Abstract
Type XV collagen occurs widely in the basement membrane zones of tissues, but its function is unknown. To understand the biological role of this protein, a null mutation in the Col15a1 gene was introduced into the germ line of mice. Despite the complete lack of type XV collagen, the mutant mice developed and reproduced normally, and they were indistinguishable from their wild-type littermates. However, Col15a1-deficient mice showed progressive histological changes characteristic for muscular diseases after 3 months of age, and they were more vulnerable than controls to exercise-induced muscle injury. Despite the antiangiogenic role of type XV collagen-derived endostatin, the development of the vasculature appeared normal in the null mice. Nevertheless, ultrastructural analyses revealed collapsed capillaries and endothelial cell degeneration in the heart and skeletal muscle. Furthermore, perfused hearts showed a diminished inotropic response, and exercise resulted in cardiac injury, changes that mimic early or mild heart disease. Thus, type XV collagen appears to function as a structural component needed to stabilize skeletal muscle cells and microvessels.
Type XV collagen belongs to the heterogeneous group of non-fibril-forming collagens and is thought to be a homotrimer consisting of three α1(XV) collagen chains (1). It is characterized by a central highly interrupted triple helical domain and large N- and C-terminal noncollagenous domains (2–4), and it has been shown to be a chondroitin sulfate proteoglycan (5). Type XV collagen mRNAs are expressed in many tissues, but the highest mRNA levels in the mouse can be detected in the heart and skeletal muscle (4). The protein is shown by immunostaining to have a widespread tissue distribution and has been localized mainly to the basement membrane zones, although it can also be found in the fibrillar collagen matrix of some tissues (6, 7). Its function is not known, however.
In terms of primary structure, type XV collagen is highly homologous with type XVIII collagen, and together they form a distinct subgroup among the collagens (1, 3). They have thrombospondin-1 sequence homology in the N terminus, seven homologous collagenous domains, and highly homologous C-terminal noncollagenous domains. Type XVIII collagen is the precursor of endostatin, which has been shown to have a potent antiangiogenic effect (8), and the highest degree of homology between collagen types XV and XVIII involves the C-terminal endostatin sequence. The corresponding fragment in type XV collagen has also been shown to have antiangiogenic activity (9, 10).
To understand the biological function and significance of type XV collagen, we generated a mouse strain lacking in α1(XV) collagen chains by site-specific Cre-loxP-mediated deletion in embryonic stem (ES) cells (11). The data suggest a structural role for type XV collagen in providing mechanical stability between cells and the extracellular matrix in skeletal muscle fibers and microvessels. Col15a1 deficiency leads to functional rather than structural changes in the myocardium, changes that are apparent after increased workload.
Materials and Methods
Generation of a Mouse Strain Lacking in Type XV Collagen.
To construct a targeting vector, a 10.2-kb genomic fragment containing exons 1 and 2, 4.5 kb of the second intron, and a 5.5-kb 5′-flanking region was subcloned into the pSP72 vector (Promega). A loxP sequence (12) was inserted into the SpeI site existing about 900 bp upstream of the first exon. A selection marker gene cassette flanked by loxP sites (12) was inserted into an ApaI site 120 bp downstream of the second exon. The linearized targeting vector was electroporated into R1-ES (13) cells and cultured on embryonic fibroblast feeder cells as described (14). Genomic DNA from G418-resistant ES clones was digested with SpeI and analyzed by Southern blot hybridization with a 5′ external probe (HP5′). Of 140 clones, seven were correctly targeted, having the selection marker genes neor and HSV-tk in the second intron of the Col15a1 gene and loxP sites flanking exons 1 and 2. Targeted ES cells were electroporated with the supercoiled Cre plasmid (pIC-Cre, a gift from Dr. Müller); after ganciclovir selection, the DNA from surviving ES clones was digested with XbaI and EcoRI, and two clones shown by Southern blotting with the IP4E probe to have an inactivated Col15a1 allele were injected into blastocysts and implanted in pseudopregnant mice (14). Chimeric founders were bred to 129sv mice to generate inbred mutant mice, which were used for analyses with sibling controls.
Histological Analysis.
Muscle samples were oriented under a microscope, frozen in isopentane cooled with liquid nitrogen, and stored at −80°C. Sections were stained with hematoxylin and eosin. The histology of the various muscle groups (soleus, gastrocnemius, quadriceps femoris, triceps brachii, diaphragm, and muscles from the neck and back) was studied at ages ranging from 3 to 84 weeks in 14 mutant and 10 wild-type sibling mice. In addition, hematoxylin and eosin-stained quadriceps femoris samples were analyzed at ages of 7–13 weeks (Col15a1+/+, n = 4; Col15a1−/−, n = 5) and 27–34 weeks (Col15a1+/+, n = 7; Col15a1−/−, n = 6). For indirect immunofluorescence staining, frozen tissue samples were sectioned, fixed, and stained as described (7).
Ultrastructural Examinations.
The samples from free wall of the left ventricles were fixed and dehydrated, and sections were viewed in a transmission electron microscope (Philips CM100). At least two samples from the heart (wild-type mice, n = 6, 12–97 weeks old; Col15a1−/− mice, n = 7, 12–129 weeks old), and the gastrocnemius and quadriceps femoris muscles (wild-type mice, n = 4, 12–97 weeks old; Col15a1−/− mice, n = 5, 12–131 weeks old) were examined per mouse. In addition, the structure of the capillaries was examined in three samples from the cerebellum and lung from each mouse (wild-type mice, n = 1, 19 weeks old; Col15a1−/− mice, n = 2, 22 and 36 weeks old).
Physical Exercise Experiments.
