Abstract
OBJECTIVE
To review current understandings of and approaches to topical psoriasis therapies and to assess their efficacies and adverse effects.
QUALITY OF EVIDENCE
Literature from 1987 to 2003, inclusive, was reviewed via MEDLINE using the search term “psoriasis” combined with “topical treatment.” Articles were prioritized based on their level of evidence, favouring double-blind, randomized controlled trials over other comparison studies. Other studies were included where level I research was unavailable. No level III research was included.
MAIN MESSAGE
Psoriasis is very common and causes substantial morbidity. Because most psoriasis is mild to moderate, patients are well suited to outpatient topical therapy. Advances in topical treatments for psoriasis have kept pace with a rapidly evolving comprehension of its pathogenesis, making a review of current therapies useful for those who treat psoriasis. While research supports continued reliance on corticosteroids as first-line therapy, comparable efficacy has been shown for vitamin D analogues and topical retinoids, albeit with a slight increase in adverse effects.
CONCLUSION
The combination of steroids and vitamin D analogues or topical retinoids is perhaps the most promising current treatment. It seems to have increased efficacy and fewer side effects.
Abstract
OBJECTIF
Faire le point sur ce que l’on sait des différentes formes de traitement topique du psoriasis et évaluer leur efficacité et leurs effets indésirables.
QUALITÉ DES PREUVES
Une recherche a été effectuée dans MEDLINE de 1987 à 2003 inclusivement à l’aide des mots-clés « psoriasis » et « topical treatment » combinés. On a classé les articles repérés selon leur niveau de preuve, en favorisant les essais randomisés à double insu plutôt que les essais comparatifs. D’autres études ont été retenues lorsqu’il n’y avait pas de recherche de niveau I. Les recherches de niveau III ont été exclues.
PRINCIPAL MESSAGE
Le psoriasis est très fréquent et c’est une importante cause de morbidité. Comme la plupart des cas sont bénins ou modérés, les patients peuvent généralement bénéficier d’un traitement topique en externe. Le traitement topique du psoriasis a connu des progrès rapides parallèlement à l’amélioration des connaissances sur la pathogenèse du psoriasis, si bien qu’il est opportun de faire le point sur les modalités thérapeutiques actuelles. Même si les données actuelles préconisent toujours les corticostéroïdes comme traitement de première ligne, on a observé que les analogues de la vitamine D et les rétinoïdes topiques ont une efficacité comparable, quoiqu’ils aient un peu plus d’effets indésirables.
CONCLUSION
La combinaison de stéroïdes et d’analogues de la vitamine D ou de rétinoïdes topiques semble être le traitement le plus prometteur actuellement. Il semble avoir une efficacité accrue et moins d’effets secondaires.
EDITOR’S KEY POINTS.
Psoriasis is a psychologically and sometimes medically disabling condition affecting 1% to 3% of the population. Most patients are managed by family physicians.
The goal of treatment is lifetime control. About 75% of patients have mild-to-moderate psoriasis, amenable to topical treatment.
Corticosteroids form the basis of treatment, with the best success and fewest side effects. Since they come in various potencies, they permit individualized treatment depending on severity of the condition and site on the body. Pulsed delivery (once weekly) can minimize side effects.
Other treatments include vitamin D analogues (calcipotriol), retinoids (tazarotene), anthralin (dithranol), tar compounds, or combinations (such as steroids and vitamin D).
POINTS DE REPÈRE DU RÉDACTEUR.
Le psoriasis, qui touche de 1% à 3% de la population, est une source de problèmes psychologiques et parfois médicaux. La plupart des cas sont traités par les médecins de famille.
Le but du traitement est un contrôle à vie. Environ 75% des patients ont une forme bénigne ou modérée de psoriasis qui répond au traitement topique.
Les corticostéroïdes sont la pierre angulaire du traitement, avec le meilleur taux de succès et le moins d’effets indésirables. Leur posologie variée permet d’adapter le traitement à la gravité de l’affection et au site anatomique touché. On peut minimiser les effets indésirables par une administration hebdomadaire unique.
Parmi les autres traitements, mentionnons les analogues de la vitamine D (calcipotriol), les rétinoïdes (tazarotène), l’anthraline (dithranol), les composés à base de goudron ou les combinaisons (par ex., stéroïdes et vitamine D).
Psoriasis is a psychosocially, and at times medically, debilitating disorder that affects 1% to 3% of the population worldwide. Although psoriasis is undoubtedly distressing, affected individuals are typically otherwise healthy and thus well suited to thoughtful outpatient care.
