Abstract
Tuberculosis (TB) continues as a major public health challenge worldwide. HIV-TB coinfection is especially concerning as it accelerates progression of infection to active disease and amplifies spread of TB including drug resistant disease. Application of molecular biology and insights from classic microbiology to TB control have resulted in important innovations in diagnosis and treatment.
Radiometric assay and, particularly, PCR, with nucleic acid probing, have reduced the time to diagnosis. Moreover, the sensitivity of these techniques is potentially log orders of magnitude more sensitive. Molecular techniques can be adapted to drug susceptibility testing.
The differential activity and post-antibiotic effect of various drugs against TB have led to highly effective briefer regimens and to directly observed therapy. Insights into basic host defense against TB and description of the M. tuberculosis genome have created optimism for developing new treatments and effective vaccines in the years to come.
TUBERCULOSIS
Introduction
Despite being treatable and, indeed, preventable, tuberculosis (TB) continues to be a major public health challenge in many parts of the world. Moreover, the global burden of TB is growing as reflected by increases in new cases and per capita incidence rates of 1.8 percent per year and 0.4 percent per year, respectively, between 1997 and 2000. (1) The facilitation of TB by HIV coinfection is now an important factor in TB worldwide. While the situation is much better in the United States and other areas of the industrialized world, many of these nations now “import” a substantial proportion of their TB cases given immigration patterns from so-called high burden countries. Containing and eliminating TB will require taking creative approaches in the clinical, scientific, and political sectors on a global basis.
Epidemiology of Tuberculosis
Worldwide, TB is second only to HIV/AIDS as a cause of death from infectious disease. There are an estimated eight to nine million new TB cases annually and an estimated two million deaths each year attributable to TB (1,2). It has been estimated that TB ranks seventh among all illnesses as a cause of disability adjusted life years (DALYs) lost, an estimate of disease morbidity, and it is projected that ranking is unlikely to change through the early part of the twenty-first century (3). This mirrors the increasing incidence of TB noted above. Tuberculosis is unevenly distributed throughout the world with 22 so-called “high burden” countries accounting for about 80 percent of all new cases; just five countries (Bangladesh, China, India, Indonesia and Pakistan) have fully half the global burden of the disease (4). Because most new cases occur in adults aged 15–49 years (3), TB has a tremendous economic impact on these countries by removing many individuals from the workforce during the most productive period of their lives. Case numbers appear to be increasing most rapidly in the former Soviet Union and in sub-Saharan Africa (4). In many of these same areas, rates of multidrug resistance (i.e., resistance to at least isoniazid and rifampin) among new TB cases are now in double digits (5,6). World-wide, the rate of multidrug resistant TB (MDR-TB) in the year 2000 was estimated at about 3.1 percent or more than a quarter of a million cases (7).
A critically important factor in the epidemiology of TB worldwide is HIV/AIDS. Because of its adverse effect on the immune system, HIV infection facilitates acquisition of tuberculosis infection and co-infection with HIV is the most powerful risk factor associated with progression of latent TB infection (LTBI) to active tuberculosis (8). In effect, HIV serves to catalyze the acquisition and progression of TB and has been shown to be an important factor in the spread of MDR-TB. Worldwide, approximately 9 percent of new TB cases in 2000 were attributable to HIV. However, this varies greatly between regions and in sub-Saharan Africa, for example, some 31 percent of case were HIV related (1).
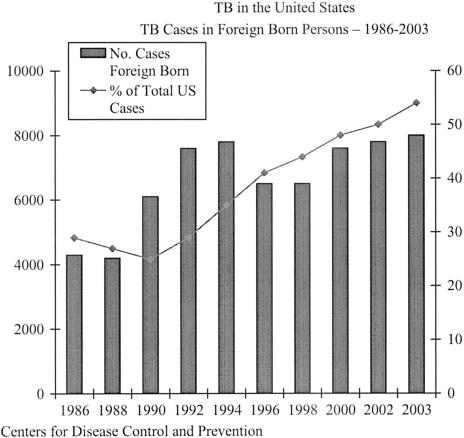
The situation in the United States and most developed nations is such that rates of TB have been declining for the past decade (9). Although total annual cases are now less than 15,000 and case rates have declined 25 percent since 1998 to 5.1 per 100,000 population (10), major challenges remain. More than a quarter of TB cases in the United States appear attributable to HIV infection (1) underscoring the importance of both TB and HIV/AIDS treatment programs. For some years the proportion of TB cases occurring in immigrants to the United States has been increasing (Figure 1). The United States now has over half of its cases occurring in foreign-born immigrants, often within the first several years after their arrival in the United States. Cases rates in 2003 of 2.7 and 23.4/100,000 in United States born and foreign-born persons respectively, reflect this phenomenon. Because drug resistance is common in some parts of the world, the potential for difficult to treat resistant disease is increased. Among U.S. born cases, the majority have traditionally been understood to arise from the activation of remotely acquired latent TB infections (LTBI). Our long-standing concept of TB held that once infected with TB an individual was vulnerable to progression or reactivation of that infection but relatively resistant to an acquiring new exogenous reinfection. Persons previously treated and cured of TB or treated for LTBI were, therefore, thought to be at low risk for new infection. Application of the tools of molecular epidemiology, however, has shown that this may not be the case, at least not to the extent previously thought (11). This has important implications for public health programs aimed at TB control. Finally, TB in the United States is very much effected by adverse social conditions, poverty and inadequate infection control practices (12,13). These situations have contributed to transmission of TB including MDR-TB, inadequate or unsustained treatment programs, and inadequate application of prevention measures. Because TB is now infrequently seen by many healthcare providers in the United States, they often do not consider it in a timely fashion with important and potentially adverse effects for patients and their contacts.