In experiment 1 (Exp1), four mutant and seven wild-type male mice, aged 7–13 weeks, ran on a motor-driven treadmill with 6° uphill tracks at a speed of 10.5 m × min−1 for 4 h. After 2 h of running, there was a 20-min rest period, during which the animals had free access to pelleted food and water. In experiment 2 (Exp2), 11 mutant, aged 27–34 weeks, and 11 age and sex-matched wild-type male mice were subjected to 6 h of running with a 6° uphill inclination at the speed of 8.5 m × min−1 with two 20-min pauses. Forty-eight hours after the cessation of exercise, the animals were killed together with unexercised mutant and wild-type controls (Exp1: unexercised control Col15a1+/+, n = 4; Col15a1−/−, n = 5; Exp2: Col15a1+/+, n = 7; Col15a1−/−, n = 7). The soleus muscles from both hind limbs and the proximal part of the quadriceps femoris (MQF) muscle from the left leg were excised and frozen by liquid nitrogen for the assays of β-glucuronidase activity. This was measured from the combined soleus and MQF samples as described (15). For histological analysis, MQF from the contralateral leg was oriented under a microscope and frozen in isopentane cooled with liquid nitrogen.
Markers of Cardiac Injury.
After the second exercise protocol (Exp2), the left ventricles were cut into three pieces for histological, mRNA, and biochemical analysis. DNA fragmentation [terminal deoxynucleotidyltransferase-mediated UTP end-labeling (TUNEL) assay] was detected from cryostat sections stained with the In Situ Cell Death Detection Kit (Boehringer Mannheim) according to the manufacturer's protocol. The mean number of TUNEL-positive nuclei per 40× objective field was counted from four sections on different planes in each sample. Pro matrix metalloproteinase 2 (MMP-2) activity was measured by zymography as described (16), using 50 μg of protein and 7.5% gels. β-Glucuronidase activity was measured in a cardiac muscle homogenate as mentioned earlier (15). For atrial natriuretic peptide (ANP) mRNA analysis, a cDNA reaction was performed according to the manufacturer's protocol (GIBCO/BRL), after which ANP mRNA levels were measured by quantitative reverse transcription-PCR analysis as described (17).
Preparation of Isolated Perfused Hearts.
Two groups of female mice at ages of 6 and 12 months were studied as described for the rat (18). The hearts were perfused at a constant flow rate of 2.5 ml/min and paced to a constant rate of 400 beats/min. To measure the isometric force of contraction, the left atrium was cut off, and an empty plastic balloon was inserted into the left ventricle, filled with 50% ethanol to give an end diastolic pressure of 2–3 mmHg (1 mmHg = 133 Pa), and the developed pressure inside the balloon was recorded with a pressure transducer (Isotec, Hugo Sachs Elektronik). The data were analyzed and recorded with an IBM PC-compatible computer using Ponemah data acquisition software (Gould, Cleveland). After 20 min of stabilization followed by a 10-min control period, the experiment was started with infusion of the first dose of isoproterenol (Sigma). The infusion was stopped after 30 s when the maximal response had been reached, and the next dose was given after a 10-min equilibration period. The contractile response to each dose was calculated as a ratio of the maximal level of contractility to the level before isoproterenol administration. To study cAMP levels, hearts from 18-month-old wild-type (n = 5) and null mice (n = 6) were perfused as mentioned above, and concentrations of cAMP were measured as described (19) in left ventricles removed 5 min after a 60-s infusion of isoproterenol (0.1 nmol/liter).
Statistics.
The Mann–Whitney U test was used to compare the means of the cardiac injury markers; Student's t test was used for the other comparisons.
Results
Generation of a Mouse Strain Lacking in Type XV Collagen.
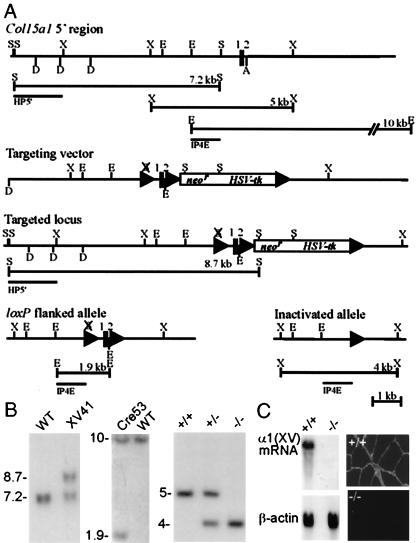
The mouse type XV collagen gene is 110 kb in size and contains 40 exons. The closely spaced first and second exons of the Col15a1 gene encode the first 33 residues of the 1,367-residue polypeptide and a split codon for the next residue (20). A knockout targeting vector was prepared with selection marker genes (neor and HSV-tk) at the 5′ end of the 120-nt second intron and loxP sites flanking the marker cassette and the first two exons (Fig. 1A). After homologous recombination, a Cre expression plasmid was electroporated into the targeted ES cell lines to generate Cre-mediated deletions. Two types of Cre-mediated recombination alleles were observed: loxP-flanked alleles identified by Southern blot analysis of EcoRI-digested genomic DNA and inactivated alleles identified by XbaI-digested genomic DNA, lacking the first two exons and the transcription start sites (20) (Fig. 1 A and B).
Figure 1.