Recent advances in our understanding of psoriasis have provided parallel advances in topical treatments. Appropriate knowledge and use of these therapies is vital to both dermatologists and family physicians caring for patients with psoriasis.
Quality of evidence
MEDLINE was searched from 1987 to 2003 using the search term “psoriasis” combined with “topical treatment.” Articles were selected based on relevance, quality, and originality; 127 abstracts were reviewed. Additional articles were identified from the reference lists of the papers selected. Thirty-eight articles were included in the final review.
Levels of evidence of the cited articles are categorized according to the Canadian Task Force on Preventative Health Care. Of the studies, 58% were level I (randomized controlled trial, systematic review, or meta-analysis) and 21% were level II (non-randomized, cohort, case-control, or epidemiologic studies). The remaining articles included four basic science papers (10.5%) and four review articles (10.5%). No level III articles were included (expert opinion or consensus statements).
Pathogenesis
The phenotypic appearance of psoriasis is due to hyperproliferation and abnormal differentiation of keratinocytes, inflammatory cell infiltration, and vascular changes (Figure 1). These changes appear to be initiated by immunologic mechanisms, namely, the T lymphocyte. A great deal of research, however, is still uncovering the details of the immune system’s involvement in pathogenesis of psoriasis.
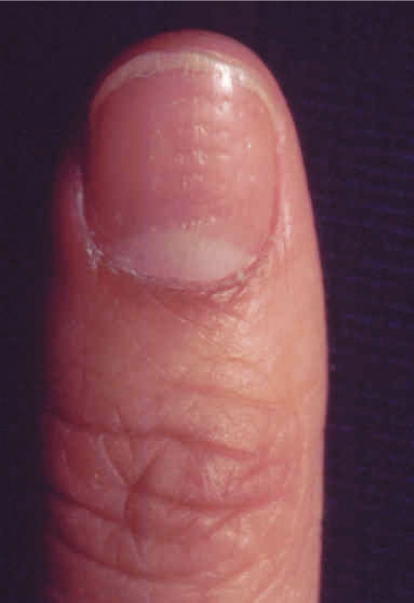
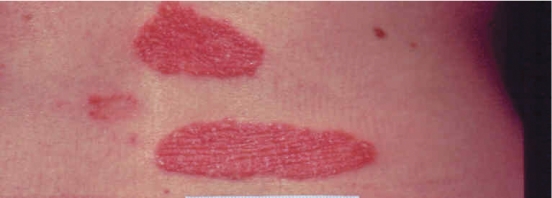
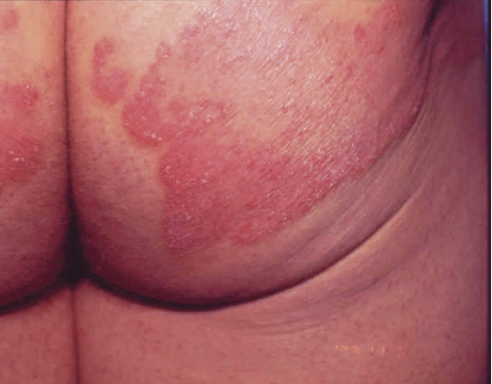
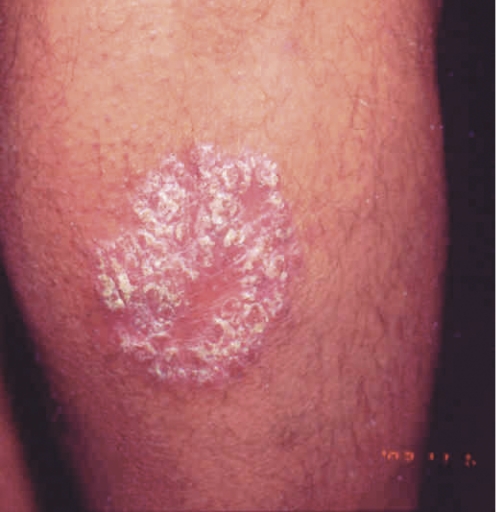
Figure 1. Appearance of psoriasis.
A) Nail pitting; B) Lesions on the back; C) Inflammatory areas on the buttocks; D) Lesion on the back of the calf.
