Gout is a common cause of acute arthritis. An ageing population, increasing obesity, and lifestyle changes will render it more common.1 Here I outline the epidemiology of gout, appraise the evidence base for its management, and suggest ways of managing idiopathic gout. Management of hyperuricaemia due to inborn errors of metabolism (for example, Lesch-Nyhan syndrome) and its prevention during cancer chemotherapy are not discussed here.
Sources and selection criteria
The material for this review draws heavily on my chapter on gout in Clinical Evidence and from my work on a recent systematic review of studies on the prevention and treatment of recurrent gout. To ensure that no relevant randomised controlled trials published since the systematic review had been overlooked, I ran a previous search strategy in PubMed and the Cochrane database of systematic reviews. I identified other relevant studies from my personal database of papers on gout, did forward and backward citation tracking from other key papers, and carried out new targeted searches of multiple electronic databases.
What is gout?
The clinical syndrome of gout arises from deposition of urate crystals in joints, where they cause an inflammatory response, and in soft tissues, where they do not. The classic symptom of gout affecting the big toe, podagra, literally a “foot catch,” has been recognised since antiquity. Crystal deposition occurs when serum becomes saturated with urate, the final breakdown product of purine metabolism. Most patients with idiopathic gout have a genetically reduced renal excretion of urate. This alone does not usually lead to hyperuricaemia. Many other factors affect serum urate concentration (box 1).
Typically, gout produces an acute monoarthritis of rapid onset, often waking patients from sleep. The most commonly affected joints are the great toe, foot, ankle, knee, wrist, finger, and elbow, possibly because urate is more likely to crystallise in cooler parts of the body. Crystal deposits (tophi) may also develop around hands, feet, elbows, and ears. Older patients, particularly very elderly patients, can develop oligoarticular or polyarticular gout, which may be less painful and may affect osteoarthritic interphalangeal joints. Without specific treatment gout usually resolves within 7-10 days.2,3 After an initial acute attack patients may be free of symptoms for months or years; but some go on to have more frequent attacks and a few eventually develop chronic tophaceaous gout or permanent joint damage, or both. The characteristic radiological changes of gout are subcortical cysts without erosions. No reliable data exist on the likely recurrence rate in patients who do not have urate lowering treatment. A retrospective case series, from before the introduction of allopurinol, reported recurrence rates after a first attack of 62% after one year, 78% after two years, and 84% after three years.4
Summary points
Acute gout is common and likely to become more so
Most cases of acute gout are usually diagnosed and treated clinically
It is possible that moderate lifestyle interventions may reduce the incidence of recurrent gout
The risks and benefits of gout treatments should be carefully considered before prescribing
Who gets gout and hyperuricaemia?
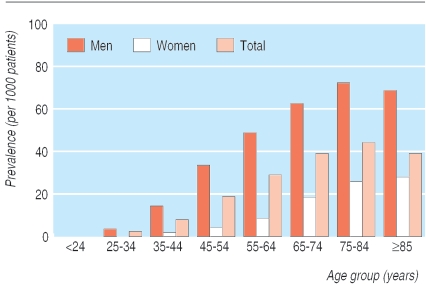
In most studies the prevalence of gout is at least 1%. In some countries it is substantially higher—for example, 3.6% in New Zealand Europeans and 6.4% in Maoris.5-7 Gout is much more common in men than in women; it is rare before menopause and more common in old age (fig 1).
Fig 1.
