostate tissue and increased PSMA expression in cancer tissue.2,3,5,6,20,21 The binding occurs in the epithelial cells of the prostate but not in the basal or stromal cells. Bostwick and colleagues22 described PSMA immunohistochemical expression in 184 prostate specimens examined, all of which had PSMA expression and demonstrated a correlation between this expression and severity of cancer. There was an increase in the percentage of PSMA staining from benign epithelial tissue (69.5% of cells positive) to high-grade prostatic intraepithelial neoplasia (77.9% of cells positive) to malignant cells (80.2% of cells positive).22
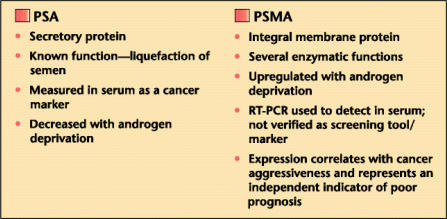
Prostate-specific antigen (PSA) and PSMA are different in several ways (Figure 2). Importantly, PSMA expression seems to be inversely related to androgen levels.23 Denmeade and colleagues24 recently examined cell lines in different states of androgen deprivation and discovered that PSMA activity in prostate cancer cell lines increased as cells became more androgen independent. Such manipulation could improve the efficacy of any antibody-directed, diagnostic/therapeutic targeting. Short-term (3-month) neoadjuvant deprivation therapy in clinically localized prostate cancer patients, however, did not increase immunohistochemical PSMA expression within prostate tissue.25
Figure 2.
Comparison of prostate-specific antigen (PSA) and prostate-specific membrane antigen (PSMA). RT-PCR, reverse transcriptase polymerase chain reaction.
Antibody binding to PSMA does not seem to be restricted absolutely to prostate tissue. Anti-PSMA mAbs consistently bind duodenal epithelial (brush border) cells and proximal tubule cells in the kidney.15,17 More excitingly, PSMA seems to be expressed in other cancers, more specifically in the neovasculature associated with these cancers.5,15 We have examined a wide range of carcinomas, including conventional (clear cell) renal cell, transitional cell of the bladder, testicular-embryonal, neuroendocrine, colon, and breast, and the different types of malignancies consistently and strongly expressed PSMA in their neovasculature.17 Interestingly, this binding of the neovasculature does not seem to occur in prostate cancer.5,17,22
Diagnostic Applications
Researchers have attempted to use PSMA as a serum-based marker, but results have been variable at best.2,26–28 Murphy and colleagues29 examined the results of a number of reverse transcriptase polymerase chain reaction (RT-PCR) studies and found that RT-PCR of serum PSMA was not accurate enough to be the basis of a decision to treat and did not independently contribute more than the currently established prognostic indicators of Gleason sum, serum PSA, or clinical stage. Current RT-PCR strategies have much to overcome, especially in the reproducibility of these techniques. Better differentiating primers need to be identified as well. As a result, PSMA is not used as a serum-based diagnostic or screening marker.
What has been clinically useful and safe is the ProstaScint® scan (Cytogen Corporation, Princeton, NJ), the US Food and Drug Administration-approved radiographic test that uses the mAb 7E11 by linking it to 111indium to produce a radiodiagnostic marker, 111indium-capromab pendetide.26,30,31 The majority of studies in high-risk metastatic prostate cancer and recurrent prostate cancer have demonstrated a sensitivity rate of 60% to 80% and a specificity rate of 70% to 90%, which are better than the accuracy of current CT scans or MRIs.31,32 A combination of algorithms, nomograms, and the ProstaScint scan was analyzed, and the combination of algorithms and ProstaScint scan provided an improved 72% positive predictive value for metastatic disease.33 A recent study also found that no minimum serum PSA value was needed to detect radiographic disease after surgery.34 Importantly, however, a recent study by Thomas and coll eagues35 revealed that the ProstaScint scan did not predict biochemical control after radiation therapy.