Topical therapies
Despite our evolving comprehension of the pathogenesis of psoriasis, no lasting cure has been found; lifetime control is often necessary. Therapeutic options abound; frequent advances have been made in topical therapies, systemic treatments, and phototherapies. Since an estimated 75% of psoriatic patients have mild-to-moderate disease,1 topical treatments remain the most widely used. These medications are efficacious in mild-to-moderate disease and provoke less concern about systemic side effects.
This review highlights the efficacies and adverse effects of topical treatments, as well as the evidence and rationale for combining therapies. It is important to note that several excellent reviews of topical psoriasis therapies have been written.2-4 The purpose of this review is to discuss additional developments, to include data comparing various treatments, and to update family physicians who treat a great number of patients with psoriasis.
Corticosteroids.
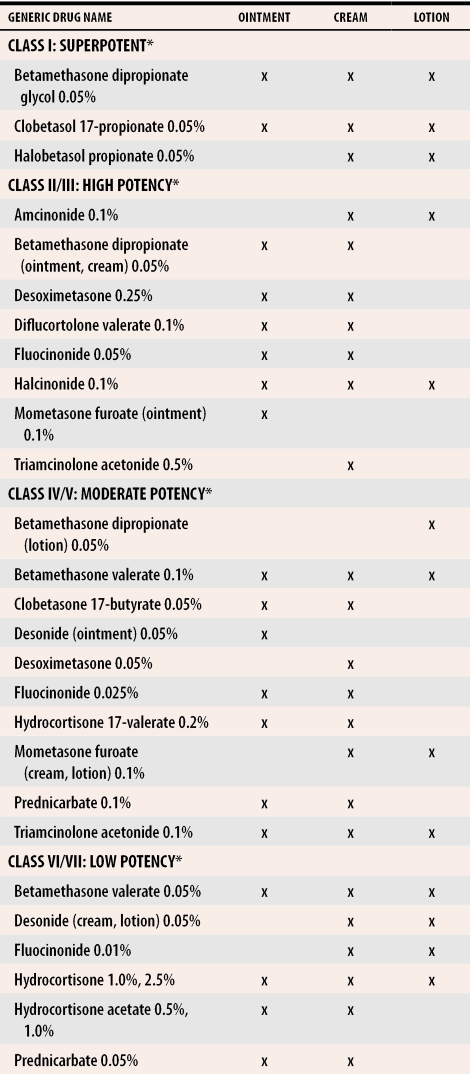
Corticosteroids form the basis of topical psoriasis treatment in North America. They are efficacious, are well tolerated, and come in a variety of forms. Several potencies are available, ranging from Class 1 (highest potency) to Class 7 (Table 15). The ability to vary strength and administration method gives steroids the versatility to tread lightly on sensitive or thinly skinned areas, such as the face and body folds, and the power to treat more resistant areas of the body, such as extensor surfaces and the soles of the feet.
Table 1.
Potency of topical corticosteroids available in Canada
*Potency class based on the Stoughton vasoconstrictor assay.5
Recently, Mason et al1 analyzed data from all randomized placebo-controlled trials involving topical psoriasis treatments and all randomized head-to-head studies involving vitamin D3 derivatives published between 1966 and 1999. The statistically pooled data spanned 3380 patients randomized in 41 placebo-controlled trials and 4898 patients in head-to-head trials. Even though all treatments (including steroids, vitamin D3 derivatives, anthralin, and tar) outperformed placebo, the very potent steroids were found to be the most efficacious (level I evidence). More recently, foam preparations of betamethasone valerate and clobetasol propionate, which were originally developed for scalp psoriasis, have been shown to be effective in treating nonscalp psoriasis as well (level I evidence).6,7 That these preparations are effective is important, because patients find them more acceptable and are thus more likely to comply with treatment. Unfortunately, the foams are not yet available in Canada.
With such clear benefits, the only limit to corticosteroids is their side effects. These include local cutaneous reactions, such as atrophy, telangiectases, striae, traumatic purpura, perioral dermatitis, hypertrichosis, and rarely, contact dermatitis. These effects are more likely to occur in sensitive areas, such as the face and intertriginous areas. Adrenal suppression has also been reported,8 but appears to be serious only with very widespread use or in infants.9
In a systematic review of randomized or double-blind trials evaluating the adverse effects of topical psoriasis treatments, Bruner et al10 found that corticosteroids had the lowest rate of adverse events (ranging from 3.2% to 23%, level I evidence). Fluticasone propionate was at the low end of this range. Dose regimens, such as pulse delivery (application of topical steroid weekly instead of daily as described below), both minimize cumulative exposure and reduce local side effects (level I evidence).11 For instance, Lebwohl et al12 found that using fluticasone propionate twice daily for 2 weeks and then once daily for 2 days of the week for 8 weeks was effective and did not cause atrophy in steroid-sensitive areas. One final problem with steroid treatment is development of tachyphylaxis (tolerance to the action of a drug after repeated doses) with prolonged use.13 Some studies have failed to find this effect.14 Nevertheless, pulsed dosing can eliminate this concern.