Fig. 1.
The horizontal axis lists years. The left vertical axis enumerates number of foreign born cases and the right vertical axis the percent of U.S. tuberculosis cases in a given year that occurred in foreign born immigrants to the U.S.
The U.S. Public Health Service has officially committed itself to a policy of tuberculosis elimination (defined as a case rate of < 1 case/million population). The Institute of Medicine estimates that even with acceleration of the decline in case rates from the current 7.5 percent/year to an annual rate of decline of 20 percent by 2013, it will still take until 2035 to achieve the goal (14). Moreover, achieving this goal will likely require development of new tools for tuberculosis control and a global perspective on TB containment. Table 1 summarizes the general approach advocated by the IOM Committee.
TABLE 1.
Approaches to Tuberculosis Elimination
| ● Maintain control of TB by adapting to a declining incidence of disease and changing systems of health care financing and management. |
| ● Develop tools needed for TB elimination, including new diagnostic tools, new treatments and an effective vaccine. |
| ● Speed the decline of TB through increased efforts in targeted tuberculin skin testing and treatment of latent infection. |
| ● Increase U.S. engagement in global TB elimination efforts. |
| ● Mobilize support for elimination and measure progress toward that goal. |
Approaches to Diagnosis
Diagnosis of TB must be thought of as having two aspects, diagnosis of TB infection and diagnosis of active disease. Diagnosis of infection typically occurs in the setting of latent infection with the intent of preventing progression to active TB. However, evidence of infection by M. tuberculosis may be pursued in the course of evaluation of suspected active TB. Molecular biology and other technical advances are having a major impact in this area, at least in resource rich developed countries.
Diagnosis of Infection
Traditionally, this has been accomplished by tuberculin skin testing (TST) with purified protein derivative (PPD). This approach dates back almost to the time of Koch's discovery of the tubercle bacillus. Infection by M. tuberculosis of an immunocompetent host results in skin test sensitization over a period of about 8–12 weeks following infection. Application of an intradermal dose of tuberculin—purified protein derivative (PPD) illicits an immune response which can be measured as induration of the skin 48–72 hours after injection. Variation of the threshold size of induration required for a positive test is used to increase the test's sensitivity (small reaction) and specificity (larger area of induration) for detecting LTBI. Although the test is cheap, safe and easy to use it has limitations (15). Tuberculin testing requires that the test subject return for reading, it is nonspecific in that it does not clearly distinguish between reactions from infection by virulent M. tuberculosis or sensitization by more benign nontuberculous mycobacteria or vaccination with Bacillus Calmette Guerin (BCG), and it does not define the likelihood of progression from latent infection to active disease. Of greatest concern, skin tests may be falsely nonreactive in the setting of overwhelming disease or immune compromise. For these and other reasons, attempts have been made to develop new tests. Recently, assays that utilize the observation that the immune response to M. tuberculosis is a Th-1 type response characterized by release of interferon gamma (IFN-γ) have been used. The QuantiFERON test has probably been most widely applied. It involves measurement of whole-blood release of IFN-γ after overnight incubation with a variety of antigens including PPD-tuberculin. The test is currently recommended for use in certain subgroups at risk for LTBI but not persons suspected to have active tuberculosis (17). Anecdotal reports suggest it may compliment TST as a method for identifying tuberculosis infection in persons who are immune compromised or otherwise anergic. The application of more specific antigens such as ESAT-6 and CFP-10 proteins coded by a portion of the M. tuberculosis genome not shared by most other mycobacteria may enhance the specificity of IFH-γ release tests in the future (18,19).
Diagnosis of Active Disease
Diagnosis of active tuberculosis has long been problematic because of the diseases' often nonspecific and protean manifestations and the slow growth of the organism making its isolation a protracted process. Perhaps no aspect of clinical tuberculosis has progressed more in recent years than diagnosis of disease.
Imaging of the chest has been substantially augmented by the application of CT scanning. An array of CT patterns (Table II) have now been correlated with stages of pulmonary tuberculosis ranging from the so-called tree-in-bud presentation of early disease to the fibrotic/bronchiectatic pattern of late stage tuberculosis scarring. These patterns may be useful in predicting the activity of pulmonary tuberculosis (20). Extrapulmonary tuberculosis has long presented a diagnostic dilemma. Specimens are often not readily available or available only through somewhat invasive means. Moreover, extrapulmonary disease often involves lower concentrations of tubercle bacilli, so-called paucibacillary disease, rendering many culture techniques less sensitive and slower in providing a definitive diagnosis. Adenosine deaminase (ADA), an enzyme produced by activated T-lymphocytes, appears to be elevated in many of these sites. Measurement of ADA levels has been most extensively studied in the setting of pleural tuberculosis and appears to be a potentially helpful adjunct to conventional diagnostic tests (21). In that setting sensitivity and specificity of ADA for the diagnosis of tuberculosis have been from 85 to 100 percent and 80 to 97 percent respectively. Efforts at detecting antibodies against a variety of mycobacterial products or at detecting specific mycobacterial antigens have shown varying results and this approach remains experimental at this time.
TABLE 2.