Targeted inactivation of the Col15a1 gene. (A) Targeting strategy and Cre-mediated recombinations. (Top) Restriction map for the 5′ portion of the Col15a1 gene wild-type allele, predicted restriction fragments, and location of the probes (HP5′ and IP4E) used for Southern blot analysis. The vertical lines represent the first and second exons (1 and 2). (Middle) The targeting vector and a targeted locus. lox-P sites are represented by triangles. The selection marker gene cassette (neor and HSV-tk) is located in the second intron. (Bottom) loxP flanked and inactivated Col15a1 alleles. Restriction sites: A, ApaI; D, DraI; E, EcoRI; S, SpeI; X, XbaI. (B) Genotypes of wild-type, targeted, lox-P flanked, and inactivated alleles analyzed by Southern blot hybridization. (Left) SpeI-digested ES cell DNA from the wild-type (WT, a 7.2-kb fragment) and a targeted cell line (XV41, an 8.7-kb fragment) hybridized to the HP5′ probe. (Center) ES cell DNA from the wild-type allele and the modified allele (Cre53) in which the first two exons are flanked by loxP sites. The IP4E probe detects a 1.9-kb EcoRI fragment derived from the loxP flanked allele (WT, 10 kb). (Right) Southern analysis of tail biopsies from wild-type (+/+), heterozygous (+/−), and homozygous (−/−) mutant mice digested with XbaI and hybridized with the IP4E probe. After Cre-mediated deletion, the IP4E probe detected a 4-kb XbaI fragment diagnostic of the inactivated allele (WT, 5 kb). (C) Northern blot hybridization (Left) and immunostainings against type XV collagen in muscle (Right). No mRNA or immunostaining for type XV collagen can be detected in a homozygous mutant mouse (−/−).
Genotyping of 345 offspring from heterozygous intercrosses showed that 21.5% were of the wild type, 53.9% were heterozygous, and 24.6% were homozygous for the null allele. Furthermore, the adult mice lacking type XV collagen displayed no obvious alteration in phenotype, were fertile, and had a normal lifespan. To confirm that the mutation leads to a loss of type XV collagen function, mRNA and protein expression was examined. Northern blot analysis of RNA derived from skeletal muscle, heart, kidney, and testis (Fig. 1C and data not shown) showed a complete absence of the type XV collagen mRNA in the homozygous mutant mice, and this was confirmed by immunostaining with antibodies against the C-terminal domain (Fig. 1C). No compensatory changes were observed in the level of expression of the homologous type XVIII collagen mRNA as analyzed by Northern blotting or with immunohistological studies of the Col15a1−/− mice (not shown).
Focal Areas of Degeneration, Regeneration, and Variation in Fiber Size in Col15a1−/− Muscles.
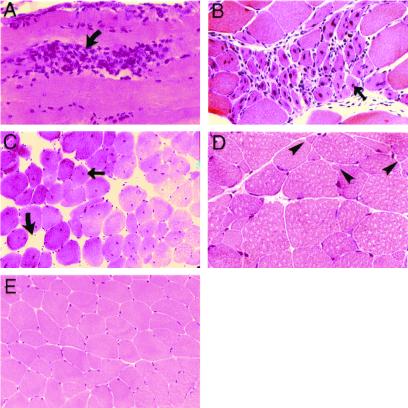
Histological screening of tissues from Col15a1−/− revealed changes in the skeletal muscle, including muscle cell degeneration, macrophage infiltration, and increased regeneration (Fig. 2). Muscle samples from the Col15a1−/− mice showed variation in fiber size ranging from mild to moderate, with atrophic and split-muscle fibers (Fig. 2D), and occasionally a group of atrophic muscle fibers was observed. The histological changes were first detected at 13 weeks of age and were more clearly seen after 26 weeks of age. The changes were focal, with the number of degenerative fibers in the samples ranging from none to a few necrotic ones. The histological changes were more frequent in the back and paraspinal muscles than in the other muscle groups analyzed. Two-thirds of the mutant mice aged over 6 months displayed these muscle abnormalities, the majority of the samples being mildly affected. No clear signs of fibrosis were observed in hematoxylin–eosin and van Gieson stainings. Antibodies against basement membrane components (lamininα2, tenascin-C, and type IV collagen) and dystrophin showed a normal pattern of immunostaining (not shown).
Figure 2.
Muscle histology: focal areas of degeneration, regeneration, and variation in fiber size. Hematoxylin and eosin-stained sections of the gastrocnemius (A), triceps brachii (B), paraspinal (C), and quadriceps (D) muscles of 6-month-old null mice showing cell degeneration (curved arrows, A and C) with macrophage infiltration (A), regenerative fibers (thin arrow, B), central nuclei (thin arrow, C), and increased variation in fiber size with atrophic muscle fibers (D, arrowheads) compared with age-matched wild-type mice (E). Original magnifications: A and D, ×300; B, ×200; C and E, ×80.
Susceptibility to Exercise-Induced Muscle Damage.
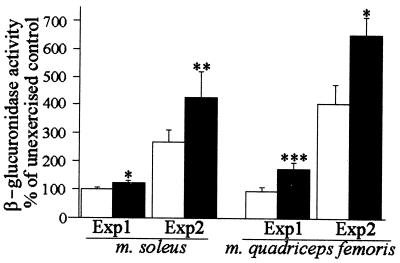
The histopathological signs of a muscular disorder led us to study the possibility that the type XV collagen deficiency may have an increased effect on susceptibility to exercise-induced damage. Mice were subjected to running on a motor-driven treadmill in two exercise protocols, the first under milder conditions (Exp1) and the second with a heavier physical load (Exp2). Both mutant and control mice sustained the exercise protocols without signs of serious exhaustion or behavioral avoidance of running. The severity of the muscle cell damage was estimated from hematoxylin–eosin and immunostained sections, and muscle β-glucuronidase activity was used as a quantitative measure of the injury, as described (21). The first running protocol did not cause any marked muscle injury in the wild-type mice, but a significant increase in the β-glucuronidase level was noted in the mutant mice as compared with the unexercised Col15a1−/− mice and the exercised Col15a1+/+ mice (Fig. 3).