Vitamin D analogues.
Topical vitamin D analogues are thought to inhibit keratinocyte growth, promote keratinocyte differentiation, and decrease inflammation in psoriatic lesions via vitamin D receptors on keratinocytes and T lymphocytes.15,16 Calcipotriol is available in North America, while calcipotriol, calcitriol, and tacalcitol are offered in Europe.
This class has fared well in efficacy studies, performing as well as midpotency steroids,17,18 but less well than superpotent steroids (level I evidence). These results are supported by the Mason and associates1 study, which found that vitamin D analogues were as effective as all but the very potent steroids in placebo-controlled trials (level I evidence). A recent randomized trial of 258 psoriasis patients treated with either calcitriol or betamethasone dipropionate 0.05% ointment found that, while betamethasone performed slightly better on two of three efficacy measures, the calcitriol group remained in remission longer (level I evidence).19 Whether or not calcipotriol also induces a longer remission period requires clarification.
Overall, vitamin D analogues are tolerated well; the most common adverse effect is a mild irritant contact dermatitis. The face and body folds are more prone to this effect, with irritation developing in up to 20% of patients treated in those areas.20 Hypercalcemia has also been reported, but this appears inconsequential in treatment doses.21 In the study by Bruner and colleagues10 of adverse events with topical therapies, vitamin D analogues were found to have the second lowest rate of adverse events (range of 4.8% to 35%, level I evidence). Tacalcitol was the least irritating.
Retinoids.
Oral retinoids have been used in the treatment of psoriasis for some time. A topical retinoid, tazarotene, is a more recent innovation. It is believed to act at the gene level, via retinoic acid receptors, which mediate keratinocyte proliferation and differentiation.22
Studies of the efficacy of tazarotene lag behind those of vitamin D analogues and corticosteroids. One placebo-controlled trial of tazarotene,23 involving 318 patients, was included in the study by Bruner and colleagues.10 Tazarotene’s performance was equivalent to the potent steroid and vitamin D groups (level I evidence). Success rates, defined as 50% improvement or more, were 52% and 70% for the 0.05% and 0.1% gels, respectively. The effects were maintained for 12 weeks after treatment. Similar results have been seen in trials published since that time.24 Although there were no head-to-head studies with vitamin D analogues to include in the study by Mason and associates,1 a comparison of 0.05% fluocinonide with tazarotene found the retinoid to have comparable efficacy and a longer remission period (level I evidence).25
Tazarotene induces a dose-dependent local irritation, with itching, burning, and erythema.23 Tazarotene was found to have a slightly higher incidence of adverse effects than corticosteroids or vitamin D analogues (range 13% to 50%), but less than anthralin or coal tar (level I evidence).10
Anthralin.
Although anthralin (dithranol) is a time-honoured treatment for psoriasis, its staining and irritating effects limit its use to recalcitrant disease. The precise efficacy of anthralin is difficult to ascertain, as few studies have adequate enrolment. Moreover, anthralin is more commonly used in conjunction with ultraviolet B phototherapy in current practice. The study by Mason and associates,1 however, did include data from five head-to-head trials with vitamin D derivatives. Analysis revealed anthralin to be less effective than other derivatives (level I evidence).
Anthralin’s adverse effects include local irritation and staining of skin, clothing, and furniture. Recent attempts to reduce these effects include a short-contact regimen (topical therapy applied to skin daily for a short period [5 to 30 minutes] then washed off),26 heat-sensitive preparations,27 and use of triethanolamine to prevent staining.28 These variations have not reduced the effectiveness of the treatment.
Tar.
Like anthralin, tar has been used on psoriasis for a long time. The main form used, coal tar, is a by-product of coal distillation and contains more than 10 000 compounds. Its effect is likely mediated by DNA suppression.29 Recent randomized controlled trials comparing coal tar and calcipotriol have shown comparable clinical efficacy (calcipotriol has a faster onset of action) and similar relapse rates (level I evidence). 30,31 Calcipotriol is better tolerated and has a better cosmetic appearance. Coal-tar preparations continue to be considerably less expensive than calcipotriol.