Chest CT Patterns Associated with Tuberculosis
| Pattern | Early Active (Pre-Therapy) | Active (On Therapy) | Late/Inactive (Post Therapy) |
|---|---|---|---|
| Tree-in-bud | ++ | + | − |
| Centrilobular nodule or branching structure | +++ | + | − |
| Lobar consolidation | ++ | − | − |
| Cavitation | ++ | + | − |
| Thickened bronchus | ++ | ++ | ++ |
| Bronchiectasis | − | ++ | ++ |
| Bronchovascular distortion and fibrotic bands | + | ++ | +++ |
+ = relative frequency.
− = generally does not occur.
Conventional agar-based tuberculosis cultures, while highly specific and useful for drug testing susceptibility, can take weeks to perform and has somewhat limited sensitivity. The application of new technologies to tuberculosis has provided an array of innovative tests that are more rapid and at least as sensitive and specific as conventional culture techniques. Radiometric assays, such as BACTEC (Becton Dickinson, Franklin Lake, N.J.), which utilize a radioisotope labeled nutrient in a liquid broth medium which is incorporated into growing mycobacteria, have reduced the time needed to identify the presence of mycobacteria to two weeks or less for many clinical specimens. Techniques for rapid speciation and for drug susceptibility testing of major drugs using these systems have provided an important new tool for diagnosis and managing TB. Radiometric assays have now been adapted to nonisotopic broth-based systems that can be more easily used by many laboratories (22). These systems are now being combined with nucleic acid probes. When used with DNA probes for highly conserved regions of the M. tuberculosis genome, most sputum AFB smear positive specimens (i.e., high concentration of organism) produce a confirmation of the organism within 10 days to 2 weeks with smear negative specimens (i.e., lower concentration of organisms) taking about one additional week. Perhaps the most exciting development with respect to identifying tubercle bacilli involves the combined application of nucleic acid amplification (NAA) techniques and genetic probes for diagnosis. These tests are far more rapid than existing systems with excellent sensitivity/specificity profiles. There are currently two Food and Drug Administration NAA tests approved for commercial use in the U.S., the Mycobacterium Direct Test/MTD (GenProbe, San Diego, California) and Amplicor (Roche, Branchburg, New Jersey). The former uses an isothermal approach to DNA amplification and is approved for use with AFB smear-negative and positive specimens. The latter uses the polymerase chain reaction (PCR) to amplify nucleic acid sequences specific to M. tuberculosis and is currently approved for use with only smear positive specimens. Both systems seem to perform with a similar sensitivity/specificity profile of about 83/97 percent (23) which is far superior to acid fast staining and which approaches the 85/99 percent characteristics long associated with conventional culture. The NAA systems are, however, much faster than conventional culture providing results within several days. The NAA test systems do have problems with naturally occurring inhibitors and, because of their high sensitivity, are more easily contaminated leading to falsely positive results. The NAA systems are also more expensive on a per test basis than conventional culture systems, particularly in laboratories processing a low volume of specimens. The CDC has provided guidance on the use of NAA tests which emphasizes their combined use with AFB staining to enhance the predictive value of results obtained with either of these rapid tests alone (24). Moreover knowledge of common alterations in the M. tuberculosis genome associated with anti-tuberculosis drug resistance is leading to the development of probes for commonly encountered genetic patterns predictive of resistance to key drugs.
Despite much progress in this area, there are still major issues to resolve. For example, many new diagnostic approaches are expensive and/or technically difficult limiting their applicability. In addition, much of our approach to such important issues as infectiousness and response to therapy is based on well studied methods such as smear and conventional culture. Finally, although NAA holds the promise of extremely sensitivity for TB diagnosis, its main contributions to date has been speed with acceptable specificity. Additional technical adaptations and experience may address these issues.
Therapy of Tuberculosis
Treatment of Active Disease
Although drugs that are effective in treating TB have been available for over half a century, the impact of such treatment, particularly in the developing world, has been far less than might have been expected. Indeed, the annual number of TB cases and deaths worldwide has changed little since the advent of the chemotherapy era. Several factors contribute to this situation including the biology of the tubercle bacillus, the emergence of drug resistant organisms, and the problems associated with HIV coinfection. Social and economic factors also limit adequate treatment in many areas.
Actively growing tubercle bacilli have a relatively long generation time compared to many bacterial pathogens. Moreover, the organism is an effective intracellular parasite, in part facilitated by its ability to utilize fatty acids as a source of energy and carbon. This is distinct from metabolic pathways involved with in vitro growth and possibly growth during early infection. Utilization of fatty acid metabolism may permit tubercle bacilli to mitigate the effect of host immune defenses such as IFN-γ (25). The net effect of these biologic realities is that effective treatment of tuberculosis has been recognized as requiring a protracted course so as to eliminate three critical subpopulations of organisms: a relatively large, extracellular population of actively multiplying organisms; a smaller, intermittently active extracellular population; a small relatively inactive population of intracellular parasites. The first of these is thought to account for infectiousness and the possibility of drug resistance and the latter two for the recurrence and ultimate failure of treatment regimens that are not sustained for sufficient time to permit eradication of the organism (26). Because antituberculosis drugs have typically worked as metabolic “poisons” and because drugs are not equally effective against these diverse populations of organisms, eradication was traditionally slow and ineffective requiring many months to produce a cure. This, in turn, created many opportunities for nonadherence to treatment, side effects, and emergence of drug resistance. More recently, the concept of combining drugs to target these subpopulations in the most efficient manner has been used to create more effective regimens which can produce cures in relatively short periods of time, so-called short-course chemotherapy (26).