Figure 3.
Increased muscle injury in mutant mice after acute exercise. Increase in β-glucuronidase activity 48 h after the cessation of experiments 1 and 2. The elevation of enzyme activity reflecting the amount of damage is presented as a percentage of the average activity measured in unexercised controls of the same mouse strain. White bars, Col15a1+/+ mice; black bars, Col15a1−/− mice. The basal levels of β-glucuronidase activities in unexercised control mice were as follows. Experiment 1: soleus+/+ 1.1 ± 0.1, soleus−/− 1.1 ± 0.1, MQF+/+ 1.0 ± 0.1, and MQF−/− 0.9 ± 0.1; in experiment 2: soleus+/+ 0.9 ± 0.1, soleus−/− 0.8 ± 0.1, MQF+/+ 0.7 ± 0.1, and MQF−/− 0.7 ± 0.1 μmol s−1⋅kg−1 protein (mean ± SEM). Significant differences between the Col15a1−/− and Col15a1+/+ mice are denoted by asterisks as follows: *, P < 0.05, **, P < 0.01, ***, P < 0.001.
After exercise session 2, using a physical load sufficient to cause marked damage in the wild-type mice, the Col15a1−/− muscle cells were affected significantly more severely, as evaluated by the increase in the β-glucuronidase activity, than the wild-type mice (Fig. 3). The severity of the histopathological findings varied between mice, but in general the mutant runners showed more degenerative fibers and more intensive inflammation (not shown). Immunostainings for dystrophin, type IV collagen, and the laminin α2 chain showed the same pattern of staining in the mutant and wild-type mice (not shown).
Ultrastructural Changes in the Heart and Skeletal Muscle Capillaries.
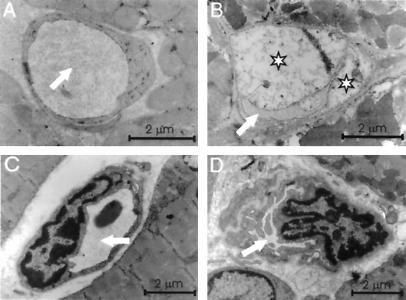
The strong expression in the heart prompted us to search for cardiac changes in the Col15a1−/− mice. Although light microscopy did not reveal any conspicuous changes compared with the controls, electron microscopy showed abnormalities encompassing the microvessels and their endothelium. Where the capillaries in the wild-type mice were round and had a wide lumen, some of those in the mutant mice were irregular in shape with intensively folded endothelial membranes (Fig. 4 A–D), and some of the endothelial cells were degenerated and swollen, having a pale cytoplasm with only a few cell organelles and having bulged into the vessel lumen (Fig. 4 A and B). In some cases, the endothelial cells were shrunken, showing a thin, electron-dense structure (not shown). These changes were detected in all null mice, but they were focal and the incidence of the abnormal capillaries varied. In two of seven mice, more than half of the heart capillaries were affected, and in the others the incidence of the capillary defects ranged between 10% and 50%. The capillary defects were also found in skeletal muscle samples, although they were more frequent in the heart. No changes were noted in the vascular basal lamina. No endothelial cell degeneration or changes in the capillary structure could be seen in the wild-type mice. The heart specimens containing swollen endothelial cells also showed focal ischemic changes in the cardiac myocytes such as intracellular edema and vacuolization (not shown). The capillaries of the lung and cerebellum are normally devoid of type XV collagen (ref. 7 and A.M., T. Väisänen, L.E., M.I. and T.P., unpublished observations), and no degenerative endothelial cells or changes in the capillary structure were observed in these tissues in the Col15a1−/− mice (not shown).
Figure 4.
Ultrastructural changes in capillaries. Electron microscopy of heart capillaries from a wild-type (A) and a null mouse (B) with swollen endothelial cells (asterisks). The lumen is indicated by a white arrow. Skeletal muscle capillaries from a wild-type (C) and a mutant mouse (D) showing luminal narrowing and folding of endothelial cells.
Heart Response to Cardiovascular Stress.
Treadmill exercise is one of the primary physiological methods used to increase hemodynamic stress and to detect cardiovascular abnormalities that may not be readily apparent at rest (22, 23). To test the hypothesis that the abnormalities in capillary morphology observed in the mutant mice could have some consequences for blood flow and lead to pathological changes after cardiovascular stress, left ventricle samples were prepared after exercise experiment 2, and the possibility of cardiac injury was studied by assessing the extent of apoptosis (24), the activities of β-glucuronidase (25) and matrix metalloproteinase 2 (26), and the mRNA levels of ANP (27). Statistically significant increases in the number of TUNEL-positive nuclei and in the activities of β-glucuronidase and proMMP-2 were detected in the Col15a1−/− mice. However, it should be noted that the basal proMMP-2 level was lower in the null mice compared with the control ones. The exercise also induced highly increased ANP mRNA levels in the 2 of 11 null mice, although in average this was not statistically significant. No changes were observed in the wild-type individuals (Table 1).
Table 1.