Tar is infrequently used for outpatients with psoriasis, as it can cause acne, folliculitis, phototoxicity, and local irritation, and it can be messy and can stain. Although occupational exposure has been linked to skin cancer, use of tar treatment has not (level II evidence).32
Combination therapies
Corticosteroids and vitamin D analogues.
In general, the combination of corticosteroids and vitamin D derivatives is more efficacious than either therapy alone and produces fewer side effects. Randomized trials have demonstrated this for several steroid-calcipotriol pairings (level I evidence).33-35 The calcipotriol and betamethasone dipropionate combination is now available premixed, providing increased convenience. Since corticosteroids are anti-inflammatory, they tend to reduce the irritation of calcipotriol. Conversely, calcipotriol can serve as a steroid-sparing agent,3 reducing its side effects. Thus, an improved side effect profile is not surprising. This theoretical advantage is not always borne out, though; some studies find a slight increase in side effects when therapies are combined (level I evidence).10
Corticosteroids and tazarotene.
Akin to the previous combination, steroids and tazarotene seem a good complement. Indeed, studies have found this combination to be more effective than monotherapy (level I evidence).36 Adding tazarotene to a steroid can also circumvent the problem of tachyphylaxis, as duration of treatment benefit and length of remission are prolonged (level I evidence).37 An interesting result of this combination is that each has the opposite effect on epidermal thickness. Whereas steroids induce atrophy, tazarotene increases epidermal thickness. Hence, the combination has been shown to reduce the degree of steroid-induced atrophy by as much as 37% (level II evidence).38 Last, tazarotene-induced irritation is reduced by the anti-inflammatory effect of a steroid.
Corticosteroids and salicylic acid.
Salicylic acid is a useful adjunct in treating psoriasis that reduces scale and softens lesions. It too has proven valuable in combination: it enhances steroid efficacy by increasing penetration (level I evidence).39 Since this might be of concern with respect to systemic toxicity, some authors suggest combining salicylic acid with a mid-potency steroid.3
Conclusion
Psoriasis is a disease without a lasting cure. Yet it is the subject of active research that provides frequent therapeutic advances. As knowledge progresses, more efficacious treatments with fewer side effects become available. Awareness of these advances in therapy is the responsibility of physicians caring for patients with psoriasis.
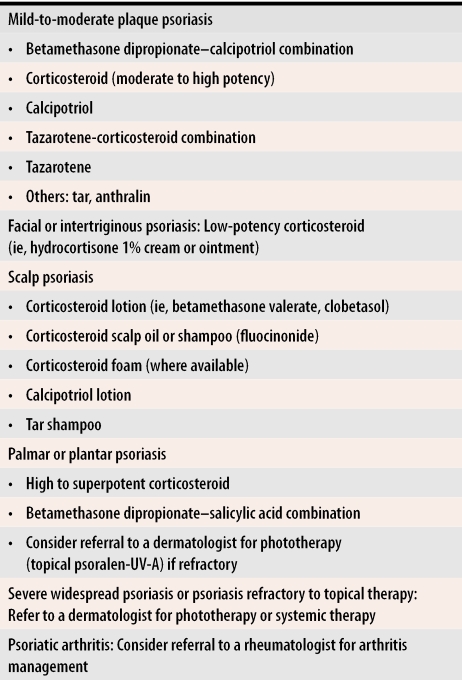
Corticosteroids remain central to treatment, but vitamin D analogues are important to use either in conjunction with or as an alternative to steroids. Owing to their ability to induce long remission periods, use of retinoids is also likely to increase over time. The combination of steroids and these two newer medications is perhaps the most promising current treatment, as increased efficacy and fewer side effects seem to result. Because in general there is wide variation in patients’ psoriatic presentations, both in terms of location and in disease severity, individualized approaches are indicated in choosing specific treatments. The scheme in Table 2 provides a framework that can be altered to suit the needs of individual patients.
Table 2.
Approach to treatment
Levels of evidence.
Level I: At least one properly conducted randomized controlled trial, systematic review, or meta-analysis
Level II: Other comparison trials, non-randomized, cohort, case-control, or epidemiologic studies, and preferably more than one study
Level III: Expert opinion or consensus statements
Biographies
Dr Afifi is a first-year dermatology resident at the University of Manitoba in Winnipeg.