Prevalence of gout. Adapted from Mikuls et al5
Box 1 Factors affecting serum urate concentration
Factors that decrease serum urate concentration
Diet: low fat dairy products
Drugs: xanthine oxidase inhibitors (allopurinol, febuxostat), uricosuric drugs (sulfinpyrazone), uricase drugs (rasburicase), coumarin anticoagulants, and oestrogens
Factors that increase serum urate concentration
Diet: meat, fish, alcohol (particularly beer and spirits), obesity, and weight gain
Drugs: including diuretics, low dose salicylates, pyrazinamide, ethambutol, cytotoxics, and lead poisoning
Disease: increased purine turnover—myeloproliferative and lymphoproliferative disorders, chronic haemolytic anaemia, haemoglobinopathies, secondary polycythaemia, thalassaemia; increased purine synthesis—glucose-6-phosphate dehydrogenase deficiency, Lesch-Nyhan syndrome; reduced renal excretion—hypertension, hypothyroidism, sickle cell anaemia, hyperparathyroidism, chronic renal disease
Box 2 American College of Rheumatology preliminary criteria for the clinical diagnosis of gout14
Six or more of these criteria are needed to make a diagnosis:
More than one attack of acute arthritis
Maximum inflammation developed within one day
Attack of monoarthritis
Redness over joints
Painful or swollen first metatarsophalangeal joint
Unilateral attack on first metatarsophalangeal joint
Unilateral attack on tarsal joint
Tophus (proved or suspected)
Hyperuricaemia
Asymmetric swelling within a joint on radiograph
Subcortical cysts without erosions on radiograph
Joint fluid culture negative for organisms during attack
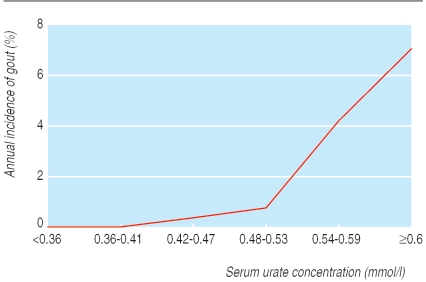
Gout occurs only when the serum is saturated with urate—that is, a concentration greater than 0.42 mmol/l. Coincidentally this is similar to many laboratories' normal range for men; the normal range is lower for premenopausal women. Asymptomatic hyperuricaemia is common; 9.4% of New Zealand European men have a serum urate concentration greater than 0.42 mmol/l and 2.2% have a serum urate concentration greater than 0.54 mmol/l.7 Only a minority of people with hyperuricaemia develop gout. Even when the urate concentration is 0.6 mmol/l or more, the annual incidence of gout is only 6% (fig 2).8
Fig 2.
Relation between serum urate concentration and annual incidence of gout. Adapted from Campion et al8
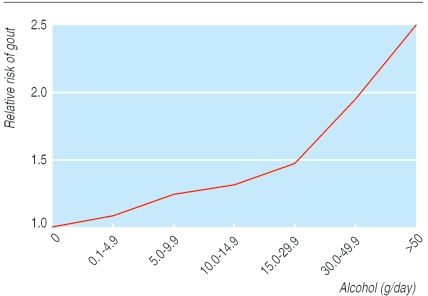
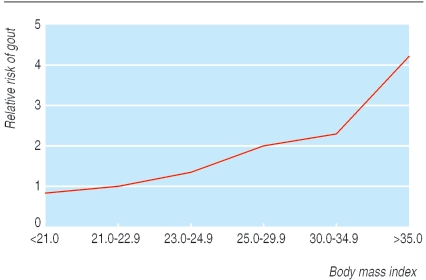
Lifestyle factors have an important effect on the incidence of gout. A 12 year cohort study of 47 150 gout free, male health professionals has quantified this. Incidence was higher in those who were obese or had a higher overall alcohol intake, or both (figs 3 and 4).9,10 Interestingly, beer intake, but not wine, increased the incidence of gout, probably because the purine content of beer has an effect independent of alcohol (table 1).1,9 Incidence was also greater in those with higher intakes of meat or fish. Purine rich vegetables had no effect whereas low fat dairy products were protective, possibly due to a uricosuric effect of casein and lactalbumin (table 1).11 Hyperuricaemia is associated with increased cardiovascular risk; it may be that for some high risk patients hyperuricaemia is an independent cardiovascular risk factor rather than just an association.12 It is worth while screening patients who present with gout for cardiovascular risk factors. A Dutch study found that in patients with gout who were not already known to have cardiovascular disease the prevalence of hypertension, hypercholesterolaemia, and diabetes was 39%, 8%, and 5%, respectively.13
Fig 3.