Markers of cardiac injury
| Treatment | GT | TUNEL | proMMP-2 | β-GUase | ANP mRNA |
|---|---|---|---|---|---|
| Control | +/+ | 0.22 ± 0.02 (6) | 1.80 ± 0.10 (7)170 | 0.19 ± 0.02 (7) | 2.27 ± 0.46 (7) |
| Control | −/− | 0.24 ± 0.09 (7)* | 1.30 ± 0.06 (7)*** | 0.22 ± 0.01 (7)** | 2.67 ± 0.55 (7) |
| Exercised | +/+ | 0.24 ± 0.02 (10) | 1.86 ± 0.06 (11) | 0.22 ± 0.02 (11) | 2.32 ± 0.32 (11) |
| Exercised | −/− | 0.72 ± 0.25 (11)† | 1.87 ± 0.09 (10) | 0.35 ± 0.05 (10)‡‡ | 3.63 ± 0.78 (11) |
Average number of TUNEL-positive nuclei in a 40× objective (TUNEL), activity of proMMP-2 (densitometric unit), activity of β-glucuronidase (β-GUase, μmol s−1⋅kg−1 protein), and ANP mRNA levels (to 18S mRNA) in the left ventricles of unexercised controls and exercised mice 48 h after the cessation of running. Values are means ± SEM, with n indicated in parentheses. Statistical significances in the Mann–Whitney U test: between the control−/− and exercised−/− mice. *, P < 0.05, **, P < 0.025, ***, P < 0.001; those between the exercised+/+ and exercised−/− mice.
, P < 0.025,
, P < 0.01; and those between the control+/+ and control−/− mice.
, P < 0.001.
Diminished Cardiac Responses to β-Adrenergic Stimulation.
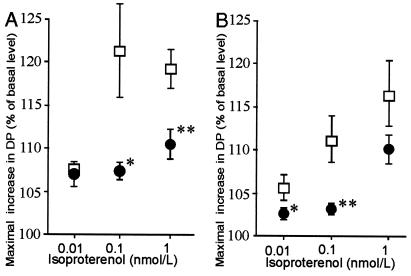
Cardiac function was studied here by using isolated perfused hearts. The developed pressure as an index of cardiac contractility was compared at the basal level and after β-adrenergic receptor stimulation with increasing doses of isoproterenol. The basal contractility in the mutant hearts did not differ significantly from that of the wild-type mice. However, the response to isoproterenol at concentrations of 0.01, 0.1, and 1 nmol/liter, measured as the maximal increase in pressure developed relative to the basal level before isoproterenol perfusion, showed significantly smaller changes in the null mice (Fig. 5). A diminished but statistically nonsignificant decrease in the left ventricular cAMP response 5 min after the administration of 0.1 nmol/liter isoproterenol was observed in the null mice compared with the wild-type mice, with figures of 5.42 ± 0.72 and 7.55 ± 1.37 pmol/mg, respectively (mean ± SEM, P = 0.098). There was no evidence for cardiac hypertrophy in Col15a1−/− mice.
Figure 5.
Effect of isoproterenol stimulation on developed pressure in (A) 6-month-old and (B) 12-month-old mutant and wild-type mice. The responses to β-agonists were measured in terms of the ratio of the maximal value of the developed pressure (DP) to the basal level before the isoproterenol perfusion in Col15a1−/− (●: A, n = 7; B, n = 11) and Col15a1+/+ (□: A, n = 7; B, n = 8) mice. The symbols represent mean ± SEM. Significant differences between the Col15a1−/− and Col15a1+/+ mice are indicated by asterisks as follows: *, P < 0.05, **, P < 0.01. The basal pressure was 31.7 ± 5.0 and 27.3 ± 5.0 mmHg for the 6-month-old control and null mice, respectively, and 20.2 ± 3.4 and 20.8 ± 2.0 mmHg for the 1-year-old mice (mean ± SEM).
Discussion
Despite the wide tissue distribution of type XV collagen, mice lacking in it are viable and fertile. Histological examinations of Col15a1−/− skeletal muscle nevertheless revealed focal changes characteristic of myopathic disorders, including the presence of degenerative fibers, increased numbers of muscle cells having central nuclei, and variations in fiber size. These changes appeared at the age of 3 months and became more apparent in older mice. In addition, the Col15a1−/− mice showed increased sensitivity to exercise-induced muscle damage.
A number of mutations in molecules involved in linkage between the muscle cell and the surrounding extracellular matrix lead to muscular diseases, notably the components of the dystrophin-associated glycoproteins-laminin α2 axis (28), the integrin α7 subunit (29), or type VI collagen (30, 31). According to a structural hypothesis, a disrupted linkage between the cytoskeleton and the matrix leads to sarcolemmal instability and muscle cell necrosis (28, 32). Type XV collagen occurs widely in the basement membrane zone and the adjacent fibrillar matrix, including the endomysium of skeletal muscle (6, 7). The increased fragility of muscle fibers in the null mice does not, however, appear to be caused by clear defects in the basement membrane, because antibodies against known basement membrane components showed normal, uninterrupted staining around degenerative as well as nonaffected muscle fibers. C-terminal fragments of type XV collagen interact in vitro with certain basement membrane and microfibrillar components (10), and thus the prominent changes seen in the type XV collagen-deficient muscle fibers could reflect a defect in linkage between the muscle cell basement membrane and the surrounding fibrillar matrix.
Mice manifesting muscle phenotypes respond differently to exercise, suggesting variations in the etiology lying behind the muscle cell damage. A single episode of excessive physical exercise is known to cause acute damage to the skeletal muscle (33) and is thought to induce focal lesions in muscle fibers, leading to cell necrosis (34–36). Dystrophin deficiency in mdx mice leads to impaired performance in running (37–39) and more pronounced muscle damage than in control mice during sudden exercise (39, 40), suggesting a structural role for this protein. Type VI collagen deficiency results in myopathy in both the human (30) and the mouse (31), although Col6a1−/− mice did not show any significant reduction in activity during a 2-month voluntary running period in wheel cages (31). Similar results have been obtained with γ-sarcoglycan null mice, which show pronounced dystrophic muscle changes (41) but tolerate rigorous swimming exercise well (42). Furthermore, the lack of γ-sarcoglycan did not lead to increased exercise-induced muscle fiber injuries, suggesting nonmechanical causes for the cell degeneration (42). The lack of type XV collagen did not affect the overall running performance of the mice in our protocols, but it did result in more prominent muscle damage as measured on histological and biochemical criteria.