The emergence of drug resistance is directly related to the number of organisms present in a focus of tuberculous disease. Drug resistant strains occur randomly and predictably in nature. Although the mathematical probability of random resistance to a single drug is such that it will likely be encountered in a previously untreated patient, especially when many tubercle bacilli are present, the likelihood of spontaneous resistance to two or more drugs is very low. For that reason, multiple antituberculosis drugs are used to prevent selection of resistant strains. However, because of the difficulties involved in correctly prescribing, taking, and sustaining regimens requiring multiple medications per dose, drug resistant and even multidrug resistant (MDR) strains are sometimes selected out and can then be transmitted to others in a community. In this way, resistant TB has become a major problem worldwide with some regions reporting prevalence of MDR-TB in excess of 20 percent. The major implication of this is the potential need for using salvage regimens that are more protracted, more toxic and more expensive than first-line regimens and which may ultimately be less effective than the preferred regimens (28). Moreover, when prevalence of resistance is high, transmission of resistant organisms is high.
Coinfection with HIV presents a particular challenge with a respect to TB treatment. Fortunately, available antituberculosis drugs appear quite effective even in the presence of HIV infection (29). On the other hand, the occurrence of TB in an HIV infected person may accelerate multiplication of the HIV virus and has been reported to be associated with a shorter survival time (30,31); treatment of TB appears to reverse this effect on HIV replication (31). For its part, HIV coinfection, by producing immune compromise, can accelerate tuberculosis disease, increase the rate of more aggressive or occult forms of disease such as miliary and meningeal tuberculosis, and increase the rate of side effects from antituberculosis treatment. Thus, well-coordinated programs to treat both diseases will be essential to control TB (32) but also for optimal HIV management.
The past several decades have seen major innovations in TB treatment that have resulted directly from the microbiologic insights noted above. By the 1960s TB treatment, when applied to susceptible organisms was effective in approximately 95 percent of patients. However, regimens required 18–24 months of daily medication and were associated with a high rate of toxicity and side effects. Building on the seminal observations of Mitchison (33) and McDermott (34) and aided by the introduction of rifampin in 1966, a series of innovative clinical trials were pursued that revolutionized TB therapy. By using multidrug regimens that contained agents with particular activity against one or another of the three populations of tubercle bacilli, investigators were able to enhance the efficacy of treatment. This increased efficiency permitted a substantial reduction in the duration of treatment. In a landmark clinical trial conducted by the British Medical Research Council, patients with limited pulmonary tuberculosis were assigned to and received either 6 or 12 months of treatment and those with the extensive disease received 9 months or 18 months of treatment. All therapy included daily isoniazid (INH) and rifampin supplemented by either streptomycin or ethambutol during the first two months of treatment. The results showed that the 9-month regimen for extensive disease produced results equivalent to the 18-month regimen and was superior to either of the briefer regimens used for more limited disease (35). Nine months of treatment, known as “short course” chemotherapy, was soon adopted as a standard regimen. Subsequent studies showed that the addition of streptomycin or ethambutol was not necessary in the regimen. However, when pyrazinamide, an agent known to be especially active against intracellular tubercle bacilli (34) was added to INH and rifampin during the initial two months of therapy, treatment efficiency was enhanced permitting a further decrease in duration of therapy from nine to six months (36). Because it was known that there was post antibiotic effect (i.e., persistent suppression) of antituberculosis agents on tubercle bacilli, investigators next attempted to give short course regimens on an intermittent rather than a daily basis using standard doses of INH and increased dosages of rifampin and pyrazinamide. The fact that there was little if any decrease in efficacy with these regimens established intermittent treatment as a reasonable option (37). Such twice-weekly therapy facilitated the establishment of directly administered and observed regimens. The implication of such an approach, which has come to be termed DOTS (Directly Observed Therapy—short course) was impressively demonstrated by Weiss and colleagues. By replacing a, then standard, daily self-administered INH-rifampin based regimen with a DOTS regimen using the same drugs, they were able to reduce treatment failures and relapse following treatment by about 75 percent. Perhaps most remarkable, within several years of instituting the DOTS program, there was a seven-fold reduction in secondary drug resistance (i.e., drug resistance in patients previously treated for TB and presenting with relapse) and primary resistance in the community was halved (38) presumably because of reduced transmission of resistant tubercle bacilli in the community. The use of DOTS, though initially somewhat more expensive than self-administered therapy, was shown to be cost effective when improved outcomes including reduced rates of TB relapses are factored in (39). For these reasons, twice-weekly DOTS using core regimens of INH and rifampin given for six months and supplemented for the first two months with pyrazinamide, has been strongly recommended for most patients with sensitive organisms in the United States (40). A similar approach is also being pursued in many other countries based on strong endorsements from the World Health Organization (WHO) and other expert bodies.
Potential New Therapies
Because treatment for six months is still difficult to sustain and because of concerns relating to the increasing prevalence of drug resistance world-wide, there is considerable interest in finding new therapeutic agents for TB. Although there has been much interest in several antibiotic classes, including the quinolones and oxazolidinones (linezolid), none of these agents is likely to represent a major breakthrough in TB treatment. Their greatest usefulness may be in treating select patients intolerant of standard therapy or resistant to other drugs. Immune modulating agents may hold greater promise. Preliminary studies using IFH-γ, interleukin-2 (IL-2) and various tumor necrosis factor (TNF) antagonists as treatment adjuncts have been conducted and are encouraging.