As a result of limitations inherent to SPECT imaging, ProstaScint imaging techniques continue to evolve in an attempt to improve accuracy and clinical utility and are discussed elsewhere in this supplement. In addition, PSMA is being used as a radiographic imaging target by newer, second-generation antibodies that bind the external portion of PSMA. Early promising results from phase I trials have demonstrated 90% correlation with conventional scans, but pathological confirmation studies are needed.36
PSMA expression might also be a predictor of disease recurrence in prostate cancer patients. In a recent series by Ross and colleagues,1 examination of postprostatectomy specimens revealed PSMA expression determined through immuno staining with the mAb 7E11 that correlated with tumor grade, pathologic stage, P SA, and aneuploidy. Importantly, in a multivariate analysis, PSMA expression independently predicted the likelihood of biochemical recurrence (Table 1).1
Table 1.
Prostate-Specific Membrane Antigen Expression Status in Prostate Cancer Treated by Radical Prostatectomy
| Non-overexpressing | Overexpressing | ||
|---|---|---|---|
| (n = 71) | (n = 65) | P | |
| Incidence of | |||
| recurrent disease | 20/71 (28%) | 37/65 (57%) | .001 |
| Mean time to | |||
| recurrence (months) | 43.75 | 34.78 | .001 |
Prostate-specific membrane antigen overexpression independently predicts disease recurrence.
Data from Ross JS et al.1
Expanding the possible role of PSMA as a radiographic marker was an incidental renal cell carcinoma discovered by 111indium-capromab pendetide scan. The scan revealed suspicious uptake in a kidney, which subsequent conventional imaging revealed to be a solid renal mass with necrosis.37 In an in vivo setting, this example might have demonstrated the recognition by the anti-PSMA mAb 7E11 of tumor-associated neovasculature. Other malignancies such as non-Hodgkin’s lymphoma, neurofibromatosis, and meningioma have been detected as well.38–40 More research is necessary to determine the in vivo activity of anti-PSMA monoclonal antibodies with regard to nonprostatic primary and metastatic malignancies.
Therapeutic Interventions
The use of PSMA as a therapeutic antigenic target for antibodies has recently become more than a hypothetical proposal. Recent studies with an anti-PSMA mAb have used linkages to radionuclides to treat metastatic prostate cancer. In an early trial, no toxicity has been noted, and as important, the antibody-radionuclide compound localized to tumor in vivo, even to bony sites of metastatic disease.36,41 This technique of combining an antibody with some type of therapeutic intervention has also been used in nonprostate cancer lesions, including renal cell cancer, in which phase II trials have combined anti-PSMA mAb with interleukin-2.42
Another type of therapy is immunotherapy, which avoids foreign DNA and uses a patient’s own cells to provide the mechanism for treatment. Currently, several novel treatment options use PSMA in this manner. Gong and colleagues43 have developed a unique approach involving creation of an artificial T cell receptor to target cells expressing PSMA. This artificial T-cell receptor incorporates a PSMA-specific single-chain antibody fused to a zeta chain signal transduction domain. Promising in vitro results demonstrate successful lysis of PSMA-positive prostate cancer cells with no effect on PSMA-negative cells. This model can successfully produce large amounts of interleukin-2. In addition, the amplified cell populations retain their antigen-specificity.44
PSMA peptides have been used to generate an immune response by infusing dendritic cells pulsed by these PSMA peptides. In a recent trial, a small number of patients with advanced or metastatic disease had a partial clinical response, defined as greater than 50% reduction in serum PSA.45
Gardner and colleagues46 recently presented findings of early studies of a newly developed vaccine based on a novel recombinant soluble PSMA protein representing the extracellular domain of PSMA.19 This vaccine was administered to patients with progressive disease following local therapy, a n d was well tolerated in this patient population. Results showed that the vaccination elicited antibodies that reacted strongly with prostate cancer cells, and that antibody levels increased with repeated dosing.
Based on the evaluation of fully human mAbs against PSMA in a preclinical setting, Ma and colleagues47 recently selected a lead fully human antibody for testing in naked, radiolabeled and toxin-conjugated forms. Focusing on novel recombinant forms of PSMA and XenoMouse™ technology (Abgenix, Fremont, CA), they evaluated these mAbs’ cytotoxic effects and their ability to deliver cytotoxic agents to PSMA-expressing tumor cells. Results showed naked mAbs induced antibody-dependent cell-mediated cytotoxicity of human prostate cells; the mAb labeled with isotope 177Lu was well tolerated and was effective in targeting PSMA and increasing survival in animals by more than 3-fold. In addition, toxin-conjugated mAbs quickly internalized and killed PSMA-expressing cells and eradicated prostate tumors in mice without toxicity.