Dr de Gannes is a fourth-year resident in dermatology at the University of British Columbia in Vancouver.
Dr Huang is an Associate Professor in Dermatology in the Department of Dermatology at Tongji Medical College of the Central China University of Science and Technology in Wuhan, Hubei Province, China.
Dr Zhou is an Assistant Professor in Dermatology in the Department of Medicine, Division of Dermatology, at the University of British Columbia.
Footnotes
Competing interests: None declared
References
- 1.Mason J, Mason AR, Cork MJ. Topical preparations for the treatment of psoriasis: a systematic review. Br J Dermatol. 2002;146:351–364. doi: 10.1046/j.1365-2133.2000.04713.x. [DOI] [PubMed] [Google Scholar]
- 2.Greaves MW, Weinstein GD. Treatment of psoriasis. Drug Ther. 1995;332(9):581–587. doi: 10.1056/NEJM199503023320907. [DOI] [PubMed] [Google Scholar]
- 3.Endzweig-Gribetz CH, Brady C, Lynde C, Sibbald D, Lebwohl M. Drug interactions in psoriasis: the pros and cons of combining topical psoriasis therapies. J Cutan Med Surg. 2002;6(3 Suppl):12–16. doi: 10.1177/12034754020060S304. [DOI] [PubMed] [Google Scholar]
- 4.Tremblay JF, Bissonnette R. Topical agents for the treatment of psoriasis, past, present and future. J Cutan Med Surg. 2002;6(3 Suppl):8–11. doi: 10.1177/12034754020060S303. [DOI] [PubMed] [Google Scholar]
- 5.Stoughton RB. Vasoconstrictor activity and percutaneous absorption of glucocorticosteroids. Arch Dermatol. 1969;99:753–756. [PubMed] [Google Scholar]
- 6.Lebwohl M, Sherer D, Washenik K, Krueger GG, Menter A, Koo J, et al. A randomized, double-blind, placebo-controlled study of clobetasol propionate 0.05% foam in the treatment of nonscalp psoriasis. Int J Dermatol. 2002;41:269–274. doi: 10.1046/j.1365-4362.2002.01431.x. [DOI] [PubMed] [Google Scholar]
- 7.Stein LF, Sherr A, Solodkina G, Gottlieb AB, Chaudhari U. Betamethasone valerate foam for treatment of nonscalp psoriasis. J Cutan Med Surg. 2001;5(4):303–307. doi: 10.1007/s10227-001-0006-0. [DOI] [PubMed] [Google Scholar]
- 8.Katz HI, Hien NT, Prawer SE. Superpotent topical steroid treatment of psoriasis vulgaris—clinical efficacy and adrenal function. J Am Acad Dermatol. 1987;16:804–811. doi: 10.1016/s0190-9622(87)70105-4. [DOI] [PubMed] [Google Scholar]
- 9.Turpeinen M, Salo OP, Leisti S. Effect of percutaneous absorption of hydrocortisone on adrenocortical responsiveness in infants with severe skin disease. Br J Dermatol. 1986;115:475–484. doi: 10.1111/j.1365-2133.1986.tb06242.x. [DOI] [PubMed] [Google Scholar]
- 10.Bruner CR, Feldman SR, Ventrapragada M, Fleischer AB., Jr A systematic review of adverse effects associated with topical treatments for psoriasis. Dermatol Online J. 2003;9(1):2. [PubMed] [Google Scholar]
- 11.Katz HI, Prawer SE, Medansky RS, Krueger GG, Mooney JJ, Jones ML, et al. Intermittent corticosteroid maintenance treatment of psoriasis: a double-blind multi-center trial of augmented betamethasone dipropionate ointment in a pulse dose treatment regimen. Dermatologica. 1991;183:269–314. doi: 10.1159/000247698. [DOI] [PubMed] [Google Scholar]
- 12.Lebwohl MG, Tan MH, Meador SL, Singer G. Limited application of fluticasone propionate ointment 0.005% in patients with psoriasis of the face and intertriginous areas. J Am Acad Dermatol. 2001;44:77–82. doi: 10.1067/mjd.2001.110046. [DOI] [PubMed] [Google Scholar]