Prevention and Treatment of Latent TB Infection
There have been two general approaches to preventing active TB, identifying and treating latent TB infection (LTBI) and vaccination with BCG to prevent infection. The former has been pursued most extensively in the United States and the latter in many other parts of the world.
Targeted tuberculin skin testing and treatment of LTBI reduce the risk of active TB by up to 90 percent. Such treatment has traditionally utilized INH. Although effective and relatively inexpensive, to gain maximum benefit it must be taken for about nine months. Side effects and toxicity from INH can be serious and increase in frequency with advancing age. Recent expert panels have provided guidelines for provision of treatment of LTBI so as to maximize benefit relative to risk (15). In essence, groups with the greatest risk of developing active disease if infected with TB are targeted for skin testing and, if found to be infected, for treatment. Moreover, the threshold for diagnosing infection, as defined by the size of the tuberculin skin test reaction, is adjusted up or down thereby modulating the sensitivity of the skin test (Table 3). A number of treatment options are available for both daily or intermittent use (15).
TABLE 3.
Criteria for Tuberculin Positivity by Risk Group
| Reaction ≥ 5 mm in Duration | Reaction ≥ 10 mm in Duration | Reaction ≥ 15 mm in Duration |
|---|---|---|
| ● HIV-seropositive persons | ● Recent immigrant high prevalence countries | ● Persons with no TB risk factor |
| ● Recent contacts TB case | ● Injection drug users | |
| ● Fibrotic changes on chest radiograph consistent with prior untreated TB | ● Residents/employees of high risk congregate settings (e.g., nursing homes, jails, etc.) | |
| ● Patients with organ transplants and other immunosuppressed persons | ● Mycobacteriology laboratory personnel | |
| ● Persons with high risk (for TB) Clinical conditions: silicosis, diabetes mellitus, chronic renal failure, leukemia/lymphoma, other select malignancies, weight loss ≥10% ideal body weight, gastrectomy/ileal bypass | ||
| ● Children younger than four years or all children/adolescents exposed to adults at high risk |
Modified from MMWR 2000; 49 (No. RR-6).
Because of the difficulty of sustaining and monitoring a protracted period of preventive treatment and because recent studies suggest that acquisition of multiple TB infections over time is possible, the application of a vaccine is extremely attractive particularly in areas with a high rates of TB transmission due to TB prevalence (41). The most widely used TB vaccine, BCG, has been extensively studied and has shown widely varying efficacy ranging from zero to about 80 percent. Indeed, the only consistent benefit that has been demonstrated is a reduction in the most severe forms of childhood tuberculosis (42). Moreover, given the continuing high prevalence of TB in countries where BCG has been widely used, it is difficult to argue that it has been of any practical value in preventing tuberculosis. Finally, because BCG uses live attenuated mycobacteria, it cannot be used in HIV infected and other immune compromised persons.
Challenges and Opportunities for the Future
Tuberculosis control in the United States continues to slowly improve and major ongoing challenges will likely revolve around dealing with subgroups of patients with psychosocial and economic impediments to treatment, drug resistance and complicating comorbid conditions, particularly HIV/AIDS. Because more than half of the new cases of TB identified each year in the United States now occur in immigrants to this country, it is essential that we closely monitor and assist in worldwide TB control efforts. The situation on a global scale is far more precarious. The WHO has set a target of detecting 70 percent of estimated infectious cases and curing 85 percent of these by 2005. While the expansion of DOTS to many parts of the world and improved access to drugs makes it appear that the latter target is achievable, case detection is lagging and was estimated at only 32 percent in 2001 (43). In addition, there is reason to believe that in many communities and countries, TB and HIV treatment programs are not well coordinated or have inadequate resources contributing to continuing propagation of both infections (44,45). Finally, socioeconomic and political forces continue to play a major role in TB control and in health generally around the world (46). Many believe that ultimately worldwide tuberculosis control and elimination will only be possible when a truly effective vaccine (preventive and/or therapeutic) becomes available. The possibility of such a vaccine, while still years in the future, appears better than in years (47).
DISCUSSION
Duma, Daytona Beach: Jeff, I enjoyed that overview and updating very much. In the southeast part of the country we've seen a large number of patients with pulmonary atypical mucobacteriosis. Many of those patients have been confused (and still are) with tuberculosis. As far as the practicing clinician is concerned, the PCR and direct studies of DNA of sputum and/or cultures have helped enormously in recognizing those people rather quickly. But some of them still carry a label of tuberculosis. This is a very tough problem in this country, a developed country as compared to underdeveloped countries. I wonder what your thoughts might be in this area. These people with atypical mycobactoriz often require prolonged therapy, a year or year and half of very complicated courses, very complicated regimens, very toxic regimens; and we're not doing much nor too well with these groups. They are often, of course the immunocompetent, I'm not talking about immune deficient individuals.
Glassroth, Madison: Nontuberculous mycobacteria, the so called “atypical” mycobacteria, are a problem in the US, and becoming more appreciated elsewhere in the world as well. Advances in microbiologic techniques developed for TB have clearly been helpful with nontuberculous organisms as well. For example, some of the same gene probe technigues have been adapted for M. avium. Likewise, some therapeutic developments for TB could also have implications for these organisms. I think some of the new knowledge and developments particularly with agents such as interferon, I think hold the greatest promise for all these organisms. Interferon-gamma appears to play an important role in containing mycobacteria, and defects in interferon-gamma receptor or interferon generation seem to be central not only to MTB but to nontuberculous mycobacteria as well. I'm hopeful that we will see adjunctive therapies that use this type of therapy whether it's aerosolized or, as Bill Martin described in animals yesterday, instilled in macrophage carriers perhaps to try to deal at least with the sickest and most refractory of these patients; we are seeing more and more of them, and they are hard to treat.
