Abstract
Just as there have been 20th century changes in our “macroecology,” including global warming, there have been alterations in our “microecology,” involving the microbial populations that colonize the human body. Helicobacter pylori, an ancient inhabitant of the human stomach, has been disappearing over the course of the 20th century. As such, by comparing H. pylori+ and H. pylori− persons, the consequences of its colonization can be determined. The presence of H. pylori is associated with increased risk for development of gastric cancer and peptic ulceration, and with decreased risk for gastroesophageal reflux disease (GERD) and its sequelae, including esophageal adenocarcinoma. The disappearance of H. pylori (especially cag+ strains), possibly contributing to the risk of these esophageal diseases, may be an indicator for changing human microecology.
Introduction
For the American Clinical and Climatological Association, it is fitting to discuss changing ecology. It is widely known that the “macroecology” of the Earth is changing. One indicator is the gradual “global warming,” a product of the “Greenhouse effect,” due to the combustion of hydrocarbons, releasing carbon dioxide. This is a change that has been occurring over the last century and probably is accelerating. In this paper, I will review evidence that an analogous process is occurring in the human body, with gradual but cumulatively important changes in our “microecology,” that is, our microbial composition. My thesis is that one of our commensal organisms, Helicobacter pylori, is disappearing, which has important consequences for health and for disease. One implication of this hypothesis is that H. pylori is an “indicator” organism for other changes in human microecology that are less easily observed. This review is based on a selection from the research that my colleagues and I have conducted since 1985 (1–45).
HOW OLD IS H. PYLORI?
Helicobacter species are spiral, gram-negative bacteria that colonize the gastrointestinal tract of many mammals. A Helicobacter species has been identified in the stomach of essentially every mammal from which it has been sought (Table 1). In general, each animal has its own Helicobacter species. Stomachs are ecological niches that have been present for an estimated 400 million years, and the great radiation of mammals occurred about 100 to 150 million years ago. As these organisms differentiated, so did their stomachs. The available evidence is most consistent with the hypothesis that Helicobacter species are ancient inhabitants of mammalian stomachs, and that as their niches diversified so did they.
TABLE 1.
Helicobacter species isolated from the stomach in several mammals
| Helicobacter species | Animal |
|---|---|
| H. felis | cat |
| H. bizzozeroni | dog |
| H. “bovis” | cow |
| H. “suis” | pig |
| H. acinonychis | cheetah |
| H. mustelae | ferret |
| H. muridarum | mouse |
H. pylori is the predominant Helicobacter species in humans, and based on the above hypothesis, its ancestors have been with us since we diverged from other mammals in the distant past. Accordingly, when humans traversed the land-bridge across the Bering Straits from Asia to North America, they should have been carrying H. pylori and their descendants today should be carrying H. pylori strains with Asian genotypes. In studies of Amerindians deep in the interior of South America, examining three independent highly polymorphic H. pylori genetic loci, we have found exactly this (1).
These findings provide evidence that H. pylori has been present in humans for at least 11,000 years, the last time the land bridge was open. Further studies indicate that all modern H. pylori strains derive from five ancient populations, possibly arising in Africa, and carried by humans for at least 60,000 years (2). In total, these observations are consistent with a very ancient origin of H. pylori in humans, perhaps since before we became humans.
Consistent with this hypothesis, H. pylori persists in the stomach for years (3), or decades (4), if not for the full life of its host. There is considerable and growing evidence that H. pylori has co-evolved with humans (5,6), and that persistence can be explained by cross-signaling between microbial populations and the host creating a dynamic equilibrium maintained by up- and down-regulatory pathways (5–8).
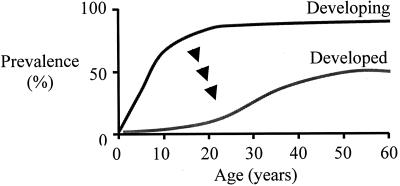
Consistent with its excellent adaptation to humans (6), persons living in developing countries nearly universally develop gastric H. pylori colonization (9). In total, these data suggest that H. pylori are the major constituents of the indigenous (“normal”) biota of the human stomach (10). However, in developed countries such as the U.S., prevalence is much lower, with much diminished acquisition (11) compared to developing countries (9). There now is substantial evidence that as countries are modernizing, H. pylori is disappearing (4) (Figure 1).