- 13.Du Vivier A, Stoughton RB. Tachyphylaxis to the action of topically applied corticosteroids. Arch Dermatol. 1975;111:581–583. [PubMed] [Google Scholar]
- 14.Miller JJ, Roling D, Margolis D, Guzzo C. Failure to demonstrate therapeutic tachyphylaxis to topically applied steroids in patients with psoriasis. J Am Acad Dermatol. 1999;41:546–549. [PubMed] [Google Scholar]
- 15.Gerritsen MJ, Rulo HF, Van Vlijmen-Willems I, Van Erp PE, van de Kerkhof PC. Topical treatment of psoriatic plaques with 1,25-dihydroxyvitamin D3: a cell biologic study. Br J Dermatol. 1993;128:666–673. doi: 10.1111/j.1365-2133.1993.tb00263.x. [DOI] [PubMed] [Google Scholar]
- 16.Reichtrath J, Perez A, Muller SM, Chen TC, Kerber A, Bahmer FA, et al. Topical calcitriol (1,25-dihydroxyvitamin D3) treatment of psoriasis: an immunohistological evaluation. Acta Derm Venereol (Stockh) 1997;77:268–272. doi: 10.2340/0001555577268272. [DOI] [PubMed] [Google Scholar]
- 17.Kragballe K, Gjertsen BT, De Hoop D, Karlsmark T, van de Kerkhof PC, Larko O, et al. Double-blind, right/left comparison of calcipotriol and betamethasone valerate in treatment of psoriasis vulgaris. Lancet. 1991;337:193–196. doi: 10.1016/0140-6736(91)92157-w. [DOI] [PubMed] [Google Scholar]
- 18.Cunliffe WJ, Berth-Jones J, Claudy A, Fairiss G, Goldin D, Gratton D, et al. Comparative study of calcipotriol (MC 903) ointment and betamethasone 17-valerate ointment in patients with psoriasis vulgaris. J Am Acad Dermatol. 1992;26:736–743. doi: 10.1016/0190-9622(92)70103-m. [DOI] [PubMed] [Google Scholar]
- 19.Camarasa JM, Ortonne JP, Dubertret L. Calcitriol shows greater persistence of treatment effect than betamethasone dipropionate in topical psoriasis therapy. J Dermatol Treat. 2003;14(1):8–13. doi: 10.1080/09546630305545. [DOI] [PubMed] [Google Scholar]
- 20.Lebwohl M, Ali S. Treatment of psoriasis. Part 1. Topical therapy and phototherapy. J Am Acad Dermatol. 2001;45:487–502. doi: 10.1067/mjd.2001.117046. [DOI] [PubMed] [Google Scholar]
- 21.Mortensen L, Kragballe K, Wegmann E, Schifter S, Risteli J, Charles P. Treatment of psoriasis vulgaris with topical calcipotriol has no short-term effect on calcium or bone metabolism: a randomized, double-blind, placebo-controlled study. Acta Derm Venereol (Stockh) 1993;73:300–304. doi: 10.2340/000155557296299. [DOI] [PubMed] [Google Scholar]
- 22.Duvic M, Nagpal S, Asano AT, Chandraratna RA. Molecular mechanisms of tazarotene action in psoriasis. J Am Acad Dermatol. 1997;37(2 Pt 3):18–24. [PubMed] [Google Scholar]
- 23.Weinstein GD, Krueger GG, Lowe NJ, Duvic M, Friedman DJ, Jegasothy BV, et al. Tazarotene gel, a new retinoid, for topical therapy of psoriasis: vehicle-controlled study of safety, efficacy, and duration of therapeutic effect. J Am Acad Dermatol. 1997;37:85–92. doi: 10.1016/s0190-9622(97)70216-0. [DOI] [PubMed] [Google Scholar]
- 24.Weinstein GD, Koo JY, Krueger GG. Tazarotene cream in the treatment of psoriasis: two multicenter, double-blind, randomized, vehicle-controlled studies of the safety and efficacy of tazarotene creams 0.05% and 0.1% applied once daily for 12 weeks. J Am Acad Dermatol. 2003;48:760–767. doi: 10.1067/mjd.2003.103. [DOI] [PubMed] [Google Scholar]
- 25.Lebwohl M, Ast E, Callen JP. Once-daily tazarotene gel versus twice-daily fluocinonide cream in the treatment of plaque psoriasis. J Am Acad Dermatol. 1998;38:705–711. doi: 10.1016/s0190-9622(98)70594-8. [DOI] [PubMed] [Google Scholar]