Effect of total alcohol intake on relative risk of first attack of gout. Adapted from Choi et al9
Fig 4.
Effect of obesity on incidence of first attack of gout. Adapted from Choi et al10
Table 1.
Effect of one additional daily portion on first attack of gout
| Portion | Relative risk (95% CI) |
|---|---|
| Alcohol: | |
| Beer 335 ml | 1.49 (1.32 to 1.70) |
| Spirits 44 ml | 1.15 (1.04 to 1.28) |
| Wine 118 ml | 1.04 (0.88 to 1.22) |
| Food: | |
| Meat | 1.21 (1.04 to 1.41) |
| Seafood (fish)* | 1.07 (1.01 to 1.12) |
| Purine rich vegetables | 0.97 (0.79 to 1.19) |
| Total dairy products | 0.82 (0.75 to 0.90) |
| Low fat dairy products | 0.79 (0.71 to 0.87) |
| High fat dairy products | 0.99 (0.89 to 1.10) |
How is gout diagnosed?
Identifying urate crystals in fluid from an affected joint is the definitive diagnostic test for the diagnosis of gout. In practice, this test is applied to only a minority of patients diagnosed as having gout: 11% in the health professionals study.9,10 Guidelines exist for clinical diagnosis without joint aspiration (box 2).14 However, patients with gout may not meet these criteria when they first present. In the acute situation it is reasonable to treat patients with suspected gout as if they had gout; inadvertent treatment for gout of patients with pseudogout or another inflammatory arthritis is unlikely to be problematic. The important differential diagnosis in the acute situation is septic arthritis. If this is suspected an immediate referral for joint aspiration is indicated.
The serum urate concentration may reduce during an acute attack; a normal urate concentration at this point does not rule out a diagnosis of gout. Table 2 summarises other suggested investigations for patients with suspected gout.
Table 2.
Investigations to consider for patients with gout
| Test | Comment |
|---|---|
| Serum urate concentration | Level may go down during an acute attack |
| Full blood count | To exclude myeloproliferative disorders; raised white cell count may indicate septic arthritis |
| Renal function | Hyperuricaemia can occur in renal failure; reduce dose of allopurinol |
| Fasting lipids, glucose, and thyroid function | Hyperlipidaemia, diabetes, hypothyroidism, and possibly hyperthyroidism are associated with gout13,15 |
| Urinary urate excretion | Uricosurics are contraindicated in patients with high urinary excretion of urate. Some authorities advise measuring this if the serum urate concentration is >0.8 mmol/l because of risk of renal stone formation |
Treatment of acute gout
Few robust data exist on the treatment of acute gout.16 Choice of treatment depends on the balance of risks and benefits.
Non-steroidal anti-inflammatory drugs
Non-steroidal anti-inflammatory drugs, specifically indometacin, are the most popular treatment for acute gout in the United Kingdom.17 Only one, low quality, placebo controlled trial has been carried out on non-steroidal anti-inflammatory drugs for gout.16 Many underpowered trials have failed to show a difference in outcome between different conventional non-steroidal anti-inflammatory drugs.16 Two large non-inferiority trials showed that clinical outcomes from indometacin and etoricoxib, a cyclo-oxygenase-2 inhibitor, were similar.16 The potential gastrointestinal and cardiovascular risks from these drugs are well documented and beyond the scope of this review. However, people who develop gout may be those at highest risk of side effects from either traditional non-steroidal anti-inflammatory drugs or cyclo-oxygenase-2 inhibitors.
Patient's story
It seems to be assumed, even among doctors, that gout is an extinct Victorian ailment due to too much port and cigars. For over 10 years at various general practitioner and hospital visits, I was repeatedly misdiagnosed as having a sprained ankle or a broken bone in my foot. I would experience extreme pain for a couple of days which would then ease off over the course of the following week.