By using different combinations of anti-PSMA antibodies or antibodies to other previously described targets such as GM2, KSA, Thomsen-Friedenreich antigen, or others yet to be identified, one could perhaps develop a more powerful and/or more precisely targeted treatment strategy for prostate cancer.48 This approach would attempt to decrease any nonspecific binding that can occur. Current antibodies used to target PSMA are not absolutely prostate-specific,27 but this has been true with current therapeutic antibodies available for cancer therapy.49,50
Finally, what we know now about PSMA is that its promotor and gene, or surrounding gene sequence, must contain transcriptional enhancer regions that selectively activate PSMA transcription. This activation seems to occur in tumor-associated neovasculature but not in benign vessels. By manipulating these sequences or better understanding the impact of certain sequences, one could develop an antiangiogenic gene therapy construct. Studies have demonstrated the effect of modulating the promotor region on PSMA expression,51 but actually utilizing this in vivo is currently hypothetical.
Conclusions
The possible diagnostic and therapeutic role of PSMA continues to evolve. In prostate cancer, PSMA continues to be a useful antigenic target that will continue to be of diagnostic and therapeutic value as newer targeting agents are developed and imaging systems and techniques continue to improve. In addition, beyond prostate cancer, PSMA might represent a unique angiogenic target for a variety of neoplasms.
Main Points.
Studies have consistently demonstrated prostate-specific membrane antigen (PSMA) expression in all types of prostate tissue and increased PSMA expression in cancer tissue.
PSMA seems to be expressed in other cancers (conventional renal cell, transitional cell of the bladder, testicular-embryonal, neuroendocrine, colon, and breast), specifically in the neovasculature associated with these cancers.
Reverse transcriptase polymerase chain reaction of serum PSMA is not accurate enough to be the basis of clinical therapy and does not independently contribute more than the currently established prognostic indicators of Gleason sum, serum prostate-specific antigen, or clinical stage.
What has been clinically useful and safe is the ProstaScint® scan (Cytogen Corporation, Princeton, NJ), the US Food and Drug Administration-approved radiographic test that uses the monoclonal antibody 7E11 by linking it to an 111indium to produce a radiodiagnostic marker, 111indium-capromab pendetide.
References
- 1.Ross JS, Sheehan CE, Fisher HAG, et al. Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer. Clin Cancer Res. 2003;9:6357–6362. [PubMed] [Google Scholar]
- 2.Horoszewicz JS, Kawinski E, Murphy GP. Monoclonal antibodies to a new antigenic marker in epithelial cells and serum of prostatic cancer patients. Anticancer Res. 1987;7:927–936. [PubMed] [Google Scholar]
- 3.Lopes AD, Davis WL, Rosenstraus MJ, et al. Immunohistochemical and pharmacokinetic characterization of the site-specific immunoconjugate CYT-356 derived from antiprostate monoclonal antibody 7E11-C5. Cancer Res. 1990;50:6423–6429. [PubMed] [Google Scholar]
- 4.Israeli RS, Miller WH, Jr, Su SL, et al. Sensitive nested reverse transcription polymerase chain reaction detection of circulating prostatic tumor cells: comparison of prostate-specific membrane antigen and prostate-specific antigen-based assays. Cancer Res. 1994;54:6306–6310. [PubMed] [Google Scholar]
- 5.Silver DA, Pellicer I, Fair WR, et al. Prostatespecific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–85. [PubMed] [Google Scholar]
- 6.Troyer JK, Beckett ML, Wright GL., Jr. Detection and characterization of the prostate-specific membrane antigen (PSMA) in tissue extracts and body fluids. Int J Cancer. 1995;62:552–558. doi: 10.1002/ijc.2910620511. [DOI] [PubMed] [Google Scholar]