- 26.Runne U, Kunze J. Short duration (“minutes”) therapy with dithranol for psoriasis: a new outpatient regimen. Br J Dermatol. 1982;106:135–139. doi: 10.1111/j.1365-2133.1982.tb00922.x. [DOI] [PubMed] [Google Scholar]
- 27.Volden G, Bjornberg A, Tegner E, Pedersen NB, Arles UB, Agren S, et al. Short-contact treatment at home with Micanol. Acta Derm Venereol Suppl (Stockh) 1992;172:20–22. [PubMed] [Google Scholar]
- 28.Ramsay B, Lawrence CM, Bruce JM, Shuster S. The effects of triethanolamine application on anthralin-induced inflammation and therapeutic effect in psoriasis. J Am Acad Dermatol. 1990;23:73–76. doi: 10.1016/0190-9622(90)70189-o. [DOI] [PubMed] [Google Scholar]
- 29.Walter JF, Stoughton RB, DeQuoy PR. Suppression of epidermal proliferation by ultraviolet light, coal tar and anthralin. Br J Dermatol. 1978;99:89–96. doi: 10.1111/j.1365-2133.1978.tb01965.x. [DOI] [PubMed] [Google Scholar]
- 30.Sharma V, Kaur I, Kumar B. Calcipotriol versus coal tar: a prospective randomized study in stable plaque psoriasis. Int J Dermatol. 2003;42:834–838. doi: 10.1046/j.1365-4362.2003.01974.x. [DOI] [PubMed] [Google Scholar]
- 31.Tzaneva S, Honigsmann H, Tanew A. Observer-blind, randomized, intrapatient comparison of a novel 1% coal tar preparation (Exorex) and calcipotriol cream in the treatment of plaque type psoriasis. Br J Dermatol. 2003;149:350–353. doi: 10.1046/j.1365-2133.2003.05421.x. [DOI] [PubMed] [Google Scholar]
- 32.Jemec GBE, Osterlind A. Cancer in patients treated with coal tar: a long-term follow up study. J Eur Acad Dermatol Venereol. 1994;3(2):153–156. [Google Scholar]
- 33.Kragballe K, Barnes L, Hamber KJ. Calcipotriol cream with or without concurrent topical corticosteroid in psoriasis: tolerability and efficacy. Br J Dermatol. 1998;139:649–654. doi: 10.1046/j.1365-2133.1998.02461.x. [DOI] [PubMed] [Google Scholar]
- 34.Austad J, Bjerke JR, Gjertsen BT. Clobetasol propionate followed by calcipotriol is superior to calcipotriol alone in topical treatment of psoriasis. J Eur Acad Dermatol Venereol. 1998;11(1):19–24. [PubMed] [Google Scholar]
- 35.Papp KA, Guenther L, Boyden B. Early onset of action and efficacy of a combination of calcipotriene and betamethasone dipropionate in the treatment of psoriasis. J Am Acad Dermatol. 2003;48:48–54. doi: 10.1067/mjd.2003.130. [DOI] [PubMed] [Google Scholar]
- 36.Lebwohl MG, Breneman DL, Goffe BS. Tazarotene 0.1% gel plus corticosteroid cream in the treatment of plaque psoriasis. J Am Acad Dermatol. 1998;39:590–596. doi: 10.1016/s0190-9622(98)70008-8. [DOI] [PubMed] [Google Scholar]
- 37.Lebwohl M, Lombardi K, Tan MH. Duration of improvement of psoriasis after treatment with tazarotene 0.1% gel plus clobetasol propionate 0.05% ointment: comparison of maintenance treatments. Int J Dermatol. 2001;40:64–66. doi: 10.1046/j.1365-4362.2001.01067-7.x. [DOI] [PubMed] [Google Scholar]
- 38.Kaidbey K, Kopper SC, Sefton J, Gibson JR. A pilot study to determine the effect of tazarotene 0.1% gel on steroid-induced epidermal atrophy. Int J Dermatol. 2001;40:468–471. doi: 10.1046/j.1365-4362.2001.01234.x. [DOI] [PubMed] [Google Scholar]
- 39.Koo J, Cuffie CA, Tanner DJ, Bressinck R. Mometasone furoate 0.1%–salicylic acid 5% ointment versus mometasone furoate 0.1% ointment in the treatment of moderate-to-severe psoriasis: a multicenter study. Clin Ther. 1998;20(2):283–291. doi: 10.1016/s0149-2918(98)80091-x. [DOI] [PubMed] [Google Scholar]