A physiotherapist treating my “sprained ankle” suggested gout might be the cause. I mentioned this to my general practitioner and, after a lot of effort searching for information, he confirmed that it probably was gout and that it was far more prevalent than he expected.
It is a debilitating pain! During a full attack just lifting the foot off the pillow it's resting on is enough to create panting and tears. Colchicine is supposed to relieve the symptoms but all I guarantee is that it causes vomiting and diarrhoea. I dare not move because of the pain, and yet I have to make repeated frantic visits to the toilet.
Since I started to take 300 mg a day of allopurinol, I have had no more attacks of gout.
Colchicine
Colchicine is the most popular treatment for acute gout in some countries, such as France.18 Although one randomised controlled trial showed that colchicine is more effective than placebo, all participants in the colchicine arm developed diarrhoea and vomiting, many before onset of pain relief.3 The high dose of colchicine, up to 6 mg, usually advised for the treatment of gout may cause unnecessary toxicity; a lower dose of 0.5 mg every eight hours may be more appropriate.19 The toxicity of intravenous colchicine is too high to justify its use.
Steroids and adrenocorticotrophic hormone
Oral, parenteral, and intra-articular steroids and adrenocorticotrophic hormone are all used to treat acute gout. No placebo controlled trials have assessed the effect of steroids or adrenocorticotrophic hormone on acute gout.16 Occasional short courses of oral steroids may be preferable to either non-steroidal anti-inflammatory drugs or colchicine, because of the lower incidence of adverse events.
Local treatments
Many cartoon images of people with gout testify to the long history of treatment by elevation and rest of the affected joint. More recently, a small controlled trial suggested benefit from the application of ice to the affected area.16
Although there is little objective evidence, clinical experience suggests that all these approaches can be effective for the treatment of acute gout. Choice should be determined by the risk of adverse events and patient preferences. For some patients with a high risk of side effects it may be appropriate to advise rest, cooling, and strong analgesics rather than any gout specific drug treatment.
Prevention of recurrent gout
Few robust data exist on the prevention of recurrent gout.16 Since only a minority of people with asymptomatic hyperuricaemia ever develop gout, asymptomatic hyperuricaemia seldom requires treatment. The two exceptions are preventive treatment for some patients being treated for malignancy; and possibly for people with a high urate concentration (> 0.8 mmol/l) and high renal excretion of urate, to prevent formation of renal stones. Consequently there is no indication for routine screening for hyperuricaemia. Nevertheless, many patients receiving allopurinol do not have a recorded diagnosis of gout.20
Box 3 Suggested quality care indicators for management of gout. Adapted from Mikuls et al21
Treatment of acute gout
Patients presenting with acute gouty arthritis who do not have significant renal impairment (creatinine clearance ≤ 50 ml/min or creatinine concentration ≥ 167 μmol/l) or peptic ulcer disease should be treated with one of the following:
A non-steroidal anti-inflammatory drug
Adrenocorticotrophic hormone or steroids (systemic or intra-articular)
Colchicine
Prevention of recurrent gout
Patients with gout who are obese (body mass index > 28), or who have one or more alcoholic drinks per day, should be advised to lose weight or decrease their alcohol consumption, or both
When starting allopurinol in patients with major renal impairment, initially use a low dose (< 300 mg/day)
When coprescribing a xanthine oxidase inhibitor with azothiaprine or 6-mercaptopurine, reduce dose of azothiaprine or 6-mercaptopirine by at least 50%
When starting a urate lowering drug in patients with gout who do not have major renal impairment (see definition above) or peptic ulcer disease, coprescribe a non-steroidal anti-inflammatory drug or colchicine to reduce the incidence of rebound gout attacks
Patients with asymptomatic hyperuricaemia do not need treatment
Uricosuric drugs should not be used in patients with significant renal impairment (see definition above) or a history of renal stones
Patients with gout and either tophaceous deposits, gouty erosive changes on radiographs, or more than two attacks per year should be offered urate lowering treatment
Patients with gout who are taking a xanthine oxidase inhibitor should have their serum urate level checked at least once during the first six months of continued use
Patients taking long term prophylactic oral colchicine who have major renal impairment (see definition above) should have a full blood count and creatine kinase checked at least once every six months
An increased serum urate concentration and recurrent joint pain is insufficient to diagnose gout. Careful consideration is required before starting lifelong prophylactic drug treatment with potentially serious side effects. If the patient does not seem to benefit from treatment the diagnosis should be reviewed (box 2).