Duma: These individuals really haven't been studied in-depth, especially those elderly females for example who have no underline lung disease and non-smokers. We cannot identify any particular immune deficiency, but I did participate in a study using inhalation interferon, and it really wasn't successful in helping these individuals, but of course maybe that needs to be studied in another way and in more depth.
Glassroth: In fact it has been studied. There's a recent paper in the Journal of Infectious Disease that actually looks at a group of immune competent individuals with nontuberculous disease and actually shows defects in interferon production. So it may be the way we administer the interferon, it may be the amount of interferon we give them, there may be subtle defects in interferon gamma receptor, which is an even tougher problem. A lot of this “story” seems to be funneling down into that pathway, and I'm sure we'll hear much more about it.
DuPont, Houston: Is BCG gone, is it of no value anywhere currently?
Glassroth: BCG is not gone, and its main value seems to be political in nature. And I don't mean that facetiously or cynically. Even though most studies, the largest one being of an elegant trial done in South India about ten years ago, showed no benefit other than perhaps to reduce the frequency of the most aggressive forms of TB. All this aside, BCG is still used because it's a very visible way of demonstrating that national health authorities are aggressive about a disease that's of great concern to the population. So it's politically expedient if you will. Most people though agree that it's not going to be the answer to TB and we're going to need much more effective vaccines using more protective, more immunogenic epitopes as the antigenic component of the vaccine. Ironically though, many feel that the ultimate answer to this disease will be a vaccine but a more effective one than BCG.
Southwick, Gainesville: You alluded to the issue of interferon, and the question I have is – is the interferon low before the disease develops? Or does the disease cause a reduction in interferon production?
Glassroth: It's a good question, and we don't know the answer. We know that some people who get mycobacterial disease and it's probably been best studied in patients with nontuberculous disease, but it seems to occur in patients with MTB as well – actually have defects in their interferon gamma receptor, and those are durable whether you treat them or simply follow the disease. So in that small sub-group there seems to be a problem in responding to interferon. Other patients seem, as I showed you, seem to have disease and make plenty of interferon and in fact they have a phenotype that has fewer cavities, smaller number of organisms; in a sense they seem to have more contained, less aggressive disease if you will. And then we have people with the most aggressive disease and they seem to have low levels of interferon as they are treated, they get better and their levels of interferon-gamma rise. Now there's still a chicken and egg kind of question in there as you implied, but the preponderance of the information seems to imply that it is not something acquired from the infection, but it is some innate aspect of the individual's host response that produces less interferon and predisposes to more aggressive disease.
Wing, Providence: Jeff, that was a very nice talk. I wonder if you'd comment on the effect of the HIV epidemic which is devastated many areas of the world, particularly sub-Saharan Africa, and overwhelmed the medical systems in those areas, so that tuberculosis has taken a back seat to the devastation of HIV. And I wonder if you would comment on the interaction of the two.
Glassroth: Thanks for the compliment. It is a huge public health challenge. Some would say a public health calamity in those parts of the world. I wouldn't say though that TB has taken a back seat. I think the World Health Organization, certainly the Gates Foundation and other groups like that, have recognized the intimate linkage between TB and HIV, and so there is a lot of money going into linked or coordinated TB/HIV projects. Some countries have actually done a terrific job with their TB/HIV programs to great advantage, and are actually containing both conditions. The problem, as I've said, is often social, political, economic – getting drugs to people. Both of these conditions are, in effect, chronic diseases. Both of them require some type of sustained medical treatment. The therapies are not cheap. And I think as much as anything that's where the problem lies. And of course, you've got governmental issues in terms of organization. I have done work in Ethiopia. They have over 100,000 cases a year. We have about 15,000 cases in the US. In Atlanta we have several hundred people dedicated to TB. In Ethiopia they have a handful of people in their central program, and some of them split their time between several different activities. And it's not unique just to TB in Ethiopia, it's HIV and it's many other diseases in many other countries.
Wolf, Boston: I just wanted to tell a story about how people can make a difference. Jim Kim and Paul Farmer and their colleague Partners-in-Health got involved in multi-drug resistant TB in Peru and then in Russia, and the countries refused to treat these people because it was too expensive. Jim Kim figured out that all the drugs, which were very old drugs, were still proprietary and they were not being made as generics. He convinced companies to make these generics and dropped the cost of treating a case of multi-drug resistant TB from $1,500 to $60. So it's now possible to treat these people through that one person's intervention.
Glassroth: Marshall, let me come back with another story. There is now a Global Bank – drug bank, so the price has come down. Some years back, a friend of mine went to Peru on sabbatical with a lot of TB drugs to administer free. The problem there was they couldn't get the drugs to the people because of the distances, the roads, and so forth. So again, I think all of this is important, but I wouldn't underestimate the social and political aspects of this. By the way, during the intervening years the situation has dramatically improved in Peru showing that progress can be made.