Fig. 1.
Prevalence of H. pylori in developed and developing country populations. In developing countries, H. pylori is acquired early in life, and most children become colonized by age 10. By adulthood, colonization is detectable in nearly all adults. In developed countries, prevalence is much lower, reflecting a “birth-cohort” phenomenon of improved “hygiene.” Since all developed countries once were developing countries, these data imply that H. pylori are disappearing, and there is much evidence supporting this hypothesis.
WHY IS H. PYLORI DISAPPEARING?
Humans are the only reservoir for H. pylori, and all transmission must ultimately stem from humans. As socio-economic conditions have improved, H. pylori transmission has diminished. There is less crowding in households, family sizes have diminished, and water supplies are cleaner. Importantly, we now are more than 60 years into the antibiotic era, and in developed countries, children regularly receive multiple courses of antibiotics for various ailments, especially otitis media. If each course of antibiotics eradicated H. pylori in 5–20% of cases, the cumulative effect of childhood antibiotic regimens would remove a substantial proportion of colonizations. Diminished transmission, together with antibiotic treatment, is likely important in the continuing disappearance of H. pylori. Among children in the U.S., fewer than 10% now are acquiring H. pylori; this is an ecologic change of large magnitude. As such, we now can consider the potential costs and benefits of H. pylori colonization. In all biological relationships, the effects of cohabitation on the host can be beneficial (symbiosis), deleterious (parasitism), or mixed, depending on context (amphibiosis).
H. PYLORI AND HOST RESPONSES
To assess whether the presence of H. pylori may lead to disease, one of the first questions is whether there is host recognition of the organism. It now is clear that there are both immunological (3,9) and tissue responses (3,12) to the presence of H. pylori in the stomach. The current evidence indicates that these are the host responses to an indigenous and persistently colonizing microbiota, but pathologists have called the tissue response “chronic gastritis.” In my view, this is analogous to calling the cellular response to the endogenous microbiota in the colon “chronic colitis,” or in the female genital tract “chronic vaginitis.” There are few if any macrophages, lymphocytes, plasma cells, or polymorphonuclear leukocytes in the lamina propria of germ-free animals, but when bacteria are introduced (“conventionalization”) there is infiltration and population of the lamina propria with such cells. Pathologists term this “normal,” and the germ-free state “abnormal.” Based on the long-term relationship of H. pylori with humans, and its much more recent disappearance, I believe that pathologists studying the stomach have reversed their nomenclature, calling the “normal” response chronic gastritis. This becomes a long, and perhaps philosophical discussion, but will become increasingly relevant if evidence is sustained of H. pylori benefit as well as cost. Regardless of what the process is called, there is universal consensus that H. pylori is the major cause for infiltration of the gastric lamina propria with cells of immune and pro-inflammatory phenotypes. Further, it is widely appreciated that this process per se, which is persistently present accompanying H. pylori usually is clinically silent beyond the first weeks of H. pylori acquisition (3).
H. PYLORI AND RISK OF DISEASE
However, it had long been recognized that “chronic gastritis” is a risk factor for the development of the most prevalent form of stomach cancer, the intestinal type of gastric adenocarcinoma. Working with colleagues at the Mayo Clinic, in Japan, and with the Japan-Hawaii study, we provided evidence that the presence of H. pylori was associated with increased risk for gastric adenocarcinoma, especially of the intestinal type (13–16). In prospective-type, nested case-control studies, the longer the interval between ascertainment of H. pylori status and diagnosis of the cancer, the stronger the association (14,16). Long intervals minimize the confounding effect of H. pylori loss during precancerous stages.
Chronic gastritis also had been recognized as a risk factor for peptic ulcer disease. As part of the Japan-Hawaii study, in nested-case-control studies, the presence of H. pylori was associated with the development of both gastric and duodenal ulcers (17,18).
DETERMINANTS OF DIFFERENTIAL RISK OF DISEASE
Although these studies showed that H. pylori presence conferred biological cost (= disease), most H. pylori-positive persons did not become ill. Thus, the next challenge was to begin to understand the factors promoting illness. The mathematical models indicated that the key factors involved the interaction between bacterium and host (5–8). Returning to the Japan-Hawaii study, we provided evidence that early life family structure was a determinant of risk, especially for gastric cancer (19). Such findings suggested that the age of acquisition reflected the early events of the interaction that then established particular patterns in the ensuing decades.