For most people with occasional attacks of gout, the risks of prophylactic treatment probably outweigh the benefits. Patient preferences play an important part in the decision to prescribe prophylactic drugs; however, these drugs should be offered to patients who experience more than two acute attacks per year, have tophi, or have radiological changes.21 Urate lowering drugs shouldn't be started during an acute attack. Unless contraindicated, prophylactic colchicine or a non-steroidal anti-inflammatory drug should be prescribed for the first three months of urate lowering treatment to prevent a rebound increase in acute gout. In one trial colchicine prophylaxis reduced the incidence of rebound gout by 1.2 attacks per person over the first three months of allopurinol treatment.22
Tips for non-specialists
Serum urate concentrations can go down during an attack of gout
Oral steroids may be a safer alternative to non-steroidal anti-inflammatory drugs or colchicine for the management of acute gout
Urate lowering drugs are usually needed only for patients with frequent attacks of gout
Asymptomatic hyperuricaemia does not require treatment
The target of interventions to reduce serum urate is to decrease the serum urate concentration to below 0.36 mmol/l, well below the level at which urate crystallises.23 At this level urate should no longer crystallise and existing deposits should be mobilised. Even if this target is not reached, any reduction in urate concentration should reduce the incidence of recurrent gout.
Medication review
Some drugs, most commonly diuretics, can cause iatrogenic hyperuricaemia. The patient's regular drugs should be reviewed and consideration given to stopping any urate raising drugs.
Lifestyle changes
No controlled trials of the effect of lifestyle change on the incidence of recurrent gout have been carried out.16 Adherence to traditional low purine diets is poor and they are not usually recommended. Data from the health professionals study, however, suggest that the following relatively simple changes may have an impact on incidence of recurrent gout9-11:
Lose weight
Eat one less portion of meat or fish a day
Drink wine instead of beer
Drink a glass of skimmed milk a day.
Xanthine oxidase inhibitors
Allopurinol has been the mainstay of gout prevention since the 1960s. Its effect on incidence of recurrent gout has not been shown in placebo controlled trials.16 Nevertheless, decades of experience by doctors and patients attest to its effectiveness. However, in two trials of a new xanthine oxidase inhibitor, febuxostat, only 21% and 22% of patients taking allopurinol achieved the target urate level of less than 0.36 mmol/l.24,25 The dose of allopurinol should be titrated, if necessary up to 900 mg/day, to achieve a serum urate concentration of less than 0.36 mmol/l. Up to one fifth of patients are unable to tolerate allopurinol; some develop potentially fatal allopurinol hypersensitivity syndrome.23 Patients with renal impairment are more likely to have adverse reactions to allopurinol and should be prescribed 300 mg or less daily.21
Febuxostat may be marketed soon. Two large randomised controlled trials have shown it to be substantially more effective than allopurinol at reducing serum urate concentration to a target level of less than 0.36 mmol/l (48% and 69% v 21% and 22%).24,25 This did not, however, result in fewer acute attacks of gout when compared with the allopurinol group.24 Because febuxostat is biochemically unrelated to allopurinol it may have a role in patients who are intolerant to allopurinol.