- 7.Leek J, Lench N, Maraj B, et al. Prostate-specific membrane antigen: evidence for the existence of a second related human gene. Br J Cancer. 1995;72:583–588. doi: 10.1038/bjc.1995.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denekamp J, Dasu A, Waites A. Vasculature and microenvironmental gradients: the missing links in novel approaches to cancer therapy? Adv Enz Regul. 1998;38:281–299. doi: 10.1016/s0065-2571(97)00015-0. [DOI] [PubMed] [Google Scholar]
- 9.O’Keefe DS, Su S, Bacich DJ, et al. Mapping, genomic organization and promoter analysis of the human prostate-specific membrane antigen gene. Biochem Biophys Acta. 1998;1443:113–127. doi: 10.1016/s0167-4781(98)00200-0. [DOI] [PubMed] [Google Scholar]
- 10.Pinto JT, Suffoletto BP, Berzin TM, et al. Prostate-specific membrane antigen: a novel folate hydrolase in human prostatic carcinoma cells. Clin Cancer Res. 1996;2:1445–1451. [PubMed] [Google Scholar]
- 11.Carter RE, Feldman AR, Coyle JT. Prostate-specific membrane antigen is a hydrolase with substrate and pharmacologic characteristics of a neuropeptidase. Proc Natl Acad Sci USA. 1996;93:749–753. doi: 10.1073/pnas.93.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halsted CH, Ling EH, Luthi-Carter R, et al. Folylpoly-gamma-glutamate carboxypeptidase from pig jejunum: molecular characterization and relation to glutamate carboxypeptidase II. J Biol Chem. 1998;273:20417–20424. doi: 10.1074/jbc.273.32.20417. [DOI] [PubMed] [Google Scholar]
- 13.Rajasekaran SA, Anilkumar G, Oshima E, et al. A novel cytoplasmic tail MXXXL motif mediates the internalization of prostate-specific membrane antigen. Mol Biol Cell. 2003;14:4835–4845. doi: 10.1091/mbc.E02-11-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Troyer JK, Beckett ML, Wright GL., Jr. Location of prostate-specific membrane antigen in the LNCaP prostate carcinoma cell line. Prostate. 1997;30:232–242. doi: 10.1002/(sici)1097-0045(19970301)30:4<232::aid-pros2>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 15.Liu H, Moy P, Kim S, et al. Monoclonal antibodies to the extracellular domain of prostatespecific membrane antigen also react with tumor vascular endothelium. Cancer Res. 1997;57:3629–3634. [PubMed] [Google Scholar]
- 16.Murphy GP, Greene TG, Tino WT, et al. Isolation and characterization of monoclonal antibodies specific for the extracellular domain of prostate specific membrane antigen. J Urol. 1998;160(6 part 2):2396–2401. doi: 10.1097/00005392-199812020-00006. [DOI] [PubMed] [Google Scholar]
- 17.Chang SS, O’Keefe DS, Bacich DJ, et al. Identification of a novel tumor-associated neovasculature marker: prostate-specific membrane antigen (PSMA) Clin Cancer Res. 1999;5:2674–2681. [PubMed] [Google Scholar]
- 18.Liu H, Rajasekaran AK, Moy P, et al. Constitutive and antibody-induced internalization of prostate-specific membrane antigen. Cancer Res. 1998;58:4055–4060. [PubMed] [Google Scholar]
- 19.Schulke N, Varlamova OA, Donovan GP, et al. The homodimer of prostate-specific membrane antigen is a functional target for cancer therapy. Proc Natl Acad Sci USA. 2003;100:12590–12595. doi: 10.1073/pnas.1735443100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang SS, Reuter VE, Heston WD, Gaudin PB. Comparison of anti-prostate-specific membrane antigen antibodies and other immunomarkers in metastatic prostate carcinoma. Urology. 2001;57:1179–1183. doi: 10.1016/s0090-4295(01)00983-9. [DOI] [PubMed] [Google Scholar]
- 21.Kawakami M, Nakayama J. Enhanced expression of prostate-specific membrane antigen gene in prostate cancer as revealed by in situ hybridization. Cancer Res. 1997;57:2321–2324. [PubMed] [Google Scholar]