However, our major focus was on variation amongst H. pylori strains. While seeking to define the nature and composition of a cytotoxin produced by H. pylori [which we eventually purified (20), cloned (21), and showed the relation to human disease (22,23)], we found that some but not all H. pylori strains produced a protein of high molecular mass (128–140 kDa) (24). The gene encoding the protein was cloned and called cagA for cytotoxin-associated gene) (25). We found that persons carrying cagA+ strains are at increased risk for peptic ulcer disease (24,18), as well as for atrophic gastritis and intestinal metaplasia (26), precursor lesions for gastric cancer, and for gastric cancer itself (27,16).
From the earliest observations (28), it has become clear that cagA+H. pylori strains inject the CagA protein into gastric epithelial cells via a type IV secretion system. The stability of serum antibodies to the CagA protein over at least 21 years (4) indicates the persistence of this phenomenon. In relation to cagA− strains, those that are cagA+ induce a more marked tissue response (24,26,29,30), higher levels of pro-inflammatory cytokines (31,29), and affect gastric epithelial cell cycle dynamics (32,33). It has become clear that cag+ and cag−H. pylori strains interact with the host in different ways, with important consequences for disease (6,34).
H. PYLORI AND THE ESOPHAGUS
If H. pylori is truly an ancient organism of humans, and if it is disappearing, and there is strong evidence for both, then it could be predicted that there would be consequences for both health and disease (35). Consistent with this view is that gastric cancer rates are declining in all developed countries, and there also is evidence for a decline in (non-iatrogenic) peptic ulcer disease. However, in the last 30 years, there has been a marked rise in the incidence of adenocarcinomas involving the distal esophagus and proximal (cardia) stomach. The major risk factor for these cancers is gastroesophageal reflux disease (GERD), an inflammatory disorder that can lead to metaplasia (Barrett's esophagus), then dysplasia, and then cancer (34). These cancers are increasing in incidence as H. pylori is disappearing (36,37), suggesting that there may be a relationship.
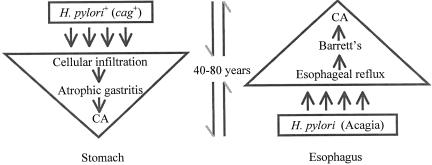
In studies with colleagues in different sites in the United States and Europe, we asked whether the presence of H. pylori was associated with GERD, Barrett's, or adenocarcinomas of the distal esophagus or gastric cardia. Distinguishing between cag+ and cag− strains was the critical element. Our studies showed that carriage of a cag+ strain was inversely associated with GERD (38–40), Barrett's (38–41), and with these adenocarcinomas (42); subsequent studies by other investigators and our on-going studies in New York add support to these findings. Our model of the relationship between H. pylori and adenocarcinomas of the stomach and esophagus is shown as Figure 2. In retrospect, it is clear that the major risks and benefits associated with H. pylori are due to the cag+ strains since these are the most interactive with the host (6,34).
Fig. 2.
Relation of H. pylori to gastric and esophageal adenocarcinomas. H. pylori colonization increases risk for atrophic gastritis and intestinal metaplasia, leading to gastric adenocarcinoma, a process requiring 40–70 years on average. Evidence now indicates that lack of H. pylori, especially cagA+ strains, a process that I have termed “acagia,” increases risk for GERD, then Barrett's esophagus, leading to dysplasia and esophageal adenocarcinoma, a process requiring at least 20 years.
CONCLUSIONS
In total, carriage of H. pylori (especially cag+ strains) confers both cost and potential benefit to humans (43). There may be other benefits of gastric colonization by H. pylori, such as increased resistance to enteric pathogens and diarrheal diseases (44). Such a benefit could have strongly selected for the presence of H. pylori when lethal diarrheal diseases were hyperendemic.