REFERENCES
- 1.Corbett EL, Watt CJ, Walker N, et al. The growing burden of tuberculosis. Global trends and interactions with the HIV epidemic. Arch Int Med. 2003;163:1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 2.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. JAMA. 1999;282:677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 3.Murray CJL, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global burden of disease study. Lancet. 1997;349:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Global tuberculosis control: surveillance, planning, financing. WHO Report 2003 (WHO/CDC/TB/2003. 316) Geneva: WHO; 2003. [Google Scholar]
- 5.World Health Organization. Anti-tuberculosis drug resistance in the world. Report No. 2. Prevalence and trends. (WHO/CDC/TB/2000. 278) Geneva: WHO; 2000. [Google Scholar]
- 6.Pablo-Mendez A, Raviglione MC, Laszlo A, et al. Global surveillance for anti-tuberculosis drug resistance, 1994–1997. World Health Organization-International Union Against Tuberculosis and Lung Disease Working Group on Antituberculosis Drug Resistance Surveillance. N Eng J Med. 1998;338:1641–1649. doi: 10.1056/NEJM199806043382301. [DOI] [PubMed] [Google Scholar]
- 7.Dye C, Espinal MA, Watt CJ, et al. Worldwide incidence of multidrug-resistant tuberculosis. J Infect Dis. 2002;185:1197–1202. doi: 10.1086/339818. [DOI] [PubMed] [Google Scholar]
- 8.Selwyn PA, Hartell D, Lewis VA, et al. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N Engl J Med. 1989;320:545–550. doi: 10.1056/NEJM198903023200901. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Trends in tuberculosis morbidity-United States, 1992–2003. MWWR Morbid Mortal Wkly Rep. 2003;52:217–220. [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Trends in tuberculosis-United States, 1998–2003. MMWR Morbid Mortal Wkly Rep. 2004;53:209–214. [PubMed] [Google Scholar]
- 11.Barnes PF, Cave MO. Molecular epidemiology of tuberculosis. N Engl J Med. 2003;349:1149–1156. doi: 10.1056/NEJMra021964. [DOI] [PubMed] [Google Scholar]
- 12.Brudney K, Dobkin J. Resurgent tuberculosis in New York City. Human immunodeficiency virus, homelessness, and the decline of tuberculosis control programs. Am Rev Respir Dis. 1991;144:745–749. doi: 10.1164/ajrccm/144.4.745. [DOI] [PubMed] [Google Scholar]
- 13.Gandy M, Zumla A. The resurgence of a disease: Social and historical perspectives on the “new” tuberculosis. Soc Sci Med. 2002;55:385–396. doi: 10.1016/s0277-9536(01)00176-9. [DOI] [PubMed] [Google Scholar]
- 14.Geiter L, editor. Institute of Medicine. Ending neglect. The elimination of tuberculosis in the United States. Washington, D.C.: National Academy Press; 2004. [PubMed] [Google Scholar]
- 15.American Thoracic Society, Centers for Disease Control and Prevention. Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med. 2000;161:S221–S247. doi: 10.1164/ajrccm.161.supplement_3.ats600. [DOI] [PubMed] [Google Scholar]
- 16.Mazurek GH, LoBue PA, Daley CI, et al. Comparison of a whole-blood interferon-gamma assay with tuberculin skin testing for detecting latent Mycobacterium tuberculosis infection. JAMA. 2001;286:1740–1747. doi: 10.1001/jama.286.14.1740. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Guidelines for using the QuantiFERON-TB test for diagnosing latent Mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep. 2003;52:15–18. [PubMed] [Google Scholar]
- 18.Mori T, Sakatani M, Yamagishi F, et al. Specific detection of tuberculosis infection. An interferon-γ-based assay using new antigens. Am J Respir Crit Care Med. 2004;170:59–64. doi: 10.1164/rccm.200402-179OC. [DOI] [PubMed] [Google Scholar]
- 19.Brock I, Weldingh K, Lillebaek T, Follmann F, Andersen P. Comparison of tuberculin skin test and new specific blood test in tuberculosis contacts. Am J Respir Crit Care Med. 2004;170:65–69. doi: 10.1164/rccm.200402-232OC. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y-H, Lin AS, Lai YF, Chao TY, Liu JW, KO SF. The high value of high-resolution computed tomography in predicting the activity of pulmonary tuberculosis. Int J Tuberc Lung Dis. 2003;7:563–568. [PubMed] [Google Scholar]
- 21.Gorguner M, Cerci M, Gorguner I. Determination of adenosine deaminase activity and its isoenzymes for diagnosis of pleural effusions. Respirology. 2000;5:321–324. [PubMed] [Google Scholar]
- 22.Kanchana MV, Cheke D, Natyshak I, et al. Evaluation of the BACTEC MGIT 960 system for the recovery of mycobacteria. Diagn Microbiol Infect Dis. 2000;37:31–36. doi: 10.1016/s0732-8893(99)00151-0. [DOI] [PubMed] [Google Scholar]
- 23.Della-Latta P, Whittier S. Comprehensive evaluation of performance, laboratory application, and clinical usefulness of two different amplification technologies for the detection of mycobacterium tuberculosis complex. Am J Clin Pathol. 1998;110:301–310. doi: 10.1093/ajcp/110.3.301. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease-Control and Prevention. Update: Nucleic acid amplification tests for tuberculosis. MMWR Morb Mortal Wkly Rep. 2000;49:593–594. [PubMed] [Google Scholar]