Of special interest was whether the lack of type XV collagen would affect blood vessel formation. Despite the antiangiogenic role of type XV collagen-derived endostatin (9, 10), we could not observe any abnormalities in the number of vessels. Instead, type XV collagen appears to play a role in the integrity of the microvessels, because its deficiency was found to lead to an apparent collapse of the capillary wall in the heart and the skeletal muscle, resulting in various degrees of narrowing or obstruction of the capillary lumen. This may lead to impaired microvascular perfusion, as witnessed by the endothelial cell swelling typical of ischemic cell damage (43). Immunostaining studies (ref. 7, A.M., T. Väisänen, L.E., M.I., and T.P., unpublished observations) have indicated that, whereas type XV collagen is associated with many capillaries, including those in the heart and the skeletal muscle, there are some tissues, including the lung and brain, in which it is not detected around the capillaries. The fact that the lung and brain capillaries of the null mice were normal in structure and no endothelial cell degeneration was observed in them further confirms that the defects seen in the heart and skeletal muscle capillaries are due to the lack of type XV collagen.
Our exercise protocols were optimized for studying degenerative changes in skeletal muscle, and the time point for analysis could cause limitations for some of the markers used to study exercise-induced cardiac injury, because the maximal responses of MMP-2 (26) and ANP (27) expressions were reached later than 48 h after acute cardiac injury and the apoptotic effects earlier (24). Interestingly, basal level proMMP-2 activities were found to be significantly lower in the Col15a1−/− mice than in the wild-type ones. Because MMP-2 is known to be expressed by endothelial cells (44), this could be suggestive of an endothelial cell defect and coincide with the ultrastructurally identified endothelial cell degeneration. As the organization and function of the heart as a continually contracting muscle differ from that of skeletal muscle, acute loading is not likely to lead to similar injuries to those affecting the latter. The abnormalities in the heart microvasculature observed at the morphological level will most probably cause marked ischemic-like damage only after loading. This is also the case with young mice lacking δ-sarcoglycan before the development of cardiomyopathy (23). Furthermore, the preservation of the histological integrity of the heart tissue in the Col15a1−/− mice supports the hypothesis that a certain degree of vascular dysfunction may be required to reach the ischemic threshold necessary to induce myocardial necrosis (23).
Reduced responsiveness to β-adrenergic receptor (β-AR) stimulation is associated with congestive heart failure (45) and with aging (46). Hearts suffering from chronic diseases have shown multiple changes in β-AR-mediated events, including the expression and function of β-adrenergic receptors, G proteins, adenylyl cyclases, and G protein receptor kinases (45). A pacing-induced animal model has suggested that the down-regulation of β1-AR receptors occurs in an early phase in the development of heart failure (47). In humans, reduced β1-AR mRNA levels (48) and β-receptor density (49) are also detected in a mild form of cardiac dysfunction, indicating that down-regulation of β-AR receptors is not restricted to severe or advanced heart disease. Moreover, histological analysis of cardiac tissue from volume-overloaded pigs indicates that decreased responsiveness to β-AR stimulation can occur without degenerative changes such as inflammation or fibrosis (50). It is not known at present, however, whether the changes in the β-AR system are causes or consequences of heart dysfunction. The present isolated perfused hearts of Col15a1−/− mice showed a decreased response to a β-AR agonist, which is a result of some change(s) in the β-adrenergic-mediated signal transduction system.
In view of its collagenous primary structure, its location in the extracellular space, and the consequences of the loss of function described here, we assume that type XV collagen functions as a structural component that is needed to stabilize cells with surrounding connective tissue, at least in the skeletal muscle and microvessels. Our data also suggest that a lack of type XV collagen will cause damage to the heart after induced cardiovascular stress. It is attractive to speculate that type XV collagen deficiency may cause mild cardiac dysfunction, detectable first as a diminished inotropic response to isoproterenol, before any clear morphological changes emerge. Furthermore, the sedentary lifestyle of Col15a1−/− mice may prevent the development of severe heart failure. Interestingly, these changes mimic early or mild heart disease, such as decreased inotropy and impaired response to exercise. The microvessel defects are more pronounced in the heart and are accompanied by ischemic changes in the endothelial cells and adjoining cardiomyocytes. Whereas the skeletal muscle defect appears to be due to myogenic degeneration, it is possible that the heart phenotype is due to impaired perfusion. This should be further investigated by generating mice with a conditional knockout in endothelial cells.
Acknowledgments
We thank Päivi Tuomaala and Anna-Liisa Oikarainen for their technical assistance, Björn Olsen and Ulrike Mayer for helpful comments on the manuscript, and Riitta Herva for advice on preparing the muscle samples. This work was supported by Finnish Centre of Excellence Program (2000–2005) of the Academy of Finland Grant 44843, the Sigrid Juselius Foundation, and FibroGen Inc. (South San Francisco, CA).