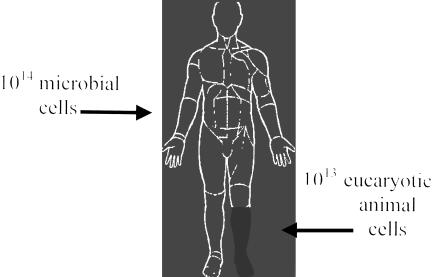
The human body contains myriad microbes (Figure 3). Many of them have not yet been described, and many others cannot be cultivated in vitro. In total, they are a metabolic compartment of the human body influencing our metabolism and physiology. If, like H. pylori, bacteria of the colon, mouth, vagina, or skin began to disappear, we probably could not detect it.
Fig. 3.
Who are we? Microbial cells in the human body outnumber human cells by about 10:1. The types of organisms present in humans show great consistency from person to person, and there is increasing evidence that, as with H. pylori, these are ancient constituents of the human body, and have co-evolved with us. Carriage of other organisms always carries biological cost to the host, but there are circumstances in which benefits may equal or exceed cost (symbiosis). Importantly, co-evolved organisms may be a part of our physiology.
The position of H. pylori in human microecology may not only rely on its strong relationship to inflammatory, endocrine, and oncogenic properties in the stomach and esophagus (6,34). Because of its dominant position in the gastric niche, we can relatively easily track its comings and goings. As such, H. pylori may become the indicator organism for changes in human microecology. We now know that there exists an indigenous microbiota in the human distal esophagus (45), and are eager to study its changes in relation to GERD.
ACKNOWLEDGMENTS
Most of the work described has been published (references 1–45), and all authors are listed. In particular, the author acknowledges that many contributions of Guillermo I. Perez-Perez, Timothy Cover, Richard Peek, John Atherton, Abraham Nomura, and Grant Stemmerman to a long series of studies that address the relationship of H. pylori to human disease.
Supported in part by NIH R01 GM62370, and by the Filomena D'Agostino Foundation.
DISCUSSION
Schiffman, Providence: Marty that was a terrific talk. How does this paradigm relate to the mucosa-associated lymphoid, tissue lymphomas, the MALT tumors, what relationship does H. pylori have and is there a similar genetic molecular biologic pathway?
Blaser, New York: The MALT lymphomas, the B-cell local lymphomas of the stomach are very uncommon conditions. They probably occur around 1 per million population per year. They clearly are associated with the presence of H. pylori, and we now know that if you treat patients who have that condition, their tumors regress. However, one of the questions about this is where is the boundary between true malignancy and a benign hyperproliferative stage. In some ways it's similar to the myoloma and the benign monoclonal gammopathy. I think, in fact, there's been overdiagnosis of what really are benign conditions which respond very well to antibiotics, which is not surprising. For the malignant conditions, it's clear that they are antigen-driven, and you can “drain the gas tank” by treating H. pylori, which definitely affects the natural history. So this is an advance as well, although it's on a much smaller scale.
Toskes, Gainesville: Marty, an excellent presentation. My question relates to the disappearance of the infectivity; certainly happening in Caucasians. But there still seems to be a high rate in minority groups like Afro-americans, hispanics, and orientals. Has anyone looked at them in terms of cag positivity and cag negativity?
Blaser: It turns out that the populations where Helicobacter is most prevalent are also the populations in which cag positivity is also in a higher proportion of those strains. And as you correctly pointed out, H. pylori have not disappeared as rapidly in some of the minority populations in the United States. Interestingly those are the populations that have the highest rates of gastric cancer. They have the highest rates of cag positivity, and their rates of esophageal disease, interestingly, are lagging, which is also part of the dose-response biological coherence in the Bradford Hill proof of causality as well. I think I'll just stop there.
Palmer, New York: I was particularly interested in your comments about the different origins of Helicobacter in connection with the migration of humans, and I was reminded that the people who study mitochondrial DNA have identified seven major groups of humans (European), referred to as the seven daughters of Eve. Is there any correlation between the typing of humans by mitochondrial DNA and their Helicobacter?
Blaser: That's a wonderful question. One of our other studies, which I didn't have time to show you, concerned a study of Amerindians in the Venezuelan Amazon. In this isolated population, by mitochondrial DNA, everyone has Amerindian mitochondrial haplotypes, which are related to the East Asian haplotype. The markers are quite consistent. In another study that we've recently published from Ladakh, also with Marc Achtman and his colleagues, looking at Buddhists who've migrated from Tibet and Muslims who have been indigenous for the last thousand years, it's become clear that whereas mitochondrial DNA can divide humans into the seven major groups, Helicobacter can divide them into a thousand groups. And if mitochondrial DNA is an hour hand of ancestry, Helicobacter is a minute or second hand. It's much more finely tuned.