- 25.McKinney JD, Honer Zu, Bentraup K, Munoz-Elias EJ, et al. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature. 2000;406:735–738. doi: 10.1038/35021074. [DOI] [PubMed] [Google Scholar]
- 26.Grosset J. Bacteriologic basis of short-course chemotherapy of tuberculosis. Clin Chest Med. 1980;1:231–241. [PubMed] [Google Scholar]
- 27.Espinal MA, Laszlo A, Simonsen L, et al. Global trends in resistance to antituberculosis drugs. N Eng J Med. 2001;344:1294–1303. doi: 10.1056/NEJM200104263441706. [DOI] [PubMed] [Google Scholar]
- 28.Chan ED, Laurel V, Strand MJ, et al. Treatment and outcome analysis of 205 patients with multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 2004;169:1103–1109. doi: 10.1164/rccm.200308-1159OC. [DOI] [PubMed] [Google Scholar]
- 29.Murray J, Sonnenberg P, Shearer SC, Godfrey-Faussett P. Human immunodeficiency virus and the outcome for new and recurrent pulmonary tuberculosis. Am J Respir Crit Care Med. 1995;151:129–135. doi: 10.1164/ajrccm.159.3.9804147. [DOI] [PubMed] [Google Scholar]
- 30.Whalen C, Horsburgh CR, Hom D, et al. Accelerated course of human immunodeficiency virus infection after tuberculosis. Am J Respir Crit care Med. 1995;151:129–135. doi: 10.1164/ajrccm.151.1.7812542. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Nakatak , Weiden M, et al. Mycobacterium tuberculosis enhances human immunodeficiency virus-1 replication by transcriptional activation at the long terminal repeat. J Clin Invest. 1995;95:2324–2331. doi: 10.1172/JCI117924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams DG, Dye C. Antiretroviral drugs for tuberculosis control in the era of HIV/AIDS. Science. 2003:1535–1537. doi: 10.1126/science.1086845. [DOI] [PubMed] [Google Scholar]
- 33.Mitchison DA, Dickinson JM. Laboratory aspects of intermittent drug therapy. Postgraduate Med J. 1971;47:737–741. doi: 10.1136/pgmj.47.553.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDermott W, Tomsett R. Activation of pyrazinamide and nicotinamide in acidic environments in vitro. Am Rev Tuberc. 1954;70:748–754. doi: 10.1164/art.1954.70.4.748. [DOI] [PubMed] [Google Scholar]
- 35.British Thoracic and Tuberculosis Association. Short course chemotherapy in tuberculosis. Lancet. 1976;2:1102–1104. [PubMed] [Google Scholar]
- 36.Combs DL, O'Brien RJ, Greiter L. USPHS tuberculosis short course trial 21: Effectiveness, toxicity and acceptability. Ann Int Med. 1990;112:397–406. doi: 10.7326/0003-4819-76-3-112-6-397. [DOI] [PubMed] [Google Scholar]
- 37.Cohn DL, Catlin BJ, Peterson KL, et al. A 62 dose, 6-month therapy for pulmonary and extrapulmonary TB. Ann Int Med. 1990;112:407–415. doi: 10.7326/0003-4819-76-3-112-6-407. [DOI] [PubMed] [Google Scholar]
- 38.Weiss SE, Slocum PC, Blais FX, et al. The effect of directly observed therapy on rates of drug resistance and relapse in TB. N Eng J Med. 1994;330:1179–1184. doi: 10.1056/NEJM199404283301702. [DOI] [PubMed] [Google Scholar]
- 39.Burman WJ, Dalton CB, Cohn DL, et al. A cost effective analysis of DOT vs. self-administered therapy for treatment of TB. Chest. 1997;112:63–70. doi: 10.1378/chest.112.1.63. [DOI] [PubMed] [Google Scholar]
- 40.American Thoracic Society/Centers for Disease Control and Prevention/Infectious Disease Society of America. Statement on treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603–662. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention. Development of new vaccines for tuberculosis: recommendations of the Advisory Council for the Elimination of Tuberculosis (A.C.E.T.) Morb Mortal Wkly Rep. 1998;47(RR-13):1–6. [PubMed] [Google Scholar]
- 42.Statement of the International Union Against Tuberculosis and Lung Disease. Criteria for discontinuation of vaccination programmes using Bacille Calmette-Guerin (BCG) in countries with a low prevalence of tuberculosis. Tuber Lung Dis. 1994;75:179–180. doi: 10.1016/0962-8479(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 43.Véron LJ, Blanc LJ, Suchi M, Raviglione MC. DOTS expansion: will we reach the 2005 targets? Int J Tuberc Lung Dis. 2004;8:139–146. [PubMed] [Google Scholar]
- 44.Girardi E, Antonucci G, Vanacore P, et al. Tuberculosis in HIV-infected persons in the context of wide availability of highly active antiretroviral therapy. Eur Respir J. 2004;24:11–17. doi: 10.1183/09031936.04.00109303. [DOI] [PubMed] [Google Scholar]
- 45.Williams BG, Dyc C. Antiretroviral drugs for tuberculosis control in the era of HIV/AIDS. Science. 2003;301:1535–1537. doi: 10.1126/science.1086845. [DOI] [PubMed] [Google Scholar]
- 46.Benator SR. Respiratory health in a globalizing world. Am J Respir Crit Care Med. 2001;163:1064–1067. doi: 10.1164/ajrccm.163.5.16354. [DOI] [PubMed] [Google Scholar]
- 47.Lewinsohn DM, Lewinsohn DA, Grotzke JE. TB vaccines at the turn of the century: Insights into immunity to M. tuberculosis and modern approaches for prevention of an ancient disease. Sem Respir Infect. 2003;18:320–338. doi: 10.1053/s0882-0546(03)00068-9. [DOI] [PubMed] [Google Scholar]