Abbreviations
- β-AR
β-adrenergic receptor
- MMP-2
matrix metalloproteinase 2
- MQF
musculus quadriceps femoris
- TUNEL
terminal deoxynucleotidyltransferase-mediated UTP end labeling
- ANP
atrial natriuretic peptide
- ES
embryonic stem
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.031444798.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.031444798
References
- 1.Rehn M, Hintikka E, Pihlajaniemi T. J Biol Chem. 1994;269:13929–13935. [PubMed] [Google Scholar]
- 2.Myers J C, Kivirikko S, Gordon M K, Dion A S, Pihlajaniemi T. Proc Natl Acad Sci USA. 1992;89:10144–10148. doi: 10.1073/pnas.89.21.10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muragaki Y, Abe N, Ninomiya Y, Olsen B R, Ooshima A. J Biol Chem. 1994;269:4042–4046. [PubMed] [Google Scholar]
- 4.Hägg P M, Horelli-Kuitunen N, Eklund L, Palotie A, Pihlajaniemi T. Genomics. 1997;45:31–34. doi: 10.1006/geno.1997.4884. [DOI] [PubMed] [Google Scholar]
- 5.Li D, Clark C C, Myers J C. J Biol Chem. 2000;275:22339–22347. doi: 10.1074/jbc.M000519200. [DOI] [PubMed] [Google Scholar]
- 6.Myers J C, Dion A S, Abraham V, Amenta P S. Cell Tissue Res. 1996;286:493–505. doi: 10.1007/s004410050719. [DOI] [PubMed] [Google Scholar]
- 7.Hägg P M, Hägg P O, Peltonen S, Autio-Harmainen H, Pihlajaniemi T. Am J Pathol. 1997;150:2075–2086. [PMC free article] [PubMed] [Google Scholar]
- 8.O'Reilly M S, Boehm T, Shing Y, Fukai N, Vasios G, Lane W S, Flynn E, Birkhead J R, Olsen B R, Folkman J. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 9.Ramchandran R, Dhanabal M, Volk R, Waterman J F, Segal M, Lu H, Knebelmann B, Vilkas P S. Biochem Biophys Res Commun. 1999;255:735–739. doi: 10.1006/bbrc.1999.0248. [DOI] [PubMed] [Google Scholar]
- 10.Sasaki T, Larsson H, Tisi D, Claesson-Welsh L, Hohenester E, Timpl R. J Mol Biol. 2000;301:1179–1190. doi: 10.1006/jmbi.2000.3996. [DOI] [PubMed] [Google Scholar]
- 11.Gu H, Marth J D, Orban P C, Mossmann H, Rajewsky K. Science. 1994;265:103–106. doi: 10.1126/science.8016642. [DOI] [PubMed] [Google Scholar]
- 12.Potocnik A J, Brakebusch C, Fässler R. Immunity. 2000;12:653–663. doi: 10.1016/s1074-7613(00)80216-2. [DOI] [PubMed] [Google Scholar]
- 13.Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder J C. Proc Natl Acad Sci USA. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fässler R, Meyer M. Genes Dev. 1995;9:1896–1908. doi: 10.1101/gad.9.15.1896. [DOI] [PubMed] [Google Scholar]
- 15.Barrett A J. In: Lysosomes: A Laboratory Handbook in Lysosomal Enzymes. Dingle J T, editor. Amsterdam: North–Holland; 1972. pp. 46–126. [Google Scholar]
- 16.Koskinen S O A, Kjaer M, Mohr T, Sorensen F B, Suuronen T, Takala T E S. Muscle Nerve. 2000;23:580–589. doi: 10.1002/(sici)1097-4598(200004)23:4<580::aid-mus18>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Majalahti-Parviainen T, Hirvinen M, Tervonen V, Ilves M, Ruskoaho H, Vuolteenaho O. Endocrinology. 2000;141:731–740. doi: 10.1210/endo.141.2.7312. [DOI] [PubMed] [Google Scholar]
- 18.Magga J, Vuolteenaho O, Marttila M, Ruskoaho H. Circulation. 1997;96:3053–3062. doi: 10.1161/01.cir.96.9.3053. [DOI] [PubMed] [Google Scholar]
- 19.Szokodi I, Kinnunen P, Tavi P, Weckstrom M, Toth M, Ruskoaho H. Circulation. 1998;97:1062–1070. doi: 10.1161/01.cir.97.11.1062. [DOI] [PubMed] [Google Scholar]
- 20.Eklund L, Muona A, Liétard J, Pihlajaniemi T. Matrix Biol. 2000;19:489–500. doi: 10.1016/s0945-053x(00)00090-1. [DOI] [PubMed] [Google Scholar]
- 21.Salminen A, Kihlström M. Muscle Nerve. 1985;8:269–279. doi: 10.1002/mus.880080402. [DOI] [PubMed] [Google Scholar]
- 22.Fewell J G, Osinska H, Klevitsky R, Ng W, Sfyris G, Bahrehmand F, Robbins J. Am J Physiol. 1997;273:H1595–H1605. doi: 10.1152/ajpheart.1997.273.3.H1595. [DOI] [PubMed] [Google Scholar]
- 23.Coral-Vazquez R, Cohn R D, Moore S A, Hill J A, Weiss R M, Davisson R L, Straub V, Barresi R, Bansal D, Hrstka R F, et al. Cell. 1999;98:465–474. doi: 10.1016/s0092-8674(00)81975-3. [DOI] [PubMed] [Google Scholar]
- 24.Kajstura J, Cheng W, Reiss K, Clark W A, Sonnenblick E H, Krajewski S, Reed J C, Olivetti G, Anversa P. Lab Invest. 1996;74:86–107. [PubMed] [Google Scholar]
- 25.Takala T E S, Kiviluoma K, Kihlström M, Rämö P, Vihko V. Int J Sports Med. 1992;13:52–55. doi: 10.1055/s-2007-1021234. [DOI] [PubMed] [Google Scholar]