Giannella, Cincinnati: Marty, from time to time one reads letters to the editors and the like of the claims of H. pylori involvement in non-gastrointestinal diseases. Would you just say a word about that?
Blaser: Since the discovery of H. pylori, finding associations has been a mini-cottage industry. Most of the associations, in my opinion, have not really panned out. The one that's quite intriguing is a recurrent association from Japan of H. pylori and ITP. There have been a number of studies now that have shown that patients who have ITP are usually H. pylori positive. If you treat them with antibiotics, platelet counts go up, and they stay up. I don't have any first-hand experience with these studies, but there's a growing literature about ITP, particularly from Asia.
Stevenson, Stanford: I'm fascinated by your last slide. I have heard some other people speak about the vastness and complexity of the microbial world. It would seem to be the case, that if we think about nature, that if something leaves the microbial scene, something else replaces it. With newer techniques we must be getting better at understanding the microbial world in which we live, and also that lives in us. Are people looking for other kinds of organisms that may, in fact, contribute to chronic diseases, and will we see a replacement like that which you described, should we decide to use antimicrobials against them?!
Blaser: Nature abhors a vacuum. So we're working with David Relman at your institution studying the gastric flora using 16S ribosomal RNA to ask what are the organisms that are present in the H. pylori positive stomach and in the H. pylori negative stomach. As you point out, there are organisms that are populating the stomach in the absence of H. pylori. They are generally lower in number, and appear to be predominantly mouth organisms. My prediction is that the next H. pylori is not going to be as benign as this one that we've had for million of years, and we may start thinking about our Helicobacter replacement therapy, especially those of us who have reflux symptoms. We are also studying the biota of the esophagus, and we've recently described that there is an indigenous biota in the human esophagus as well. We now are beginning to study the relationship in normal esophagus and GERD, with the hypothesis that microbial populations will change in consequence to GERD and possibly contribute to the pathogenesis there as well. I just might add one last thing, which I also didn't have time to mention, and that is that the stomach is an endocrine organ, in addition to producing gastrin gastric and somatostatin, it produces leptin and it produces ghrelin. There now is evidence that H. pylori positive people produce more leptin and less ghrelin than people who don't have H. pylori. This has implications for obesity and diabetes as well.
Stevenson: I'm going to push you to the edge. There's an old belief that there are sterile compartments in the body? Are there any sterile compartments in the body?
Blaser: Yes, it's a very good question. The work on H. pylori has moved me from studying pathogens to studying commensals. And I could easily spend the next ten careers examining commensals of the human body, which could include bacteria, protozoa, viral commensals like the herpes viruses and endogenous retroviruses. Commensalism is a very complex and interesting ecological relationship, appropriate to a society like ours.
REFERENCES