- 22.Bostwick DG, Pacelli A, Blute M, et al. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: a study of 184 cases. Cancer. 1998;82:2256–2261. doi: 10.1002/(sici)1097-0142(19980601)82:11<2256::aid-cncr22>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 23.Wright GL, Jr, Grob BM, Haley C, et al. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology. 1996;48:326–334. doi: 10.1016/s0090-4295(96)00184-7. [DOI] [PubMed] [Google Scholar]
- 24.Denmeade SR, Sokoll LJ, Dalrymple S, et al. Dissociation between androgen responsiveness for malignant growth vs. expression of prostate specific differentiation markers PSA, hK2, and PSMA in human prostate cancer models. Prostate. 2003;54:249–257. doi: 10.1002/pros.10199. [DOI] [PubMed] [Google Scholar]
- 25.Chang SS, Bander NH, Heston WDW. Monoclonal antibodies: will they become an integral part of the evaluation and treatment of prostate cancer? Curr Opin Urol. 2000;9:391–395. doi: 10.1097/00042307-199909000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Barren RJ, 3rd, Holmes EH, Boynton AL, et al. Method for identifying prostate cells in semen using flow cytometry. Prostate. 1998;36:181–188. doi: 10.1002/(sici)1097-0045(19980801)36:3<181::aid-pros6>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 27.Beckett ML, Cazares LH, Vlahou A, et al. Prostate-specific membrane antigen levels in sera from healthy men and patients with benign prostate hyperplasia or prostate cancer. Clin Cancer Res. 1999;5:4034–4040. [PubMed] [Google Scholar]
- 28.Moreno JG, Croce CM, Fischer R, et al. Detection of hematogenous micrometastases in patients with prostate cancer. Cancer Res. 1992;52:6110–6112. [PubMed] [Google Scholar]
- 29.Murphy GP, Snow PB, Brandt J, et al. Evaluation of prostate cancer patients receiving multiple staging tests, including ProstaScint scintiscans. Prostate. 2000;42:145–149. doi: 10.1002/(sici)1097-0045(20000201)42:2<145::aid-pros9>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 30.Petronis JD, Regan F, Lin K. Indium-111 capromab pendetide (ProstaScint) imaging to detect recurrent and metastatic prostate cancer. Clin Nucl Med. 1998;23:672–677. doi: 10.1097/00003072-199810000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Hinkle GH, Burgers JK, Neal CE, et al. Multicenter radioimmunoscintigraphic evaluation of patients with prostate carcinoma using indium-111 capromab pendetide. Cancer. 1998;83:739–747. [PubMed] [Google Scholar]
- 32.Kahn D, Williams RD, Seldin DW, et al. Radioimmunoscintigraphy with 111indium labeled CYT-356 for the detection of occult prostate cancer recurrence. J Urol. 1994;152(5 part 1):1490–1495. doi: 10.1016/s0022-5347(17)32453-9. [DOI] [PubMed] [Google Scholar]
- 33.Polascik TJ, Manyak MJ, Haseman MK, et al. Comparison of clinical staging algorithms and 111In-capromab pendetide immunoscintigraphy to predict lymph node involvement in high-risk prostate cancer patients. Cancer. 1999;85:1586–1592. [PubMed] [Google Scholar]
- 34.Raj GV, Partin AW, Polascik TJ. Clinical utility of indium 111-capromab pendetide immunoscintigraphy in the detection of early, recurrent prostate carcinoma after radical prostatectomy. Cancer. 2002;94:987–996. [PubMed] [Google Scholar]
- 35.Thomas CT, Bradshaw PT, Pollock BH, et al. Indium-111-capromab pendetide radio-immunoscintigraphy and prognosis for durable biochemical response to salvage radiation therapy in men after failed prostatectomy. J Clin Oncol. 2003;21:1715–1721. doi: 10.1200/JCO.2003.05.138. [DOI] [PubMed] [Google Scholar]
- 36.Bander NH, Trabulsi EJ, Kostakoglu L, et al. Targeting metastatic prostate cancer with radiolabeled monoclonal antibody J591 to the extracellular domain of prostate specific membrane antigen. J Urol. 2003;170:1717–1721. doi: 10.1097/01.ju.0000091655.77601.0c. [DOI] [PubMed] [Google Scholar]