- 26.Cleutjens J P, Kandala J C, Guarda E, Guntaka R V, Weber K T. J Mol Cell Cardiol. 1995;27:1281–1292. doi: 10.1016/s0022-2828(05)82390-9. [DOI] [PubMed] [Google Scholar]
- 27.Hama N, Itoh H, Shirakami G, Nakagawa O, Suga S, Ogawa Y, Masuda I, Nakanishi K, Yoshimasa T, Hashimoto Y, et al. Circulation. 1995;92:1558–1564. doi: 10.1161/01.cir.92.6.1558. [DOI] [PubMed] [Google Scholar]
- 28.Campbell K P. Cell. 1995;80:675–679. doi: 10.1016/0092-8674(95)90344-5. [DOI] [PubMed] [Google Scholar]
- 29.Mayer U, Saher G, Fassler R, Bornemann A, Echtermeyer F, von der Mark H, Miosge N, Poschl E, von der Mark K. Nat Genet. 1997;17:318–323. doi: 10.1038/ng1197-318. [DOI] [PubMed] [Google Scholar]
- 30.Jöbsis G J, Keizers H, Vreijling J P, de Visser M, Speer M C, Wolterman R A, Baas F, Bolhuis P A. Nat Genet. 1996;14:113–115. doi: 10.1038/ng0996-113. [DOI] [PubMed] [Google Scholar]
- 31.Bonaldo P, Braghetta P, Zanetti M, Piccolo S, Volpi D, Bressan G M. Hum Mol Genet. 1998;7:2135–2140. doi: 10.1093/hmg/7.13.2135. [DOI] [PubMed] [Google Scholar]
- 32.Engel A G, Yamamoto M, Fischbeck K H. In: Myology: Basic and Clinical. Engel A G, Franzini-Amstrong C, editors. New York: McGraw–Hill; 1994. pp. 1133–1187. [Google Scholar]
- 33.Komulainen J, Vihko V. In: in Oxidative Stress in Skeletal Muscle. Reznick A Z, Packer L, Sen C K, Holloszy O, Jackson M J, editors. Basel: Birkhäuser; 1998. pp. 59–74. [Google Scholar]
- 34.Komulainen J, Takala T E S, Kuipers H, Hesselink M K C. Pflügers Arch Eur J Physiol. 1998;436:735–741. doi: 10.1007/s004240050696. [DOI] [PubMed] [Google Scholar]
- 35.Armstrong R B, Ogilvie R W, Schwane J A. J Appl Physiol. 1983;54:80–93. doi: 10.1152/jappl.1983.54.1.80. [DOI] [PubMed] [Google Scholar]
- 36.McNeil P L, Khakee R. Am J Pathol. 1992;140:1097–1109. [PMC free article] [PubMed] [Google Scholar]
- 37.Carter G T, Wineinger M A, Walsh S A, Horasek S J, Abresch R T, Fowler W M., Jr Neuromuscul Disord. 1995;5:323–332. doi: 10.1016/0960-8966(94)00063-f. [DOI] [PubMed] [Google Scholar]
- 38.Wineinger M A, Abresch R T, Walsh S A, Cartet G T. Am J Phys Med Rehabil. 1998;77:20–27. doi: 10.1097/00002060-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Vilquin J T, Brussee V, Asselin I, Kinoshita I, Gingras M, Tremblay J P. Muscle Nerve. 1998;21:567–576. doi: 10.1002/(sici)1097-4598(199805)21:5<567::aid-mus2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 40.Brussee V, Tardif F, Tremblay J P. Neuromuscul Disord. 1997;7:487–492. doi: 10.1016/s0960-8966(97)00115-6. [DOI] [PubMed] [Google Scholar]
- 41.Hack A A, Jiang F, Clending C J, Sigrist K S, Wollmann R L, McNally E M. J Cell Biol. 1998;142:1279–1287. doi: 10.1083/jcb.142.5.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hack A A, Cordier L, Shoturma D I, Lam M Y, Sweeny H L, McNally E M. Proc Natl Acad Sci USA. 1999;96:10723–10728. doi: 10.1073/pnas.96.19.10723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Armiger L C, Gavin J B. Lab Invest. 1975;33:51–56. [PubMed] [Google Scholar]
- 44.Lewalle J M, Munaut C, Pichot B, Cataldo D, Baramova E, Foidart J M. J Cell Physiol. 1995;165:475–483. doi: 10.1002/jcp.1041650305. [DOI] [PubMed] [Google Scholar]
- 45.Post S R, Hammond H K, Insel P A. Annu Rev Pharmacol Toxicol. 1999;39:343–360. doi: 10.1146/annurev.pharmtox.39.1.343. [DOI] [PubMed] [Google Scholar]
- 46.Lakatta E G. J Am Geriatr Soc. 1999;47:613–625. doi: 10.1111/j.1532-5415.1999.tb02579.x. [DOI] [PubMed] [Google Scholar]
- 47.Kiuchi K, Shannon R P, Komamura K, Cohen D J, Bianchi C, Homcy C J, Vatner S F, Vatner D E. J Clin Invest. 1993;91:907–914. doi: 10.1172/JCI116312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Engelhardt S, Böhm M, Erdmann E, Lohse M J. J Am Coll Cardiol. 1996;27:146–154. doi: 10.1016/0735-1097(95)00425-4. [DOI] [PubMed] [Google Scholar]
- 49.Fowler M B, Laser J A, Hopkins G L, Minobe W, Bristow M R. Circulation. 1986;74:1290–1302. doi: 10.1161/01.cir.74.6.1290. [DOI] [PubMed] [Google Scholar]
- 50.Hammond H K, Roth D A, Insel P A, Ford C E, White F C, Maisel A S, Ziegler M G, Bloor C M. Circulation. 1992;85:269–280. doi: 10.1161/01.cir.85.1.269. [DOI] [PubMed] [Google Scholar]