- 1.Ghose C, Perez-Perez GI, Dominguez-Bello MG, Pride DT, Bravi CM, Blaser MJ. East Asian genotypes of Helicobacter pylori: strains in Amerindians provide evidence for its ancient human carriage. Proceedings of the National Academy of Science, USA. 2002;99:15107–15111. doi: 10.1073/pnas.242574599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falush D, Wirth T, Linz B, Pritchard JK, Stephens M, Kidd M, Blaser MJ, Graham DY, Vacher S, Perez-Perez GI, Yamaoka Y, Negraud F, Otto K, Reichard U, Katzowitsch E, Wang X, Achtman M, Suerbaum S. Traces of human migration in Helicobacter pylori populations. Science. 2003;299:1582–1585. doi: 10.1126/science.1080857. [DOI] [PubMed] [Google Scholar]
- 3.Morris AJ, Ali MR, Nicholson GI, Pérez-Pérez GI, Blaser MJ. Long term follow-up of voluntary ingestion of Helicobacter pylori. Ann Intern Med. 1991;114:662–663. doi: 10.7326/0003-4819-114-8-662. [DOI] [PubMed] [Google Scholar]
- 4.Perez-Perez GI, Salomaa A, Kosunen TU, Daverman B, Rautelin H, Aromaa A, Knekt P, Blaser MJ. Evidence that cagA+Helicobacter pylori strains are disappearing more rapidly than cagA− strains. Gut. 2002;50:295–298. doi: 10.1136/gut.50.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaser MJ. Hypotheses on the pathogenesis and natural history of Helicobacter pylori-induced inflammation. Gastroenterology. 1992;102:720–727. doi: 10.1016/0016-5085(92)90126-j. [DOI] [PubMed] [Google Scholar]
- 6.Blaser MJ, Atherton J. Helicobacter pylori persistence: biology and disease. J Clin Invest. 2004;113:321–333. doi: 10.1172/JCI20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirschner DE, Blaser MJ. The dynamics of Helicobacter pylori infection of the human stomach. J Theoret Biol. 1995;176:281–290. doi: 10.1006/jtbi.1995.0198. [DOI] [PubMed] [Google Scholar]
- 8.Blaser MJ, Kirschner D. Dynamics of Helicobacter pylori colonization in relation to the host response. Proc Nat Acad Sci, USA. 1999;96:8359–8364. doi: 10.1073/pnas.96.15.8359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pérez-Pérez GI, Dworkin BM, Chodos JE, Blaser MJ. Campylobacter pylori antibodies in humans. Ann Intern Med. 1988;109:11–17. doi: 10.7326/0003-4819-109-1-11. [DOI] [PubMed] [Google Scholar]
- 10.Blaser MJ. Helicobacters are indigenous to the human stomach: duodenal ulceration is due to changes in gastric microecology in the modern era. Gut. 1998;43:721–727. doi: 10.1136/gut.43.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pérez-Pérez GI, Bodhidatta L, Wongsrichanalai J, Taylor DN, Baze WB, Dunn BE, Echeverria PD, Blaser MJ. Seroprevalence of Helicobacter pylori infections in Thailand. J Infect Dis. 1990;161:1237–1241. doi: 10.1093/infdis/161.6.1237. [DOI] [PubMed] [Google Scholar]
- 12.Dooley CP, Fitzgibbons PL, Cohen H, Appleman MD, Pérez-Pérez GI, Blaser MJ. Prevalence of Helicobacter pylori infection and histologic gastritis in asymptomatic persons. N Engl J Med. 1989;321:1562–1566. doi: 10.1056/NEJM198912073212302. [DOI] [PubMed] [Google Scholar]
- 13.Talley NJ, Zinsmeister AR, DiMagno EP, Weaver A, Carpenter HA, Pérez-Pérez GI, Blaser MJ. Gastric adenocarcinoma and Helicobacter pylori infection. J Nat Cancer Instit. 1991;83:1734–1739. doi: 10.1093/jnci/83.23.1734. [DOI] [PubMed] [Google Scholar]
- 14.Nomura A, Stemmerman GN, Chyou P-H, Kato I, Pérez-Pérez GI, Blaser MJ. Helicobacter pylori infection and gastric carcinoma in a population of Japanese-Americans in Hawaii. N Engl J Med. 1991;325:1132–1136. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- 15.Blaser MJ, Kobayashi K, Cover TL, Cao P, Feurer ID, Pérez-Pérez GI. Helicobacter pylori infection in Japanese patients with adenocarcinoma of the stomach. Int J Cancer. 1993;55:799–802. doi: 10.1002/ijc.2910550518. [DOI] [PubMed] [Google Scholar]
- 16.Nomura AMY, Lee J, Stemmerman G, Nomura RY, Perez-Perez GI, Blaser MJ. Helicobacter pylori cagA seropositivity and gastric carcinoma risk in a Japanese American population. J Infect Dis. 2002;186:1138–1144. doi: 10.1086/343808. [DOI] [PubMed] [Google Scholar]