- 37.Michaels EK, Blend M, Quintana JC. Indiumcapromab pendetide unexpectedly localizes to renal cell carcinoma. J Urol. 1999;161:597–598. [PubMed] [Google Scholar]
- 38.Zanzi I, Stark R. Detection of a non-Hodgkin’s lymphoma by capromab pendetide scintigraphy (ProstaScint) in a patient with prostate cancer. Urology. 2002;60:514ix–514xi. doi: 10.1016/s0090-4295(02)01835-6. [DOI] [PubMed] [Google Scholar]
- 39.Khan A, Caride VJ. Indium-111 capromab pendetide (ProstaScint) uptake in neurofibromatosis. Urology. 2000;56:154xxii–154xxiv. doi: 10.1016/s0090-4295(00)00531-8. [DOI] [PubMed] [Google Scholar]
- 40.Zucker RJ, Bradley YC. Indium-111 capromab pendetide (ProstaScint) uptake in a meningioma. Clin Nucl Med. 2001;26:568–569. doi: 10.1097/00003072-200106000-00026. [DOI] [PubMed] [Google Scholar]
- 41.Smith-Jones PM, Vallabhajosula S, Navarro V, et al. Radiolabeled monoclonal antibodies specific to the extracellular domain of prostatespecific membrane antigen: preclinical studies in nude mice bearing LNCaP human prostate tumor. J Nucl Med. 2003;44:610–617. [PubMed] [Google Scholar]
- 42.Nanus DM, Milowsky MI, Kostakoglu L, et al. Clinical use of monoclonal antibody HuJ591 therapy: targeting prostate specific membrane antigen. J Urol. 2003;170(6 part 2):S84–S88. doi: 10.1097/01.ju.0000095151.97404.7c. discussion S88–S89. [DOI] [PubMed] [Google Scholar]
- 43.Gong MC, Chang SS, Sadelain M, et al. Prostatespecific membrane antigen (PSMA)-specific monoclonal antibodies in the treatment of prostate and other cancers. Cancer Metastasis Rev. 1999;18:483–490. doi: 10.1023/a:1006308826967. [DOI] [PubMed] [Google Scholar]
- 44.Maher J, Brentjens RJ, Gunset G, et al. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta/CD28 receptor. Nat Biotechnol. 2002;20:70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 45.Salgaller ML, Lodge PA, McLean JG, et al. Report of immune monitoring of prostate cancer patients undergoing T-cell therapy using dendritic cells pulsed with HLA- A2-specific peptides from prostate-specific membrane antigen (PSMA) Prostate. 1998;35:144–151. doi: 10.1002/(sici)1097-0045(19980501)35:2<144::aid-pros8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 46.Gardner JP, Slovin SF, Morrissey DM, et al. Recombinant soluble prostate-specific membrane antigen (rsPSMA) vaccine: preliminary findings of a Phase I safety/immunogenicity trial [abstract] J Clin Oncol. 2004;22(14S):2584. [Google Scholar]
- 47.Ma D, Gardner JP, Hopf CE, et al. Fully human monoclonal antibodies to PSMA selectively target cytotoxins, radiotoxins, and host immunity to prostate cancer [abstract] J Clin Oncol. 2004;22(14S):2546. [Google Scholar]
- 48.Weijerman PC, Zhang Y, Shen J, et al. Expression of prostatic factors measured by reverse transcription polymerase chain reaction in human papillomavirus type 18 deoxyribonucleic acid immortalized prostate cell lines. Urology. 1998;51:657–662. doi: 10.1016/s0090-4295(97)00696-1. [DOI] [PubMed] [Google Scholar]
- 49.Gottlinger HG, Funke I, Johnson JP, et al. The epithelial cell surface antigen 17-1A, a target for antibody-mediated tumor therapy: its biochemical nature, tissue distribution, and recognition by different monoclonal antibodies. Int J Cancer. 1986;38:47–53. doi: 10.1002/ijc.2910380109. [DOI] [PubMed] [Google Scholar]
- 50.Pegram MD, Lipton A, Hayes DF, et al. Phase II study of receptor-enhanced chemosensitivity using recombinant humanized p185HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J Clin Oncol. 1998;16:2659–2671. doi: 10.1200/JCO.1998.16.8.2659. [DOI] [PubMed] [Google Scholar]
- 51.Noss KR, Wolfe SA, Grimes SR. Upregulation of prostate specific membrane antigen/folate hydrolase transcription by an enhancer. Gene. 2002;285:247–256. doi: 10.1016/s0378-1119(02)00397-9. [DOI] [PubMed] [Google Scholar]