Uricosurics
The only uricosuric routinely available in the United Kingdom is sulfinpyrazone. It works by blocking renal tubular reabsorption of urate, consequentially increasing risk of renal stones. It is unsuitable for patients with impaired renal function or a high renal excretion of urate. Evidence for its long term effect on urate concentration and recurrent gout is sparse.16
Colchicine
No controlled trials of colchicine's use as a single agent for prevention of recurrent gout have been carried out.16 However, it reduces rebound attacks for up to six months in patients starting allopurinol, indirectly supporting its use in long term prevention.22
Non-steroidal anti-inflammatory drugs
Long term treatment with non-steroidal anti-inflammatory drugs is sometimes used to prevent recurrent gout. Data from controlled trials are lacking.16
Other urate lowering drugs
Both losartan and flubiprofen may reduce serum urate concentration. Data from controlled trials on their use to prevent recurrent gout are lacking.16
Conclusion
Until recently there has been little new information to inform the diagnosis and management of gout. Now there is a resurgence of interest in improving its management. In existence are new high quality epidemiological data; systematic reviews of existing evidence for treatment; evidence based quality of care indicators (box 3); new high quality controlled trials; and the first new gout specific drug to become generally available is likely to come on the market soon. Uricase drugs that break down urate are already available for specific indications; in future similar preparations may become more generally available for patients with intractable gout.
Additional educational resources
Clinical Evidence (www.clinicalevidence.com/ceweb/conditionpdf/1120.pdf)—a review of the evidence for the treatment and prevention of recurrent gout Drug and Therapeutics Bulletin (NHS Athens login) (http://nhsia-lin-01.niss.ac.uk/idtb/content/db/pdf0405)—review of the primary care management of gout
Evidence Based Rheumatology (www.blackwellpublishing.com/medicine/bmj/rheumatology/pdfs/gout.pdf)—book chapter on the evidence based treatment of gout
Information for patients
Arthritis Research Campaign (UK) (www.arc.org.uk/about_arth/booklets/6015/6015.htm)—provides an information booklet for patients UK Gout Society (www.ukgoutsociety.org/about.htm)—a charity that provides information and campaigns to improve the management of gout
Ongoing research
More trials are under way comparing cyclo-oxygenase-2 inhibitors with indometacin for acute gout
Ongoing studies are examining the use of pegylated recombinant mammalian uricase
The long term safety of febuxostat is being studied
Unanswered research questions
How effective are non-steroidal anti-inflammatory drugs and prednisolone for the treatment of acute gout when compared with each other and with placebo?
Are simple lifestyle changes effective at preventing gout?
How effective is allopurinol at preventing gout in different patient groups?
I thank Dawn Carnes and Lynette Edwards for their comments on earlier versions of this paper.
Competing interests: MU has received speaker fees from Pfizer, the manufacturer of valdecoxib and celecoxib and from Menarini, the manufacturer of ketoprofen and dexketoprofen. He is a current or recent applicant on research projects funded in excess of £100 000 by the NHS health technology assessment programme, Arthritis Research Campaign, and the UK Medical Research Council.
References
- 1.Choi HK, Curhan G. Gout: epidemiology and lifestyle choices. Curr Opin Rheumatol 2005;17: 341-5. [PubMed] [Google Scholar]
- 2.Bellamy N, Downie WW, Buchanan WW. Observations on spontaneous improvement in patients with podagra: implications for therapeutic trials of non-steroidal anti-inflammatory drugs. Br J Clin Pharmacol 1987;24: 33-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahern MJ, Reid C, Gordon TP, McCredie M, Brooks PM, Jones M. Does colchicine work? The results of the first controlled study in acute gout. Aust NZ J Med 1987;17: 301-4. [DOI] [PubMed] [Google Scholar]