- 17.Nomura A, Stemmerman GN, Chyou P-H, Pérez-Pérez GI, Blaser MJ. Helicobacter pylori infection and the risk for duodenal and gastric ulceration. Ann Intern Med. 1994;120:977–981. doi: 10.7326/0003-4819-120-12-199406150-00001. [DOI] [PubMed] [Google Scholar]
- 18.Nomura AMY, Perez-Perez GI, Lee J, Stemmerman G, Blaser MJ. Relationship between H. pylori cagA status and risk of peptic ulcer disease. Am J Epidemiol. 2002;155:1054–1059. doi: 10.1093/aje/155.11.1054. [DOI] [PubMed] [Google Scholar]
- 19.Blaser MJ, Chyou PH, Nomura A. Age at establishment of Helicobacter pylori infection and gastric carcinoma, gastric ulcer, and duodenal ulcer risk. Cancer Research. 1995;55:562–565. [PubMed] [Google Scholar]
- 20.Cover TL, Blaser MJ. Purification and characterization of the vacuolating toxin from Helicobacter pylori. J Biol Chem. 1992;267:10570–10575. [PubMed] [Google Scholar]
- 21.Cover TL, Tummuru MKR, Cao P, Thompson SA, Blaser MJ. Divergence of genetic sequences for the vacuolating cytotoxin among Helicobacter pylori strains. J Biol Chem. 1994;269:10566–10573. [PubMed] [Google Scholar]
- 22.Atherton J, Cao P, Peek RM, Tummuru MKR, Blaser MJ, Cover TC. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori: association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995;270:17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- 23.Atherton JC, Peek RM, Tham KT, Cover TL, Blaser MJ. The clinical and pathological importance of heterogeneity in vacA, encoding the vacuolating cytotoxin of Helicobacter pylori. Gastroenterology. 1997;112:92–99. doi: 10.1016/s0016-5085(97)70223-3. [DOI] [PubMed] [Google Scholar]
- 24.Cover TL, Dooley CP, Blaser MJ. Characterization and human serologic response to proteins in Helicobacter pylori broth culture supernatants with vacuolizing cytotoxin activity. Infect Immun. 1990;58:603–610. doi: 10.1128/iai.58.3.603-610.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tummuru MKR, Cover TL, Blaser MJ. Cloning and expression of a high molecular weight major antigen of Helicobacter pylori: Evidence of linkage to cytotoxin production. Infect Immun. 1993;61:1799–1809. doi: 10.1128/iai.61.5.1799-1809.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuipers EJ, Pérez-Pérez GI, Meuwissen SGM, Blaser MJ. Helicobacter pylori and atrophic gastritis: importance of the cagA status. J Nat Cancer Instit. 1995;87:1777–1780. doi: 10.1093/jnci/87.23.1777. [DOI] [PubMed] [Google Scholar]
- 27.Blaser MJ, Pérez-Pérez GI, Kleanthous H, Cover TL, Peek RM, Chyou PH, Stemmermann GN, Nomura A. Infection with Helicobacter pylori strains possessing cagA associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Research. 1995;55:2111–2115. [PubMed] [Google Scholar]
- 28.Tummuru MKR, Sharma SA, Blaser MJ. Helicobacter pylori picB, a homologue of the Bordetella pertussis toxin secretion protein, is required for induction of IL-8 in gastric epithelial cells. Mol Microbiol. 1995;18:867–876. doi: 10.1111/j.1365-2958.1995.18050867.x. [DOI] [PubMed] [Google Scholar]
- 29.Peek RM, Miller GG, Tham KT, Pérez-Pérez GI, Zhao X, Atherton JC, Blaser MJ. Heightened inflammatory response and cytokine expression to cagA+Helicobacter pylori strains. Laboratory Investigation. 1995;73:760–770. [PubMed] [Google Scholar]
- 30.Tham KT, Peek RM, Atherton JC, Cover TL, Perez-Perez GI, Shyr Y, Blaser MJ. Helicobacter pylori genotypes, host factors, and gastric mucosal histopathology in peptic ulcer disease. Human Pathology. 2001;32:264–273. doi: 10.1053/hupa.2001.21136. [DOI] [PubMed] [Google Scholar]
- 31.Sharma SA, Tummuru MKR, Miller GG, Blaser MJ. Interleukin-8 response of gastric epithelial cell lines to Helicobacter pylori stimulation in vitro. Infect Immun. 1995;63:1681–1687. doi: 10.1128/iai.63.5.1681-1687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peek RM, Moss SF, Tham KT, Pérez-Pérez GI, Wang S, Miller GG, Atherton JC, Holt PR, Blaser MJ. Infection with H. pylori cagA+ strains dissociates gastric epithelial cell proliferation from apoptosis. J Nat Cancer Instit. 1997;89:863–868. doi: 10.1093/jnci/89.12.863. [DOI] [PubMed] [Google Scholar]
- 33.Peek RM, Blaser MJ, Bays DJ, Forsyth MH, Cover TL, Song SY, Pietenpol JA. Helicobacter pylori strain-specific genotypes and modulation of the gastric epithelial cell cycle. Cancer Research. 1999;59:6124–6131. [PubMed] [Google Scholar]
- 34.Peek RM, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nature Reviews Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 35.Blaser MJ. Not all Helicobacter pylori strains are created equal: should all be eliminated? Lancet. 1997;349:1020–1022. doi: 10.1016/S0140-6736(96)09133-7. [DOI] [PubMed] [Google Scholar]
- 36.Blaser MJ. The changing relationships of Helicobacter pylori and humans: implications for health and disease. J Infect Dis. 1999;179:1523–1530. doi: 10.1086/314785. [DOI] [PubMed] [Google Scholar]
- 37.Blaser MJ, Saito D. Trends in reported adenocarcinoma of the oesophagus and gastric cardia in Japan. Eur J Gastroenterol Hepatol. 2002;14:107–113. doi: 10.1097/00042737-200202000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Vicari JJ, Peek RM, Falk GW, Goldblum JR, Easley KA, Schnell J, Pérez-Pérez GI, Halter SA, Rice TW, Blaser MJ, Richter JE. The seroprevalence of cagA positive Helicobacter pylori strains in the spectrum of gastroesophageal reflux disease. Gastroenterology. 1998;115:50–57. doi: 10.1016/s0016-5085(98)70364-6. [DOI] [PubMed] [Google Scholar]
- 39.Peek RM, Vaezi MF, Falkow S, Goldblum JR, Pérez-Pérez GI, Richter JE, Blaser MJ. The role of Helicobacter pylori cagA+ strains and specific host immune responses on the development of premalignant and malignant lesions of the gastric cardia. Int J Cancer. 1999;82:520–524. doi: 10.1002/(sici)1097-0215(19990812)82:4<520::aid-ijc9>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 40.Loffeld RJLF, Werdmuller BFM, Kusters JG, Blaser MJ, Pérez-Pérez GI, Kuipers EJ. Colonization with cagA-positive H. pylori strains inversely associated with reflux oesophagitis and Barrett's oesophagitis. Digestion. 2000;62:95–99. doi: 10.1159/000007801. [DOI] [PubMed] [Google Scholar]
- 41.Vaezi MF, Falk GW, Peek RM, Vicari JJ, Goldblum JR, Perez-Perez GI, Rice TW, Blaser MJ, Richter JE. cagA-positive strains of Helicobacter pylori may protect against Barrett's esophagus. Am J Gastroenterol. 2000;95:2206–2211. doi: 10.1111/j.1572-0241.2000.02305.x. [DOI] [PubMed] [Google Scholar]
- 42.Chow W-H, Blaser MJ, Blot WJ, Gammon MD, Vaughan TL, Risch HA, Pérez-Pérez GI, Schoenberg JB, Stanford JL, Rotterdam H, West AB, Fraumeni JF. An inverse relation between cagA+ strains of Helicobacter pylori infection and risk of esophageal and gastric cardia adenocarcinoma. Cancer Research. 1998;58:588–590. [PubMed] [Google Scholar]
- 43.Blaser MJ. In a world of black and white, Helicobacter pylori is gray. Ann Intern Med. 1999;130:695–697. doi: 10.7326/0003-4819-130-8-199904200-00019. [DOI] [PubMed] [Google Scholar]
- 44.Rothenbacher D, Blaser MJ, Bode G, Brenner H. An inverse relationship between gastric colonization by Helicobacter pylori and diarrheal illnesses in children: results of a population-based cross-sectional study. J Infect Dis. 2000;182:1446–1449. doi: 10.1086/315887. [DOI] [PubMed] [Google Scholar]
- 45.Pei Z, Bini EJ, Yang L, Zhou M, Francois F, Blaser MJ. Bacterial biota in the human distal esophagus. Proc Nat Acad Sci USA. 2004;101:4250–4255. doi: 10.1073/pnas.0306398101. [DOI] [PMC free article] [PubMed] [Google Scholar]