- 4.Yu TF, Gutman AB. Efficacy of colchicine prophylaxis in gout. Ann Intern Med 1961;55: 179-92. [DOI] [PubMed] [Google Scholar]
- 5.Mikuls TR, Farrar JT, Bilker WB, Fernandes S, Schumacher HR Jr, Saag KG. Gout epidemiology: results from the UK general practice research database, 1990-1999. Ann Rheum Dis 2005;64: 267-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bieber JD, Terkeltaub RA. Gout: on the brink of novel therapeutic options for an ancient disease. Arthritis Rheum 2004;50: 2400-14. [DOI] [PubMed] [Google Scholar]
- 7.Klemp P, Stansfield SA, Castle B, Robertson MC. Gout is on the increase in New Zealand. Ann Rheum Dis 1997;56: 22-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campion EW, Glynn RJ, DeLabry LO. Asymptomatic hyperuricemia. Risks and consequences in the normative aging study. Am J Med 1987;82: 421-6. [DOI] [PubMed] [Google Scholar]
- 9.Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Alcohol intake and risk of incident gout in men: a prospective study. Lancet 2004;363: 1277-81. [DOI] [PubMed] [Google Scholar]
- 10.Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Arch Intern Med 2005;165: 742-8. [DOI] [PubMed] [Google Scholar]
- 11.Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med 2004;350: 1093-103. [DOI] [PubMed] [Google Scholar]
- 12.Baker JF, Krishnan E, Chen L, Schumacher HR. Serum uric acid and cardiovascular disease: recent developments, and where do they leave us? Am J Med 2005;118: 816-26. [DOI] [PubMed] [Google Scholar]
- 13.Janssens HJ, van de Lisdonk EH, Bor H, van den Hoogen HJ, Janssen M. Gout, just a nasty event or a cardiovascular signal? A study from primary care. Fam Pract 2003;20: 413-6. [DOI] [PubMed] [Google Scholar]
- 14.Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yu TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum 1977;20: 895-900. [DOI] [PubMed] [Google Scholar]
- 15.Giordano N, Santacroce C, Mattii G, Geraci S, Amendola A, Gennari C. Hyperuricemia and gout in thyroid endocrine disorders. Clin Exp Rheumatol 2001;19: 661-5. [PubMed] [Google Scholar]
- 16.Sutaria, S, Katbamna R, Underwood M. Effectiveness of interventions for the treatment of acute and prevention of recurrent gout: a systematic review. Rheumatology Advance Access published April 21, 2006.doi:10.1093/rheumatology/ke1071. [DOI] [PubMed]
- 17.Gout in primary care. Drug Ther Bull 2004;42: 37-40. [DOI] [PubMed] [Google Scholar]
- 18.Rozenberg S, Lang T, Laatar A, Koeger AC, Orcel P, Bourgerois P. Diversity of opinions on the management of gout in France. A survey of 750 rheumatologists. Rev Rhum Engl Ed 1996;63: 255-61. [PubMed] [Google Scholar]
- 19.Morris I, Varughese G, Mattingly P. Colchicine in acute gout. BMJ 2003;327: 1275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mikuls TR, Farrar JT, Bilker WB, Fernandes S, Saag KG. Suboptimal physician adherence to quality indicators for the management of gout and asymptomatic hyperuricaemia: results from the UK general practice research database (GPRD). Rheumatology (Oxford) 2005;44: 1038-42. [DOI] [PubMed] [Google Scholar]
- 21.Mikuls TR, MacLean CH, Olivieri J, Patino F, Allison JJ, Farrar JT, et al. Quality of care indicators for gout management. Arthritis Rheum 2004;50: 937-43. [DOI] [PubMed] [Google Scholar]
- 22.Borstad GC, Bryant LR, Abel MP, Scroggie DA, Harris MD, Alloway JA. Colchicine for prophylaxis of acute flares when initiating allopurinol for chronic gouty arthritis. J Rheumatol 2004;31: 2429-32. [PubMed] [Google Scholar]
- 23.Wortmann RL. Recent advances in the management of gout and hyperuricemia. Curr Opin Rheumatol 2005;17: 319-24. [DOI] [PubMed] [Google Scholar]
- 24.Becker MA, Schumacher HR Jr, Wortmann RL, MacDonald PA, Eustace D, Palo WA, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med 2005;353: 2450-61. [DOI] [PubMed] [Google Scholar]
- 25.Schumacher HR, Becker MA, Wortmann RL, Macdonald PA, Hunt B, Streit J, et al. Febuxostat vs allopurinol and placebo in subjects with hyperuricaemia and gout: the 28-week APEX study. Annual scientific meeting of the American College of Rheumatology, San Diego, Nov 2005.